Processability and Degradability of PHA-Based Composites in Terrestrial Environments
Abstract
1. Introduction
2. Results and Discussion
2.1. Composite Processing
2.2. Composite Characterization
2.3. Biodegradability in Lab-Scale Terrestrial Environments
2.3.1. Mineralization
2.3.2. Disintegration
Degradation of Pots in Soil
3. Material and Methods
3.1. Materials
3.2. Composite Preparation
3.3. Composite Characterization
3.4. Biodegradation in Terrestrial Environment
3.4.1. Mineralization Test in Compost
3.4.2. Disintegration Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Studies on mechanical, thermal, and morphological characteristics of biocomposites from biodegradable polymer blends and natural fibers. In Biocomposites; Elsevier: Amsterdam, The Netherlands, 2015; pp. 93–140. ISBN 9781782423737. [Google Scholar]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. On-line Characterization of Physiological State in Poly(β-Hydroxybutyrate) Production by Wautersia eutropha. Appl. Biochem. Biotechnol. 2009, 157, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M.G. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; George, S.M.; Thomas, S. Recycling of polymer blends. In Recent Developments in Polymer Recycling; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2011; Volume 37, pp. 187–214. ISBN 978-81-7895-524-7. [Google Scholar]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Chiellini, F.; Imam, S.H. Environmentally Degradable Bio-Based Polymeric Blends and Composites. Macromol. Biosci. 2004, 4, 218–231. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Padamati, R.B.