Platelet-Rich Plasma in Treatment of Temporomandibular Joint Dysfunctions: Narrative Review
Abstract
1. Introduction
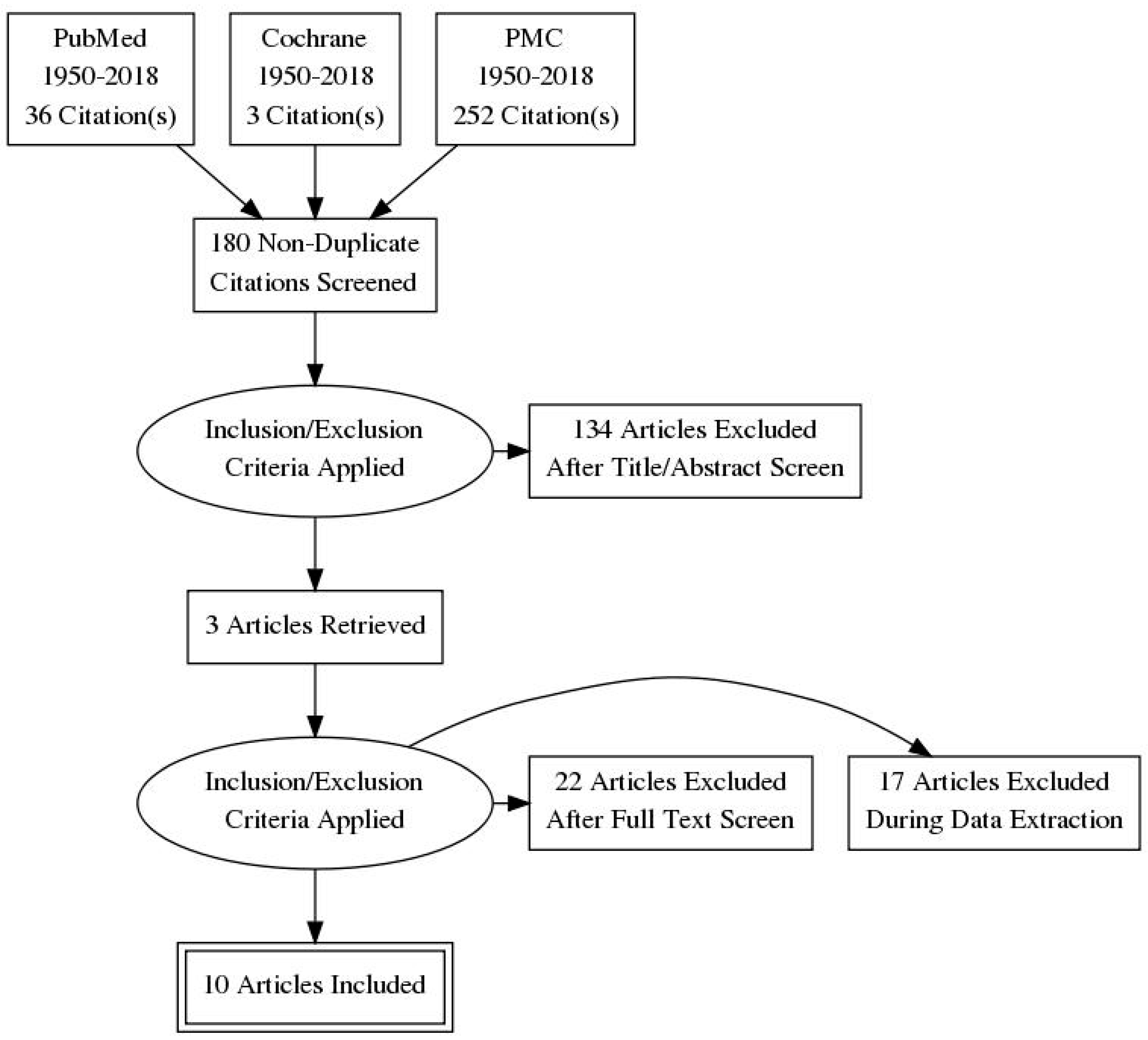
2. Research Methods
3. Results
3.1. Outcome of Pain Improvement
3.2. Outcome of Joint Sound
3.3. Mandibular Motion Outcome
3.4. Risk of Bias Assessment
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gencer, Z.K.; Özkiriş, M.; Okur, A.; Korkmaz, M.; Saydam, L. A comparative study on the impact of intra-articular injections of hyaluronic acid, tenoxicam and betametazon on the relief of temporomandibular joint disorder complaints. J. Cranio-Maxillofac. Surg. 2014, 42, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Al-Delayme, R.M.A.; Alnuamy, S.H.; Hamid, F.T.; Azzamily, T.J.; Ismaeel, S.A.; Sammir, R.; Hadeel, M.; Nabeel, J.; Shwan, R.; Alfalahi, S.J.; et al. The Efficacy of Platelets Rich Plasma Injection in the Superior Joint Space of the Tempromandibular Joint Guided by Ultra Sound in Patients with Non-reducing Disk Displacement. J. Maxillofac. Oral Surg. 2017, 16, 43–47. [Google Scholar] [CrossRef]
- Gay-Escoda, C. Eminectomy associated with redirectioning of the temporal muscle for treatment of recurrent TMJ dislocation. J. Cranio-Maxillo-Facial Surg. 1987, 15, 355–358. [Google Scholar] [CrossRef]
- Güven, O. Inappropriate treatments in temporomandibular joint chronic recurrent dislocation: A literature review presenting three particular cases. J. Craniofacial Surg. 2005, 16, 449–452. [Google Scholar] [CrossRef]
- Vasconcelos, B.-C.; Porto, G.-G.; Neto, J.-P.-M.-R.; Vasconcelos, C.-F. Treatment of chronic mandibular dislocations by eminectomy: Follow-up of 10 cases and literature review. Med. Oral Patol. Oral Cir. Bucal 2009, 14, e593–e596. [Google Scholar] [CrossRef]
- Koh, K.-J.; Park, H.-N.; Kim, K.-A. Relationship between anterior disc displacement with/without reduction and effusion in temporomandibular disorder patients using magnetic resonance imaging. Imaging Sci. Dent. 2013, 43, 245. [Google Scholar] [CrossRef] [PubMed]
- Taşkaya-Yilmaz, N.; Oğütcen-Toller, M. Magnetic resonance imaging evaluation of temporomandibular joint disc deformities in relation to type of disc displacement. J. Oral Maxillofac. Surg. 2001, 59, 860–865. [Google Scholar] [CrossRef]
- Comert Kilic, S.; Gungormus, M.; Cömert Kiliç, S.; Güngörmüş, M. Is Arthrocentesis Plus Platelet-Rich Plasma Superior to Arthrocentesis Alone in the Treatment of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2016, 45, 1538–1544. [Google Scholar] [CrossRef]
- Cömert Kiliç, S.; Güngörmüş, M.; Sümbüllü, M.A. Is Arthrocentesis Plus Platelet-Rich Plasma Superior to Arthrocentesis Alone in the Treatment of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2015, 73, 1473–1483. [Google Scholar]
