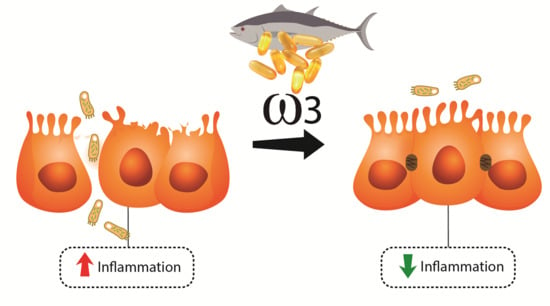
Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview
Abstract
1. Introduction
2. Results
3. Discussion
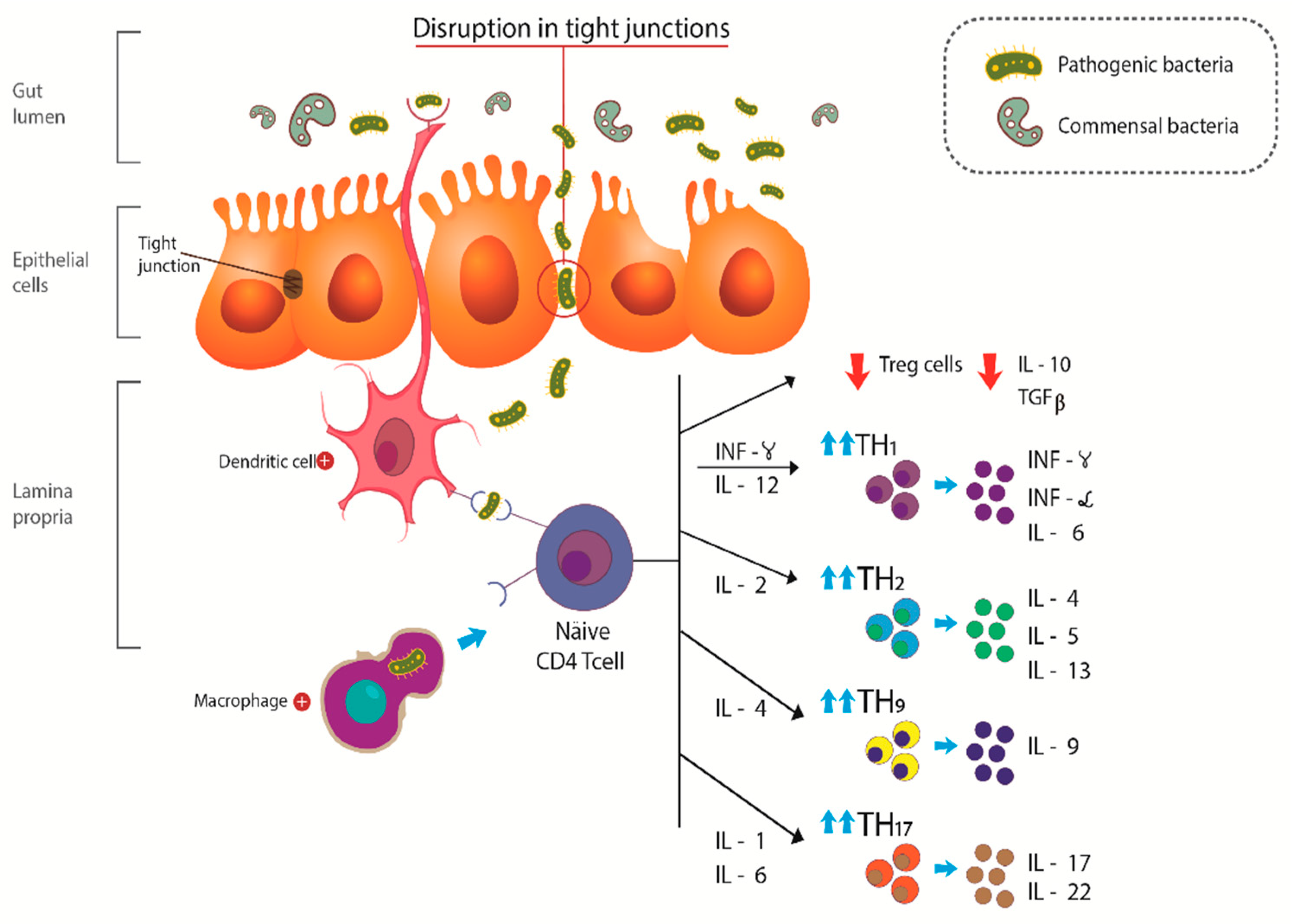
3.1. IBD: Pathophysiologic Aspects
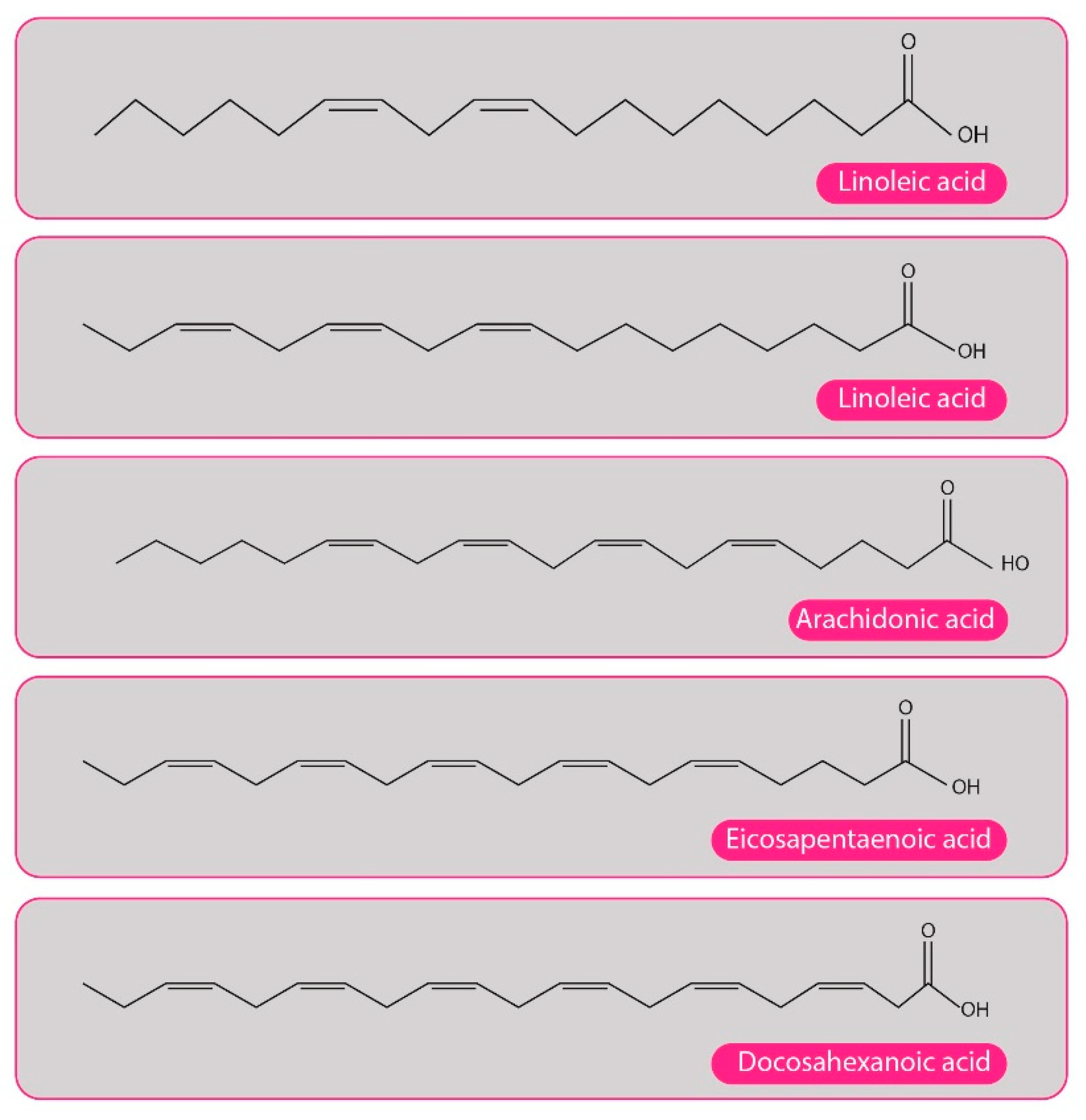
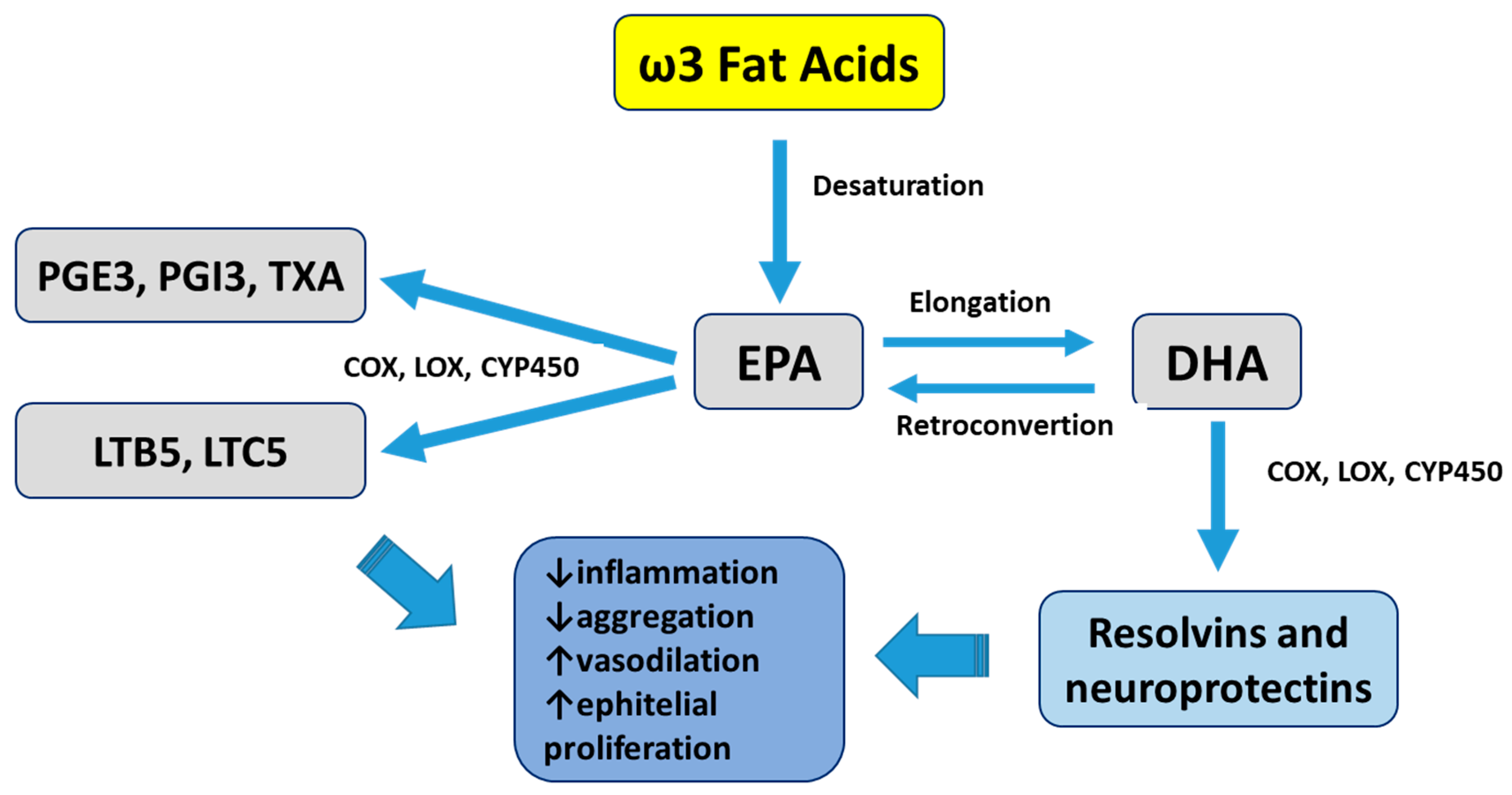
3.2. ω3 Fat Acids
3.3. ω3 Fatty Acids and IBD
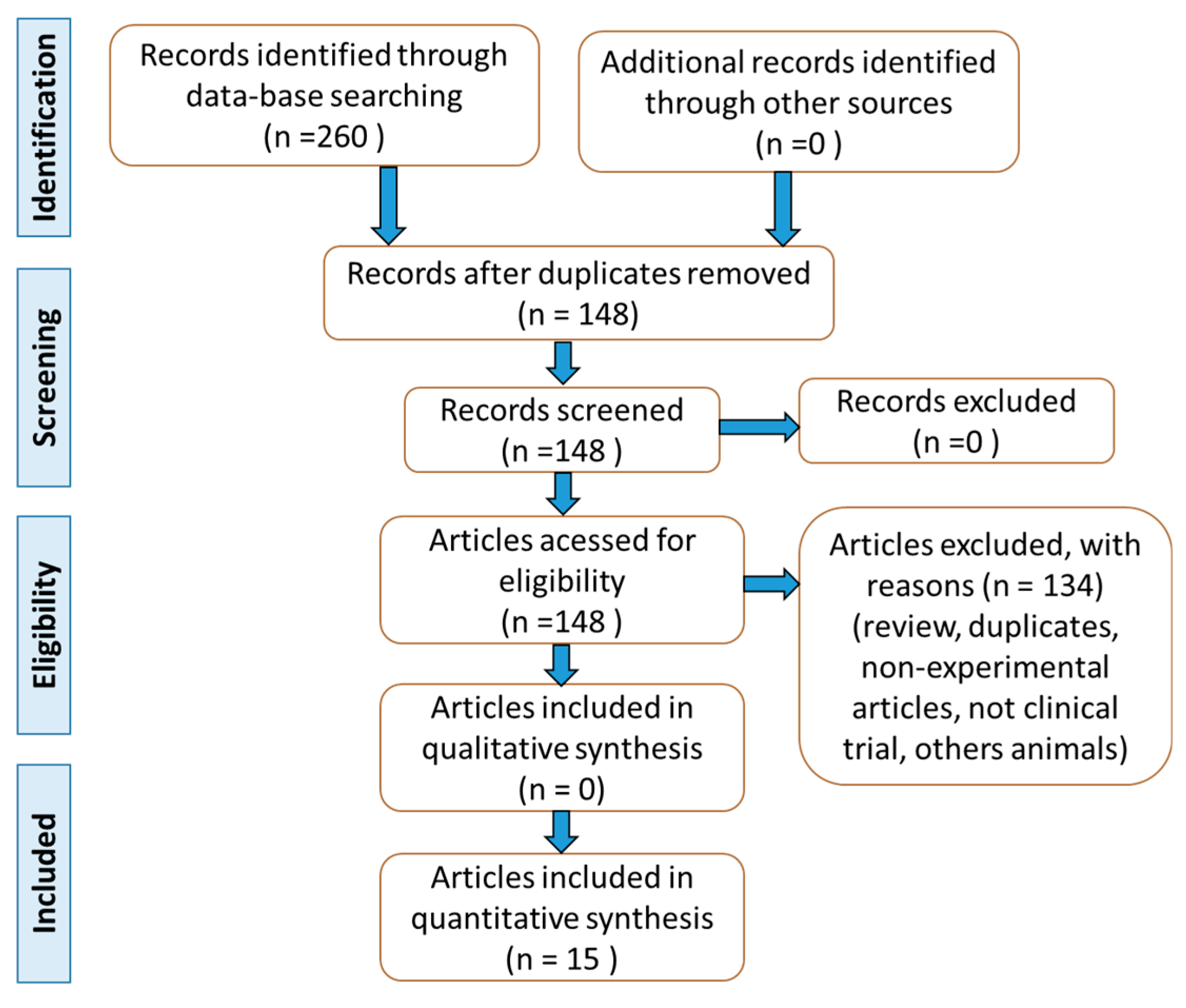
4. Methods
4.1. Data Sources
4.2. Research
4.3. Eligible criteria and Study Selection
4.4. Extraction of Data
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdolmaleki, F.; Kovanen, P.T.; Mardani, R.; Gheibi-Hayat, S.M.; Bo, S.; Sahebkar, A. Resolvins: Emerging players in autoimmune and inflammatory diseases. Clin. Rev. Allergy Immunol. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; de Alvares Goulart, R.; Quesada, K.; Bechara, M.D.; de Carvalho Ade, C. Inflammatory bowel disease: Can omega-3 fatty acids really help? Ann. Gastroenterol. 2016, 29, 37–43. [Google Scholar] [PubMed]
- Shamoon, M.; Martin, N.M.; O’Brien, C.L. Recent advances in gut Microbiota mediated therapeutic targets in inflammatory bowel diseases: Emerging modalities for future pharmacological implications. Pharmacol. Res. 2019, 148, 104344. [Google Scholar] [CrossRef] [PubMed]
- Diab, J.; Al-Mahdi, R.; Gouveia-Figueira, S.; Hansen, T.; Jensen, E.; Goll, R.; Moritz, T.; Florholmen, J.; Forsdahl, G. A quantitative analysis of colonic mucosal oxylipins and endocannabinoids in treatment-naïve and deep remission ulcerative colitis patients and the potential link with cytokine gene expression. Inflamm. Bowel. Dis. 2019, 25, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kaur, R.; Kaushik, K.; Kaushal, N. Redox modulatory protective effects of ω-3 fatty acids rich fish oil against experimental colitis. Toxicol. Mech. Methods 2019, 29, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Onuki, M.; Watanabe, M.; Ishihara, N.; Suzuki, K.; Takizawa, K.; Hirota, M.; Yamada, T.; Egawa, A.; Shibahara, O.; Nishii, M.; et al. A partial agonist for retinoid X receptor mitigates experimental colitis. Int. Immunol. 2019, 31, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lan, S. Implications of antioxidant systems in inflammatory bowel disease. Biomed. Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Rashvand, S.; Somi, M.H.; Rashidkhani, B.; Hekmatdoost, A. Dietary fatty acid intakes are related to the risk of ulcerative colitis: A case-control study. Int. J. Colorectal. Dis. 2015, 30, 1255–1260. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Nimptsch, K.; Wu, K.; Chan, A.T. High school diet and risk of Crohn’s disease and ulcerative colitis. Inflamm. Bowel. Dis. 2015, 21, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, C.; Chan, R.; Salameh, E.; Mbodji, K.; Ueno, A.; Coëffier, M.; Guérin, C.; Ghosh, S.; Savoye, G.; Marion-Letellier, R. Dietary n-3 PUFA may attenuate experimental colitis. Mediators Inflamm. 2018, 2018, 8430614. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Izumi, Y.; Yoshida, M. Alterations in docosahexaenoic acid-related lipid cascades in inflammatory bowel disease model mice. Dig. Dis. Sci. 2018, 63, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; Tacconi, C.; Massimino, L.; Corsetto, P.A.; Correale, C.; Fonteyne, P.; Piontini, A.; Garzarelli, V.; Calcaterra, F.; Della Bella, S.; et al. MFSD2A promotes endothelial generation of inflammation-resolving lipid mediators and reduces colitis in mice. Gastroenterology 2017, 153, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lu, Y.; Zhi, M.; Hu, P.; Wu, W.; Gao, X. Dietary n-3 polyunsaturated fatty acids ameliorate Crohn’s disease in rats by modulating the expression of PPAR-γ/NFAT. Mol. Med. Rep. 2017, 16, 8315–8322. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Goulart, R.D.A.; Aranão, A.L.D.C.; de Oliveira, P.G.C. Inflammatory bowel diseases and fermentable oligosaccharides, disaccharides, monosaccharides, and polyols: An overview. J. Med. Food 2018, 21, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Scaioli, E.; Sartini, A.; Bellanova, M.; Campieri, M.; Festi, D.; Bazzoli, F.; Belluzzi, A. Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Prossomariti, A.; Scaioli, E.; Piazzi, G.; Fazio, C.; Bellanova, M.; Biagi, E.; Candela, M.; Brigidi, P.; Consolandi, C.; Balbi, T.; et al. Short term treatment with eicosapentaenoic acid improves inflammation and affectscolonic differentiation markers and microbiota in patients with ulcerative colitis. Sci. Rep. 2017, 7, 7458. [Google Scholar] [CrossRef] [PubMed]
- Yasueda, A.; Shinzaki, S.; Iijima, H.; Mizushima, T.; Nishimura, J.; Hiyama, S.; Ohno, S.; Ito, T. Safety of emulsifying lipid formulation containing Omega-3 polyunsaturated fatty acids for patients with Crohn’s disease. Anticancer Res. 2016, 36, 3753–3759. [Google Scholar] [PubMed]
- Wiese, D.M.; Horst, S.N.; Brown, C.T.; Allaman, M.M.; Hodges, M.E.; Slaughter, J.C.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T.; et al. Serum fatty acids are correlated with inflammatory cytokines in ulcerative colitis. PLoS ONE 2016, 11, e0156387. [Google Scholar] [CrossRef]
- Scaioli, E.; Cardamone, C.; Liverani, E.; Munarini, A.; Hull, M.A.; Belluzzi, A. The pharmacokinetic profile of a new gastroresistant capsule preparation of eicosapentaenoic acid as the free fatty acid. Biomed. Res. Int. 2015, 2015, 360825. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Luben, R.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Lindgren, S.; Grip, O.; Bergmann, M.M.; Boeing, H.; Hallmans, G.; et al. Association between high dietary intake of the n-3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 39, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Costea, I.; Mack, D.R.; Lemaitre, R.N.; Israel, D.; Marcil, V.; Ahmad, A.; Amre, D.K. Interactions between the dietary polyunsaturated fatty acid ratio and genetic factors determine susceptibility to pediatric Crohn’s disease. Gastroenterology 2014, 146, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Pearl, D.S.; Masoodi, M.; Eiden, M.; Brümmer, J.; Gullick, D.; McKeever, T.; Whittaker, M.A.; Nitch-Smith, H.; Brown, J.F.; Shute, J.K.; et al. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. J. Crohns Colitis 2014, 8, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Hontecillas, R.; Horne, W.T.; Sandridge, M.; Herfarth, H.H.; Bloomfeld, R.; Isaacs, K.L. Conjugated linoleic acid modulates immune responses in patients with mild to moderately active Crohn’s disease. Clin. Nutr. 2012, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.L.; Casson, D.H.; Terry, A.; Burdge, G.C.; El-Matary, W.; Dalzell, A.M. Enteral feeding therapy for newly diagnosed pediatric Crohn’s disease: A double-blind randomized controlled trial with two years follow-up. Inflamm. Bowel. Dis. 2012, 18, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wiese, D.M.; Lashner, B.A.; Lerner, E.; DeMichele, S.J.; Seidner, D.L. The effects of an oral supplement enriched with fish oil, prebiotics, and antioxidants on nutrition status in Crohn’s disease patients. Nutr. Clin. Pract. 2011, 26, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, T.; Berge, R.K.; Bohov, P.; Skorve, J.; Gøransson, L.; Omdal, R.; Aasprong, O.G.; Haugen, M.; Meltzer, H.M.; Hausken, T. Salmon diet in patients with active ulcerative colitis reduced the simple clinical colitisactivity index and increased the anti-inflammatory fatty acid index—A pilot study. Scand. J. Clin. Lab. Investig. 2011, 71, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Nakamura, M.; Odahara, S.; Koido, S.; Katahira, K.; Shiraishi, H.; Ohkusa, T.; Fujise, K.; Tajiri, H. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm. Bowel. Dis. 2010, 16, 1696–1707. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, K.; Bednarz-Misa, I.; Walecka-Zacharska, E.; Wierzbicki, J.; Agrawal, A.; Gamian, A.; Krzystek-Korpacka, M. Oversecretion and overexpression of nicotinamide Phosphoribosyltransferase/Pre-B colony-enhancing factor/Visfatin in inflammatory bowel disease reflects the disease activity, severity of inflammatory response and hypoxia. Int. J. Mol. Sci. 2019, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ney, M.; Eslamparast, T.; Vandermeer, B.; Ismond, K.P.; Kroeker, K.; Halloran, B.; Raman, M.; Tandon, P. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, Z.; Li, H.; Liu, X.; Zheng, S.; Su, W. IL-38: A new player in inflammatory autoimmune disorders. Biomolecules 2019, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Goulart, R.A.; Gasparini, R.G. Associations between inflammatory bowel diseases and vitamin D. Crit. Rev. Food Sci. Nutr. 2019, 59, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.; Daneshzad, E.; Larijani, B.; Bellissimo, N.; Azadbakht, L. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Matz, A.; Klemashevich, C.; Rosenberg, D.W. Dietary walnut supplementation alters mucosal metabolite profiles during DSS-induced colonic ulceration. Nutrients 2019, 11, 1118. [Google Scholar] [CrossRef] [PubMed]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The imbalance between n-6/n-3 polyunsaturated fatty acids and inflammatory bowel disease: A comprehensive review and future therapeutic perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef]
- Hawthorne, A.B.; Daneshmend, T.K.; Hawkey, C.J.; Belluzzi, A.; Everitt, S.J.; Holmes, G.K.; Malkinson, C.; Shaheen, M.Z.; Willars, J.E. Treatment of ulcerative colitis with fish oil supplementation: A prospective 12 month randomised controlled trial. Gut 1992, 33, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Mate, J.; Castanos, R.; Garcia-Samaniego, J.; Pajares, J.M. Does dietary fish oil maintain the remission of Crohn’s disease (CD): A study case control. Gastroenterology 1993, 100, 228. [Google Scholar]
