Targeting SUMO Modification of the Non-Structural Protein 5 of Zika Virus as a Host-Targeting Antiviral Strategy
Abstract
1. Introduction
2. Results
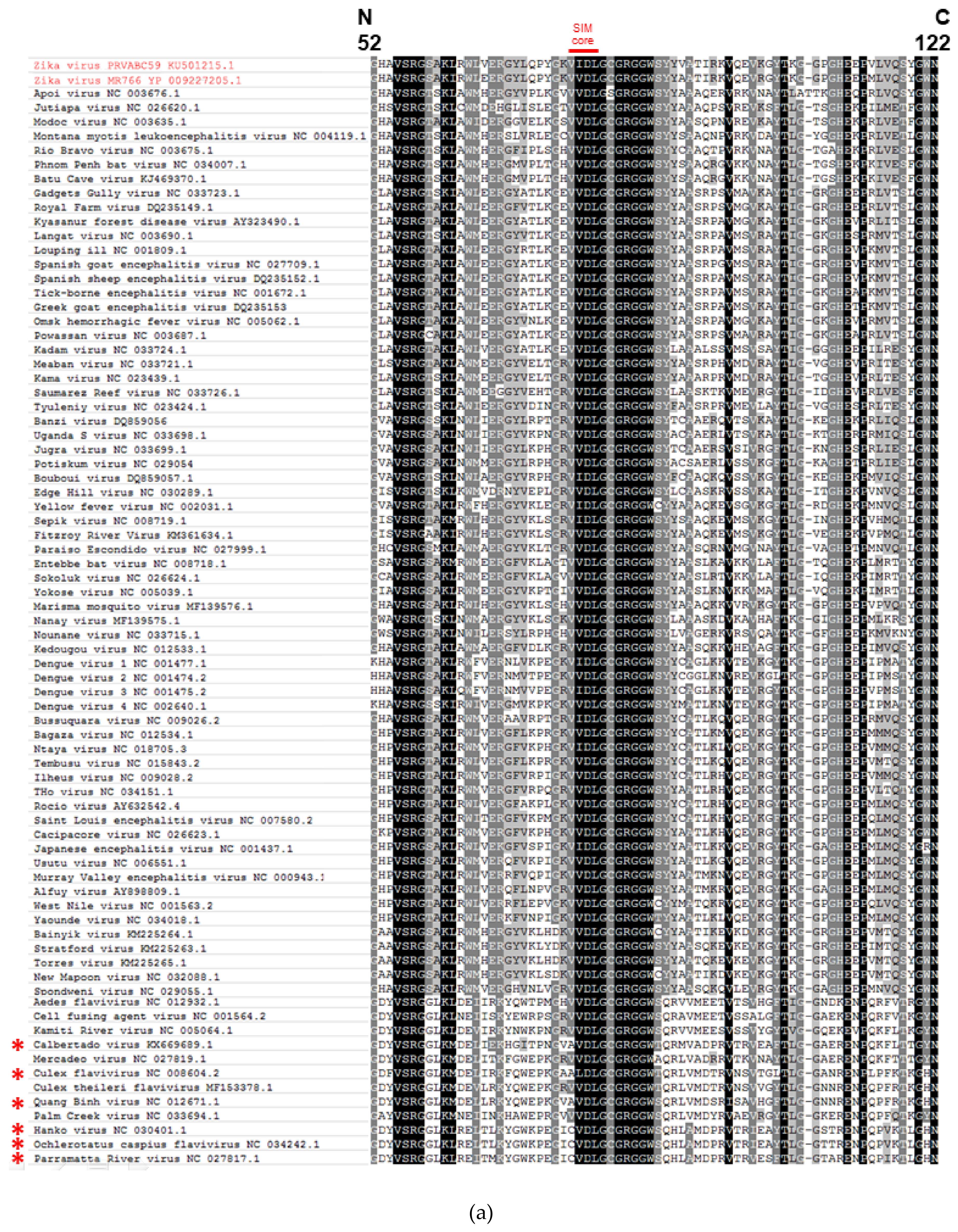
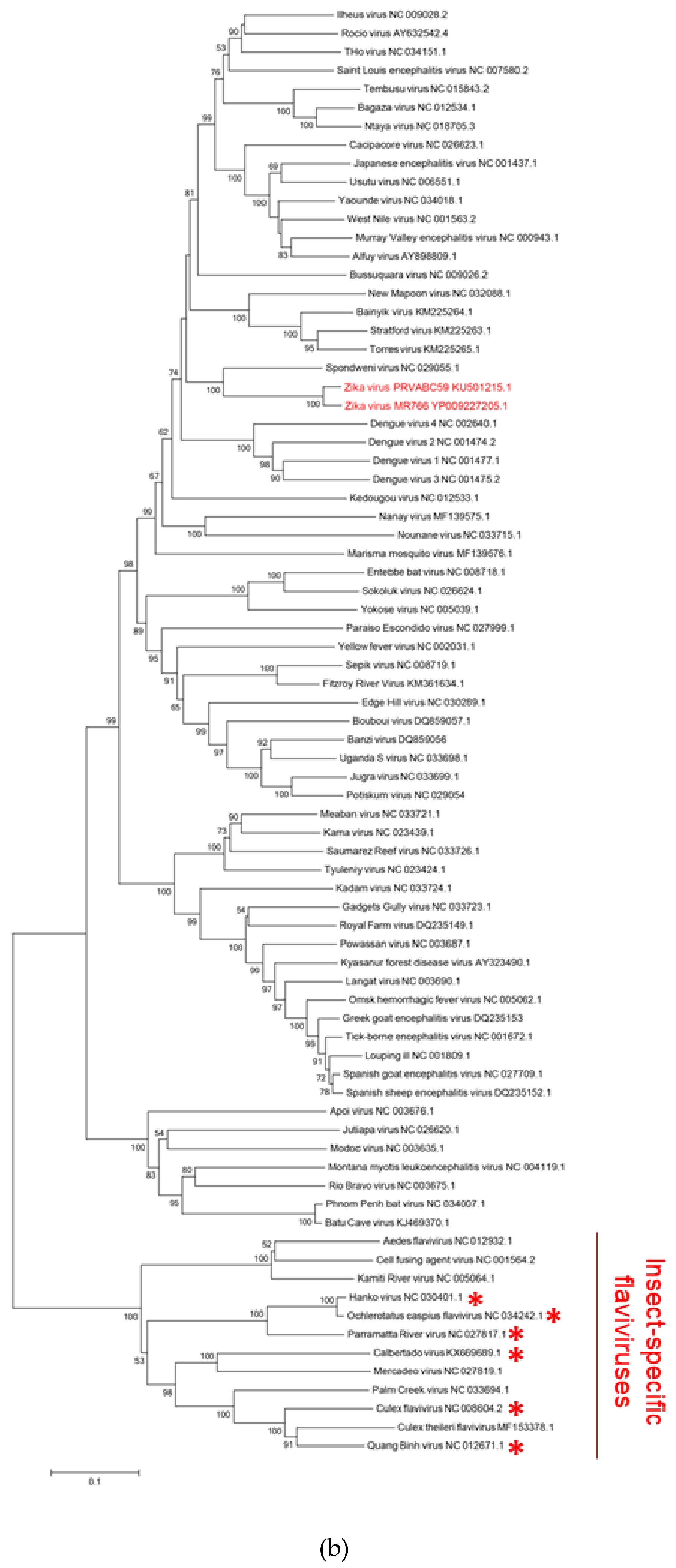
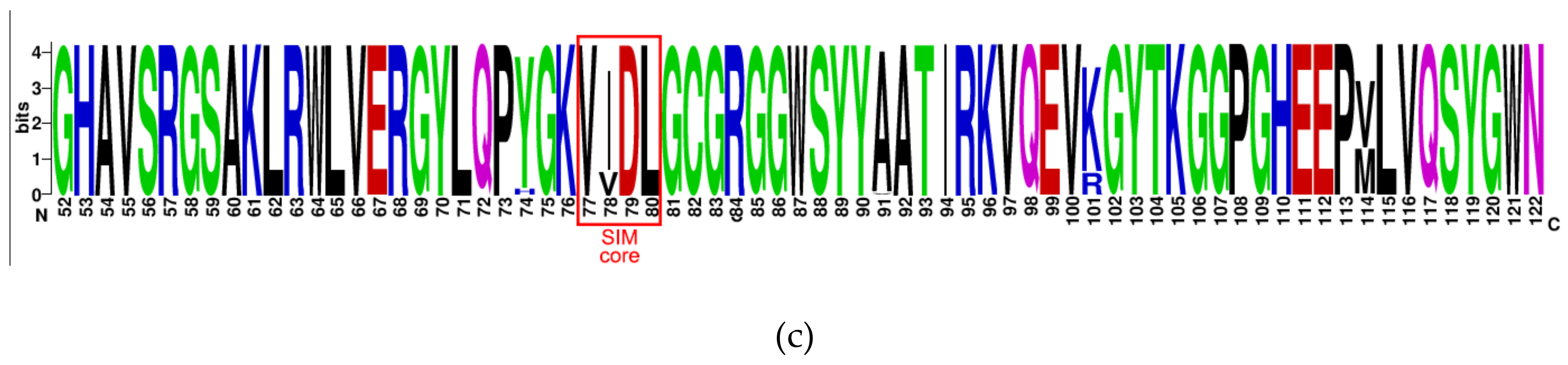
2.1. The Putative SIM at the N-Terminal Domain of NS5 Protein Is Highly Conserved among Flaviviruses
