Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro
Abstract
1. Introduction
2. Results
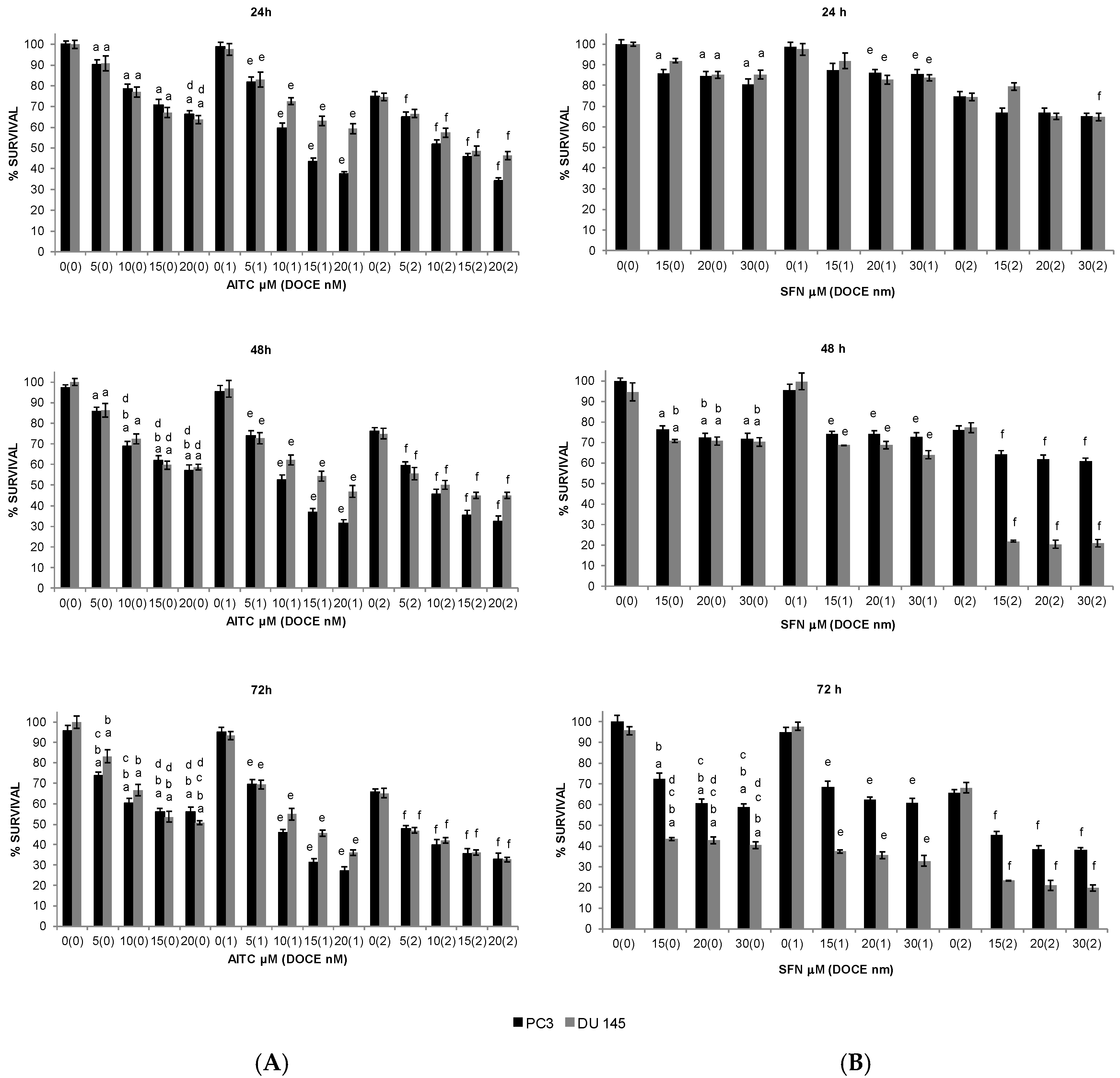
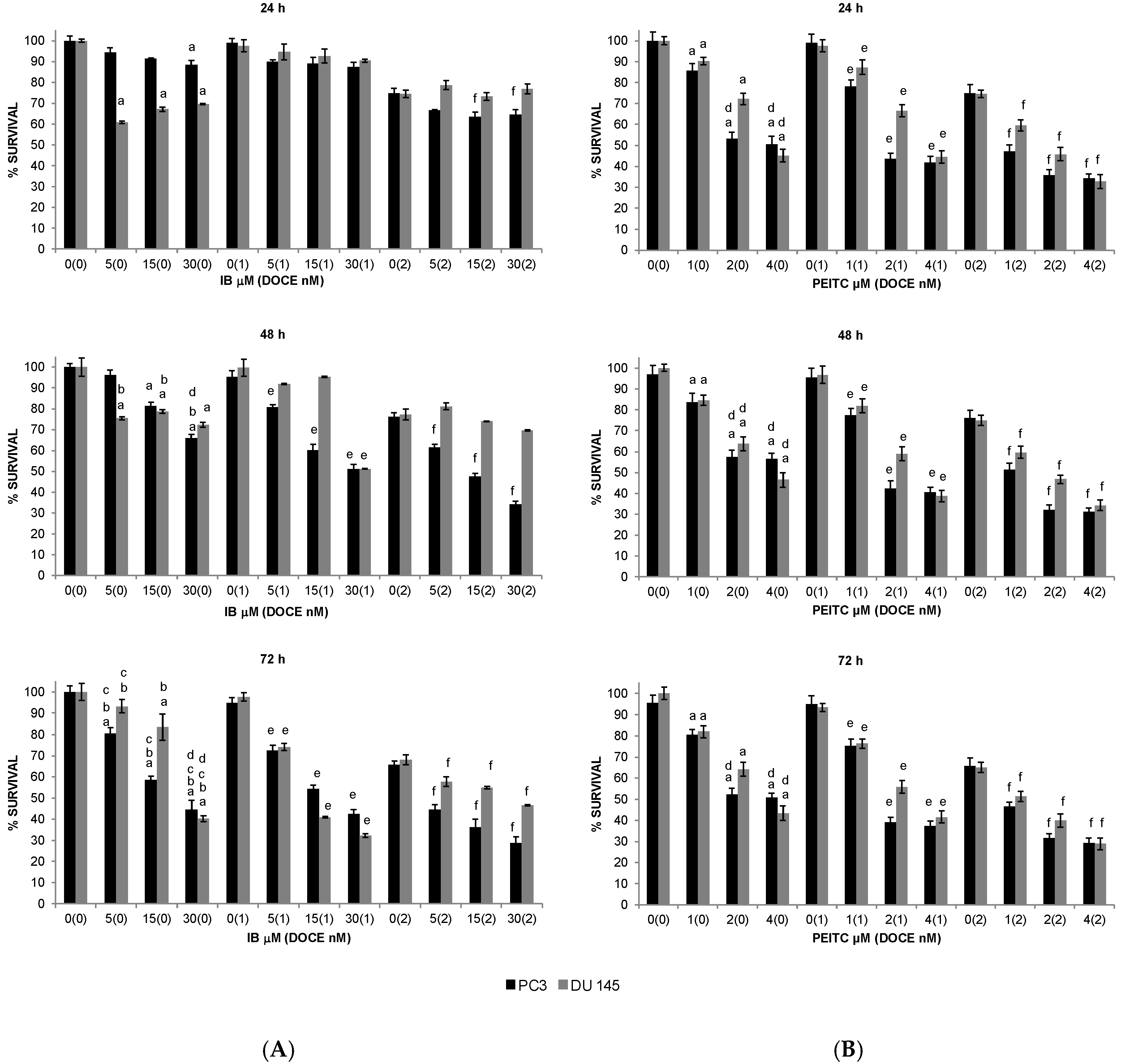
2.1. Inhibition of PC Cells Proliferation by GS Degradation-Products and/or DOCE
2.2. Sensitization of PC Cells to Growth Suppression by DOCE
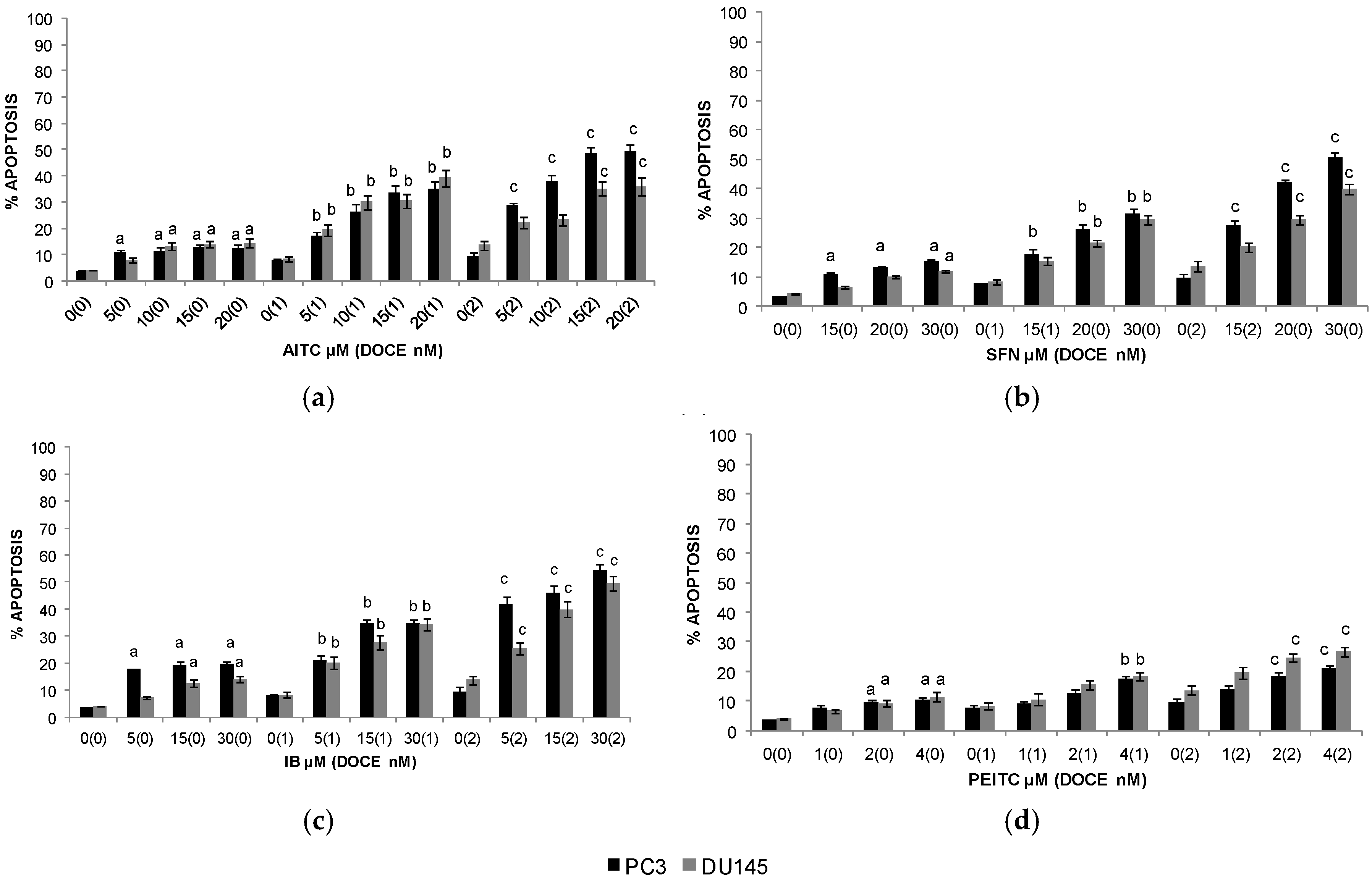
