Pituitary Hormones (FSH, LH, PRL, and GH) Differentially Regulate AQP5 Expression in Porcine Ovarian Follicular Cells
Abstract
1. Introduction
2. Results
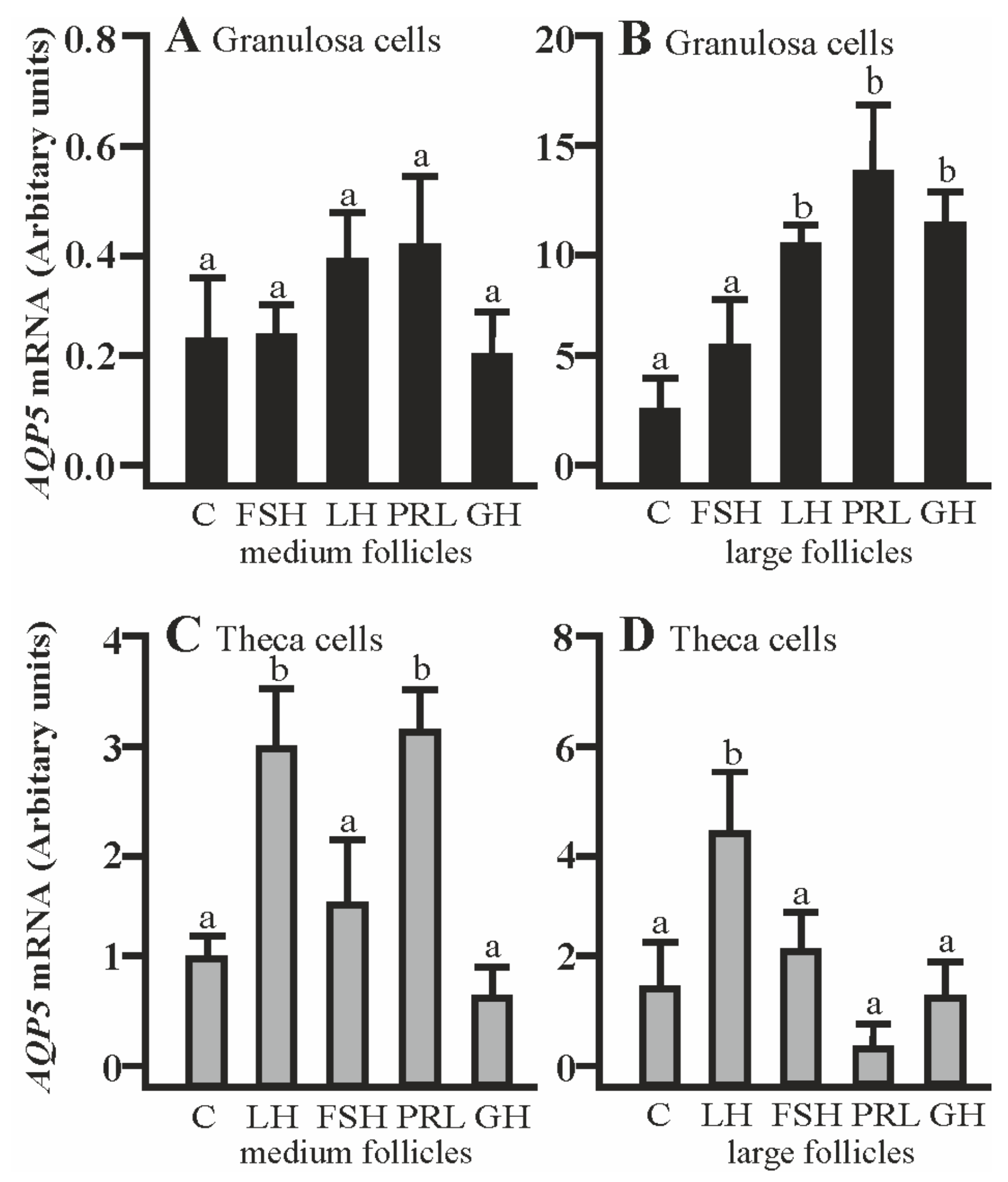
2.1. The Effects of FSH, LH, PRL, and GH on AQP5 mRNA Expression in Gc and Tc Cells from the Medium (MF) and Large (LF) Follicles
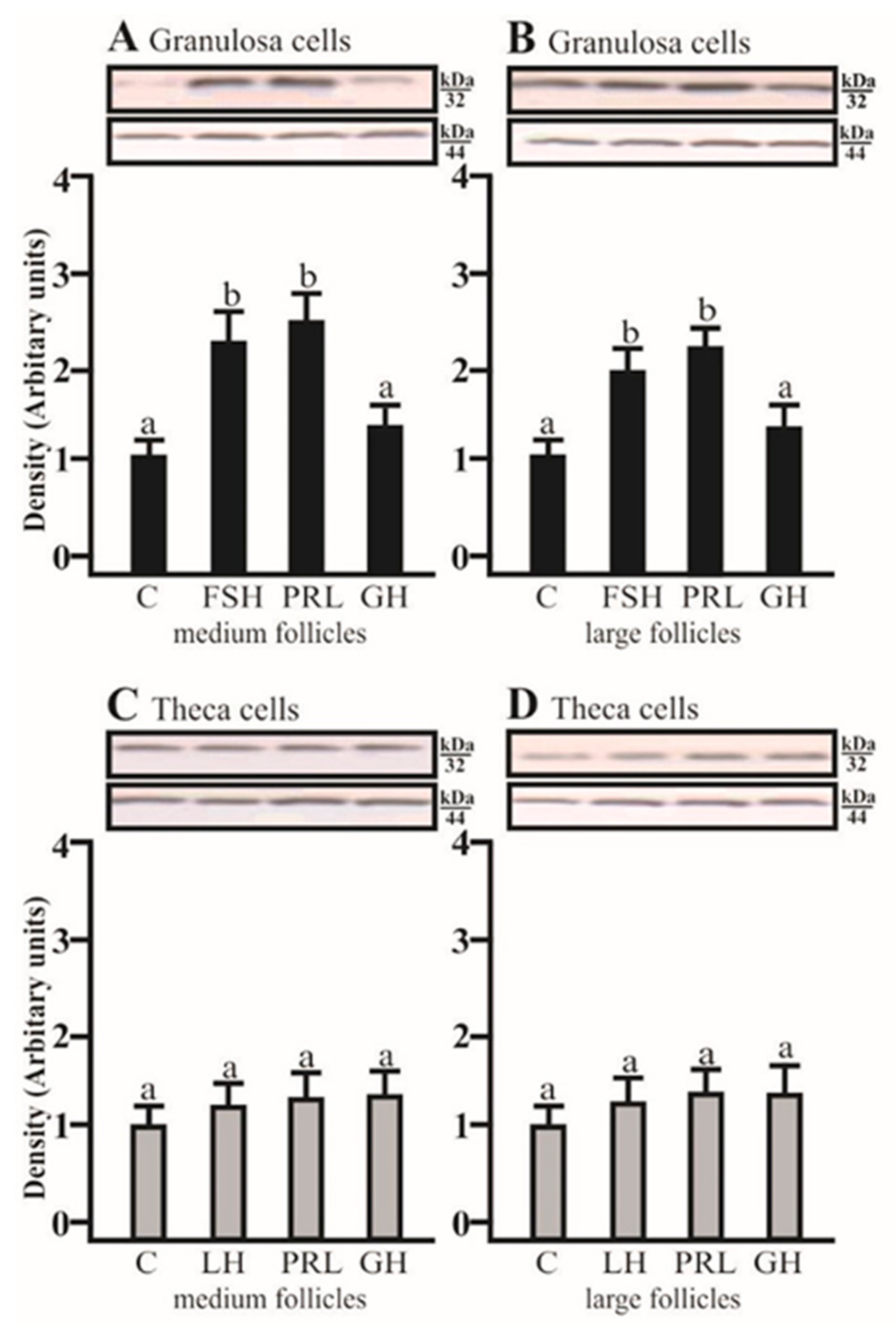
2.2. The Effects of FSH, LH, PRL, and GH on AQP5 Protein Expression in the Gc and Tc Cells from MF and LF
2.3. The Effects of FSH, LH, PRL, and GH on AQP5 mRNA Expression in the Co-Culture of Gc and Tc Cells from MF and LF
2.4. The Effects of FSH, LH, PRL, and GH on AQP5 Protein Expression in the Co-Culture of Gc and Tc Cells from MF and LF
2.5. The Effects of FSH, LH, PRL, and GH on AQP5 Protein Expression in the Culture of Gc and Tc Cells from MF and LF
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Experimental Design
4.2. RNA Extraction and Real-Time qPCR
4.3. SDS-PAGE and Western Blot Analysis
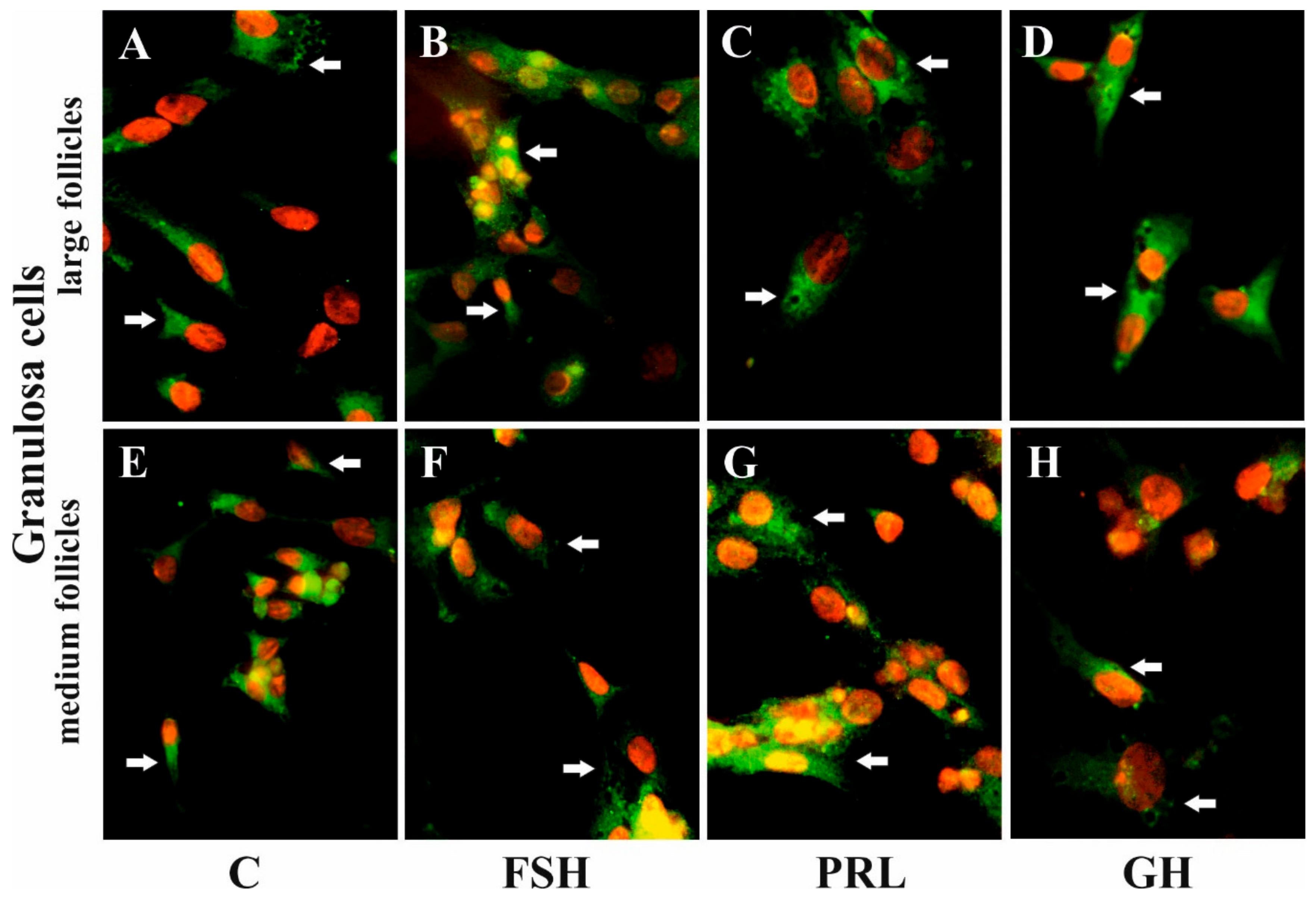
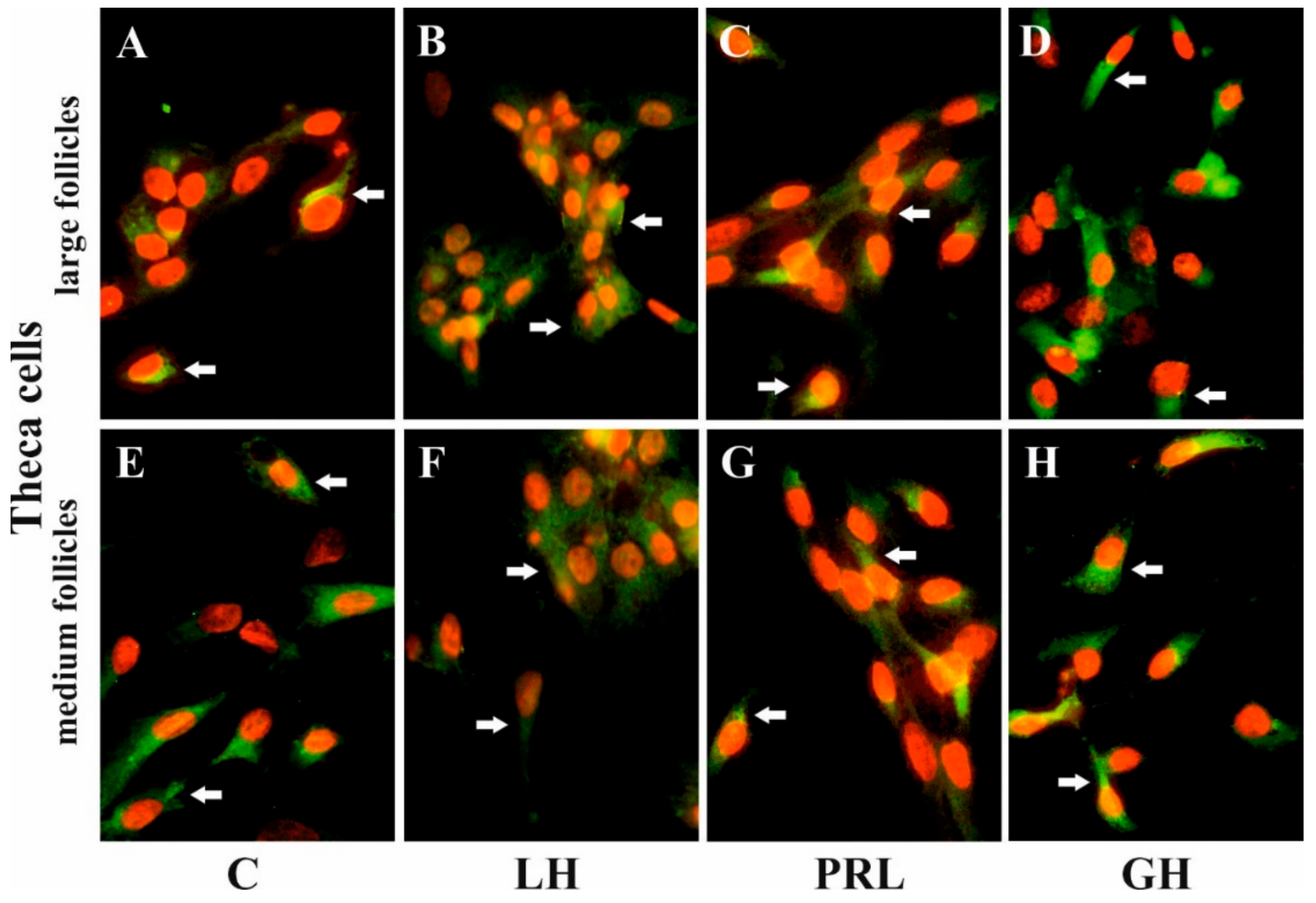
4.4. Immunofluorescence
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AQP5 | Aquaporin 5 |
| FSH | Follicle-stimulating hormone |
| LH | Luteinising hormone |
| PRL | Prolactin |
| GH | Growth hormone |
| Gc | Granulosa cells |
| Tc | Theca cells |
| MF | Medium follicles |
| LF | Large follicles |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| PPIA | Cyclophylin A |
References
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins. Curr. Biol. 2013, 23, R52–R55. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Renal. Physiol. 2000, 278, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Carbrey, J.M.; Agre, P. Discovery of the aquaporins and development of the field. Handb. Exp. Pharm. 2009, 3–28. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Lebeck, J.; Rojek, A.; Praetorius, J.; Füchtbauer, E.M.; Frøkiaer, J.; Nielsen, S. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: implications in glycerol metabolism. Am. J. Physiol. Renal. Physiol. 2007, 292, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.M.; Skowronski, M.T.; Füchtbauer, E.M.; Füchtbauer, A.C.; Fenton, R.A.; Agre, P.; Frøkiaer, J.; Nielsen, S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. USA 2007, 104, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Shono, M.; Ishikawa, Y. Mechanisms Underlying Activation of α1-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions. Int. J. Mol. Sci. 2017, 17, 1022. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Koide, S.S. The water channel gene in human uterus. Biochem. Mol. Biol. Int. 1994, 32, 371–377. [Google Scholar] [PubMed]
- Escobar, J.; Gormaz, M.; Arduini, A.; Gosens, K.; Martinez, A.; Perales, A.; Escrig, R.; Tormos, E.; Roselló, M.; Orellana, C.; et al. Expression of aquaporins early in human pregnancy. Early Human Develop. 2012, 88, 589–594. [Google Scholar] [CrossRef]
- Klein, C.; Troedsson, M.H.; Rutllant, J. Expression of aquaporin water channels in equine endometrium is differentially regulated during the oestrous cycle and early pregnancy. Reprod. Domest. Anim. 2012, 48, 529–537. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takahashi, E.; Miyagawa, S.; Watanabe, H.; Iguichi, T. Chromatin immunoprecipitation mediated target identification proved aquaporin 5 is regulated directly by estrogen in the uterus. Genes Cells 2006, 11, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.A.; Murphy, C.R. Redistribution of aquaporins 1 and 5 in the rat uterus is dependent on progesterone: a study with light and electron microscopy. Reprod 2006, 131, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Gao, J.; Brown, N.; Reese, J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology 2003, 144, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, E.M.; McConnell, N.A.; Hughes, F.M., Jr.; Huet-Hudson, Y.M. Estrogen regulation of aquaporins in the mouse uterus: potential roles in uterine water movement. Biol. Reprod. 