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Law, K.H.; Cheng, Y.C.; Leung, Y.C.; Lo, W.H.; Chua, H.; Yu, H.F. Construction of recombinant Bacillus subtilis strains for polyhydroxyalkanoates synthesis. Biochem. Eng. J. 2003, 16, 203–208. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Koller, M.; Marsalek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed culture: Recent trends and biotechnological importance. Biotechnol. Adv. 2004, 22, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Hideki, A.; Tadahisa, I. Biodegradability of Poly(hydroxyalkanoate) Materials. Materials 2009, 2, 1104–1126. [Google Scholar] [CrossRef]
- Breulmann, M.; Künkel, A.; Philipp, S.; Reimer, V.; Siegenthaler, K.O.; Skupin, G.; Yamamoto, M. Polymers, Biodegradable. In Ullmann’s Encyclopedia of Industrial Chemistry; VCH Publishers: New York, NY, USA, 2009. [Google Scholar]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Padovani, G.; Carlozzi, P.; Seggiani, M.; Cinelli, P. PHB-Rich Biomass and BioH 2 Production by Means of Photosynthetic Microorganisms. Chem. Eng. Trans. 2016, 49, 55–60. [Google Scholar] [CrossRef]
- Deroiné, M.; Le Duigou, A.; Corre, Y.-M.; Le Gac, P.-Y.; Davies, P.; César, G.; Bruzaud, S. Seawater accelerated ageing of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polym. Degrad. Stab. 2014, 105, 237–247. [Google Scholar] [CrossRef]
- Volova, T.G.; Boyandin, A.N.; Vasiliev, A.D.; Karpov, V.A.; Prudnikova, S.V.; Mishukova, O.V.; Boyarskikh, U.A.; Filipenko, M.L.; Rudnev, V.P.; Bá Xuân, B.; et al. Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym. Degrad. Stab. 2010, 95, 2350–2359. [Google Scholar] [CrossRef]
