Infection of Embryonic Callus with Agrobacterium Enables High-Speed Transformation of Maize
Abstract
1. Introduction
2. Results
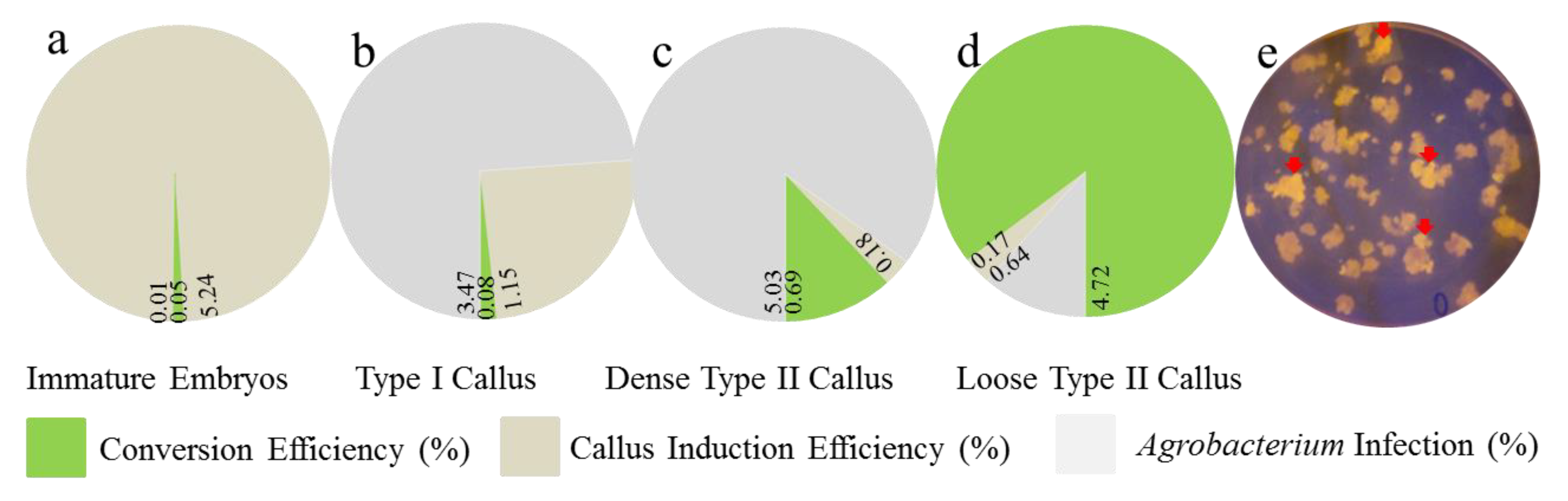
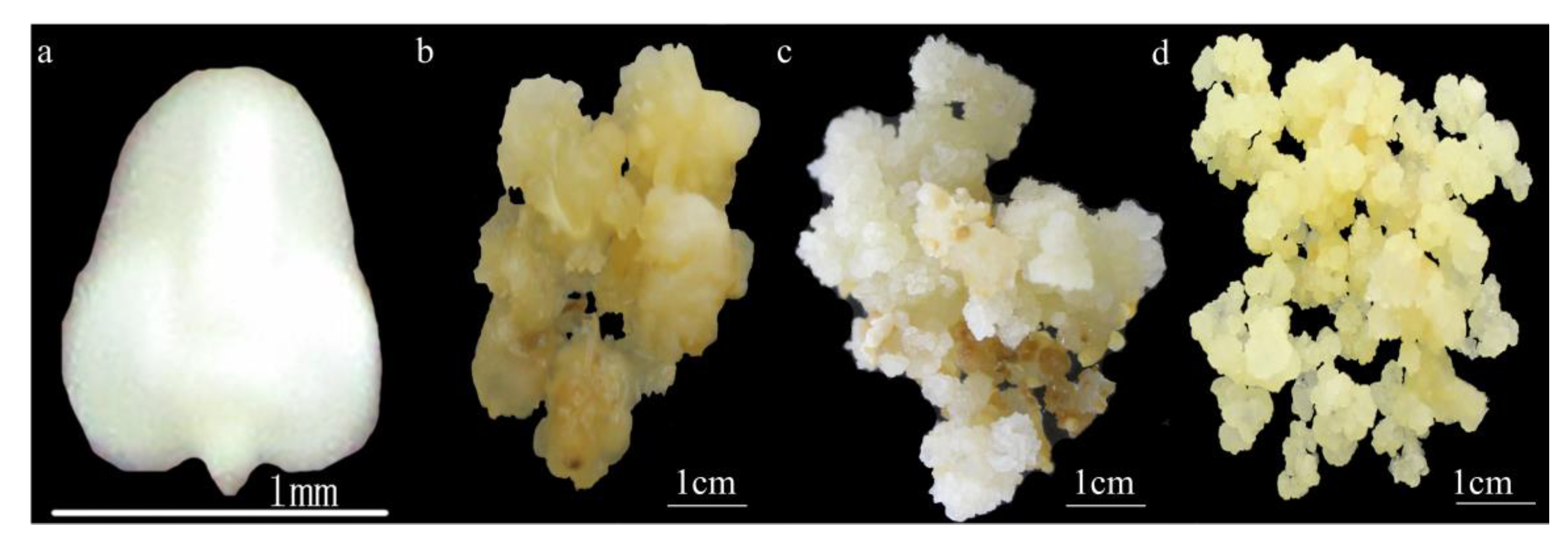
2.1. Agrobacterium Transformation Efficiency in Calli at Different Stages of Development
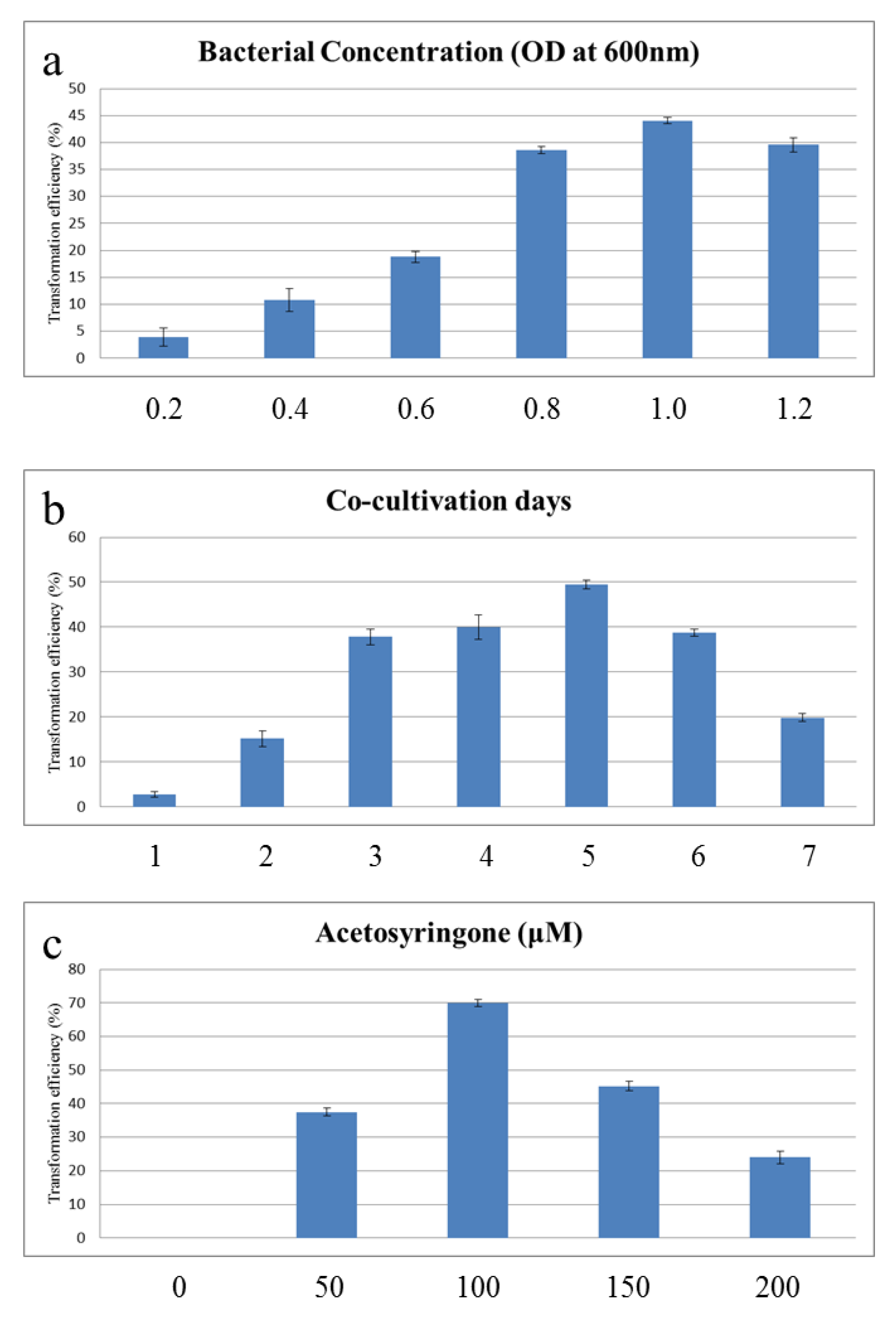
2.2. Optimization of the Agrobacterium Transgenic System on Calli
2.3. Effect of Pretreatment with a Mixed Lytic Enzyme Solution
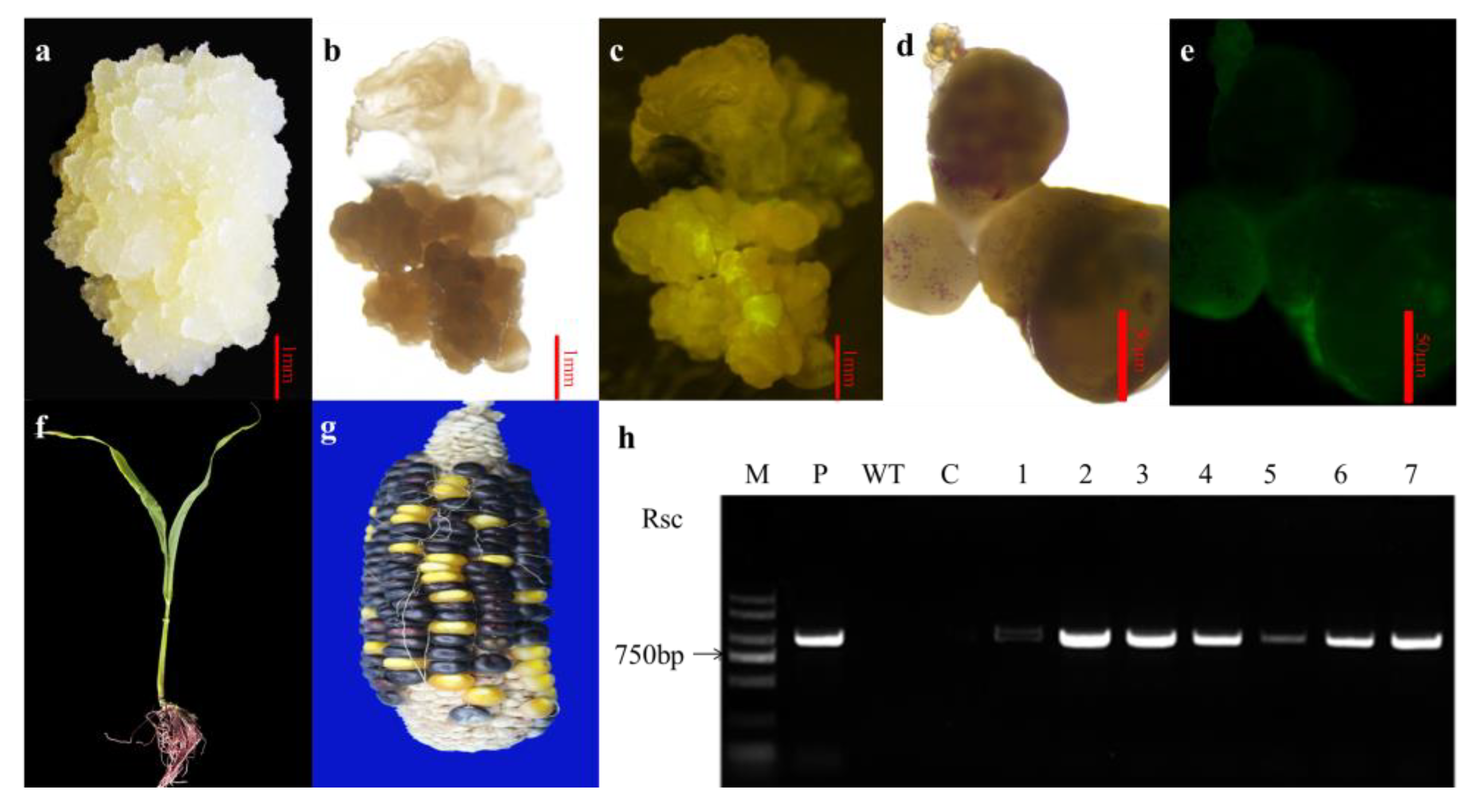
2.4. Transformation and Regeneration of Putative Transformed Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Media
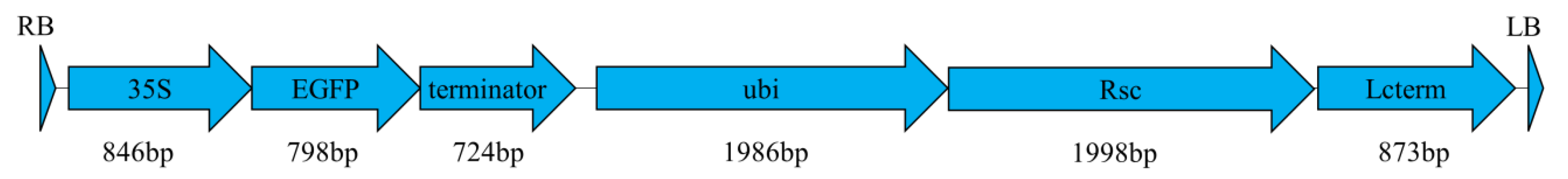
4.3. Morphogenic Vector Design and Agrobacterium Strains
4.4. Plant Transformation and Optimization of the Agrobacterium Transgenic System on Calli
4.5. GFP Fluorescence Assay
4.6. Pretreatment with Lytic Enzyme Solution
4.7. Transgene Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Casas, A.M.; Kononowicz, A.K.; Zehr, U.B.; Tomes, D.T.; Axtell, J.D.; Butler, L.G.; Bressan, R.A.; Hasegawa, P.M. Transgenic sorghum plants via microprojectile bombardment. Proc. Natl. Acad. Sci. USA 1993, 90, 11212–11216. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Fry, J.E.; Pang, S.; Zhou, H.; Hironaka, C.M.; Duncan, D.R.; Conner, T.W.; Wan, Y. Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol. 1997, 115, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zheng, P.; Marmey, P.; Zhang, S.; Tian, W.; Chen, S.; Beachy, R.N.; Fauquet, C. Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol. Breed. 2001, 7, 25–33. [Google Scholar] [CrossRef]
- Gordon-Kamm, W.J.; Spencer, T.M.; Mangano, M.L.; Adams, T.R.; Daines, R.J.; Start, W.G.; O’Brien, J.V.; Chambers, S.A.; Adams, W.R., Jr.; Willetts, N.G.; et al. Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 1990, 2, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Hiei, Y.; Komari, T.; Kubo, T. Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol. Biol. 1997, 35, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Popelka, J.; Altpeter, F. Agrobacterium tumefaciens-mediated genetic transformation of rye (Secale cereale L.). Mol. Biol. 2003, 11, 203–211. [Google Scholar]