2003, 69, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, A.; Lalitkumar, L.; Nielsen, S.; Genzell-Danilsson, K.; Stavreus- Evers, A. Expression of aquaporin 2 in human endometrium. Fertil. Steril. 2006, 86, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.B.; Zhang, R.J.; Tan, Y.J.; Ding, G.L.; Shi, S.; Zhang, D.; He, R.H.; Liu, A.X.; Wang, T.T.; Leung, P.C.K.; et al. Identification of Estrogen Response Element in the Aquaporin-2 Gene That Mediates Estrogen-Induced Cell Migration and Invasion in Human Endometrial Carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Ducza, E.; Seres, A.B.; Hajagos-Tóth, J.; Falkay, G.; Gáspár, R. Oxytocin regulates the expression of aquaporin 5 in the late-pregnant rat uterus. Mol. Reprod. Dev. 2014, 81, 524–530. [Google Scholar] [CrossRef]
- Csányi, A.; Bóta, J.; Falkay, G.; Gáspár, R.; Ducza, E. The Effects of Female Sexual Hormones on the Expression of Aquaporin 5 in the Late-Pregnant Rat Uterus. Int. J. Mol. Sci. 2016, 17, 1300. [Google Scholar] [CrossRef]
- Ducza, E.; Csányi, A.; Gáspár, R. Aquaporins during Pregnancy: Their Function and Significance. Int. J. Mol. Sci. 2017, 18, 2593. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Kwon, T.H.; Nielsen, S. Immunolocalization of aquaporin 1, 5 and 9 in the female pig reproductive system. J. Histochem. Cytochem. 2009, 57, 61–67. [Google Scholar] [CrossRef]
- Lindsay, L.A.; Murphy, C.R. Aquaporins are upregulated in glandular epithelium at the time of implantation in the rat. J. Mol. Hist. 2007, 38, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Thoroddsen, A.; Dahm-Kähler, P.; Lind, A.K.; Weijdegård, B.; Lindenthal, B.; Müller, J.; Brännström, M. The water permeability channels aquaporins 1–4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J. Clin. Endocrinol. Metab. 