2.2. The Putative SIM at the N-Terminal Domain of the NS5 Protein is Conserved among Pre-Epidemic and Epidemic ZIKV Strains
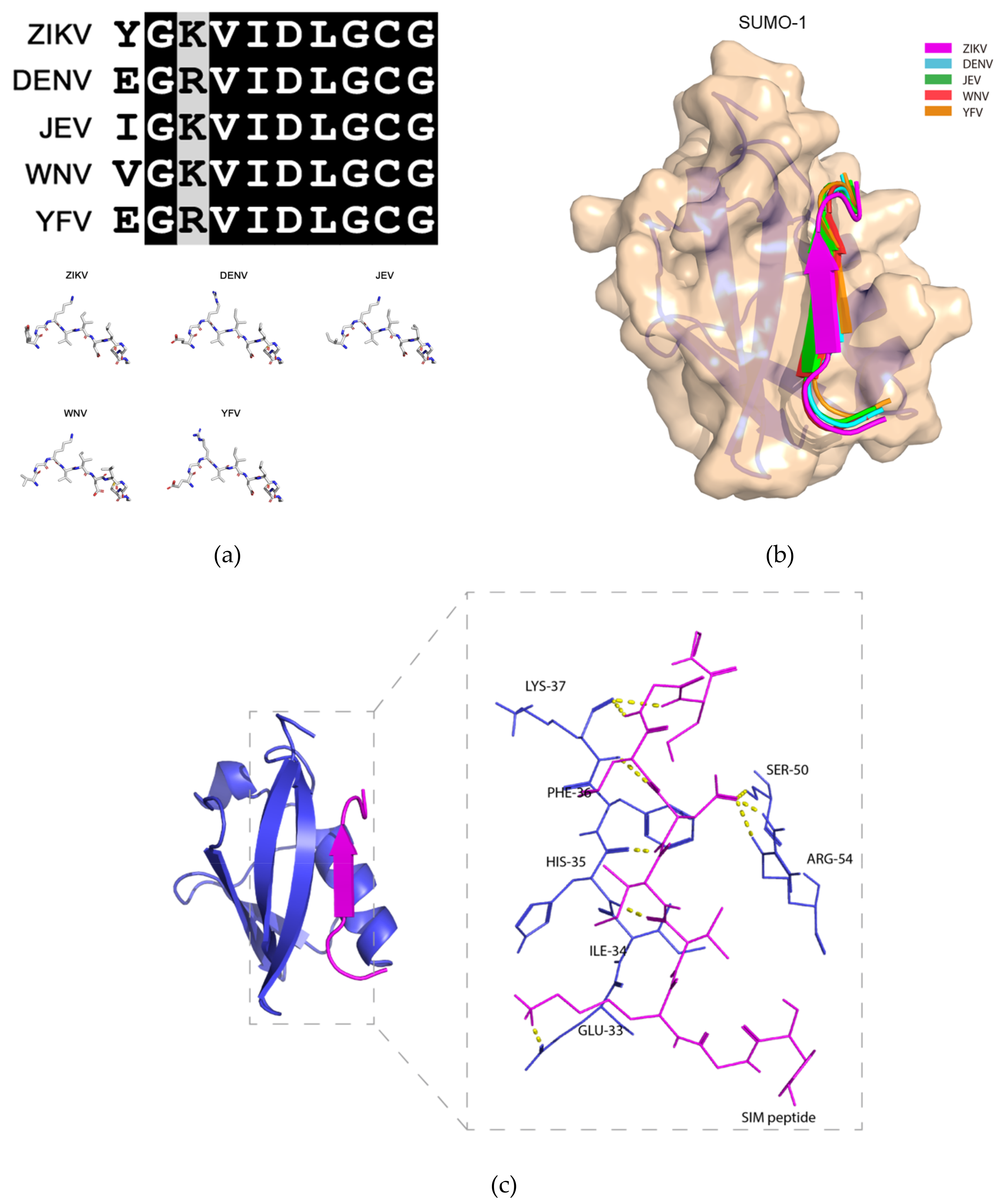
2.3. Molecular Docking Model of the Binding between the Putative ZIKV NS5 SIM and SUMO-1 Protein
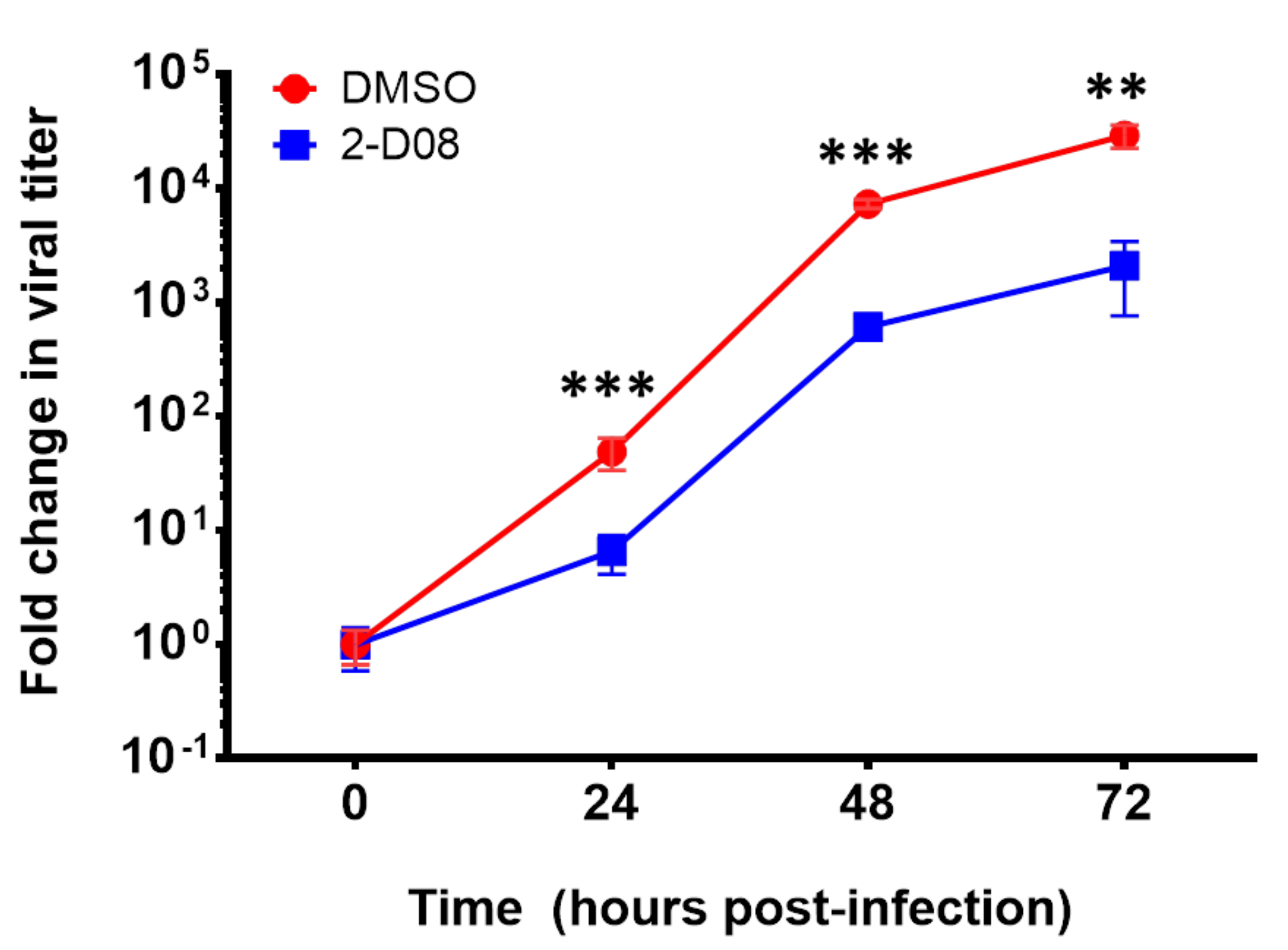
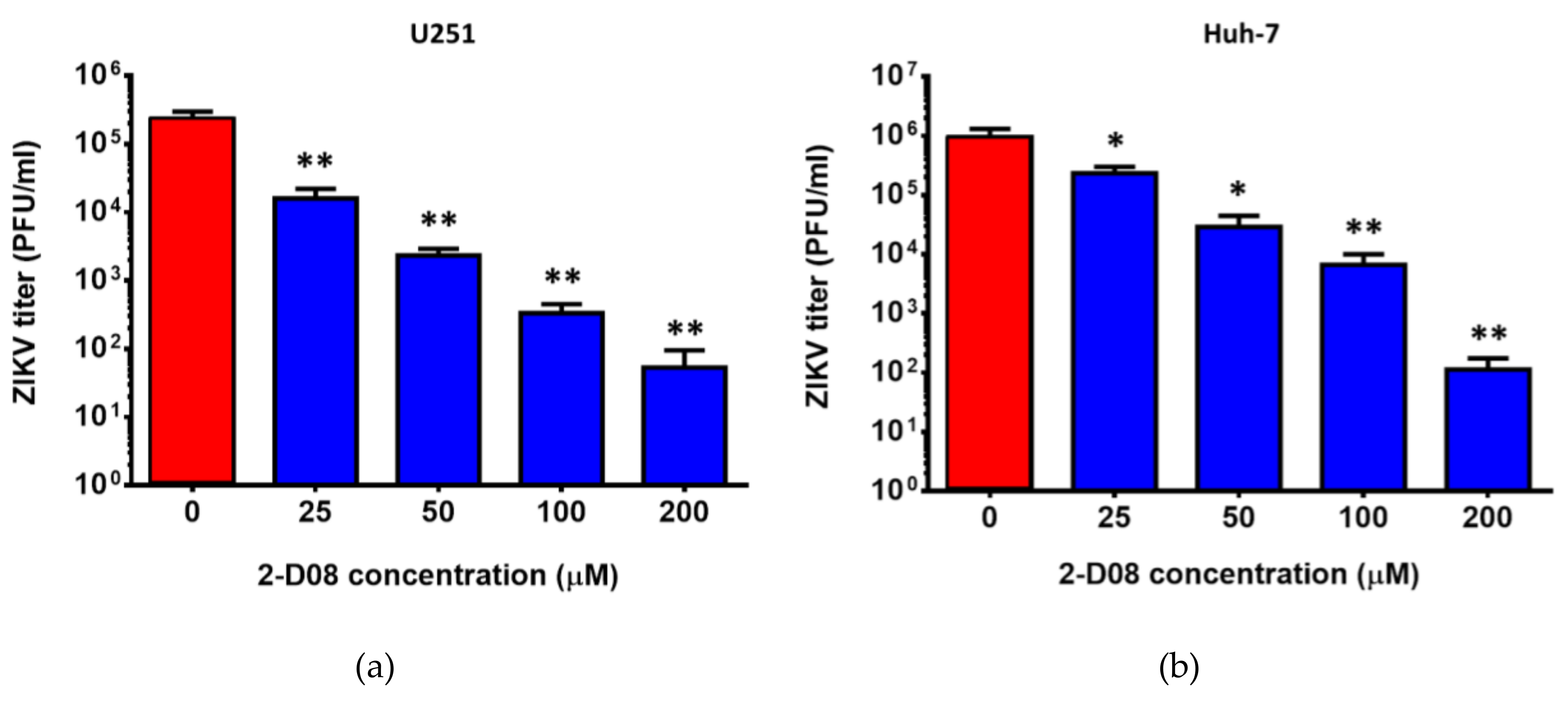
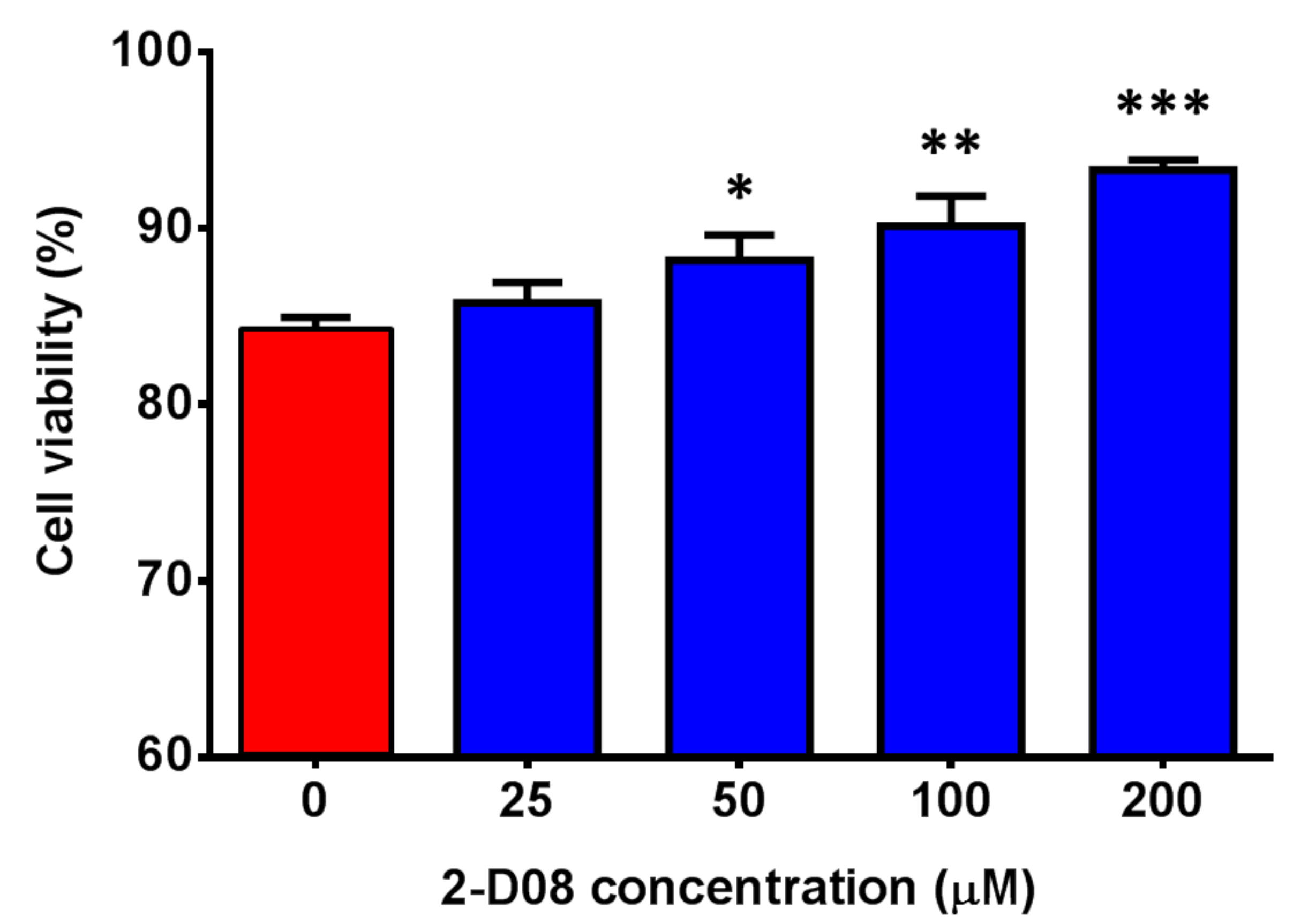