- Ritala, A.; Aspegren, K.; Kurtén, U.; Salmenkallio-Marttila, M.; Mannonen, L.; Hannus, R.; Kauppinen, V.; Teeri, T.; Enari, T.-M. Fertile transgenic barley by particle bombardment of immature embryos. Plant Mol. Biol. 1994, 24, 317–325. [Google Scholar] [CrossRef]
- Somers, D.A.; Rines, H.W.; Gu, W.; Kaeppler, H.F.; Bushnell, W.R. Fertile, transgenic oat plants. Nat. Biotechnol. 1992, 10, 1589–1594. [Google Scholar] [CrossRef]
- Tingay, S.; McElroy, D.; Kalla, R.; Fieg, S.; Wang, M.; Thornton, S.; Brettell, R. Agrobacterium tumefaciens-mediated barley transformation. Plant J. 1997, 11, 1369–1376. [Google Scholar] [CrossRef]
- Vasil, V.; Castillo, A.M.; Fromm, M.E.; Vasil, I.K. Herbicide resistant fertile transgenic wheat plants obtained by micropro-jectile bombardment of regenerable embryogenic callus. Nat. Biotechnol. 1992, 10, 667–674. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Cai, T.; Tagliani, L.; Miller, M.; Wang, N.; Pang, H.; Rudert, M.; Schroeder, S.; Hondred, D.; Seltzer, J.; et al. Agrobacterium-mediated sorghum transformation. Plant Mol. Biol. 2000, 44, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.; Brutnell, T.P.; Citovsky, V.; Conrad, L.J.; Gelvin, S.B.; Jackson, D.P.; Kausch, A.P.; et al. Advancing crop transformation in the era of genome editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Mark Cigan, A. Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.E.; Taylor, L.P.; Walbot, V. Stable transformation of maize after gene transfer by electroporation. Nature 1986, 319, 791. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.; Taylor, L.P.; Walbot, V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc. Natl. Acad. Sci. USA 1985, 82, 5824–5828. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.A.; Lowe, K.S.; Ruby, K.L. Plant regeneration from protoplasts isolated from embryogenic maize cell cultures. Nat. Biotechnol. 1988, 6, 56. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Green, C.E. Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 1985, 164, 207–214. [Google Scholar] [CrossRef]
- Frame, B.R.; Shou, H.; Chikwamba, R.K.; Zhang, Z.; Xiang, C.; Fonger, T.M.; Pegg, S.E.; Li, B.; Nettleton, D.S.; Pei, D.; et al. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 2002, 129, 13–22. [Google Scholar] [CrossRef]