- Lorenz, R.; Weber, P.C.; Szimnau, P.; Heldwein, W.; Strasser, T.; Loeschke, K. Supplementation with n-3 fatty acids from fish oil in chronic inflammatory bowel disease: A randomized placebo-controlled double-blind cross-over trial. J. Intern. Med. Suppl. 1989, 225, 225–232. [Google Scholar] [CrossRef]
- Turner, D.; Steinhart, A.H.; Griffiths, A.M. Omega 3 fatty acids (fish oil) for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2007, 3. [Google Scholar] [CrossRef]
- Tjonneland, A.; Overvad, K.; Bergmann, M.M.; Nagel, G.; Linseisen, J.; Hallmans, G.; Berglund, G.; Lindgren, S.; Grip, O.; Palli, D.; et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: A nested case-control study within a European prospective cohort study. Gut 2009, 58, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Luben, R.; Shrestha, S.S.; Welch, A.; Khaw, K.T.; Hart, A.R. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: A UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Damiano, G.; Altamore, R.; Palumbo, V.D.; Tomascello, G.; Buscemi, S.; Monte, A.I. Dietary patterns influence the gut microbiota and prevent inflammatory bowel disease. Nutrients 2014, 6. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
| Reference | Model | Investigation or Intervention | Major Findings | Conclusions |
|---|---|---|---|---|
| Diab et al. 2019 [4] | Controlled clinical trial. Colon biopsies were taken from 15 treatment-naïve UC patients (6 women, 9 men; 14–69 years), five deep remission UC patients (5 men; 41–70 years), and 10 healthy controls (4 women, 6 men; 25–86 years). | Thirty-five oxylipins and 11 eCBs were quantified. A reverse transcription-polymerase chain reaction measured levels of mRNA for ten cytokines. | Levels of ω6-related oxylipins were significantly elevated in treatment-naïve patients compared with controls, whereas the levels of ω3 eCBs were lower. 15S-HETrE was significantly upregulated in UC deep remission patients. All investigated cytokines had significantly higher mRNA levels in the inflamed mucosa of treatment-naïve UC patients. Cytokine gene expression was positively correlated with several ω6 AA-related oxylipins, whereas a negative correlation was found with lipoxin, prostacyclin, and the eCBs. | Increased levels of ω6-related oxylipins and decreased levels of ω3-related eCBs are associated with the debut of UC. This highlights the altered balance between pro- and anti-inflammatory lipid mediators in IBD and suggests potential targets for intervention. |
| Scaioli et al. 2018 [17] | Randomized clinical trial with sixty UC patients (29 men and 31 women, 18 years and older), with a partial Mayo score < 2 and FC ≥ 150 μg/g, in stable therapy for at least three months. | Patients were randomly divided into groups with EPA-FFA (500 mg, twice/d) or placebo for six months. Evaluation with colonoscopy was taken at baseline. Assessments of fecal calprotectin and clinical parameters were performed at baseline of the study, after 3 months, and after 6 months. Relapse UC patients underwent another colonoscopy. | Calprotectin showed a reduction of 100 points at six months from the baseline (63.3% in the EPA-FFA group and 13.3% in the placebo group). It was observed the maintenance of clinical remission at six months (76.7% in the EPA-FFA group and 50% in the placebo group). No serious adverse events were observed. | The use of EPA-FFA was essential to reduce FC and no serious adverse events were observed. This agent can be used to induce and maintain symptom-free remission in UC patients. |