2.4. The SUMO Inhibitor 2-D08 Significantly Inhibited the Replication of ZIKV and Other Medically Important Flaviviruses In Vitro
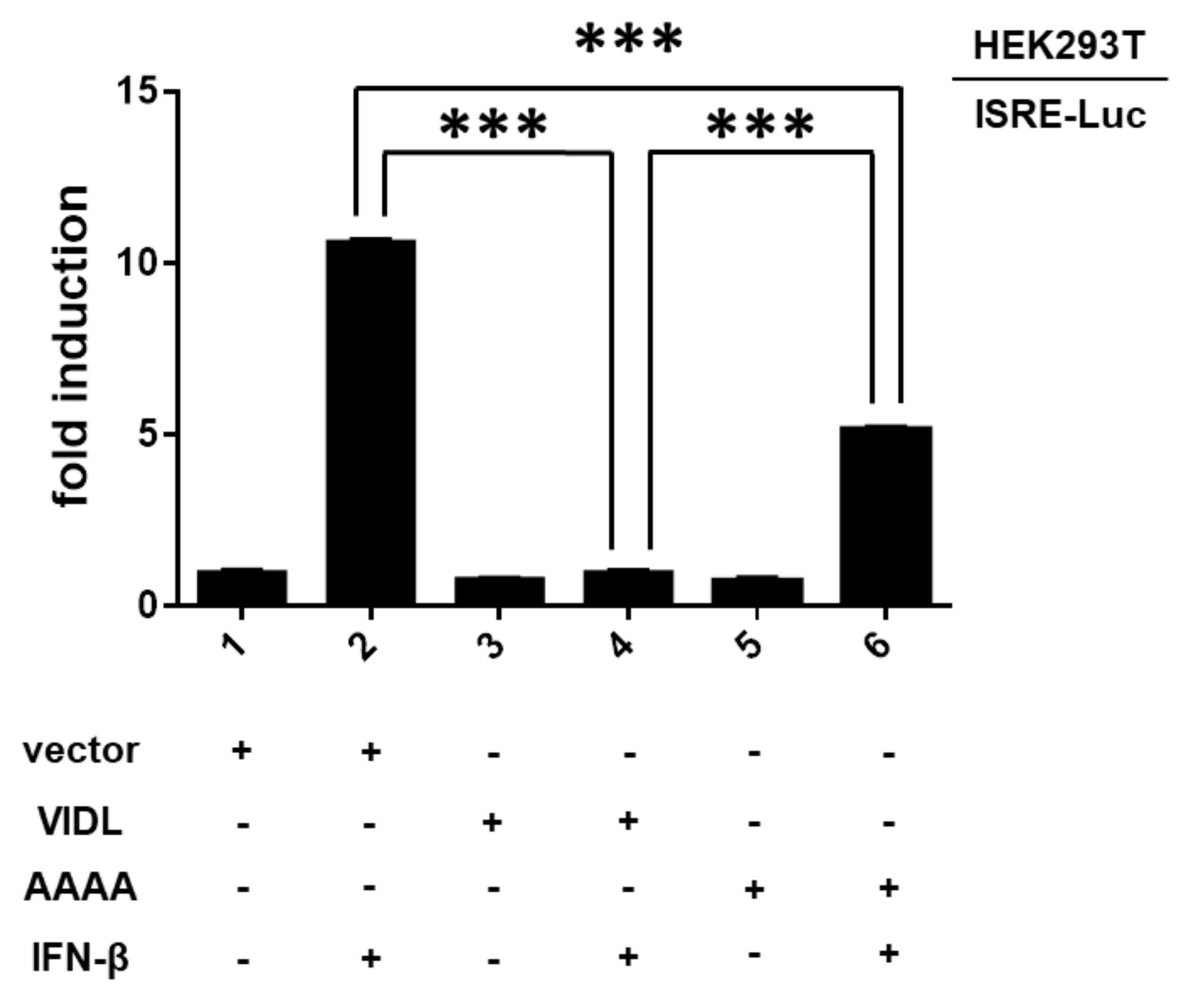
2.5. SUMO Modification of the ZIKV NS5 Protein Is Required for NS5-Mediated Type I Interferon Signaling
3. Discussion
4. Materials and Methods
4.1. Genomic Characterization and Phylogenetic Analysis
4.2. ZIKV NS5 SIM and SUMO-1 Molecular Docking
4.3. Virus Strains, Cell Lines, and Drug Compounds
4.4. CellTiter-Glo® luminescent Cell Viability Assay
4.5. Viral Load Reduction Assay and Plaque Assay
4.6. Luciferase Reporter Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| HEK293T | Human embryonic kidney epithelial cell line |
| Huh-7 | Human hepatoma cell line |
| ISRE | Interferon-stimulated response element |
| MOI | Multiplicity of infection |
| SIM | SUMO-interacting motif |
| STAT2 | Signal transducer and activator of transcription 2 |
| SUMO | Small ubiquitin-like modifier |
| U251 | Human astrocytoma cell line |
| ZIKV | Zika virus |
References
- Zhu, Z.; Chan, J.F.; Tee, K.M.; Choi, G.K.; Lau, S.K.; Woo, P.C.; Tse, H.; Yuen, K.Y. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg. Microbes Infect. 2016, 5, e22. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Chan, J.F.; Choi, G.K.; Yip, C.C.; Cheng, V.C.; Yuen, K.Y. Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. J. Infect. 2016, 72, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Zika Situation Report. 10 March 2017. Available online: http://who.int/emergencies/zika-virus/situation-report/10-march-2017/en/ (Accessed on 1 July 2018).