- Frame, B.R.; McMurray, J.M.; Fonger, T.M.; Main, M.L.; Taylor, K.W.; Torney, F.J.; Paz, M.M.; Wang, K. Improved Agrobacterium-mediated transformation of three maize inbred lines using MS salts. Plant Cell Rep. 2006, 25, 1024–1034. [Google Scholar] [CrossRef]
- Ishida, Y.; Satto, H.; Ohta, S.; Hiei, Y.; Komari, T.; Kumashiro, T. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat. Biotechnol. 1996, 14, 745–750. [Google Scholar] [CrossRef]
- Ishida, Y.; Hiei, Y.; Komari, T. Agrobacterium-mediated transformation of maize. Nat. Protocol. 2007, 2, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.A.; Vetsch, C.S.; Potts, D.E.; Lundquist, R.C. Transformation and inheritance of a hygromycin phosphotransferase gene in maize plants. Plant Mol. Biol. 1992, 18, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, A.; Song, C.; Li, N.; Zhang, J. Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotechnol. J. 2008, 6, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, H.; Zhang, Y.; Kang, T.; Zhang, L.; Tong, J.; Xiao, L.; Zhang, H. Constitutive expression of cell wall invertase genes increases grain yield and starch content in maize. Plant Biotechnol. J. 2013, 11, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.; Bowen, B.; Hoerster, G.; Margit, R.; Bond, D.; Pierce, D.; Gordon-Kamm, B. Germline transformation of maize following manipulation of chimeric shoot meristems. Nat. Biotechnol. 1995, 13, 677. [Google Scholar] [CrossRef]
- Raman, K.; Walden, D.B.; Greyson, R.I. Propagation of Zea mays L. by shoot tip culture: A feasibility study. Ann. Bot. 1980, 45, 183–189. [Google Scholar] [CrossRef]
- Du, D.X.; Geng, C.; Zhang, X.; Zhang, Z.; Zheng, Y.; Zhang, F.; Lin, Y.; Qiu, F. Transgenic maize lines expressing a cry1C* gene are resistant to insect pests. Plant Mol. Biol. Rep. 2014, 32, 549–557. [Google Scholar] [CrossRef]
- Hiei, Y.; Komari, T. Improved protocols for transformation of indica rice mediated by Agrobacterium tumefaciens. Plant Cell Tissue Org. Cult. 2006, 85, 271–283. [Google Scholar] [CrossRef]