- Musioł, M.; Sikorska, W.; Janeczek, H.; Wałach, W.; Hercog, A.; Johnston, B.; Rydz, J. (Bio)degradable polymeric materials for a sustainable future—Part 1. Organic recycling of PLA/PBAT blends in the form of prototype packages with long shelf-life. Waste Manag. 2018, 1–8. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of nucleating agents on crystallinity and properties of poly (lactic acid) (PLA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, J.; Smith, A.C.; Carvalho, C.W.P.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Godbole, S.; Gote, S.; Latkar, M.; Chakrabarti, T. Preparation and characterization of biodegradable poly-3-hydroxybutyrate–starch blend films. Bioresour. Technol. 2003, 86, 33–37. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Preparation and properties of polyhydroxybutyrate blended with different types of starch. J. Appl. Polym. Sci. 2009, 116, 688–694. [Google Scholar] [CrossRef]
- Seggiani, M.; Cinelli, P.; Mallegni, N.; Balestri, E.; Puccini, M.; Vitolo, S.; Lardicci, C.; Lazzeri, A. New Bio-Composites Based on Polyhydroxyalkanoates and Posidonia oceanica Fibres for Applications in a Marine Environment. Materials 2017, 10, 326. [Google Scholar] [CrossRef]
- Seggiani, M.; Cinelli, P.; Verstichel, S.; Puccini, M.; Anguillesi, I.; Lazzeri, A. Development of Fibres-Reinforced Biodegradable Composites. Chem. Eng. Trans. 2015, 43, 1813–1818. [Google Scholar] [CrossRef]