2.3. Apoptosis
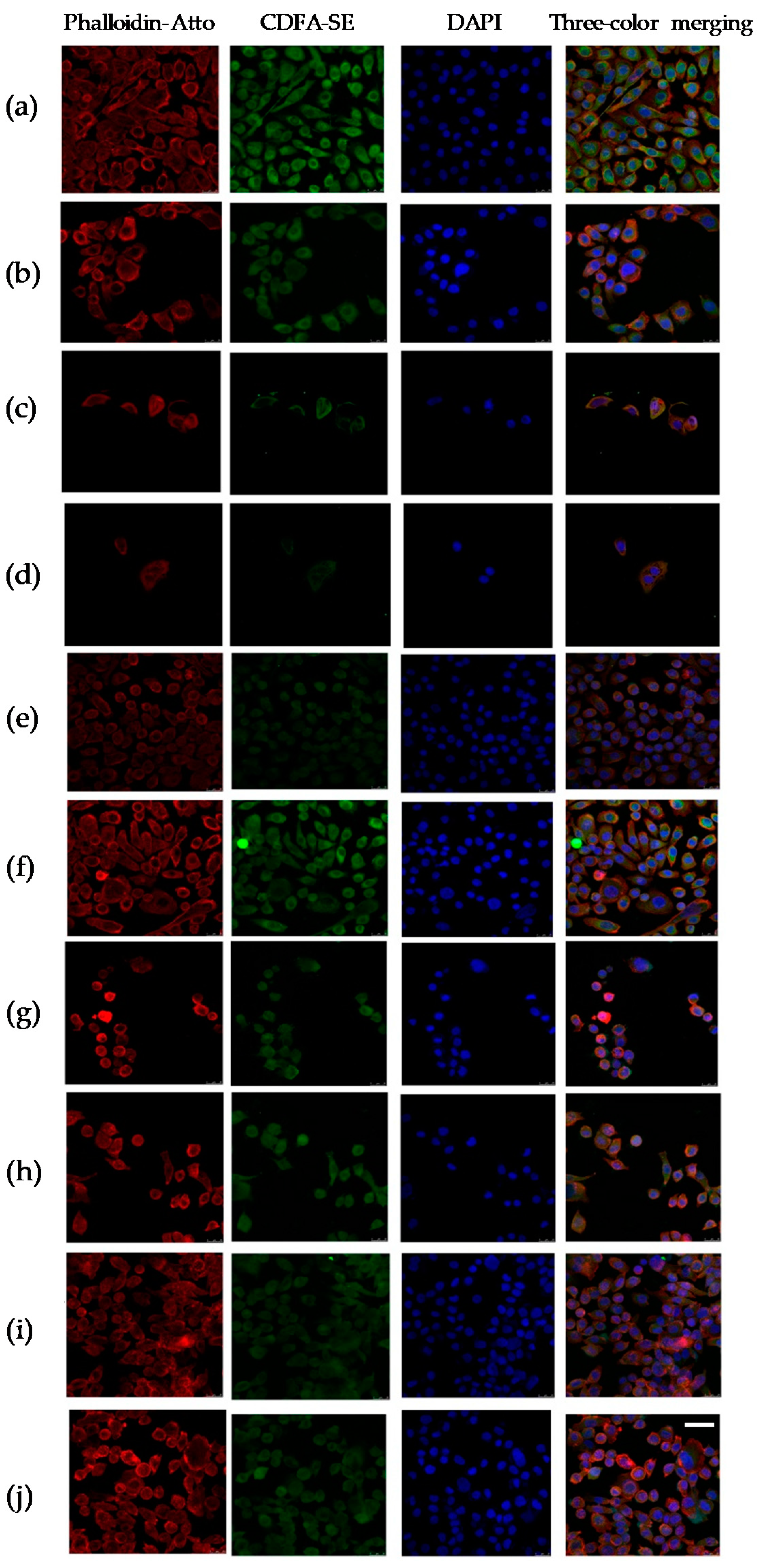
2.4. Cytopathic Changes
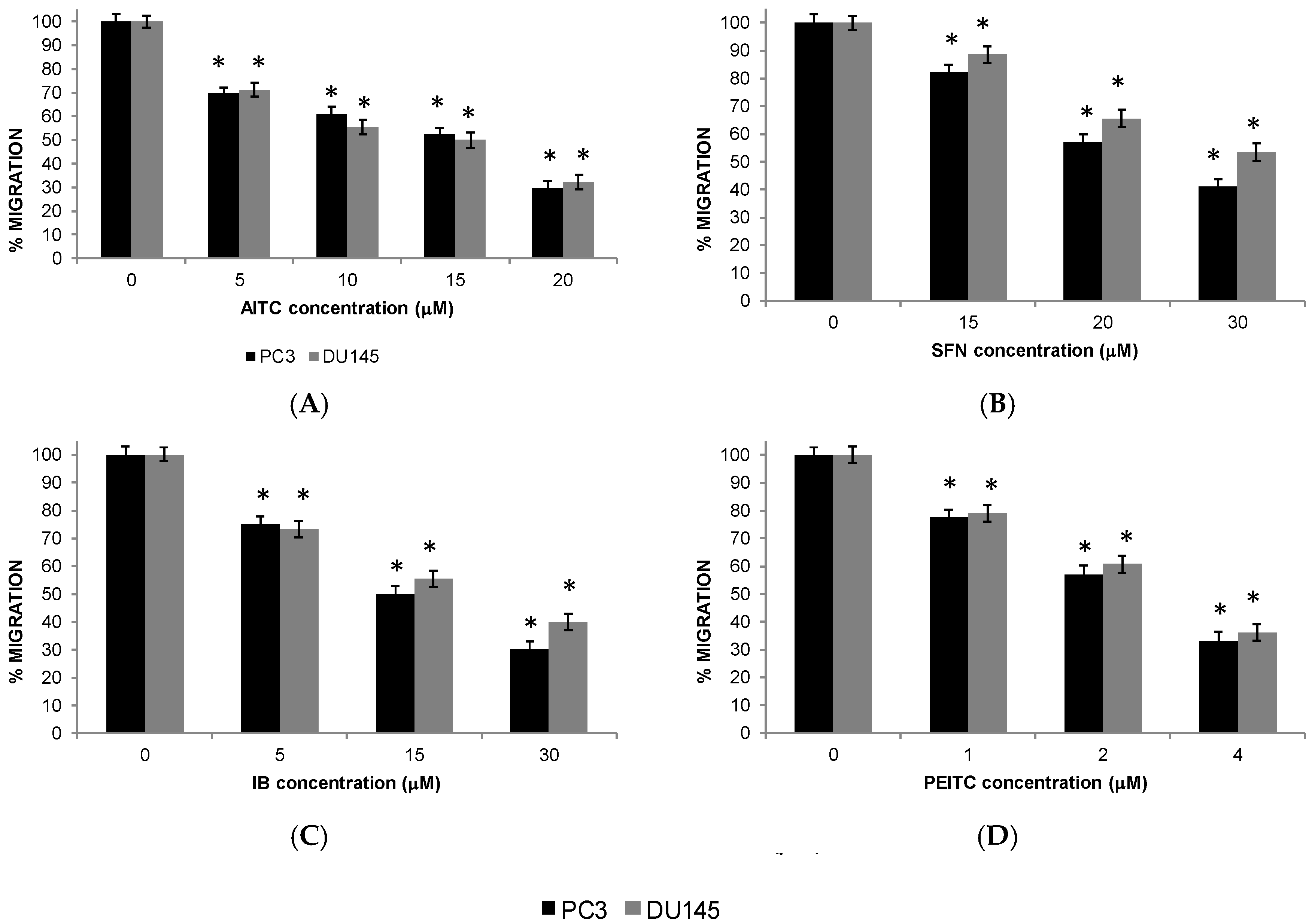
2.5. Migration of PC Cells
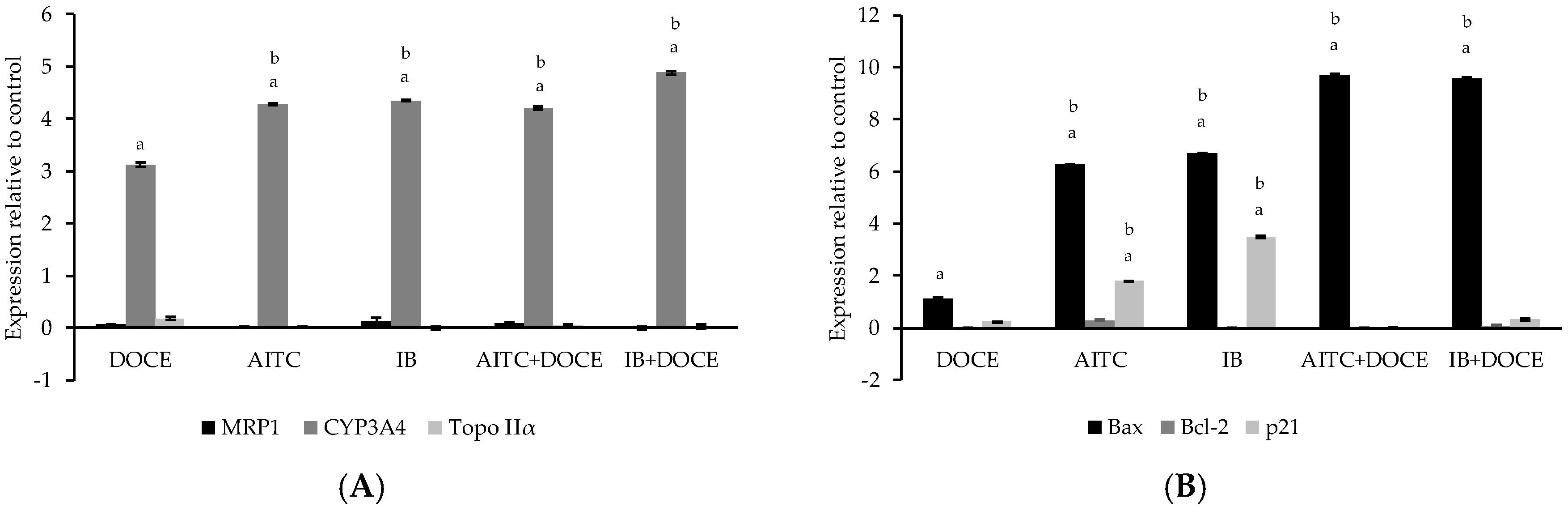
2.6. RT-qPCR
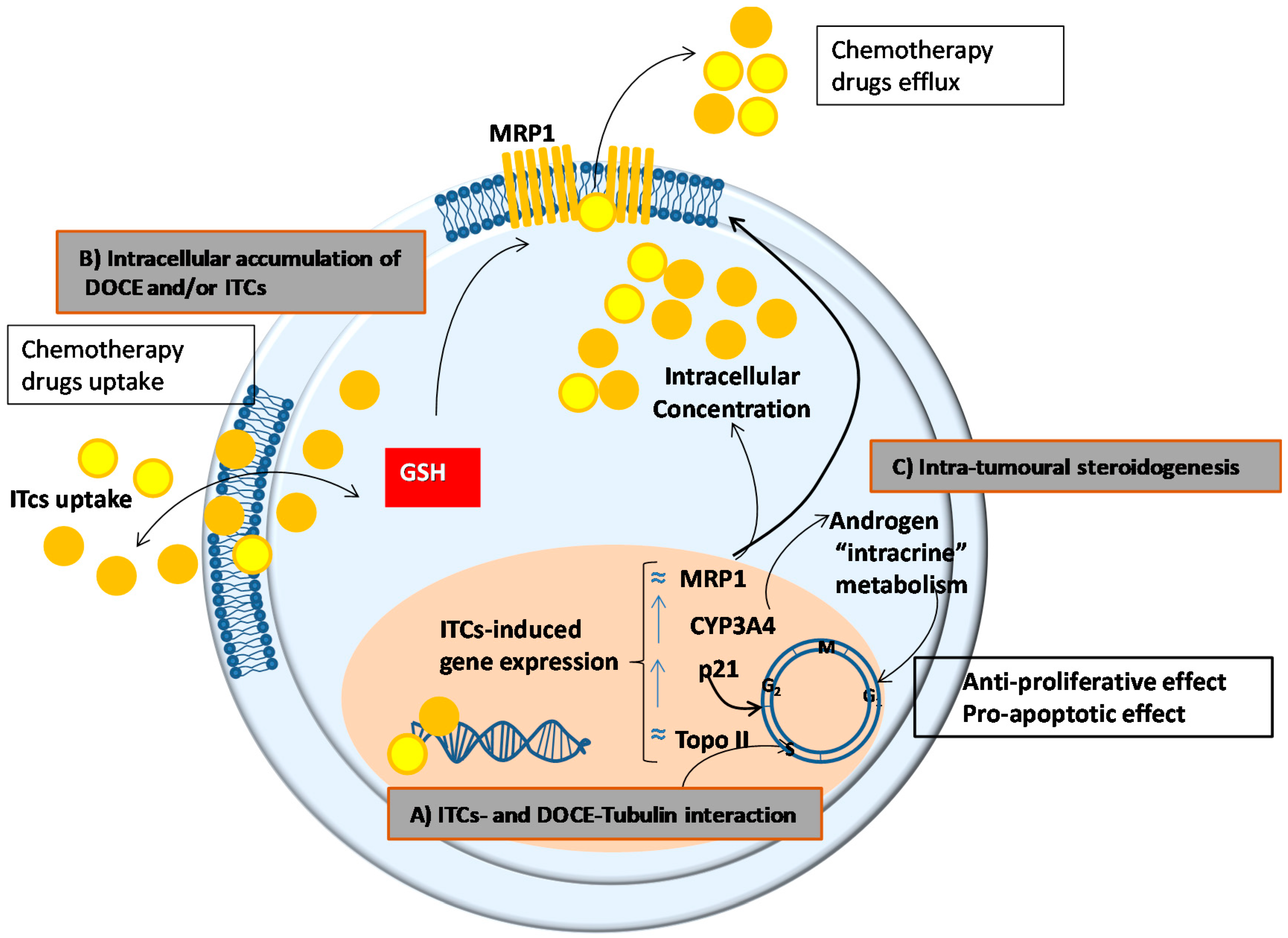
3. Discussion
- (a)
- A common mechanism of action for ITCs and DOCE at the microtubules. DOCE, a known microtubule-targeting agent, binds to microtubules and causes not only their stabilization but also the inhibition of their depolymerization which exerts anti-proliferative and pro-apoptotic effects, respectively [21]. Likewise, some authors claim that the anti-proliferative and pro-apoptotic effects of ITCs are due to the degradation and polymerization of α and β tubulin [22].