- Hamilton, C.M.; Frary, A.; Lewis, C.; Tanksley, S.D. Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc. Natl. Acad. Sci. USA 1996, 93, 9975–9979. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Beachy, R.N.; Fauquet, C.M. A protocol for consistent, large-scale production of fertile transgenic rice plants. Plant Cell Rep. 1998, 18, 25–31. [Google Scholar] [CrossRef]
- Roy, M.; Jain, R.K.; Rohila, J.S.; Wu, R. Production of agronomically superior transgenic rice plants using Agrobacterium transformation methods: Present status and future perspectives. Curr. Sci. 2000, 79, 954–960. [Google Scholar]
- Komari, T.; Kubo, T. Methods of genetic transformation: Agrobacterium tumefaciens. In Molecular Improvement of Cereal Crops; Vasil, I.K., Ed.; Springer: Dordrecht, The Netherlands, 1999; Volume 5, pp. 43–82. [Google Scholar]
- Shou, H.; Frame, B.R.; Whitham, S.A.; Wang, K. Assessment of transgenic maize events produced by particle bombardment or Agrobacterium-mediated transformation. Mol. Breed. 2004, 13, 201–208. [Google Scholar] [CrossRef]
- Cheng, M.; Lowe, B.A.; Spencer, T.M.; Ye, X.; Armstrong, C.A. Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 31–45. [Google Scholar] [CrossRef]
- Li, X.Q.; Liu, C.N.; Ritchie, S.W.; Peng, J.-Y.; Gelvin, S.B.; Hodges, T.K. Factors influencing Agrobacterium-mediated transient expression of gusA in rice. Plant Mol. Biol. 1992, 20, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Park, S.; Srivatanakul, M.; Smith, R.H. Temperature influence on stable T-DNA integration in plant cells. Plant Cell Rep. 2001, 20, 701–705. [Google Scholar]
- Yu, C.; Huang, S.; Chen, C.; Deng, Z.; Ling, P.; Gmitter, F.G. Factors affecting Agrobacterium-mediated transformation and regeneration of sweet orange and citrange. Plant Cell Tissue Org. Cult. 2002, 71, 147–155. [Google Scholar] [CrossRef]
- Zambre, M.; Terryn, N.; De Clercq, J.; De Buck, S.; Dillen, W.; Van Montagu, M.; Van der Straeten, D.; Angenoon, G. Light strongly promotes gene transfer from Agrobacterium tumefaciens to plant cells. Planta 2003, 216, 580–586. [Google Scholar] [PubMed]
- Knogge, W. Fungal infection of plants. Plant Cell 1996, 8, 1711–1722. [Google Scholar] [CrossRef]
- Osbourn, A.E. Preforned antimicrobial compounds and plant defense against fungal attack. Plant Cell 1996, 8, 1821–1831. [Google Scholar] [CrossRef]
- Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front. Plant Sci. 2015, 6, 84. [Google Scholar] [CrossRef]
- Suárez, M.H.; Hernández, A.I.M.; Galdón, B.R.; Rodríguez, L.H.; Cabrera, C.E.M.; Mesa, D.R.; Romero, C.D. Application of multidimensional scaling technique to differentiate sweet potato (Ipomoea batatas (L.) Lam) cultivars according to their chemical composition. J. Food Compost. Anal. 2016, 46, 43–49. [Google Scholar] [CrossRef]
- Pitzschke, A.; Hirt, H. New insights into an old story: Agrobacterium-induced tumour formation in plants by plant transformation. EMBO J. 2010, 29, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Szabala, B.M.; Osipowski, P.; Malepszy, S. Transgenic crops: The present state and new ways of genetic modification. J. Appl. Genet. 2014, 55, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.M.; Kornstein, L.; Sanford, J.C.; Fromm, M.E. Genetic transformation of maize cells by particle bombardment. Plant Physiol. 1989, 91, 440–444. [Google Scholar] [CrossRef]
- Koziel, M.G.; Beland, G.L.; Bowman, C.; Carozzi, N.B.; Crenshaw, R.; Crossland, L.; Dawson, J.; Desai, N.; Hill, M.; Kadwell, S.; et al. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Nat. Biotechnol. 1993, 11, 194. [Google Scholar] [CrossRef]
- Manju, S.; Arti, R.; Pankaj, K.V.; Prashant, M.; Sonali, D.; Smita, K.; Sikha, V.; Neelam, G.; Rudra, D.T.; Prabodh, K.T.; et al. An improved Agrobacterium-mediated transformation of recalcitrant indica rice (Oryza sativa L.) cultivars. Protoplasma 2013, 250, 631–636. [Google Scholar]
- Huang, X.Q.; Wei, Z.M. Successful Agrobacterium-mediated genetic transformation of maize elite inbred lines. Plant Cell Tissue Org. Cult. 2005, 83, 187–200. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Gu, W.N.; Cai, T.S.; Tagliani, L.; Hondred, D.; Bond, D.; Schroeder, S.; Rudert, M.; Pierce, D. High throughput genetic transformation mediated by Agrobacterium tumefaciens in maize. Mol. Breed. 2001, 8, 323–333. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, G.Y.; Zhang, X.H.; Zhao, H.J. Agrobacterium tumefaciens mediated maize transformation. J. Agric. Biotechnol. 2001, 9, 45–48. [Google Scholar]