- Imam, S.H.; Cinelli, P.; Gordon, S.H.; Chiellini, E. Characterization of Biodegradable Composite Films Prepared from Blends of Poly(Vinyl Alcohol), Cornstarch, and Lignocellulosic Fiber. J. Polym. Environ. 2005, 13, 47–55. [Google Scholar] [CrossRef]
- Seggiani, M.; Cinelli, P.; Geicu, M.; Elen, P.M.; Puccini, M.; Lazzeri, A. Microbiological valorisation of bio-composites based on polylactic acid and wood fibres. Chem. Eng. Trans. 2016, 49, 127–132. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Imam, S.H.; Mao, L. Composite Films Based on Biorelated Agro-Industrial Waste and Poly(vinyl alcohol). Preparation and Mechanical Properties Characterization. Biomacromolecules 2001, 2, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.K.; Nagarajan, V.; Mohanty, A.K.; Misra, M. 1—Commercial potential and competitiveness of natural fiber composites. In Woodhead Publishing Series in Composites Science and Engineering; Misra, M., Pandey, J.K., Mohanty, A.K.B.T.-B., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–15. ISBN 978-1-78242-373-7. [Google Scholar]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A review on the degradability of polymeric composites based on natural fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef]
- Herrera-Franco, P.J.; Valadez-Gonzalez, A. A study of the mechanical properties of short natural-fiber reinforced composites. Compos. Part B Eng. 2005, 36, 597–608. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Arizaga, G.G.C.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Gigante, V.; Aliotta, L.; Phuong, V.T.