- Hanc, M.; Karamese, M.; Tosun, Z.; Murad, T.; Hancı, M.; Karamese, M.; Tosun, Z.; Aktan, T.M.; Duman, S.; Savaci, N. Intra-articular platelet-rich plasma injection for the treatment of temporomandibular disorders and a comparison with arthrocentesis. J. Cranio-Maxillofac. Surg. 2015, 43, 162–166. [Google Scholar] [CrossRef]
- Mehrotra, D. TMJ Bioengineering: A review. J. Oral Biol. Craniofacial Res. 2013, 3, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Weng, Y.; Guo, S.; Zhang, Y.; Zhou, T.; Zhang, M.; Wang, L.; Ma, J. Platelet-rich plasma inhibits RANKL-induced osteoclast differentiation through activation of Wnt pathway during bone remodeling. Int. J. Mol. Med. 2018, 41, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Robotti, G.; Canepa, M.G.; Bortolotto, C.; Draghi, F. Interventional musculoskeletal US: An update on materials and methods. J. Ultrasound 2013, 16, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.T.; Borg-Stein, J.; McInnis, K. Applications of Platelet-Rich Plasma in Musculoskeletal and Sports Medicine: An Evidence-Based Approach. PmR 2011, 3, 226–250. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.F.; Ali, H.E.; Elmasry, M.; Khallaf, M.G. Platelet-Rich Plasma Injection as an Effective Treatment for Temporomandibular Joint Osteoarthritis. J. Oral Maxillofac. Surg. 2015, 73, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Escoda-Francolí, J.; Vázquez-Delgado, E.; Gay-Escoda, C. Scientific evidence on the usefulness of intraarticular hyaluronic acid injection in the management of temporomandibular dysfunction. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e644–e648. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chen, G.; Cheng, A.H.; Cheng, Y.; Deng, M.; Cai, H.; Meng, Q. A Randomized Controlled Trial of Superior and Inferior Temporomandibular Joint Space Injection With Hyaluronic Acid in Treatment of Anterior Disc Displacement Without Reduction. J. Oral Maxillofac. Surg. 2009, 67, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Morey-Mas, M.-A.; Caubet-Biayna, J.; Varela-Sende, L.; Iriarte-Ortabe, J.-I. Sodium Hyaluronate Improves Outcomes After Arthroscopic Lysis and Lavage in Patients With Wilkes Stage III and IV Disease. J. Oral Maxillofac. Surg. 2010, 68, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Altman, D.G. Systematic Reviews in Health Care: Meta-Analysis in Context; BMJ Books; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 0470693142. [Google Scholar]
- Giacomello, M.; Giacomello, A.; Mortellaro, C.; Gallesio, G.; Mozzati, M. Temporomandibular joint disorders treated with articular injection: The effectiveness of plasma rich in growth factors-Endoret. J. Craniofacial Surg. 2015, 26, 709–713. [Google Scholar] [CrossRef]