- (b)
- A higher intracellular accumulation of DOCE and/or ITCs, since their efficacy depends on their intracellular accumulation which in turn is determined by the balance between uptake and efflux processes. In this sense, and taking into account that ITCs can inhibit cellular export of anticancer agents [23], we studied the expression of the unidirectional efflux transporter MRP1, which plays a role in the development of drug resistance of various types of cancer [24]. However, the expression of MRP1 was not modified by the treatments tested in the present study.
- (c)
- A modulation of the intracrine metabolism of androgens mediated CYP3A4. CYP3A4 increases the bioavailability of growth-promoting androgens to PC cells [25], so it has special relevance in clinical practice. Low levels of expression of CYP3A4 has been associated with poor prognosis in PC patients such as metastasis, high Gleason score, and reduced survival [26]. This mechanism could not explain the anti-proliferative, pro-apoptotic, and anti-migratory effects of AITC or IB on PC-3 cells shown in this study since we used AIPC cells. However, the intra-tumoral steroidogenesis could affect the surrounding tissue and indirectly influence tumour cell behaviour [27]. Concretely, it has been shown that de novo synthesized steroids by cancer cells play an important role in the establishment of metastasis and the induction of castration resistance in PC cells by affecting androgen receptor positive cells in the tumour microenvironment, this is in stroma fibroblasts, smooth muscle cells, endothelial cells, osteoblasts, and inflammatory cells.
- (d)
- The intracellular level/activity of glutathione (GSH). ITC cell uptake and intracellular accumulation are conditioned by the intracellular levels/activity of GSH, since their uptake occurs via binding with cysteine sulfhydryl groups of GSH [18]. Taking into account that high levels/activity of GSH favour the uptake of ITC [18], the higher cytotoxic effect of SFN on PC-3 cells than on DU 145 cells shown in the present study, could be explained by the higher levels of GSH observed in PC-3 in comparison with DU 145 cells [28].
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assay
4.3. Determination of Apoptosis
4.4. Cytophatic Changes
4.5. In Vitro Invasion Assay
4.6. Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4PI | 4-pentenyl-isothiocyanate |
| ADT | Androgen deprivation therapy |
| AIPC | Androgen-insensitive human prostate cancer |
| AITC | Allyl-isothiocyanate |
| CRPC | Castration-resistant prostate cancer |
| DOCE | Docetaxel |
| FBS | Fetal bovine serum |
| GS | Glucosinolates |
| GS-degradation products | Glucosinolates-degradation products |
| GSH | Glutathione |
| I3C | Indole-3-carbinol |
| IB | Iberin |
| ITC | Isothiocyanate |
| PC | Prostate cancer |
| PEC | Prostate epithelial cells |
| PEITC | Phenethyl-isothiocyanate |
| SFN | Sulforaphane |
Appendix A
| Docetaxel | AITC | Combination Treatment | Index 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | MSR 1 | p2,* | Dose | MSR 1 | p2,* | Expected 3 | Observed 4 | p2,♦ | ||
| 24 h | 1 | 0.99 | >0.05 | 5 | 0.9 | <0.05 | 0.89 | 0.82 | >0.05 | 1.09 |
| 1 | 0.99 | >0.05 | 10 | 0.78 | <0.05 | 0.77 | 0.6 | <0.05 | 1.28 | |
| 1 | 0.99 | >0.05 | 15 | 0.71 | <0.05 | 0.7 | 0.44 | <0.05 | 1.59 | |
| 1 | 0.99 | >0.05 | 20 | 0.66 | <0.05 | 0.65 | 0.37 | <0.05 | 1.76 | |
| 2 | 0.75 | <0.05 | 5 | 0.9 | <0.05 | 0.68 | 0.65 | >0.05 | 1.05 | |
| 2 | 0.75 | <0.05 | 10 | 0.78 | <0.05 | 0.59 | 0.52 | >0.05 | 1.13 | |
| 2 | 0.75 | <0.05 | 15 | 0.71 | <0.05 | 0.53 | 0.46 | <0.05 | 1.15 | |
| 2 | 0.75 | <0.05 | 20 | 0.66 | <0.05 | 0.5 | 0.34 | <0.05 | 1.47 | |
| 48 h | 1 | 0.95 | >0.05 | 5 | 0.86 | <0.05 | 0.82 | 0.74 | >0.05 | 1.11 |
| 1 | 0.95 | >0.05 | 10 | 0.69 | <0.05 | 0.66 | 0.53 | <0.05 | 1.25 | |
| 1 | 0.95 | >0.05 | 15 | 0.62 | <0.05 | 0.59 | 0.37 | <0.05 | 1.59 | |
| 1 | 0.95 | >0.05 | 20 | 0.57 | <0.05 | 0.54 | 0.31 | <0.05 | 1.74 | |
| 2 | 0.76 | <0.05 | 5 | 0.86 | <0.05 | 0.65 | 0.59 | >0.05 | 1.1 | |
| 2 | 0.76 | <0.05 | 10 | 0.69 | <0.05 | 0.52 | 0.46 | >0.05 | 1.13 | |
| 2 | 0.76 | <0.05 | 15 | 0.62 | <0.05 | 0.47 | 0.35 | <0.05 | 1.34 | |
| 2 | 0.76 | <0.05 | 20 | 0.57 | <0.05 | 0.43 | 0.32 | <0.05 | 1.34 | |
| 72 h | 1 | 0.95 | >0.05 | 5 | 0.74 | <0.05 | 0.7 | 0.69 | >0.05 | 1.01 |
| 1 | 0.95 | >0.05 | 10 | 0.6 | <0.05 | 0.57 | 0.46 | <0.05 | 1.24 | |
| 1 | 0.95 | >0.05 | 15 | 0.56 | <0.05 | 0.53 | 0.31 | <0.05 | 1.71 | |
| 1 | 0.95 | >0.05 | 20 | 0.56 | <0.05 | 0.53 | 0.27 | <0.05 | 1.96 | |
| 2 | 0.66 | <0.05 | 5 | 0.74 | <0.05 | 0.49 | 0.48 | >0.05 | 1.02 | |
| 2 | 0.66 | <0.05 | 10 | 0.6 | <0.05 | 0.4 | 0.4 | >0.05 | 1 | |
| 2 | 0.66 | <0.05 | 15 | 0.56 | <0.05 | 0.37 | 0.35 | >0.05 | 1.06 | |
| 2 | 0.66 | <0.05 | 20 | 0.56 | <0.05 | 0.37 | 0.33 | >0.05 | 1.12 | |
| Docetaxel | AITC | Combination Treatment | Index 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | MSR 1 | p2,* | Dose | MSR 1 | p2,* | Expected 3 | Observed 4 | p2,♦ | ||
| 24 h | 1 | 0.98 | >0.05 | 5 | 0.91 | <0.05 | 0.89 | 0.83 | >0.05 | 1.07 |