- Chan, J.F.; Yip, C.C.; Tsang, J.O.; Tee, K.M.; Cai, J.P.; Chik, K.K.; Zhu, Z.; Chan, C.C.; Choi, G.K.; Sridhar, S.; et al. Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg. Microbes Infect. 2016, 5, e93. [Google Scholar] [CrossRef] [PubMed]
- Culshaw, A.; Mongkolsapaya, J.; Screaton, G.R. The immunopathology of dengue and Zika virus infections. Curr. Opin. Immunol. 2017, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hannoun, Z.; Maarifi, G.; Chelbi-Alix, M.K. The implication of SUMO in intrinsic and innate immunity. Cytokine Growth Factor Rev. 2016, 29, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S. Protein modification by SUMO. Annu. Rev. Biochem. 2004, 73, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Melchior, F. SUMO—nonclassical ubiquitin. Ann. Rev. Cell Dev. Biol. 2000, 16, 591–626. [Google Scholar] [CrossRef] [PubMed]
- Geiss-Friedlander, R.; Melchior, F. Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 2007, 8, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Kerscher, O. SUMO junction-what’s your function? New insights through SUMO-interacting motifs. EMBO Rep. 2007, 8, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Boggio, R.; Chiocca, S. Viruses and sumoylation: recent highlights. Curr. Opin. Microbiol. 2006, 9, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.D. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene. 2001, 20, 7266–7273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Sumoylation regulates diverse biological processes. Cell Mol. Life Sci. 2007, 64, 3017–3033. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Deyrieux, A.F.; Wilson, V.G. Papillomaviruses and the host SUMOylation system. Biochem. Soc. Trans. 2007, 35, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Jeng, K.S.; Lai, M.M. The SUMOylation of matrix protein M1 modulates the assembly and morphogenesis of influenza A virus. J. Virol. 2011, 85, 6618–6628. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Cheng, T.S.; Shu, C.Y.; Jeng, K.S.; Lai, M.M. Modification of small hepatitis delta virus antigen by SUMO protein. J. Virol. 2010, 84, 918–927. [Google Scholar] [CrossRef]
- Su, C.I.; Tseng, C.H.; Yu, C.Y.; Lai, M.M.C. SUMO Modification Stabilizes Dengue Virus Nonstructural Protein 5 To Support Virus Replication. J. Virol. 2016, 90, 4308–4319. [Google Scholar] [CrossRef]
- Ureña, E.; Pirone, L.; Chafino, S.; Pérez, C.; Sutherland, J.D.; Lang, V.; Rodriguez, M.S.; Lopitz-Otsoa, F.; Blanco, F.J.; Barrio, R.; Martín, D.; et al. Evolution of SUMO Function and Chain Formation in Insects. Mol. Bio. Evol. 2016, 33, 568–584. [Google Scholar] [CrossRef]
- Kim, Y.S.; Nagy, K.; Keyser, S.; Schneekloth, J.S. Jr. An electrophoretic mobility shift assay identifies a mechanistically unique inhibitor of protein sumoylation. Chem. Biol. 2013, 20, 604–613. [Google Scholar] [CrossRef]
- Chaudhary, V.; Yuen, K.S.; Chan, J.F.; Chan, C.P.; Wang, P.H.; Cai, J.P.; Zhang, S.; Liang, M.; Kok, K.H.; Chan, C.P.; et al. Selective Activation of Type II Interferon Signaling by Zika Virus NS5 Protein. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Fu, X.Y.; Schindler, C.; Improta, T.; Aebersold, R.; Darnell, J.E. Jr. The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc. Natl. Acad. Sci. USA 1992, 89, 7840–7843. [Google Scholar] [CrossRef] [PubMed]
- Rengachari, S.; Groiss, S.; Devos, J.M.; Caron, E.; Grandvaux, N.; Panne, D. Structural basis of STAT2 recognition by IRF9 reveals molecular insights into ISGF3 function. Proc. Natl. Acad. Sci. USA 2018, 115, E601–E609. [Google Scholar] [CrossRef]
- Saisawang, C.; Kuadkitkan, A.; Auewarakul, P.; Smith, D.R.; Ketterman, A.J. Glutathionylation of dengue and Zika NS5 proteins affects guanylyltransferase and RNA dependent RNA polymerase activities. PLoS One. 2018, 13, e0193133. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.S.; Dargemont, C.; Hay, R.T. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J. Biol. Chem. 2001, 276, 12654–12659. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 2012, 13, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lim, Y.S.; Park, E.M.; Baek, S.H.; Hwang, S.B. SUMOylation of nonstructural 5A protein regulates hepatitis C virus replication. J. Viral Hepat. 2014, 21, e108–e117. [Google Scholar] [CrossRef]
- Chan, J.F.; Chik, K.K.; Yuan, S.; Yip, C.C.; Zhu, Z.; Tee, K.M.; Tsang, J.O.; Chan, C.C.; Poon, V.K.; Lu, G.; et al. Novel antiviral activity and mechanism of bromocriptine as a Zika virus NS2B-NS3 protease inhibitor. Antiviral Res. 2017, 141, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Zhang, A.J.; Chan, C.C.; Yip, C.C.; Mak, W.W.; Zhu, H.; Poon, V.K.; Tee, K.M.; Zhu, Z.; Cai, J.P.; et al. Zika Virus Infection in Dexamethasone-immunosuppressed Mice Demonstrating Disseminated Infection with Multi-organ Involvement Including Orchitis Effectively Treated by Recombinant Type I Interferons. EBioMedicine. 2016, 14, 112–122. [Google Scholar] [CrossRef]