- Du, H.W.; Wu, H.X.; Yan, J.B.; Li, J.S. Effects of basal media, salt concentrations, antioxidant supplements and co-effects on the Agrobacterium-mediated transformation efficiency in maize. Afr. J. Biotechnol. 2010, 9. [Google Scholar]
- Amoah, B.K.; Wu, H.; Sparks, C.; Jones, H.D. Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J. Exp. Bot. 2001, 52, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Olhoft, P.M.; Lin, K.; Galbraith, J.; Nielsen, N.C.; Somers, D.A. The role of thiol compounds in increasing Agrobacterium-mediated transformation of soybean cotyledonary-node cells. Plant Cell Rep. 2001, 20, 731–737. [Google Scholar]
- Suzuki, S.; Nakano, M. Agrobaterium-mediated production of transgenic plants of Muscari armeniacum Leichtl. ex Bak. Plant Cell Rep. 2002, 20, 835–841. [Google Scholar]
- Vega, J.M.; Yu, W.C.; Kennon, A.R.; Chen, X.; Zhang, Z.J. Improvement of Agrobacterium-mediated transformation in Hi-II maize (Zea mays) using standard binary vectors. Plant Cell Rep. 2008, 27, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Metz, M.; Monks, D.E.; Lockwood Mullaney, M.; Hall, T.; Nester, E.W. Purine synthesis and increased Agrobacterium tumefaciens transformation of yeast and plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6634–6639. [Google Scholar] [CrossRef]
- Ditt, R.F.; Nester, E.; Comai, L. The plant cell defense and Agrobacterium tumefaciens. FEMS Microbiol. Lett. 2005, 247, 207–213. [Google Scholar] [CrossRef]
- Lacroix, B.; Tzfira, T.; Vainstein, A.; Citovsky, V. A case of promiscuity: Agrobacterium’s endless hunt for new partners. Trends Genet. 2006, 22, 29–37. [Google Scholar] [CrossRef]
- Tzfira, T.; Citovsky, V. Agrobacterium-mediated genetic transformation of plants: Biology and biotechnology. Curr. Opin. Biotechnol. 2006, 17, 147–154. [Google Scholar] [CrossRef]
- Schoonbeek, H.J.; Wang, H.H.; Stefanato, F.L.; Craze, M.; Bowden, S.; Wallington, E.; Ridout, C.J. Arabidopsis EF-Tu receptor enhances bacterial disease resistance in transgenic wheat. New Phytol. 2015, 206, 606–613. [Google Scholar] [CrossRef]
- Que, Q.; Elumalai, S.; Li, X.; Zhong, H.; Nalapalli, S.; Schweiner, M.; Fei, X.; Nuccio, M.; Kelliher, T.; Gu, W.; et al. Maize transformation technology development for commercial event generation. Front. Plant Sci. 2014, 5, 379. [Google Scholar] [CrossRef]