- Fernández-Ferro, M.; Fernández-Sanromán, J.; Blanco-Carrión, A.; Costas-López, A.; López-Betancourt, A.; Arenaz-Bua, J.; Stavaru Marinescu, B.; Nishimura, M.; Segami, N.; Kaneyama, K.; et al. Comparison of intra-articular injection of plasma rich in growth factors versus hyaluronic acid following arthroscopy in the treatment of temporomandibular dysfunction: A randomised prospective study. J. Cranio-Maxillofac. Surg. 2017, 45, 449–454. [Google Scholar]
- Pihut, M.; Szuta, M.; Ferendiuk, E.; Zeńczak-Więckiewicz, D. Evaluation of Pain Regression in Patients with Temporomandibular Dysfunction Treated by Intra-Articular Platelet-Rich Plasma Injections: A Preliminary Report. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Huang, Y.-C.; Wu, S.-L.; Ko, S.-Y.; Tsai, C.-C. Clinical efficacy of a centric relation occlusal splint and intra-articular liquid phase concentrated growth factor injection for the treatment of temporomandibular disorders. Medicine 2017, 96, e6302. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-L.; Tsai, C.-C.; Wu, S.-L.; Ko, S.-Y.; Chiang, W.-F.; Yang, J.W. Effect of arthrocentesis plus platelet-rich plasma and platelet-rich plasma alone in the treatment of temporomandibular joint osteoarthritis: A retrospective matched cohort study (A STROBE-compliant article). Medicine 2018, 97, e0477. [Google Scholar] [CrossRef] [PubMed]
- Talmaceanu, D.; Lenghel, L.M.; Bolog, N.; Hedesiu, M.; Buduru, S.; Rotar, H.; Baciut, M.; Baciut, G. Imaging modalities for temporomandibular joint disorders: An update. Clujul Med. 2018, 91, 280. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofacial Implants 1999, 14, 529–535. [Google Scholar]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Fiz, N.; Azofra, J.; Usabiaga, J.; Aduriz Recalde, E.; Garcia Gutierrez, A.; Albillos, J.; Gárate, R.; Aguirre, J.J.; Padilla, S.; et al. A Randomized Clinical Trial Evaluating Plasma Rich in Growth Factors (PRGF-Endoret) Versus Hyaluronic Acid in the Short-Term Treatment of Symptomatic Knee Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Lei, X.X.; Xuan, L.; Tang, J.B.; Cheng, B. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and anti-aging proteins in platelet-rich plasma. Platelets 2018, 1–7. [Google Scholar] [CrossRef]
- Lim, W.; Park, S.H.; Kim, B.; Kang, S.W.; Lee, J.W.; Moon, Y.L. Relationship of cytokine levels and clinical effect on platelet-rich plasma-treated lateral epicondylitis. J. Orthop. Res. 2017, 36, 913–920. [Google Scholar] [CrossRef]
- Peerbooms, J.C.; Sluimer, J.; Bruijn, D.J.; Gosens, T. Positive Effect of an Autologous Platelet Concentrate in Lateral Epicondylitis in a Double-Blind Randomized Controlled Trial. Am. J. Sports Med. 2010, 38, 255–262. [Google Scholar] [CrossRef]
- F Hegab, A.; Shuman, M.A. Efficacy of Platelet-Rich Plasma in Reduction of the Resorption of the Alveolar Cleft Bone Graft. A Comparative Study. Dentistry 2012, 2. [Google Scholar] [CrossRef]
- Jacobson, A. Orofacial Pain: Guidelines for Assessment, Diagnosis and Management, 4th ed.; de Leeuw, R., Ed.; Quintessence: Chicago, IL, USA, 2008; p. 318. [Google Scholar]