2011, 96, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- McConnell, N.A.; Yunus, R.S.; Gross, S.A.; Bost, K.L.; Clemens, M.G.; Hughes, F.M., Jr. Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology 2002, 143, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, A.; Grzesiak, M.; Mobasheri, A.; Szoltys, M. Immunolocalization of aquaporin 5 during rat ovarian follicle development and expansion of the preovulatory cumulus oophorus. Acta. Histochem. 2014, 116, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Irving-Rodgers, H.F. Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 2010, 82, 1021–1029. [Google Scholar] [CrossRef]
- Skowronski, M.T. Distribution and quantitative changes in amounts of aquaporin 1, 5 and 9 in the pig uterus during the estrous cycle and early pregnancy. Reprod. Biol. Endocrinol. 2010, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Skowronska, A.; Nielsen, S. Fluctuation of aquaporin 1, 5, and 9 expression in the pig oviduct during the estrous cycle and early pregnancy. J. Histochem. Cytochem. 2011, 59, 419–427. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Mlotkowska, P.; Tanski, D.; Lepiarczyk, E.; Oklinski, M.K.; Nielsen, S.; Skowronska, A. Pituitary Gonadotropins, Prolactin and Growth Hormone Differentially Regulate AQP1 Expression in the Porcine Ovarian Follicular Cells. Int. J. Mol. Sci. 2018, 19, 5. [Google Scholar] [CrossRef]
- Britt, K.L.; Findlay, J.K. Estrogen actions in the ovary revisited. J. Endocrinol. 2002, 175, 269–276. [Google Scholar] [CrossRef]
- Filicori, M.; Cognigni, G.E.; Gamberini, E.; Parmegiani, L.; Troilo, E.; Roset, B. Efficacy of low-dose human chorionic gonadotropin alone to complete controlled ovarian stimulation. Fertil. Steril. 2005, 84, 394–401. [Google Scholar] [CrossRef]