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A. Effects of waviness on fiber-length distribution and interfacial shear strength of natural fibers reinforced composites. Compos. Sci. Technol. 2017, 152, 129–138. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Yotinwattanakumtorn, C.; Thongpin, C. Influence of type and concentration of maleic anhydride grafted polypropylene and impact modifiers on mechanical properties of PP/wood sawdust composites. J. Appl. Polym. Sci. 2005, 97, 475–484. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Chaochanchaikul, K. Average mixing torque, tensile and impact properties, and thermal stability of poly(vinyl chloride)/sawdust composites with different silane coupling agents. J. Appl. Polym. Sci. 2005, 96, 213–221. [Google Scholar] [CrossRef]
- Seggiani, M.; Cinelli, P.; Balestri, E.; Mallegni, N.; Stefanelli, E.; Rossi, A.; Lardicci, C.; Lazzeri, A. Novel Sustainable Composites Based on Poly(hydroxybutyrate-co-hydroxyvalerate) and Seagrass Beach-CAST Fibers: Performance and Degradability in Marine Environments. Materials 2018, 11, 772. [Google Scholar] [CrossRef]
- Melik, H.D.; Schechtman, A.L. Biopolyester melt behavior by torque rheometry. Polym. Eng. Sci. 1995, 35, 1795–1806. [Google Scholar] [CrossRef]
- Baysal, E.; Deveci, I.; Turkoglu, T.; Toker, H. Thermal analysis of oriental beech sawdust treated with some commercial wood preservatives. Maderas. Cienc. Tecnol. 2017, 19, 329–338. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Reihmane, S.; Gassan, J. Properties and modification methods for vegetable fibers for natural fiber composites. J. Appl. Polym. Sci. 1996, 59, 1329–1336. [Google Scholar] [CrossRef]