- Woodell-May, J.E.; Pietrzak, W.S. Platelet-Rich Plasma in Orthopedics. In Musculoskeletal Tissue Regeneration; Humana Press: Totowa, NJ, USA, 2008; pp. 547–568. [Google Scholar]
- Woodall, J.; Tucci, M.; Mishra, A.; Asfour, A.; Benghuzzi, H. Cellular effects of platelet rich plasmainterleukin1 release from prp treated macrophages. Biomed. Sci. Instrum. 2008, 44, 489–494. [Google Scholar] [PubMed]
- Kutuk, N.; Bas, B.; Soylu, E.; Gonen, Z.B.; Yilmaz, C.; Balcioglu, E.; Ozdamar, S.; Alkan, A.; Kütük, N.; Baş, B.; et al. Effect of platelet-rich plasma on fibrocartilage, cartilage, and bone repair in temporomandibular joint. J. Oral Maxillofac. Surg. 2014, 72, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Buda, R.; Filardo, G.; Di Martino, A.; Timoncini, A.; Cenacchi, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma: Intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Sampson, S.; Reed, M.; Silvers, H.; Meng, M.; Mandelbaum, B. Injection of Platelet-Rich Plasma in Patients with Primary and Secondary Knee Osteoarthritis. Am. J. Phys. Med. Rehabil. 2010, 89, 961–969. [Google Scholar] [CrossRef]
- Spaková, T.; Rosocha, J.; Lacko, M.; Harvanová, D.; Gharaibeh, A. Treatment of Knee Joint Osteoarthritis with Autologous Platelet-Rich Plasma in Comparison with Hyaluronic Acid. Am. J. Phys. Med. Rehabil. 2012, 91, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Raeissadat, S.A.; Rayegani, S.M.; Hassanabadi, H.; Fathi, M.; Ghorbani, E.; Babaee, M.; Azma, K. Knee Osteoarthritis Injection Choices: Platelet-Rich Plasma (PRP) versus Hyaluronic Acid (A one-year randomized clinical trial). Clin. Med. Insights Arthr. Musculoskelet. Disord. 2015, 8, CMAMD.S17894. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Moskowitz, R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: A randomized clinical trial. Hyalgan Study Group. J. Rheumatol. 1998, 25, 2203–2212. [Google Scholar]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthr. Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Nurden, A.T.; Zalduendo, M.M.; de la Fuente, M.; Azofra, J.; Andia, I. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology 2007, 46, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
| Step | General Activities | Specific Activities |
|---|---|---|
| I | Formation of working group | One maxillofacial surgeon expert in arthrocentesis of TMJ, as clinical and methodological operator |
| One medical doctor expert in head and neck anathomy and PRP, as clinical and methodological operator | ||
| One researcher expert in TMJ disorders, as methodological operator | ||
| II | Formulation of the review questions | Evaluation of the state of art inTMJ osteoarthritis and arthrocentesis. Analysis of main effects of osteoarthritis in TMJ and its treatment |