| Prossomariti et al., 2017 [18] | Pilot Study with twenty UC patients (13 men and 7 women, 18–70 years), in stable clinical remission and with FC > 150 µg/g. | Supplementation with EPA-FFA 2 g/daily for 90 days. | EPA-FFA intervention decreased the levels of FC at 90 days. Individuals with FC > 150 µg/g (at 90 days) were classified as nonresponders. EPA-FFA ameliorated endoscopic and histological inflammation and induced expression of IL-10, HES1, SOCS3, and KLF4 in compatible patients. EPA-FFA partially redressed long-term UC-driven microbiota composition. | The supplementation with EPA-FFA decreased mucosal inflammation, induced differentiation of goblet cells, and modulated the composition of the intestinal microbiota in patients with long-standing UC. |
| Yasueda et al. 2016 [19] | Open-label clinical trial with five CD patients in remission (4 men and one woman; 29–60 years). | Intake of 100 mL of ω3 emulsifying formulation test (IMARK S®) daily/28 d. After a month washout period, patients drunk two bottles of the formulation/daily/28 days. Anthropometric and biochemical evaluations were performed before and after each intervention. | The formulation was safe with minimal side effects. Bodyweight and body mass index were not modified. CD activity index scores decreased after ingestion of one bottle of the formulation. Blood evaluations did not show severe side effects. | Supplementation with the formulation test can be safe and useful for maintaining remission in CD patients. |
| Wiese et al. 2016 [20] | Controlled clinical trial with 101 UC subjects (45 men and 56 women, 27–58 years) and 23 controls (9 men and 14 women, 37–65 years). | Dietetic inquiries, serum, and colonic tissue samples were collected. Histologic injury and the activity index (Mayo Disease) were assessed. Cytokines from serum and tissue were measured. Serum FA were evaluated. | UC individuals showed increased total OA and decreased AA intake when compared with the control group. Reduced percentages of SFA and AA, and higher MUFA, OA, EPA, and DPA were also observed. The levels of cytokine in the tissue were directly associated with SFA and inversely correlated with PUFA, EPA, and DPA in UC subjects, but not with controls. 5-aminosalicylic acid therapy blunted these associations. | There were differences in serum FA in individuals with UC that correlated with proinflammatory tissue cytokines suggesting that fatty acids can affect the production of cytokines and therefore be immunomodulators in UC. |