| 1 | 0.98 | >0.05 | 10 | 0.77 | <0.05 | 0.75 | 0.73 | >0.05 | 1.03 | |
| 1 | 0.98 | >0.05 | 15 | 0.67 | <0.05 | 0.66 | 0.63 | >0.05 | 1.05 | |
| 1 | 0.98 | >0.05 | 20 | 0.64 | <0.05 | 0.63 | 0.59 | >0.05 | 1.07 | |
| 2 | 0.75 | <0.05 | 5 | 0.91 | <0.05 | 0.68 | 0.67 | >0.05 | 1.01 | |
| 2 | 0.75 | <0.05 | 10 | 0.77 | <0.05 | 0.58 | 0.57 | >0.05 | 1.02 | |
| 2 | 0.75 | <0.05 | 15 | 0.67 | <0.05 | 0.5 | 0.49 | >0.05 | 1.02 | |
| 2 | 0.75 | <0.05 | 20 | 0.64 | <0.05 | 0.48 | 0.46 | >0.05 | 1.04 | |
| 48 h | 1 | 0.97 | >0.05 | 5 | 0.86 | <0.05 | 0.83 | 0.73 | <0.05 | 1.14 |
| 1 | 0.97 | >0.05 | 10 | 0.72 | <0.05 | 0.7 | 0.62 | <0.05 | 1.13 | |
| 1 | 0.97 | >0.05 | 15 | 0.6 | <0.05 | 0.58 | 0.54 | >0.05 | 1.07 | |
| 1 | 0.97 | >0.05 | 20 | 0.59 | <0.05 | 0.57 | 0.47 | <0.05 | 1.21 | |
| 2 | 0.75 | <0.05 | 5 | 0.86 | <0.05 | 0.65 | 0.56 | <0.05 | 1.16 | |
| 2 | 0.75 | <0.05 | 10 | 0.72 | <0.05 | 0.54 | 0.5 | >0.05 | 1.08 | |
| 2 | 0.75 | <0.05 | 15 | 0.6 | <0.05 | 0.45 | 0.45 | >0.05 | 1 | |
| 2 | 0.75 | <0.05 | 20 | 0.59 | <0.05 | 0.44 | 0.45 | >0.05 | 0.98 | |
| 72 h | 1 | 0.93 | >0.05 | 5 | 0.83 | <0.05 | 0.77 | 0.69 | <0.05 | 1.12 |
| 1 | 0.93 | >0.05 | 10 | 0.67 | <0.05 | 0.62 | 0.55 | <0.05 | 1.13 | |
| 1 | 0.93 | >0.05 | 15 | 0.54 | <0.05 | 0.5 | 0.46 | >0.05 | 1.09 | |
| 1 | 0.93 | >0.05 | 20 | 0.51 | <0.05 | 0.47 | 0.36 | <0.05 | 1.31 | |
| 2 | 0.65 | <0.05 | 5 | 0.83 | <0.05 | 0.54 | 0.47 | <0.05 | 1.15 | |
| 2 | 0.65 | <0.05 | 10 | 0.67 | <0.05 | 0.44 | 0.42 | >0.05 | 1.05 | |
| 2 | 0.65 | <0.05 | 15 | 0.54 | <0.05 | 0.35 | 0.36 | >0.05 | 0.97 | |
| 2 | 0.65 | <0.05 | 20 | 0.51 | <0.05 | 0.33 | 0.33 | >0.05 | 1 | |
| Docetaxel | SFN | Combination Treatment | Index 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | MSR 1 | p2,* | Dose | MSR 1 | p2,* | Expected 3 | Observed 4 | p2,♦ | ||
| 24 h | 1 | 0.99 | >0.05 | 15 | 0.86 | <0.05 | 0.85 | 0.87 | >0.05 | 0.98 |
| 1 | 0.99 | >0.05 | 20 | 0.85 | <0.05 | 0.84 | 0.86 | >0.05 | 0.98 | |
| 1 | 0.99 | >0.05 | 30 | 0.81 | <0.05 | 0.8 | 0.86 | >0.05 | 0.93 | |
| 2 | 0.75 | <0.05 | 15 | 0.86 | <0.05 | 0.65 | 0.67 | >0.05 | 0.97 | |
| 2 | 0.75 | <0.05 | 20 | 0.85 | <0.05 | 0.64 | 0.67 | >0.05 | 0.96 | |
| 2 | 0.75 | <0.05 | 30 | 0.81 | <0.05 | 0.61 | 0.65 | >0.05 | 0.94 | |
| 48 h | 1 | 0.95 | >0.05 | 15 | 0.76 | <0.05 | 0.72 | 0.74 | >0.05 | 0.97 |
| 1 | 0.95 | >0.05 | 20 | 0.72 | <0.05 | 0.68 | 0.74 | >0.05 | 0.92 | |
| 1 | 0.95 | >0.05 | 30 | 0.72 | <0.05 | 0.68 | 0.73 | >0.05 | 0.93 | |
| 2 | 0.76 | <0.05 | 15 | 0.76 | <0.05 | 0.58 | 0.64 | <0.05 | 0.91 | |
| 2 | 0.76 | <0.05 | 20 | 0.72 | <0.05 | 0.55 | 0.62 | <0.05 | 0.89 | |
| 72 h | 1 | 0.95 | >0.05 | 15 | 0.72 | <0.05 | 0.68 | 0.68 | >0.05 | 1 |
| 1 | 0.95 | >0.05 | 20 | 0.61 | <0.05 | 0.58 | 0.62 | >0.05 | 0.94 | |
| 1 | 0.95 | >0.05 | 30 | 0.59 | <0.05 | 0.56 | 0.61 | >0.05 | 0.92 | |
| 2 | 0.66 | <0.05 | 15 | 0.72 | <0.05 | 0.48 | 0.45 | >0.05 | 1.07 | |
| 2 | 0.66 | <0.05 | 20 | 0.61 | <0.05 | 0.4 | 0.39 | >0.05 | 1.03 | |
| 2 | 0.66 | <0.05 | 30 | 0.59 | <0.05 | 0.39 | 0.38 | >0.05 | 1.03 | |
| Docetaxel | SFN | Combination Treatment | Index 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | MSR 1 | p2,* | Dose | MSR 1 | p2,* | Observed 4 | MSR 1 | p2,♦ | ||
| 24 h | 1 | 0.98 | >0.05 | 15 | 0.92 | >0.05 | 0.9 | 0.92 | >0.05 | 0.98 |
| 1 | 0.98 | >0.05 | 20 | 0.85 | <0.05 | 0.83 | 0.83 | >0.05 | 1 | |
| 1 | 0.98 | >0.05 | 30 | 0.85 | <0.05 | 0.83 | 0.84 | >0.05 | 0.99 | |
| 2 | 0.75 | <0.05 | 15 | 0.92 | >0.05 | 0.69 | 0.79 | <0.05 | 0.87 | |
| 2 | 0.75 | <0.05 | 20 | 0.85 | <0.05 | 0.64 | 0.65 | >0.05 | 0.98 | |
| 2 | 0.75 | <0.05 | 30 | 0.85 | <0.05 | 0.64 | 0.65 | >0.05 | 0.98 | |
| 48 h | 1 | 1 | >0.05 | 15 | 0.71 | <0.05 | 0.71 | 0.69 | >0.05 | 1.03 |
| 1 | 1 | >0.05 | 20 | 0.71 | <0.05 | 0.71 | 0.69 | >0.05 | 1.03 | |
| 1 | 1 | >0.05 | 30 | 0.7 | <0.05 | 0.7 | 0.64 | >0.05 | 1.09 | |
| 2 | 0.77 | <0.05 | 15 | 0.71 | <0.05 | 0.55 | 0.22 | <0.05 | 2.5 | |
| 2 | 0.77 | <0.05 | 20 | 0.71 | <0.05 | 0.55 | 0.2 | <0.05 | 2.75 | |
| 72 h | 1 | 0.98 | >0.05 | 15 | 0.43 | <0.05 | 0.42 | 0.37 | >0.05 | 1.14 |
| 1 | 0.98 | >0.05 | 20 | 0.43 | <0.05 | 0.42 | 0.36 | >0.05 | 1.17 | |
| 1 | 0.98 | >0.05 | 30 | 0.4 | <0.05 | 0.39 | 0.33 | >0.05 | 1.18 | |
| 2 | 0.68 | <0.05 | 15 | 0.43 | <0.05 | 0.29 | 0.23 | >0.05 | 1.26 | |
| 2 | 0.68 | <0.05 | 20 | 0.43 | <0.05 | 0.29 | 0.21 | >0.05 | 1.38 | |
| 2 | 0.68 | <0.05 | 30 | 0.4 | <0.05 | 0.27 | 0.2 | >0.05 | 1.35 | |
| Docetaxel | IB | Combination Treatment | Index 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | MSR 1 | p2,* | Dose | MSR 1 | p2,* | Expected 3 | Observed 4 | p2,♦ | ||
| 24 h | 1 | 0.99 | >0.05 | 5 | 0.94 | >0.05 | 0.93 | 0.9 | >0.05 | 1.03 |
| 1 | 0.99 | >0.05 | 15 | 0.91 | >0.05 | 0.9 | 0.89 | >0.05 | 1.01 | |
| 1 | 0.99 | >0.05 | 30 | 0.89 | <0.05 | 0.88 | 0.88 | >0.05 | 1 | |
| 2 | 0.75 | <0.05 | 5 | 0.94 | >0.05 | 0.71 | 0.66 | >0.05 | 1.08 | |
| 2 | 0.75 | <0.05 | 15 | 0.91 | >0.05 | 0.68 | 0.64 | >0.05 | 1.06 | |
| 2 | 0.75 | <0.05 | 30 | 0.89 | <0.05 | 0.67 | 0.65 | >0.05 | 1.03 | |