| III | Identification of relevant studies on PubMed, PMC, Cochrane | 1. Identification of keywords in the field of interest 2. Use of Boolean operators (AND; OR; NOT) 3. Advanced search (Table 2 for serach strategy) 4. Inclusion criteria: papers published from 1950 to 2018; language: english; all types of full text articles 5. Elimination of duplicate 6. Manual search through the references in selected articles |
| IV | Anaysis and presentation | Data extrapolated from all revised studies were shown in tables in form of narrative review |
| Search | Database |
|---|---|
| ((“platelet-rich plasma”[MeSH Terms] OR (“platelet-rich”[All Fields] AND “plasma”[All Fields]) OR “platelet-rich plasma”[All Fields] OR (“platelet”[All Fields] AND “rich”[All Fields] AND “plasma”[All Fields]) OR “platelet rich plasma”[All Fields]) AND (“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields] OR “tmj”[All Fields])) NOT (extraction[All Fields] AND (“tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields])) | PMC |
| (((“joint diseases”[MeSH Terms] OR (“joint”[All Fields] AND “diseases”[All Fields]) OR “joint diseases”[All Fields] OR “arthrosis”[All Fields]) AND (“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields] OR “tmj”[All Fields])) AND ((“arthritis”[MeSH Terms] OR “arthritis”[All Fields]) AND (“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields] OR “tmj”[All Fields]))) AND (“platelet-rich plasma”[MeSH Terms] OR (“platelet-rich”[All Fields] AND “plasma”[All Fields]) OR “platelet-rich plasma”[All Fields] OR (“platelet”[All Fields] AND “rich”[All Fields] AND “plasma”[All Fields]) OR “platelet rich plasma”[All Fields]) | PMC |
| ((((“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields]) AND (“osteoarthritis”[MeSH Terms] OR “osteoarthritis”[All Fields])) AND (“osteoarthritis”[MeSH Terms] OR “osteoarthritis”[All Fields] OR “osteoarthrosis”[All Fields])) AND (disk[All Fields] AND (“displacement (psychology)”[MeSH Terms] OR (“displacement”[All Fields] AND “(psychology)”[All Fields]) OR “displacement (psychology)”[All Fields] OR “displacement”[All Fields]))) AND (“platelet-rich plasma”[MeSH Terms] OR (“platelet-rich”[All Fields] AND “plasma”[All Fields]) OR “platelet-rich plasma”[All Fields] OR (“platelet”[All Fields] AND “rich”[All Fields] AND “plasma”[All Fields]) OR “platelet rich plasma”[All Fields]) | PMC |
| (((“joint diseases”[MeSH Terms] OR (“joint”[All Fields] AND “diseases”[All Fields]) OR “joint diseases”[All Fields] OR “arthrosis”[All Fields]) AND (“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields] OR “tmj”[All Fields])) AND ((“arthritis”[MeSH Terms] OR “arthritis”[All Fields]) AND (“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields] OR “tmj”[All Fields]))) AND (“platelet-rich plasma”[MeSH Terms] OR (“platelet-rich”[All Fields] AND “plasma”[All Fields]) OR “platelet-rich plasma”[All Fields] OR (“platelet”[All Fields] AND “rich”[All Fields] AND “plasma”[All Fields]) OR “platelet rich plasma”[All Fields]) | PubMed |