| Scaioli, et al. 2015 [21] | Controlled clinical rial with ten UC (4 men and 6 women, 24–44 years) and 10 CD individuals (6 men and 4 women, 28–50 years) and 15 HV (5 male and 10 female, 20–36 years). | Patients received 2 g/d of EPA-FFA for eight weeks. | There was rapid incorporation of EPA into plasma phospholipids by two weeks and high incorporation into membranes of RBC (4% total FA content). There was a concomitant reduction in relative ω6 PUFA content. The elongation and desaturation of EPA (into DHA) was apparent. DHA content also augmented in the membranes. EPA-FFA was well tolerated, and no significant differences in the pharmacokinetic profile of ω3 PUFA incorporation were found between IBD patients and HV. | EPA can be considered the “universal donor” concerning key ω3 PUFAs and the enteric-coated formulation test allows long term treatment with a good level of compliance. |
| Chan et al. 2014 [22] | Multi-center prospective cohort study with 229,702 healthy participants (20–74 years) from nine European centers between 1991–1998 and monitored until 2004 (data on gender were not precise). None of the controls possessed UC, microscopic or uncertain colitis. | Dietary intake of DHA was measured at baseline (validated food frequency questionnaires). The population of the study was monitored to identify participants who developed CD. | Seventy-three individuals developed CD (64% woman), which is equivalent to 4 new cases/100,000 inhabitants/year. The consumption of ω3 may influence the development of CD | The intake of DHA was statistically significant inverse related to the development of CD. |
| Ananthakrishnan et al. 2014 [23] | Prospective Cohort study with 170,805 women over 26 years and 3,317,338 person-years of follow-up. | Diet was prospectively evaluated using a validated semi-quantitative food frequency questionnaire. Self-reporting of UC or CD was confirmed through medical report review. | Among the women sample, 338 incident cases of UC (10 new case/100,000/year) and 269 incident cases of CD (8/100,000/year). Intake of ω6 and ω3 was not associated with the risk of developing CD or UC. On the other hand, high long-term intake of trans-unsaturated FA was related to an augmented incidence of UC. | Saturated or unsaturated fat, total fat, or individual PUFA intake does not influence the risk of CD. Trans-unsaturated FA is correlated with UC. |
| Costea et al. 2014 [24] | Randomized controlled trial with 182 children recently diagnosed with CD (111 men and 71 women, 10–16 years), diagnosed before age 19, and 250 controls (Caucasians) (122 men and 128 women, 10–16 years). | The children’s responses to the questionnaire on daily consumption were evaluated. The raw intakes of EPA, DPA, and DHA and AA (ω6) were adjusted for energy intake. The ratio of AA/(EPA, DPA, DHA) was calculated. | Children who intake higher dietary ratio of ω6/ω3 were vulnerable for developing CD if they also presented specific variants of CYP4F3 and FADS2 genes. The findings implicate diet–gene interactions in the pathogenesis of CD. | These findings suggest that preventive dietary intervention could be targeted to specific subgroups (based on PUFA metabolic genes) of IBD. |
