Strong Signs for a Weak Wall in Tricuspid Aortic Valve Associated Aneurysms and a Role for Osteopontin in Bicuspid Aortic Valve Associated Aneurysms
Abstract
1. Introduction
2. Results
2.1. Study Population
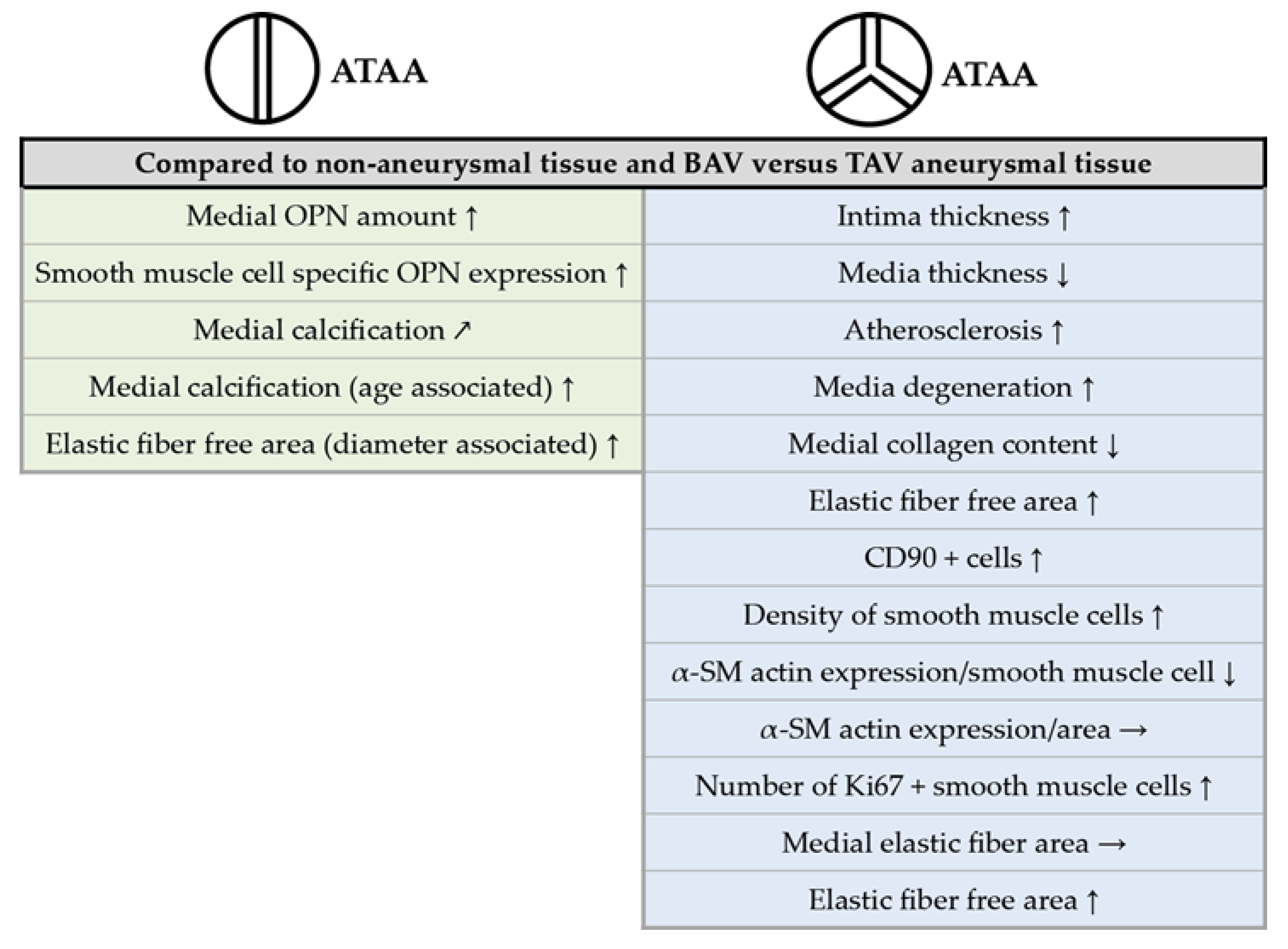
2.2. The TAV-ATAA Wall is Characterized by a Thinner Aortic Media, Atherosclerotic Changes, a Pronounced