| ((Therapy/Broad[filter] AND ((“platelet-rich plasma”[MeSH Terms] OR (“platelet-rich”[All Fields] AND “plasma”[All Fields]) OR “platelet-rich plasma”[All Fields] OR (“platelet”[All Fields] AND “rich”[All Fields] AND “plasma”[All Fields]) OR “platelet rich plasma”[All Fields]) AND maxillofacial[All Fields])) NOT extraction[All Fields]) AND ((“osteonecrosis”[MeSH Terms] OR “osteonecrosis”[All Fields]) AND (“jaw”[MeSH Terms] OR “jaw”[All Fields])) | PubMed |
| (((“platelet-rich plasma”[MeSH Terms] OR (“platelet-rich”[All Fields] AND “plasma”[All Fields]) OR “platelet-rich plasma”[All Fields] OR (“platelet”[All Fields] AND “rich”[All Fields] AND “plasma”[All Fields]) OR “platelet rich plasma”[All Fields]) AND (“temporomandibular joint disorders”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields] AND “disorders”[All Fields]) OR “temporomandibular joint disorders”[All Fields] OR (“tmj”[All Fields] AND “disorders”[All Fields]) OR “tmj disorders”[All Fields])) AND maxillofacial[All Fields]) NOT (extraction[All Fields] AND (“tooth”[MeSH Terms] OR “tooth”[All Fields] OR “teeth”[All Fields])) | PubMed |
| ((“temporomandibular joint”[MeSH Terms] OR (“temporomandibular”[All Fields] AND “joint”[All Fields]) OR “temporomandibular joint”[All Fields] OR “tmj”[All Fields]) AND (“Pharmacol Res Perspect”[Journal] OR “prp”[All Fields])) AND (“surgery”[Subheading] OR “surgery”[All Fields] OR “surgical procedures, operative”[MeSH Terms] OR (“surgical”[All Fields] AND “procedures”[All Fields] AND “operative”[All Fields]) OR “operative surgical procedures”[All Fields] OR “surgery”[All Fields] OR “general surgery”[MeSH Terms] OR (“general”[All Fields] AND “surgery”[All Fields]) OR “general surgery”[All Fields]) | PubMed |
| tmj, arthrocentesis, prp, osteoarthritis:ti, ab, kw (Word variations have been searched) | Cochrane |
| Subgroups | Outcome | Topic | Specific Keywords Searched in Papers |
|---|---|---|---|
| 1 | Signs and symptoms improvement | Pain, Mandibular motion, Joint sounds | “Pain”; “Maximum mouth opening”; “VAS scale”; “Joint sound”; “Chewing”; “Stiff”, “Mandibular mobility” |
| 2 | Effectiveness of PRP-associated arthrocentesis | Arthrocentesis alone, Arthrocentesis hyaluronic acid (HA) associated, Arthrocentesis PRP associated, Arthrocentesis associated with other biomaterials or drugs | “Arthrocentesis”; “Platelet-rich-plasma”; “Hyaluronic acid”, “Corticosteroid”, “Growing factors” |
| Studies | Affection | Study Design | Diagnostic Criteria | Intervention | Sample | Endpoint | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|