- Renner, K.; Kenyó, C.; Móczó, J.; Pukánszky, B. Micromechanical deformation processes in PP/wood composites: Particle characteristics, adhesion, mechanisms. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1653–1661. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Graupner, N.; Müssig, J. A comparison of the mechanical characteristics of kenaf and lyocell fibre reinforced poly(lactic acid) (PLA) and poly(3-hydroxybutyrate) (PHB) composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2010–2019. [Google Scholar] [CrossRef]
- Ganster, J.; Fink, H.-P. Novel cellulose fibre reinforced thermoplastic materials. Cellulose 2006, 13, 271–280. [Google Scholar] [CrossRef]
- Ku, H.; Wang, H.; Pattarachaiyakoop, N.; Trada, M. A review on the tensile properties of natural fiber reinforced polymer composites. Compos. Part B Eng. 2011, 42, 856–873. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Recent advances in bio-based polymers and composites: Preface to the BiPoCo 2012 Special Section. Eur. Polym. J. 2013, 49, 1146–1150. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined Effect of Poly(hydroxybutyrate) and Plasticizers on Polylactic acid Properties for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Lin, Y.; Chan, C.-M. 3—Calcium Carbonate Nanocomposites. Advances in Polymer Nanocomposites, Types and Applications; Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2012; pp. 55–90. ISBN 978-1-84569-940-6. [Google Scholar]