| Pearl et al., 2014 [25] | 69 patients with active UC, (35 men and 34 women, 44–47 years), 16 with UC in remission (8 men and 8 women, 43–50 years) and 69 control subjects matched by age and gender (35 men and 34 women 45–48 years). Age 16–80 years. Exclusion criteria: age less than 16 or greater than 80 years. | Biopsies from colonic mucosa were obtained from UC patients and controls. Inflammation was analyzed endoscopically and histologically. FA (esterified and non-esterified) were evaluated. | Inflamed mucosa showed higher AA (p < 0.001), lower EPA (p < 0.010); higher DPA and DHA, and lower LA and α-LNA contents (all p < 0.001), compared to controls. There was a significant association between the severity of inflammation and contents of AA, DPA, and DHA (positive correlations), and LA, α-LNA, and EPA (negative correlations). | Increased AA, AA: EPA ratio, DPA and DHA and lower LA, α-LNA, and EPA were observed in inflamed mucosa and correlate with severity of inflammation, suggesting a modification in FA metabolism. This finding may offer a novel target for intervention. |
| Bassaganya-Riera et al., 2012 [26] | Double-blind, placebo-controlled, randomized trial with thirteen CD patients (2 men and 11 women) with mild to moderately active pattern. Age 25–61 years. | Patients used CLA 6 g/day/12 weeks. Mononuclear cells and cytokines were analyzed at baseline, six, and twelve weeks after initiation of the treatment; CDAI and IBDQ were also performed. | The use of CLA significantly reduced the production of TNF-α, IFN-γ, IL-17, and lymphoproliferation at week 12. There was a significant reduction in CDAI and increase in IBDQ on week 12. | The oral use of CLA was well-tolerated and reduced the capacity of peripheral blood T cells to release pro-inflammatory cytokines, increased quality of life, and reduced disease activity, index. |
| Grogan et al. 2012 [27] | Double-blind randomized controlled trial with 34 children (20 boys and 14 girls) newly diagnosed CD Age 5–16 years. | Children were randomized to the elemental formula (EF), where the diet came from amino acid sources and had a higher concentration of LA, or polymeric formulation (PF), where the dietary source was whole protein and had higher concentration of ALA/six weeks. Change in the PCDAI, FC, and plasma FA were measured at 0 and six weeks. Patients were followed up for two years. Time and treatment choice for the first relapse were documented. | Ninety-three percent of the patients achieved remission in the EF group and 79% in the PF group. One-third of the patients maintained remission for two years. With PF, an increase of EPA and ALA was found with a reciprocal decrease in AA. EF, EPA, and AA levels decreased with a significant reduction in DHA. FC decreased significantly but did not normalize at the end of week 6. | Results did not present a significant difference between the use of EF or PF in promoting remission. Changes in plasma PUFA status were subtle and relevant. |
| Wiese et al., 2011 [28] | Randomized controlled trial with 20 patients with active CD and stable medication. Eligible patients were 18 years or older (4 men and 16 women). | Patients received 16 oz of IBDNF/day/four months. | The results showed that a dietary supplement enriched with fish oil, prebiotics, and antioxidants in CD resulted in increased fat-free and fat mass deposition, improved vitamin D status, and led to an improvement in quality of life and lower disease activity. | IBDNF exhibits potential to be used as an adjuvant in the treatment of CD patients. |