- Ignacimuthu, S.; Arockiasamy, S.; Terada, R. Genetic transformation of rice: Current status and future prospects. Curr. Sci. 2000, 79, 186–195. [Google Scholar]
| Material | Receptor Number | GFP Positives | GFP Positives | GFP Positives | Average | Standard Deviation |
|---|---|---|---|---|---|---|
| Immature Embryos | 900 | 40 | 67 | 38 | 0.053 | 0.018 |
| Type I Callus | 90 g | 6.3 g | 3.51 g | 2.88 g | 0.047 | 0.021 |
| Dense Type II Callus | 90 g | 5.94 g | 5.67 g | 4.32 g | 0.059 | 0.010 |
| Loose Type II Callus | 90 g | 4.23 g | 5.22 g | 5.49 g | 0.055 | 0.007 |
| Material | Conversion Efficiency (g) | Callus Induction Loose (g) | Agrobacterium Infection Loose (g) |
|---|---|---|---|
| Immature Embryos | 0.05 ± 0.04 | 5.24 ± 1.78 | 0.01 ± 0.01 |
| Type I Callus | 0.08 ± 0.02 | 1.15 ± 0.20 | 3.47 ± 2.07 |
| Dense Type II Callus | 0.69 ± 0.04 | 0.18 ± 0.03 | 5.03 ± 0.93 |
| Loose Type II Callus | 4.72 ± 0.50 | 0.19 ± 0.03 | 0.86 ± 0.64 |
| Mixed Enzyme Solution Concentration (g/mL) | Mixed Enzyme Treatment Time (min) | ||||
|---|---|---|---|---|---|
| Concentration | Frequency of Callus Rate | Frequency of Stable Transformation | Time | Frequency of Callus Rate | Frequency of Stable Transformation |
| 0 | 73.19 ± 3.51 | 15.57 ± 1.89 | 0 | 76.46 ± 3.21 | 15.22 ± 0.60 |
| 0.01 | 64.50 ± 3.06 | 22.67 ± 1.42 | 3 | 71.00 ± 2.00 | 20.70 ± 0.31 |
| 0.02 | 53.56 ± 3.60 | 29.37 ± 1.96 | 6 | 63. 03 ± 8.66 | 25.02 ± 0.28 |
| 0.03 | 48.56 ± 3.60 | 37.48 ± 0.49 | 9 | 53.75 ± 1.52 | 38.33 ± 0.56 |
| 0.04 | 34.59 ± 3.78 | 21.28 ± 1.22 | 12 | 42.89 ± 11.71 | 12.39 ± 1.28 |
| 0.05 | 18.47 ± 1.15 | 10.54 ± 0.99 | 15 | 43.05 ± 3.60 | 10.15 ± 0.52 |
| 0 min 2 | 3 min | 6 min | 9 min | 12 min | 15 min | |
|---|---|---|---|---|---|---|
| 0 g/m 1 | 15.57 ± 1.89 3 | 21.17 ± 2.57 | 25.53 ± 3.10 | 39.23 ± 4.77 | 12.61 ± 1.53 | 10.27 ± 1.25 |
| 0.01 g/mL | 22.67 ± 1.42 | 30.83 ± 1.94 | 37.18 ± 2.33 | 57.13 ± 3.58 | 18.36 ± 1.15 | 14.96 ± 0.93 |
| 0.02 g/mL | 29.37 ± 1.95 | 39.95 ± 2.66 | 48.17 ± 3.20 | 74.02 ± 4.92 | 23.79 ± 1.58 | 19.39 ± 1.29 |
| 0.03 g/mL | 37.48 ± 0.49 | 50.98 ± 0.66 | 61.47 ± 0.80 | 94.46 ± 1.23 | 30.36 ± 0.39 | 24.74 ± 0.32 |