| 48 h | 1 | 0.95 | >0.05 | 5 | 0.96 | >0.05 | 0.91 | 0.81 | <0.05 | 1.12 |
| 1 | 0.95 | >0.05 | 15 | 0.81 | <0.05 | 0.77 | 0.6 | <0.05 | 1.28 | |
| 1 | 0.95 | >0.05 | 30 | 0.66 | <0.05 | 0.63 | 0.51 | <0.05 | 1.24 | |
| 2 | 0.76 | <0.05 | 5 | 0.96 | >0.05 | 0.73 | 0.62 | <0.05 | 1.18 | |
| 2 | 0.76 | <0.05 | 15 | 0.81 | <0.05 | 0.62 | 0.48 | <0.05 | 1.29 | |
| 2 | 0.76 | <0.05 | 30 | 0.66 | <0.05 | 0.5 | 0.34 | <0.05 | 1.47 | |
| 72 h | 1 | 0.95 | >0.05 | 5 | 0.81 | <0.05 | 0.77 | 0.73 | >0.05 | 1.05 |
| 1 | 0.95 | >0.05 | 15 | 0.58 | <0.05 | 0.55 | 0.54 | >0.05 | 1.02 | |
| 1 | 0.95 | >0.05 | 30 | 0.45 | <0.05 | 0.43 | 0.42 | >0.05 | 1.02 | |
| 2 | 0.66 | <0.05 | 5 | 0.81 | <0.05 | 0.53 | 0.45 | >0.05 | 1.18 | |
| 2 | 0.66 | <0.05 | 15 | 0.58 | <0.05 | 0.38 | 0.36 | >0.05 | 1.06 | |
| 2 | 0.66 | <0.05 | 30 | 0.45 | <0.05 | 0.3 | 0.29 | >0.05 | 1.03 | |
| Docetaxel | IB | Combination Treatment | Index 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | MSR 1 | p2,* | Dose | MSR 1 | p2,* | Expected 3 | Observed 4 | p2,♦ | ||
| 24 h | 1 | 0.98 | >0.05 | 5 | 0.61 | <0.05 | 0.6 | 0.95 | <0.05 | 0.63 |
| 1 | 0.98 | >0.05 | 15 | 0.67 | <0.05 | 0.66 | 0.93 | <0.05 | 0.71 | |
| 1 | 0.98 | >0.05 | 30 | 0.7 | <0.05 | 0.69 | 0.9 | <0.05 | 0.77 | |
| 2 | 0.75 | <0.05 | 5 | 0.61 | <0.05 | 0.46 | 0.79 | <0.05 | 0.58 | |
| 2 | 0.75 | <0.05 | 15 | 0.67 | <0.05 | 0.5 | 0.73 | <0.05 | 0.68 | |
| 2 | 0.75 | <0.05 | 30 | 0.7 | <0.05 | 0.53 | 0.77 | <0.05 | 0.69 | |
| 48 h | 1 | 1 | >0.05 | 5 | 0.75 | <0.05 | 0.75 | 0.92 | <0.05 | 0.82 |
| 1 | 1 | >0.05 | 15 | 0.79 | <0.05 | 0.79 | 0.95 | <0.05 | 0.83 | |
| 1 | 1 | >0.05 | 30 | 0.72 | <0.05 | 0.72 | 0.51 | <0.05 | 1.41 | |
| 2 | 0.77 | <0.05 | 5 | 0.75 | <0.05 | 0.58 | 0.81 | <0.05 | 0.72 | |
| 2 | 0.77 | <0.05 | 15 | 0.79 | <0.05 | 0.61 | 0.74 | <0.05 | 0.82 | |
| 2 | 0.77 | <0.05 | 30 | 0.72 | <0.05 | 0.55 | 0.7 | <0.05 | 0.79 | |
| 72 h | 1 | 0.98 | >0.05 | 5 | 0.93 | >0.05 | 0.91 | 0.74 | <0.05 | 1.23 |
| 1 | 0.98 | >0.05 | 15 | 0.84 | <0.05 | 0.82 | 0.41 | <0.05 | 2 | |
| 1 | 0.98 | >0.05 | 30 | 0.4 | <0.05 | 0.39 | 0.32 | >0.05 | 1.22 | |
| 2 | 0.68 | <0.05 | 5 | 0.93 | >0.05 | 0.63 | 0.58 | >0.05 | 1.09 | |
| 2 | 0.68 | <0.05 | 15 | 0.84 | <0.05 | 0.57 | 0.55 | >0.05 | 1.04 | |
| 2 | 0.68 | <0.05 | 30 | 0.4 | <0.05 | 0.27 | 0.47 | <0.05 | 0.57 | |
| Docetaxel | PEITC | Combination Treatment | Index 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | MSR 1 | p2,* | Dose | MSR 1 | p2,* | Expected 3 | Observed 4 | p2,♦ | ||
| 24 h | 1 | 0.99 | >0.05 | 1 | 0.86 | <0.05 | 0.85 | 0.78 | >0.05 | 1.09 |
| 1 | 0.99 | >0.05 | 2 | 0.53 | <0.05 | 0.52 | 0.44 | <0.05 | 1.18 | |
| 1 | 0.99 | >0.05 | 4 | 0.51 | <0.05 | 0.5 | 0.42 | <0.05 | 1.19 | |
| 2 | 0.75 | <0.05 | 1 | 0.86 | <0.05 | 0.65 | 0.47 | <0.05 | 1.38 | |
| 2 | 0.75 | <0.05 | 2 | 0.53 | <0.05 | 0.4 | 0.36 | >0.05 | 1.11 | |
| 2 | 0.75 | <0.05 | 4 | 0.51 | <0.05 | 0.38 | 0.34 | >0.05 | 1.12 | |
| 48 h | 1 | 0.95 | >0.05 | 1 | 0.84 | <0.05 | 0.8 | 0.77 | >0.05 | 1.04 |
| 1 | 0.95 | >0.05 | 2 | 0.58 | <0.05 | 0.55 | 0.42 | <0.05 | 1.31 | |
| 1 | 0.95 | >0.05 | 4 | 0.56 | <0.05 | 0.53 | 0.41 | <0.05 | 1.29 | |
| 2 | 0.76 | <0.05 | 1 | 0.84 | <0.05 | 0.64 | 0.51 | <0.05 | 1.25 | |
| 2 | 0.76 | <0.05 | 2 | 0.58 | <0.05 | 0.44 | 0.32 | <0.05 | 1.38 | |
| 2 | 0.76 | <0.05 | 4 | 0.56 | <0.05 | 0.43 | 0.31 | <0.05 | 1.39 | |
| 72 h | 1 | 0.95 | >0.05 | 1 | 0.81 | <0.05 | 0.77 | 0.75 | >0.05 | 1.03 |
| 1 | 0.95 | >0.05 | 2 | 0.52 | <0.05 | 0.49 | 0.39 | <0.05 | 1.26 | |
| 1 | 0.95 | >0.05 | 4 | 0.51 | <0.05 | 0.48 | 0.37 | <0.05 | 1.3 | |
| 2 | 0.66 | <0.05 | 1 | 0.81 | <0.05 | 0.53 | 0.47 | <0.05 | 1.13 | |
| 2 | 0.66 | <0.05 | 2 | 0.52 | <0.05 | 0.34 | 0.32 | >0.05 | 1.06 | |
| 2 | 0.66 | <0.05 | 4 | 0.51 | <0.05 | 0.34 | 0.29 | >0.05 | 1.17 | |
| Docetaxel | PEITC | Combination Treatment | Index 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | MSR 1 | p2,* | Dose | MSR 1 | p2,* | Expected 3 | Observed 4 | p2,♦ | ||
| 24 h | 1 | 0.98 | >0.05 | 1 | 0.9 | <0.05 | 0.88 | 0.87 | >0.05 | 1.01 |
| 1 | 0.98 | >0.05 | 2 | 0.72 | <0.05 | 0.71 | 0.67 | >0.05 | 1.06 | |
| 1 | 0.98 | >0.05 | 4 | 0.45 | <0.05 | 0.44 | 0.44 | >0.05 | 1 | |
| 2 | 0.75 | <0.05 | 1 | 0.9 | <0.05 | 0.68 | 0.6 | >0.05 | 1.13 | |
| 2 | 0.75 | <0.05 | 2 | 0.72 | <0.05 | 0.54 | 0.46 | <0.05 | 1.17 | |
| 2 | 0.75 | <0.05 | 4 | 0.45 | <0.05 | 0.34 | 0.33 | >0.05 | 1.03 | |
| 48 h | 1 | 1 | >0.05 | 1 | 0.85 | <0.05 | 0.85 | 0.84 | >0.05 | 1.01 |
| 1 | 1 | >0.05 | 2 | 0.66 | <0.05 | 0.66 | 0.61 | >0.05 | 1.08 | |
| 1 | 1 | >0.05 | 4 | 0.48 | <0.05 | 0.48 | 0.4 | >0.05 | 1.2 | |
| 2 | 0.77 | <0.05 | 1 | 0.85 | <0.05 | 0.65 | 0.61 | >0.05 | 1.07 | |
| 2 | 0.77 | <0.05 | 2 | 0.66 | <0.05 | 0.51 | 0.48 | >0.05 | 1.06 | |
| 2 | 0.77 | <0.05 | 4 | 0.48 | <0.05 | 0.37 | 0.35 | >0.05 | 1.06 | |
| 72 h | 1 | 0.98 | >0.05 | 1 | 0.82 | <0.05 | 0.8 | 0.8 | >0.05 | 1 |
| 1 | 0.98 | >0.05 | 2 | 0.67 | <0.05 | 0.66 | 0.58 | <0.05 | 1.14 | |
| 1 | 0.98 | >0.05 | 4 | 0.45 | <0.05 | 0.44 | 0.44 | >0.05 | 1 | |