- Skowronska, A.; Mlotkowska, P.; Okrasa, S.; Nielsen, S.; Skowronski, M.T. Modulatory effects of steroid hormones, oxytocin, arachidonic acid, forskolin and cyclic AMP on the expression of aquaporin 1 and aquaporin 5 in the porcine uterus during placentation. J. Physiol. Pharmacol. 2016, 67, 311–319. [Google Scholar] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Majewski, M.; Nielsen, S.; Skowronski, M.T. Expression of aquaporin 1 and 5 and their regulation by ovarian hormones, arachidonic acid, forskolin and cAMP during implantation in pigs. Physiol. Res. 2016, 65, 637–650. [Google Scholar]
- Chen, Q.; Peng, H.; Lei, L.; Zhang, Y.; Kuang, H.; Cao, Y.; Shi, Q.X.; Ma, T.; Duan, E. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011, 21, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Zhang, J.; Fan, Y.; Ding, J.H.; Sha, J.H.; Hu, G. Aquaporin-4 deficiency induces subfertility in female mice. Fertil. Steril. 2008, 92, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Qiao, Y.; Yi, F.; Guan, X.; Zhang, D.; Zhang, S.; Hao, F.; Xiao, Y.; Zhang, H.; Guo, L.; et al. Increased female fertility in aquaporin 8-deficient mice. IUBMB Life 2010, 62, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.Y.; Xiong, Z.F.; Liu, H.S.; Zheng, Z.; Ma, T.H. Pregnant phenotype in aquaporin 8-deficient mice. Acta Pharmacol. Sin. 2011, 32, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Eliszewski, M.; Nielsen, S.; Skowronski, M.T. Expression of aquaporin 1, 5 and 9 in the ovarian follicles of cycling and early pregnant pigs. Physiol. Res. 2015, 64, 237–245. [Google Scholar] [PubMed]
- Zhu, C.; Jiang, Z.; Bazer, F.W.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Aquaporins in the female reproductive system of mammals. Front Biosci. (Landmark Ed) 2015, 20, 838–871. [Google Scholar] [PubMed]
- Madej, A.; Lang, A.; Brandt, Y.; Kindhal, H.; Madens, M.T.; Einarsson, S. Factors regulating ovarian function in pigs. Domest. Anim. Endocrinol. 2005, 29, 347–361. [Google Scholar] [CrossRef]
- Gregoraszczuk, E.L.; Gertler, A.; Bylica, A. Response of porcine theca and granulosa cells to GH during short-term in vitro culture. Anim. Reprod. 2000, 58, 113–125. [Google Scholar] [CrossRef]