| Gicomello et al., 2014 | Osteoarthritis, Non-reducing Anterior Displacement | Observational Study | Ortopantomography and magnetic resonance imaging (MRI) | 2 PRP injections at 30 days | SG, N = 13, Mean age: 47.64 | 6 months | Pain (visual analogue scale, VAS); Mandibular opening (MMO) | Pain improvement from 7.69 ± 1.9 to 0.23 ± 0.63 (*). MMO improvement from 30.15 ± 4.44 to 39.54 ± 4.55 (*) |
| Hanci et al., 2014 | Reducing Anterior Displacement | Randomized Controlled Trial (RCT) | MRI | SG: 1 singole PRP Injection, CG: Arthrocentesis | SG, N = 10, Mean age: 27.2. CG, N = 10, Mean age: 25.4 | 6 months | Pain (VAS); Minimal Interincisal Opening (MIO); Joint Sound (Number of Joints affected) | Pain improvement: SG from 6.69 ± 2.21 to 0.07 ± 0.27. CG: from 6.52 ± 2.29 to 2.76 ± 1.48 (*). MIO improvement: SG from 32 ± 8.53 to 39.7 ± 10.39. CG from 30.2 ± 9.41 to 36.3 ± 5.51 (**). Joint Sound improvement: SG: from 12 to 2. CG from 12 to 5 (*) |
| Pihut et al., 2014 | Temporomandibular disfunctions | Clinical Study (Preliminary) | Clinical (RDC/TMD questionnaire) | PRP injection | SG, N = 10, Mean age: 37.6 | 6 weeks | Pain (VAS); Mandibular Motion (MM); Joint Sound (Number of patients) | Pain improvement: from 6.5 to 0.6 (*); MM decrease to 1 (not clear); Joint Sound: from 4 to 1 |
| Hegab et al., 2015 | Osteoarthritis | RCT | Radiography or MRI | SG: 3 PRP Injections, CG: 3 HA Injections | SG: N = 25, Mean age: 39. CG: N = 25, Mean age: 38.2 | 12 months | Pain (VAS); Mouth Voluntary Opening (MVMO); Joint Sound (Number of Joints affected) | Pain improvement: SG from 7.36 ± 1.14 to 0.4 ± 0.763. CG: from 6.96 ± 1.24 to 1.64 ± 1.35 (*). MVMO improvement: SG from 33.88 ± 3.08 to 41.56 ± 2.31. CG from 32.40 ± 2.72 to 39.28 ± 2.80 (*). Joint Sound improvement: SG > CG at 1 month, SG = CG at 12 months (**) |
| Al-Delayme et al., 2016 | Non-reducing Anterior Displacement | Observational Study | Bilateral palpation and measurement of mouth opening | 2 PRP injections | SG: N = 44, Mean age: 36.6 | 6 months | Pain (VAS); Maximum Mouth Opening (MMO); Joint Sound (VAS) | Pain improvement from 33.5 ± 22.4 to 18.3 ± 17.9 (*). MMO improvement from 26.4 ± 11.3 to 41.5 ± 8.65 (*). Joint Sound imprvement from 79.3 ± 12.8 to 2.9 ± 15 (*) |
| Kiliç et al., 2015 | Osteoarthritis | RCT | Clinical (DC/TMDs) and CBCT | SG: Arthrocentesis + PRP 4 monthly PRP Injections, CG: Arthrocentesis | SG: N = 18, 32 Joints, Mean age: 32.22. CG: N = 12, 15 Joints, Mean age: 35.08 | 12 months | Pain (VAS); Maximum Mouth Opening (MMO) (VAS); Joint Sound (VAS) | Pain improvement: SG from 5.70 ± 1.35 to 1.02 ± 1.88. CG: from 6.83 ± 2.28 to 2.43 ± 4.08 (*). MMO improvement: SG: from 38.72 ± 7.84 to 38.39 ± 8.02 (**). Joint Sound: SG from 5.48 ± 3.46 to 0.70 ± 0.85. CG from 5.45 ± 3.27 to 0.75 ± 1.42 (*) |