| 2 | 0.68 | <0.05 | 1 | 0.82 | <0.05 | 0.56 | 0.54 | >0.05 | 1.04 | |
| 2 | 0.68 | <0.05 | 2 | 0.67 | <0.05 | 0.46 | 0.42 | >0.05 | 1.1 | |
| 2 | 0.68 | <0.05 | 4 | 0.45 | <0.05 | 0.31 | 0.3 | >0.05 | 1.03 | |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.; Sweeney, C.; Antonarakis, E.S.; Armstrong, A.J. The evolving landscape of metastatic hormone-sensitive prostate cancer: A critical review of the evidence for adding docetaxel or abiraterone to androgen deprivation. Prostate Cancer Prostatic Dis. 2018, 21, 306–318. [Google Scholar] [CrossRef]
- Tonyali, S.; Haberal, H.B.; Sogutdelen, E. Toxicity, adverse events, and quality of life associated with the treatment of metastatic castration-resistant prostate cancer. Curr. Urol. 2017, 10, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Novío, S.; Freire-Garabal, M.; Núñez, M.J. Target driven preclinical screening for new antimitotic chemotherapy agents. Curr. Top. Med. Chem. 2014, 14, 2263–2271. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kopeć, A.; Piatkowska, E.; Borczak, B.; Leszczyńska, T. The beneficial effects of Brassica vegetables on human health. Rocz. Panstw. Zakl. Hig. 2012, 63, 389–395. [Google Scholar] [PubMed]
- Abdull, R.; Ahmad, F.; Noor, N.M. Cruciferous vegetables: Dietary phytochemicals for cancer prevention. Asian Pac. J. Cancer Prev. 2013, 14, 1565–1570. [Google Scholar] [CrossRef]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health promoting effects of brassica-derived phytochemicals: From chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxid. Med. Cell Longev. 2013, 2013, 964539:1–964539:12. [Google Scholar] [CrossRef]
- Novio, S.; Cartea, M.E.; Soengas, P.; Freire-Garabal, M.; Nunez-Iglesias, M.J. Effects of Brassicaceae isothiocyanates on prostate cancer. Molecules 2016, 21, 626. [Google Scholar] [CrossRef]
- Xiao, D.; Singh, S.V. Phenethyl isothiocyanate sensitizes androgen-independent human prostate cancer cells to docetaxel-induced apoptosis in vitro and in vivo. Pharm. Res. 2010, 27, 722–731. [Google Scholar] [CrossRef]
- Núñez-Iglesias, M.J.; Novio, S.; García-Santiago, C.; Cartea, M.E.; Soengas, P.; Velasco, P.; Freire-Garabal, M. Effects of 3-butenyl isothiocyanate on phenotypically different prostate cancer cells. Int. J. Oncol. 2018, 53, 2213–2223. [Google Scholar]
- Lee, H.Y.; Oh, S.H.; Suh, Y.A.; Baek, J.H.; Papadimitrakopoulou, V.; Huang, S.; Hong, W.K. Response of non-small cell lung cancer cells to the inhibitors of phosphatidylinositol 3-kinase/Akt- and MAPK kinase 4/c-Jun NH2-terminal kinase pathways: An effective therapeutic strategy for lung cancer. Clin. Cancer Res. 2005, 11, 6065–6074. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, L.; Zieren, R.C.; Xue, W.; de Reijke, T.M.; Pienta, K.J. Metastatic prostate cancer remains incurable, why? Asian J. Urol. 2019, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Srivastava, S.K.; Lew, K.L.; Zeng, Y.; Hershberger, P.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 2003, 24, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.Y.; Huang, Y.T.; Yu, C.S.; Ko, Y.C.; Wu, S.H.; Ji, B.C.; Yang, J.S.; Yang, J.L.; Hsia, T.C.; Chen, Y.Y.; et al. Phenethyl isothiocyanate (PEITC) promotes G2/M phase arrest via p53 expression and induces apoptosis through caspase- and mitochondria-dependent signaling pathways in human prostate cancer DU 145 cells. Anticancer Res. 2011, 31, 1691–1702. [Google Scholar] [PubMed]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Chandra-Kuntal, K.; Desai, D.; Amin, S.; Singh, S.V. Notch activation is dispensable for D, L-sulforaphane-mediated inhibition of human prostate cancer cell migration. PLoS ONE 2012, 7, e44957. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Ganapathy, S.; Srivastava, R.K. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin. Cancer Res. 2008, 14, 6855–6866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis 2012, 33, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, B.; Usluer, S. p21 in cancer research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 2011, 1813, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Gan, N.; Cheema, A.; Dakshanamurthy, S.; Wang, X.; Yang, D.C.; Chung, F.L. Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes. J. Biol. Chem. 2009, 284, 17039–17051. [Google Scholar] [CrossRef] [PubMed]
- Tseng, E.; Kamath, A.; Morris, M.E. Effect of organic isothiocyanates on the P-glycoprotein- and MRP1-mediated transport of daunomycin and vinblastine. Pharm. Res. 2002, 19, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef] [PubMed]
- Maguire, O.; Pollock, C.; Martin, P.; Owen, A.; Smyth, T.; Doherty, D.; Campbell, M.J.; McClean, S.; Thompson, P. Regulation of CYP3A4 and CYP3A5 expression and modulation of “intracrine” metabolism of androgens in prostate cells by liganded vitamin D receptor. Mol. Cell Endocrinol. 2012, 364, 54–64. [Google Scholar] [CrossRef]