- American Society for Testing and Materials ASTM D5338. Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials under Controlled Composting Conditions; American Society for Testing and Materials: West Conshohocken, PA, USA, 1998. [Google Scholar]
- International Organization for Standardization ISO 20200. Plastics Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Vaverková, M.; Toman, F.; Adamcová, D.; Kotovicová, J. Study of the Biodegrability of Degradable/Biodegradable Plastic Material in a Controlled Composting Environment. Ecol. Chem. Eng. S 2012, 19, 347–358. [Google Scholar] [CrossRef]
- International Organization for Standardization ISO 3310. Test Sieves—Technical Requirements and Testing—Part 1: Test Sieves of Metal Wire Cloth; International Organization for Standardization: Geneva, Switzerland, 2016; pp. 1–15. [Google Scholar]
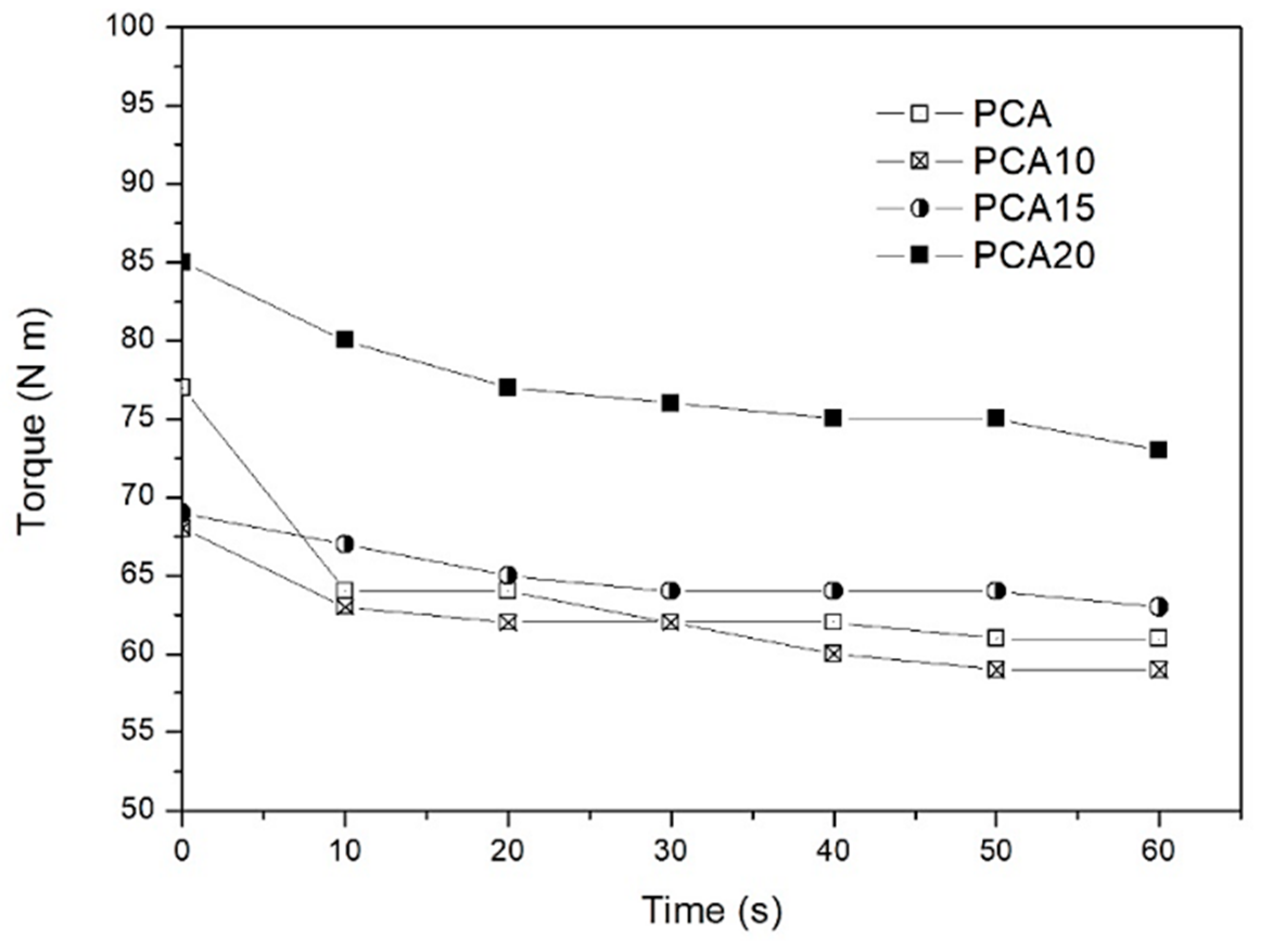

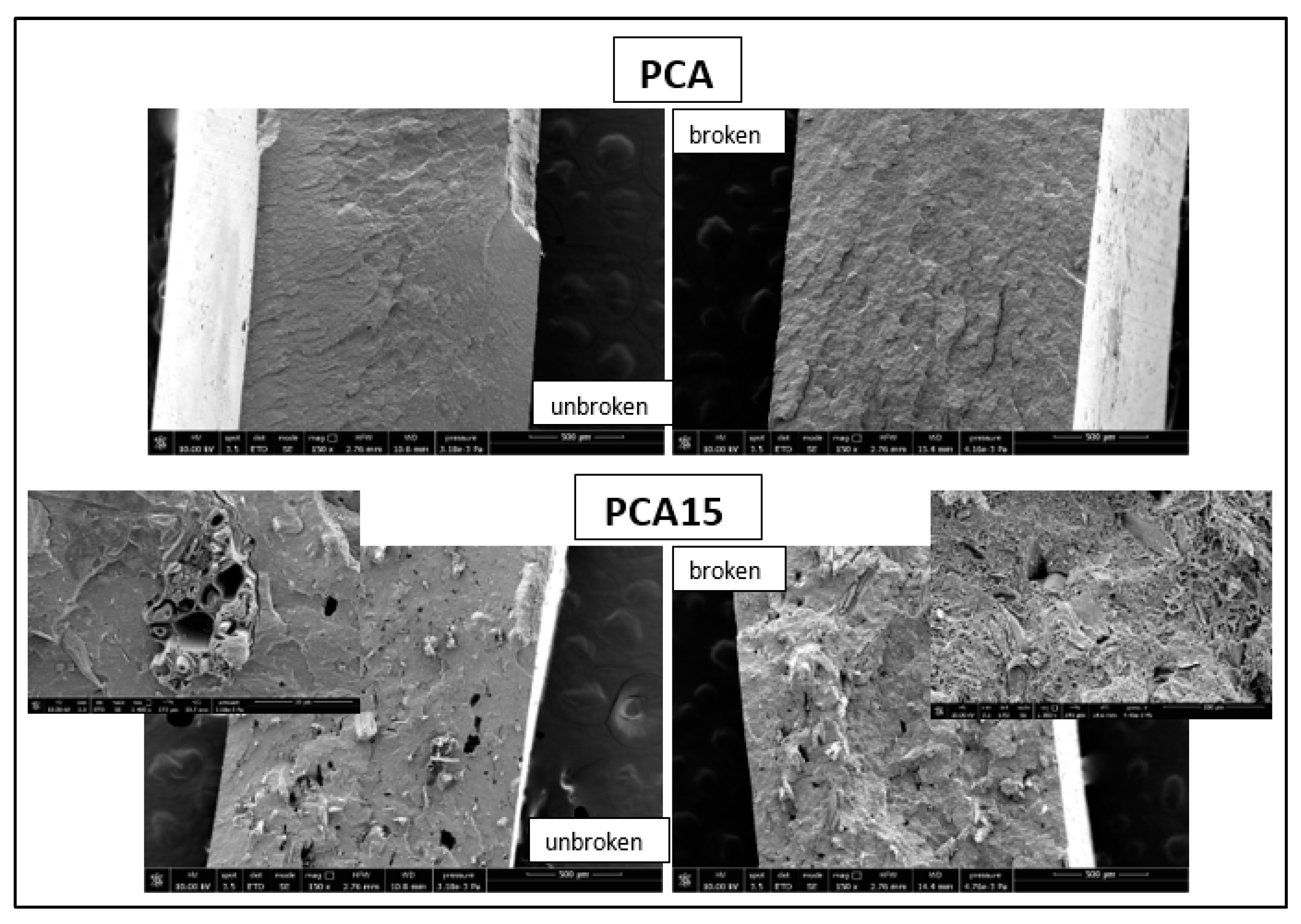
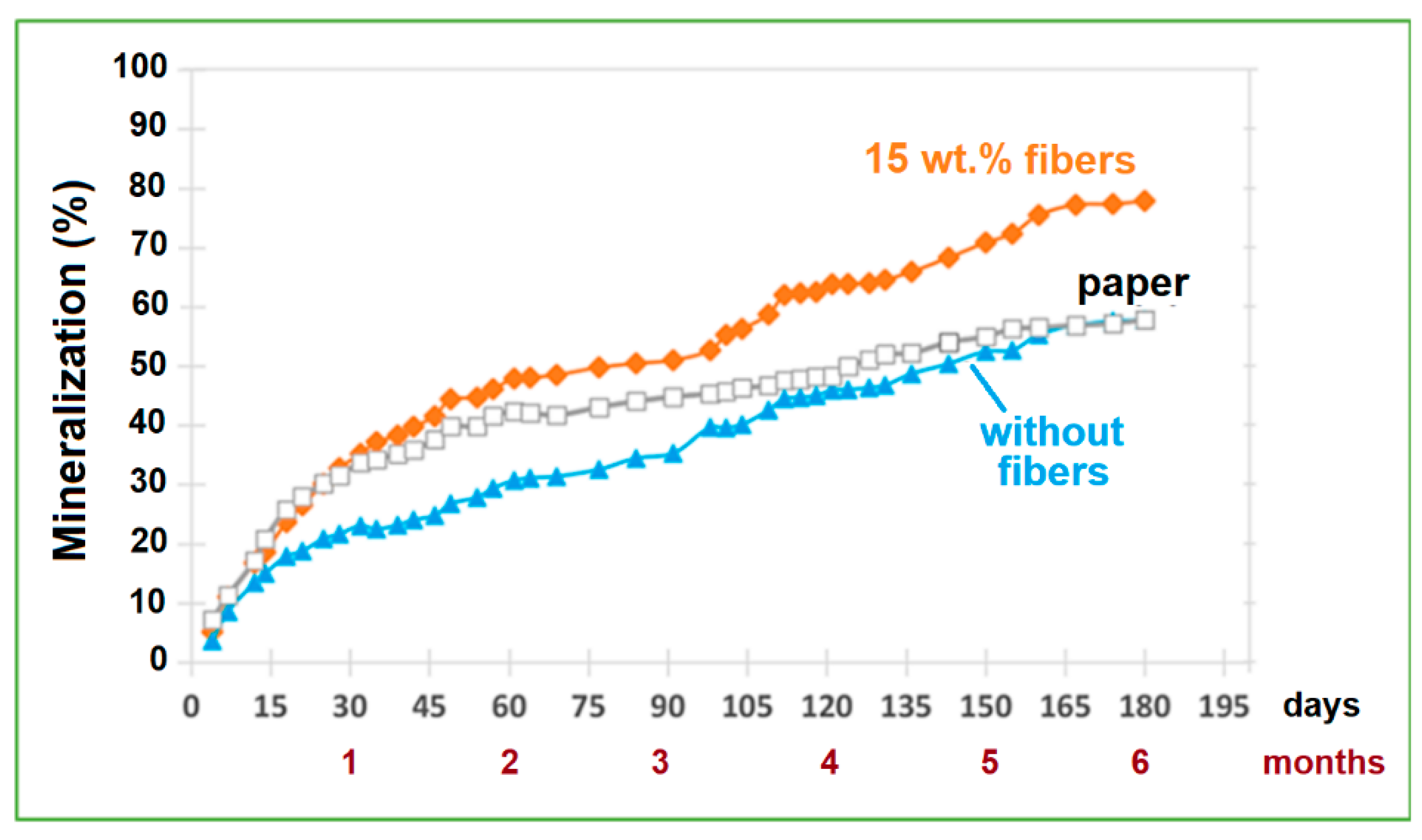
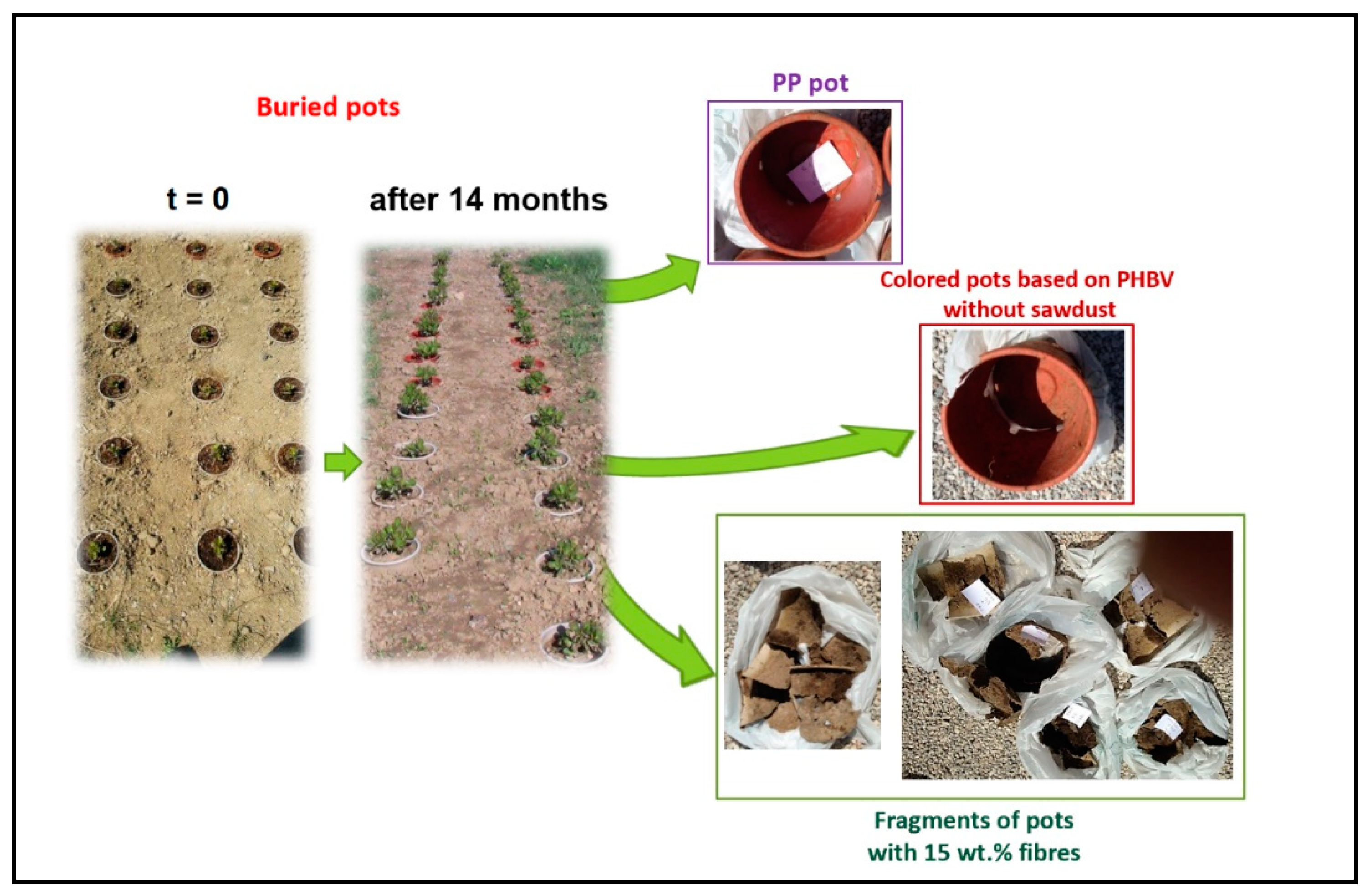
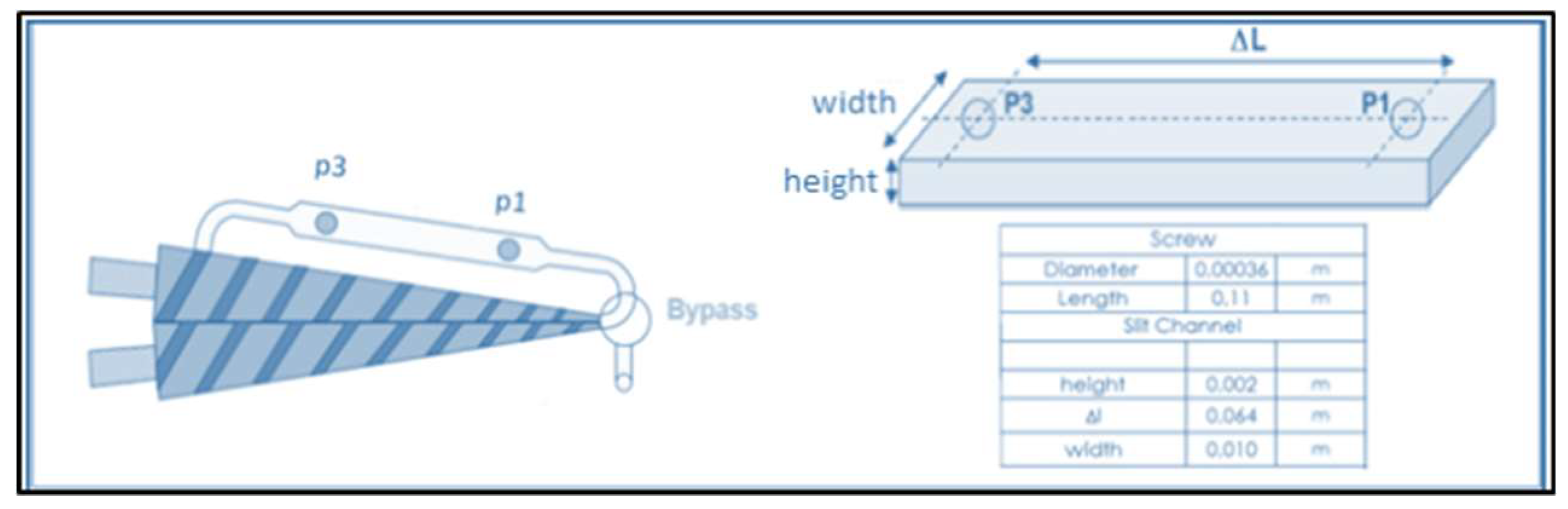
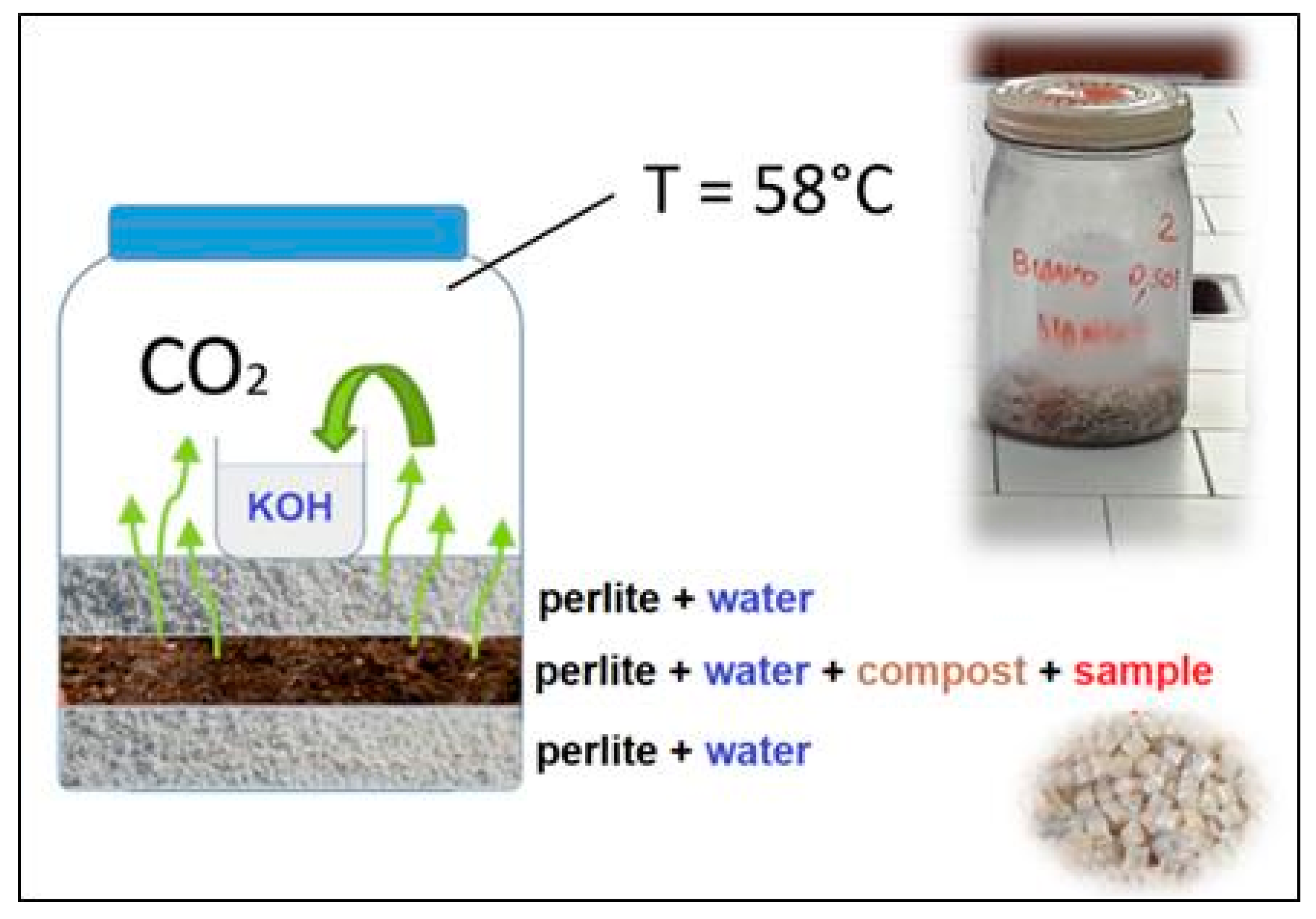

| Sample | Young’s Modulus (GPa) | Stress at Break (MPa) | Elongation (%) | Charpy Impact Energy (kJ/m2) |
|---|---|---|---|---|
| PCA | 2.64 ± 0.28 | 25.62 ± 2.11 | 2.14 ± 0.50 | 3.57 ± 0.36 |
| PCA10 | 2.35 ± 0.24 | 21.02 ± 0.94 | 2.05 ± 0.28 | 6.17 ± 0.24 |
| PCA15 | 2.52 ± 0.15 | 18.52 ± 0.84 | 1.35 ± 0.14 | 12.24 ± 0.50 |
| PCA20 | 2.94 ± 0.35 | 20.93 ± 1.57 | 1.35 ± 0.13 | 5.91 ± 0.40 |
| Sample | Sample No. | Disintegration (%) | Average Disintegration (%) |
|---|---|---|---|
| PCA | 1 | 87.2 | 92.6 |
| 2 | 94.3 | ||
| 3 | 96.4 | ||
| PCA10 | 1 | 83.4 | 93.2 |