| Grimstad et al., 2011 [29] | Open study, nonrandomized, clinical trial with 12 UC patients (5 men and 7 women, 35– 65 years. | 600 g of salmon was consumed weekly/8 weeks) and a dietary questionnaire, SCCAI, sigmoidoscopy evaluation, FC serum inflammatory markers, and rectal biopsy and plasma FA profiles were performed before and after the dietary intervention. | The levels of C20:4 ω-6 AA in biopsies after intervention were associated with endoscopy and histology and scores. The levels of ω3 PUFAs, C20:5 ω3EPA, C22:6 ω3DHA, and the ω3/ω6 ratio increased in plasma and rectal biopsies. The AIFAI increased both in plasma and biopsies accompanied by a significant reduction in SCCAI. | The intake of salmon reduced SCCAI and AIFAI and showed a tendency to decrease the levels of CRP and homocysteine. For these reasons, it may have beneficial effects on disease activity in patients with mild UC. |
| Uchiyama et al., 2010 [30] | Controlled clinical trial with 20 initial-onset IBD patients (12 UC patients (3 men, 9 women, mean age of 32.9 years) and 8 CD patients (5 men, 3 women, mean age: 29.0 years) who had not undergone any dietary intervention, and after 230 patients (168 UC patients (90 men, 78 women, mean age: 36.0 years) and 62 CD patients (46 men, 16 women, mean age: 34.6 years) in remission for 12 months | The intake of FA in the erythrocyte membrane was evaluated before and after the intervention with diet therapy involving the use of a ω3DP. This regimen, to achieve a ω3/ω6 ratio of 1 V6 PUFA intake was restricted to 50% of the mean intake, and ω3 PUFA intake was increased 2-fold in comparison with the mean, after which the activity of the disease was evaluated after 12–18 months in remission patients. | In the 20 initial-onset patients, the ω-3/ω-6 ratio significantly augmented after the intervention. The ratio in the remission group (n = 145) in the follow-up group was significantly higher than in the relapse group (n = 85). The ratio was significantly decreased in patients who suffered a relapse after the beginning of the treatment. | ω3DP significantly decreased the erythrocyte membrane ω3/ω6 ratio in IBD individuals and was significantly higher in the remission group. These findings suggest that ω3DP alters the FA composition of the cell membrane and influences clinical activity in IBD patients. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marton, L.T.; Goulart, R.d.A.; Carvalho, A.C.A.d.; Barbalho, S.M. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. Int. J. Mol. Sci. 2019, 20, 4851. https://doi.org/10.3390/ijms20194851
Marton LT, Goulart RdA, Carvalho ACAd, Barbalho SM. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. International Journal of Molecular Sciences. 2019; 20(19):4851. https://doi.org/10.3390/ijms20194851
Chicago/Turabian StyleMarton, Ledyane Taynara, Ricardo de Alvares Goulart, Antonelly Cassio Alves de Carvalho, and Sandra Maria Barbalho. 2019. "Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview" International Journal of Molecular Sciences 20, no. 19: 4851. https://doi.org/10.3390/ijms20194851
APA StyleMarton, L. T., Goulart, R. d. A., Carvalho, A. C. A. d., & Barbalho, S. M. (2019). Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. International Journal of Molecular Sciences, 20(19), 4851. https://doi.org/10.3390/ijms20194851