- Fujimura, T.; Takahashi, S.; Urano, T.; Kumagai, J.; Murata, T.; Takayama, K.; Ogushi, T.; Horie-Inoue, K.; Ouchi, Y.; Kitamura, T.; et al. Expression of cytochrome P450 3A4 and its clinical significance in human prostate cancer. Urology 2009, 74, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, E.; Bergh, A.; Wikström, P. Clinical relevance of androgen receptor alterations in prostate cancer. Endocr. Connect. 2017, 6, R146–R161. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Kunwar, A.; Sandur, S.K.; Pandey, B.N.; Chaubey, R.C. Differential response of DU 145 and PC3 prostate cancer cells to ionizing radiation: Role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim. Biophys. Acta 2014, 1840, 485–494. [Google Scholar] [CrossRef]
- Xiao, D.; Vogel, V.; Singh, S.V. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol. Cancer Ther. 2006, 5, 2931–2945. [Google Scholar] [CrossRef]
- Hejchman, E.; Taciak, P.; Kowalski, S.; Maciejewska, D.; Czajkowska, A.; Borowska, J.; Śladowski, D.; Młynarczuk-Biały, I. Synthesis and anticancer activity of 7-hydroxycoumarinyl gallates. Pharmacol. Rep. 2015, 67, 236–244. [Google Scholar] [CrossRef]
- Mao, L.; Yang, C.; Wang, J.; Li, W.; Wen, R.; Chen, J.; Zheng, J. SATB1 is overexpressed in metastatic prostate cancer and promotes prostate cancer cell growth and invasion. J. Transl. Med. 2013, 11, 111:1–111:10. [Google Scholar] [CrossRef] [PubMed]
| Common Name | Scientific Name | Main GSs | GSs Type |
|---|---|---|---|
| Broccoli | Brassica oleracea var. italica | Glucoraphanin, Sinigrin, Glucobrassicin | Aliphatic and indolic |
| Cauliflower | B. oleracea var. botrytis | Sinigrin, Glucoraphanin, Glucoiberin, Glucobrassicin | Aliphatic and indolic |
| Brussels sprouts | B. oleracea var. gemmifera | Sinigrin, Progoitrin, Glucoraphanin, Glucoiberin, Glucobrassicin | Aliphatic and indolic |
| Cabbage | B. oleracea var. capitata | Sinigrin, Glucoiberin, Progoitrin, Glucobrassicin | Aliphatic and indolic |
| Kale | B. oleracea var. acephala | Sinigrin, Glucoiberin, Glucobrassicin | Aliphatic and indolic |
| Chinese cabbage | B. oleracea var. pekinensis | Sinigrin, Progoitrin, Glucobrassicin | Aliphatic and indolic |
| Kohlrabi | B. oleracea var. gongylodes | Gluconapin, Glucoerucin, Glucoraphanin, Glucobrassicin | Aliphatic and indolic |
| Turnip | Brassica rapa | Gluconapin, Glucobrassicanapin | Aliphatic |
| Rutabaga | Brassica napus var. napobrassica | Sinigrin, Gluconapin, Progoitrin, Glucoerucin, Gluconasturtiin | Aliphatic and aromatic |
| Nabicol | B. napus var. pabularia | Glucobrassicanapin, Progoitrin | Aliphatic |
| Mustard black | Brassica nigra | Sinigrin, Gluconapin, Gluconasturtiin | Aliphatic |
| Mustard brown | Brassica juncea | Sinigrin, Progoitrin, Gluconapin, Glucobrassicanapin | Aliphatic |
| Mustard white | Sinapis alba | Glucosinalbin | Aliphatic |
| Garden cress | Lepidium sativum | Glucotropaeolin | Aromatic |
| Watercress | Nasturtium officinale | Glucoiberin, Glucobrassicin, Gluconasturtiin | Aliphatic, indolic and aromatic |
| Rocket | Eruca sativa Diplotaxis sp. | Glucoerucin, Glucoraphanin | Aliphatic |
| Radish | Raphanus sativa | Sinigrin, Glucoerucin, Glucotropaeolin, Gluconasturtiin, Glucobrassicin | Aliphatic, indolic and aromatic |
| Horseradish | Armoracia lapathifolia | Sinigrin, Gluconapin, Gluconasturtiin | Aliphatic and aromatic |
| Gen/Gene-Related Mechanism | Primes RT-qPCR (5′–3′) | |
|---|---|---|
| MRP1 Drug-transporter gene | F: TGTGGACGCTCAGAGGTTCA R: CATCGCCATCACAGCATTG | |
| CYP3A4 Drug-metabolism gene Migration gen | F: GGGAAGCAGAGACAGGCAA R: AAGGGGTCTTGTGGATTGTTG | |
| Topo IIα Target genes | F: ATTCAGAGGGGATATGATTCGG R: GGTTAAATACCAAAGGGGCATA | |
| Cell cycle related genes/apoptosis- related genes | Bax | F: AGGATGCGTCCACCAAGAAG R: TGAAGTTGCCGTCAGAAAACA |
| Bcl-2 | F: ATGTGTGTGGAGAGCGTCAACC R: TGAGCAGAGTCTTCAGAGACAGCC | |
| p21 | F: CCCGTGAGCGATGGAACT R: CGAGGCACAAGGGTACAAGA | |
| GAPDH | F: GAAGACTGTGGATGGCCCCTC R: GTTGAGGGCAATGCCAGCCCC | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Iglesias, M.J.; Novío, S.; García, C.; Pérez-Muñuzuri, E.; Soengas, P.; Cartea, E.; Velasco, P.; Freire-Garabal, M. Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro. Int. J. Mol. Sci. 2019, 20, 4977. https://doi.org/10.3390/ijms20204977
Núñez-Iglesias MJ, Novío S, García C, Pérez-Muñuzuri E, Soengas P, Cartea E, Velasco P, Freire-Garabal M. Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro. International Journal of Molecular Sciences. 2019; 20(20):4977. https://doi.org/10.3390/ijms20204977
Chicago/Turabian StyleNúñez-Iglesias, María Jesús, Silvia Novío, Carlota García, Elena Pérez-Muñuzuri, Pilar Soengas, Elena Cartea, Pablo Velasco, and Manuel Freire-Garabal. 2019. "Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro" International Journal of Molecular Sciences 20, no. 20: 4977. https://doi.org/10.3390/ijms20204977
APA StyleNúñez-Iglesias, M. J., Novío, S., García, C., Pérez-Muñuzuri, E., Soengas, P., Cartea, E., Velasco, P., & Freire-Garabal, M. (2019). Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro. International Journal of Molecular Sciences, 20(20), 4977. https://doi.org/10.3390/ijms20204977