- Kolodziejczyk, J.; Gertler, A.; Leibovich, H.; Rzasa, J.; Gregoraszczuk, E.L. Synergistic action of growth hormone and insulin-like growth factor I (IGF-I) on proliferation and estradiol secretion in porcine granulosa and theca cells cultured alone or in coculture. Theriogenology 2003, 60, 559–570. [Google Scholar] [CrossRef]
- Devesa, J.; Caicedo, D. The Role of Growth Hormone on Ovarian Functioning and Ovarian Angiogenesis. Front Endocrinol. (Lausanne) 2019, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Q.; Yang, T.; Li, D.; Ding, F.; Sun, H.; Bai, G. RNA interference-mediated silencing of aquaporin (AQP)-5 hinders angiogenesis of colorectal tumor by suppressing the production of vascular endothelial growth factor. Neoplasma 2018, 65, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Breves, J.; Inokuchi, M.; Yamaguchi, Y.; Seale, A.; Watanabe, S.; Lerner, D.; Kaneko, T.; Grau, E. Hormonal regulation of aquaporin 3: Opposing actions of prolactin and cortisol in tilapia gill. J. Endocrinol. 2016, 230, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Squillacioti, C.; Ali, S.; Liguori, G.; Mirabella, N. Cellular distribution of aquaporins in testes of normal and cryptorchid dogs: A preliminary study on dynamic roles. Anim. Reprod. Sci. 2019, 204, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, L.; Zhang, X.; Zhou, Q.; Li, J.M.; Berger, S.; Borok, Z.; Zhou, B.; Xiao, Z.; Yin, H.; et al. Aqp5 is a new transcriptional target of Dot1a and a regulator of Aqp2. PLoS ONE 2013, 8, e53342. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys Acta. 2015, 1848, 2576–2583. [Google Scholar] [CrossRef]
- Madeira, A.; Mósca, A.F.; Moura, T.F.; Soveral, G. Aquaporin-5 is expressed in adipocytes with implications in adipose differentiation. IUBMB Life 2015, 67, 54–60. [Google Scholar] [CrossRef]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968, 10, 1953–1957. [Google Scholar]