| Kiliç et al., 2016 | Osteoarthritis | RCT | Clinical (DC/TMDs) and CBCT | SG: Artrocentesis+PRP and 4 PRP Injections. CG: 1 singole Arthrocentesis + HA | SG: N = 18, 32 Joints, Mean age: 32.22. CG: N = 13, 17 Joints, Mean age: 28.08 | 12 months | Pain (VAS); Maximum Mouth Opening (MMO) (VAS); Joint Sound (VAS) | Pain improvement: SG from 5.70 ± 1.35 to 1.02 ± 1.88. CG: from 5.71 ± 2.54 to 0.54 ± 0.87 (*). MMO imrovement: SG from 38.72 ± 7.84 to 38.39 ± 8.02. CG from 44.23 ± 8.14 to 43.77 ± 6.39 (*). Joint Sound: SG from 5.48 ± 3.46 to 0.70 ± 0.85. CG from 5.81 ± 3.16 to 1.81 ± 3.04 (*). |
| Fernandez-Ferro et al., 2017 | Osteoarthritis, Reducing or Non-reducing disc displacement | Randomized Prospective Study | MRI | SG: PRP Injection; CG: HA Injection | SG: N = 50, Mean age: 38.4. CG:N = 50, Mean age: 33.2 | 18 months | Pain (VAS); Mouth Opening (MO) | Pain improvement: SG from 8.35 ± 0.90 to 1.55 ± 1.90. CG from 8.14 ± 0.60 to 2.20 ± 1.43 (*). MO improvement: SG from 27.74 ± 4.65 to 37.23 ± 4.94. CG from 27.92 ± 5.08 to 36.54 ± 5.78 (**) |
| Yang et al., 2017 | Non-reducing disc displacement | Retrospective Cohort Study | Clinical (DC/TMDs) and MRI | SG: LPCGF Injection + Centric Relation Occlusal Splint (Cros) | SG: N = 29, Mean age: 39.55 | 24 monts | Pain (VAS); Joint Sound (Number of joints affetcted) | Pain improvement from 4.72 ± 2.58 to 1.10 ± 1.72. Joint Sound improvement from 36 to 10 (*). |
| Lin et al., 2018 | Osteoarthritis | Retrospective Study | Clinical (DC/TMDs) and CBCT | SG: Arthrocentesis + PRP: CG: PRP Injection | SG: N = 30, Mean age: 42.73; CG: N = 60, Mean age: 38.73 | 12 months | Pain (VAS); Range motion (>6 mm); Mouth Assisted Opening (MAO); Joint Crepitus Sound (Number of patients) | Pain improvement: SG = CG no improvement. Range Motion: SG from 47% to 0%, CG from 20% to 2% (*). MAO: SG and CG no improvementJoint crepitus sound: SG from 100% to 47% (**). SG vs. CG: differences not statistically significant. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotti, F.; Albanese, M.; Rodella, L.F.; Nocini, P.F. Platelet-Rich Plasma in Treatment of Temporomandibular Joint Dysfunctions: Narrative Review. Int. J. Mol. Sci. 2019, 20, 277. https://doi.org/10.3390/ijms20020277
Zotti F, Albanese M, Rodella LF, Nocini PF. Platelet-Rich Plasma in Treatment of Temporomandibular Joint Dysfunctions: Narrative Review. International Journal of Molecular Sciences. 2019; 20(2):277. https://doi.org/10.3390/ijms20020277
Chicago/Turabian StyleZotti, Francesca, Massimo Albanese, Luigi Fabrizio Rodella, and Pier Francesco Nocini. 2019. "Platelet-Rich Plasma in Treatment of Temporomandibular Joint Dysfunctions: Narrative Review" International Journal of Molecular Sciences 20, no. 2: 277. https://doi.org/10.3390/ijms20020277
APA StyleZotti, F., Albanese, M., Rodella, L. F., & Nocini, P. F. (2019). Platelet-Rich Plasma in Treatment of Temporomandibular Joint Dysfunctions: Narrative Review. International Journal of Molecular Sciences, 20(2), 277. https://doi.org/10.3390/ijms20020277