- Zhao, B.; Yi, G.; Du, F.; Chuang, Y.C.; Vaughan, R.C.; Sankaran, B.; Kao, C.C.; Li, P. Structure and function of the Zika virus full-length NS5 protein. Nat. Commun. 2017, 8, 14762. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.D.; Stephens, R.M. Sequence logos: A new way to display consensus sequences. Nucleic Acids Res. 1990, 18, 6097–6100. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Namanja, A.T.; Li, Y.J.; Su, Y.; Wong, S.; Lu, J.; Colson, L.T.; Wu, C.; Li, S.S.; Chen, Y. Insights into high affinity small ubiquitin-like modifier (SUMO) recognition by SUMO-interacting motifs (SIMs) revealed by a combination of NMR and peptide array analysis. J. Biol. Chem. 2012, 287, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Zhu, Z.; Chu, H.; Yuan, S.; Chik, K.K.; Chan, C.C.; Poon, V.K.; Yip, C.C.; Zhang, X.; Tsang, J.O.; et al. The celecoxib derivative kinase inhibitor AR-12 (OSU-03012) inhibits Zika virus via down-regulation of the PI3K/Akt pathway and protects Zika virus-infected A129 mice: A host-targeting treatment strategy. Antiviral Res. 2018, 160, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chan, J.F.; den-Haan, H.; Chik, K.K.; Zhang, A.J.; Chan, C.C.; Poon, V.K.; Yip, C.C.; Mak, W.W.; Zhu, Z.; et al. Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antiviral Res. 2017, 145, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chu, H.; Chan, J.F.; Ye, Z.W.; Wen, L.; Yan, B.; Lai, P.M.; Tee, K.M.; Huang, J.; Chen, D.; et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Yip, C.C.; Tee, K.M.; Zhu, Z.; Tsang, J.O.; Chik, K.K.; Tsang, T.G.; Chan, C.C.; Poon, V.K.; Sridhar, S.; et al. Improved detection of Zika virus RNA in human and animal specimens by a novel, highly sensitive and specific real-time RT-PCR assay targeting the 5'-untranslated region of Zika virus. Trop. Med. Int. Health. 2017, 22, 594–603. [Google Scholar] [CrossRef]
- Alm, E.; Lesko, B.; Lindegren, G.; Ahlm, C.; Söderholm, S.; Falk, K.I.; Lagerqvist, N. Universal single-probe RT-PCR assay for diagnosis of dengue virus infections. PLoS Negl. Trop. Dis. 2014, 8, e3416. [Google Scholar] [CrossRef]
- Vázquez, A.; Herrero, L.; Negredo, A.; Hernández, L.; Sánchez-Seco, M.P.; Tenorio, A. Real time PCR assay for detection of all known lineages of West Nile virus. J. Virol. Methods. 2016, 236, 266–270. [Google Scholar] [CrossRef]
- Chao, D.Y.; Davis, B.S.; Chang, G.J. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medically important flaviviruses in mosquitoes. J. Clin. Microbiol. 2007, 45, 584–589. [Google Scholar] [CrossRef]
- Chaudhary, V.; Zhang, S.; Yuen, K.S.; Li, C.; Lui, P.Y.; Fung, S.Y.; Wang, P.H.; Chan, C.P.; Li, D.; Kok, K.H.; et al. Suppression of type I and type III IFN signalling by NSs protein of severe fever with thrombocytopenia syndrome virus through inhibition of STAT1 phosphorylation and activation. J. Gen. Virol. 2015, 96, 3204–3211. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; Gao, W.W.; Chan, C.P.; Cheng, Y.; Deng, J.J.; Yuen, K.S.; Iha, H.; Jin, D.Y. SIRT1 Suppresses Human T-Cell Leukemia Virus Type 1 Transcription. J. Virol. 2015, 89, 8623–8631. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chan, J.F.; Poon, V.K.; Wu, S.; Chan, C.C.; Hou, L.; Yip, C.C.; Ren, C.; Cai, J.P.; Zhao, M.; et al. Immunization with a Novel Human type 5 Adenovirus-Vectored Vaccine Expressing the Premembrane and Envelope Proteins of Zika Virus Provides Consistent and Sterilizing Protection in Multiple Immunocompetent and Immunocompromised Animal Models. J. Infect. Dis. 2018, 218, 365–377. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Chu, H.; Wen, L.; Yuan, S.; Chik, K.K.-H.; Yuen, T.T.-T.; Yip, C.C.-Y.; Wang, D.; Zhou, J.; Yin, F.; et al. Targeting SUMO Modification of the Non-Structural Protein 5 of Zika Virus as a Host-Targeting Antiviral Strategy. Int. J. Mol. Sci. 2019, 20, 392. https://doi.org/10.3390/ijms20020392
Zhu Z, Chu H, Wen L, Yuan S, Chik KK-H, Yuen TT-T, Yip CC-Y, Wang D, Zhou J, Yin F, et al. Targeting SUMO Modification of the Non-Structural Protein 5 of Zika Virus as a Host-Targeting Antiviral Strategy. International Journal of Molecular Sciences. 2019; 20(2):392. https://doi.org/10.3390/ijms20020392
Chicago/Turabian StyleZhu, Zheng, Hin Chu, Lei Wen, Shuofeng Yuan, Kenn Ka-Heng Chik, Terrence Tsz-Tai Yuen, Cyril Chik-Yan Yip, Dong Wang, Jie Zhou, Feifei Yin, and et al. 2019. "Targeting SUMO Modification of the Non-Structural Protein 5 of Zika Virus as a Host-Targeting Antiviral Strategy" International Journal of Molecular Sciences 20, no. 2: 392. https://doi.org/10.3390/ijms20020392
APA StyleZhu, Z., Chu, H., Wen, L., Yuan, S., Chik, K. K.-H., Yuen, T. T.-T., Yip, C. C.-Y., Wang, D., Zhou, J., Yin, F., Jin, D.-Y., Kok, K.-H., Yuen, K.-Y., & Chan, J. F.-W. (2019). Targeting SUMO Modification of the Non-Structural Protein 5 of Zika Virus as a Host-Targeting Antiviral Strategy. International Journal of Molecular Sciences, 20(2), 392. https://doi.org/10.3390/ijms20020392