| 0.04 g/mL | 21.28 ± 1.22 | 28.95 ± 1.66 | 34.90 ± 2.00 | 53.63 ± 3.08 | 17.24 ± 0.99 | 14.05 ± 0.80 |
| 0.05 g/mL | 10.54 ± 0.99 | 14.34 ± 1.35 | 17.29 ± 1.63 | 26.57 ± 2.51 | 8.54 ± 0.80 | 6.96 ± 0.65 |
| Medium | Composition |
|---|---|
| LB (solid) | Yeast extract 5 g/L, NaCl 10 g/L, peptone 10 g/L, agar 15 g/L, pH 6.8 |
| LB (liquid) | Yeast extract 5 g/L, NaCl 10 g/L, peptone 10 g/L, pH 6.8 |
| Infection (N-I) | N61 2 g/L, 2,4-D1 2.0 mg/L, L-proline 0.7 g/L, sucrose 68.4 g/L, D-glucose2 36 g/L, MES1 0.5 g/L, myo-inositol 0.1 g/L, As1,2 200 μM, pH 5.2 |
| Co-cultivation (N-C) | N6 4 g/L, 2,4-D 2.0 mg/L, L-proline 0.7 g/L, sucrose 30 g/L, MES 0.5 g/L, myo-inositol 0.1 g/L, CuSO41,2 0.05 µM, DTT1,2 1 M, L-cysteine 0.4 g/L, As 100 μM, agar 8 g/L, pH 5.8 |
| Resting (N-R) Selection (N-S) Regeneration (MS-D) Rooting (MS-R) | N6 4 g/L, 2,4-D 2.0 mg/L, L-proline 0.7 g/L, sucrose 30 g/L, MES 0.5 g/L, myo-inositol 0.1 g/L, AgNO31,2 0.85 mg/L, carbenicillin1,2 0.1 g/L, gelrite 2.5 g/L, pH 5.8 Resting medium without carbenicillin, pH 5.8 MS1 4.3 g/L, sucrose 30 g/L, myo-inositol 0.1 g/L, 6-BA 3.5 mg/L, gelrite 3.0 g/L, pH 5.8 MS 4.3 g/L, sucrose 25 g/L, NAA 0.5 mg/L, gelrite 2 g/L, pH 5.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, D.; Jin, R.; Guo, J.; Zhang, F. Infection of Embryonic Callus with Agrobacterium Enables High-Speed Transformation of Maize. Int. J. Mol. Sci. 2019, 20, 279. https://doi.org/10.3390/ijms20020279
Du D, Jin R, Guo J, Zhang F. Infection of Embryonic Callus with Agrobacterium Enables High-Speed Transformation of Maize. International Journal of Molecular Sciences. 2019; 20(2):279. https://doi.org/10.3390/ijms20020279
Chicago/Turabian StyleDu, Dengxiang, Ruchang Jin, Jinjie Guo, and Fangdong Zhang. 2019. "Infection of Embryonic Callus with Agrobacterium Enables High-Speed Transformation of Maize" International Journal of Molecular Sciences 20, no. 2: 279. https://doi.org/10.3390/ijms20020279
APA StyleDu, D., Jin, R., Guo, J., & Zhang, F. (2019). Infection of Embryonic Callus with Agrobacterium Enables High-Speed Transformation of Maize. International Journal of Molecular Sciences, 20(2), 279. https://doi.org/10.3390/ijms20020279