| 2 | 100.0 | ||
| 3 | 96.2 | ||
| PCA15 | 1 | 96.3 | 94.2 |
| 2 | 88.6 | ||
| 3 | 97.7 |
| Weight Percentage | ||||
|---|---|---|---|---|
| Composite | PHB-HV | CaCO3 | ATBC | Sawdust Fibers |
| PCA | 80 | 10.0 | 10.0 | 0 |
| PCA10 | 72 | 9.0 | 9.0 | 10 |
| PCA15 | 68 | 8.5 | 8.5 | 15 |
| PCA20 | 64 | 8.0 | 8.0 | 20 |
| Material | Dry Mass % |
|---|---|
| Sawdust | 40 |
| Rabbit-feed | 30 |
| Ripe compost | 10 |
| Corn starch | 10 |
| Saccharose | 5 |
| Cornseed oil | 4 |
| Urea | 1 |
| Total | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinelli, P.; Seggiani, M.; Mallegni, N.; Gigante, V.; Lazzeri, A. Processability and Degradability of PHA-Based Composites in Terrestrial Environments. Int. J. Mol. Sci. 2019, 20, 284. https://doi.org/10.3390/ijms20020284
Cinelli P, Seggiani M, Mallegni N, Gigante V, Lazzeri A. Processability and Degradability of PHA-Based Composites in Terrestrial Environments. International Journal of Molecular Sciences. 2019; 20(2):284. https://doi.org/10.3390/ijms20020284
Chicago/Turabian StyleCinelli, Patrizia, Maurizia Seggiani, Norma Mallegni, Vito Gigante, and Andrea Lazzeri. 2019. "Processability and Degradability of PHA-Based Composites in Terrestrial Environments" International Journal of Molecular Sciences 20, no. 2: 284. https://doi.org/10.3390/ijms20020284
APA StyleCinelli, P., Seggiani, M., Mallegni, N., Gigante, V., & Lazzeri, A. (2019). Processability and Degradability of PHA-Based Composites in Terrestrial Environments. International Journal of Molecular Sciences, 20(2), 284. https://doi.org/10.3390/ijms20020284