- Stokłosowa, S.; Bahr, J.; Gregoraszczuk, E.L. Some morphological and characteristics of cells of the porcine theca interna in tissue culture. Biol. Reprod. 1978, 19, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Nynca, A.; Jablonska, O.; Slomczynska, M.; Petroff, B.K.; Ciereszko, R.E. Effects of phytoestrogen daidzein and estradiol on steroidogenesis and expression of estrogen receptors in porcine luteinized granulosa cells from large follicles. J. Physiol. Pharmacol. 2009, 60, 95–105. [Google Scholar] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowronski, M.T.; Mlotkowska, P.; Tanski, D.; Lepiarczyk, E.; Kempisty, B.; Jaskiewicz, L.; Pareek, C.S.; Skowronska, A. Pituitary Hormones (FSH, LH, PRL, and GH) Differentially Regulate AQP5 Expression in Porcine Ovarian Follicular Cells. Int. J. Mol. Sci. 2019, 20, 4914. https://doi.org/10.3390/ijms20194914
Skowronski MT, Mlotkowska P, Tanski D, Lepiarczyk E, Kempisty B, Jaskiewicz L, Pareek CS, Skowronska A. Pituitary Hormones (FSH, LH, PRL, and GH) Differentially Regulate AQP5 Expression in Porcine Ovarian Follicular Cells. International Journal of Molecular Sciences. 2019; 20(19):4914. https://doi.org/10.3390/ijms20194914
Chicago/Turabian StyleSkowronski, Mariusz T., Patrycja Mlotkowska, Damian Tanski, Ewa Lepiarczyk, Bartosz Kempisty, Lukasz Jaskiewicz, Chandra S. Pareek, and Agnieszka Skowronska. 2019. "Pituitary Hormones (FSH, LH, PRL, and GH) Differentially Regulate AQP5 Expression in Porcine Ovarian Follicular Cells" International Journal of Molecular Sciences 20, no. 19: 4914. https://doi.org/10.3390/ijms20194914
APA StyleSkowronski, M. T., Mlotkowska, P., Tanski, D., Lepiarczyk, E., Kempisty, B., Jaskiewicz, L., Pareek, C. S., & Skowronska, A. (2019). Pituitary Hormones (FSH, LH, PRL, and GH) Differentially Regulate AQP5 Expression in Porcine Ovarian Follicular Cells. International Journal of Molecular Sciences, 20(19), 4914. https://doi.org/10.3390/ijms20194914