Media Degeneration, and Fibroblast Accumulation
2.3. Focal Elastic Fiber Loss and Decreased Collagen Content in TAV-ATAAs
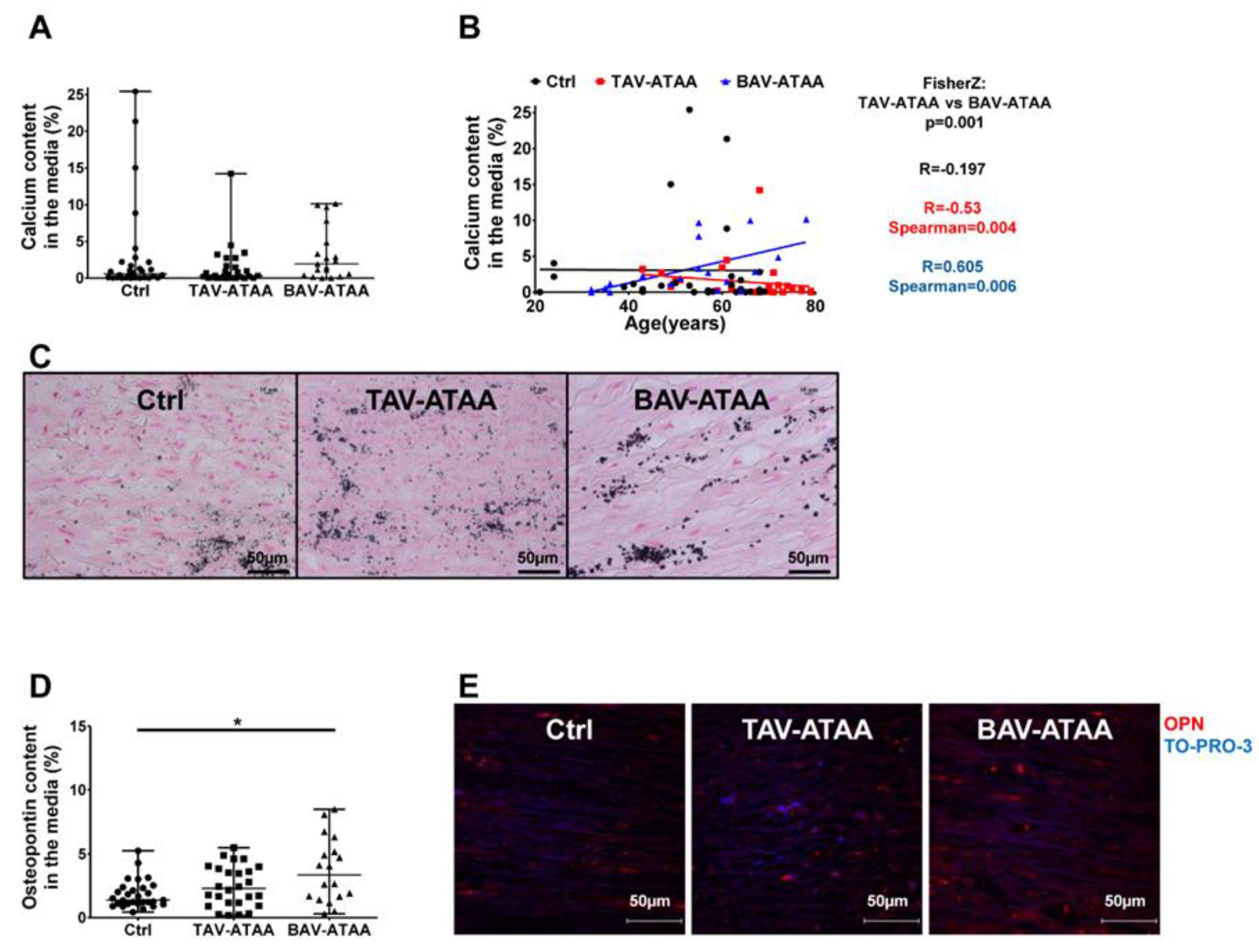
2.4. Age-Dependent Calcification and Increased OPN Expression in BAV-ATAAs
2.5. The Increased Amount of OPN Within the Medial Layer of BAV Patients is Caused by Smooth Muscle Cells
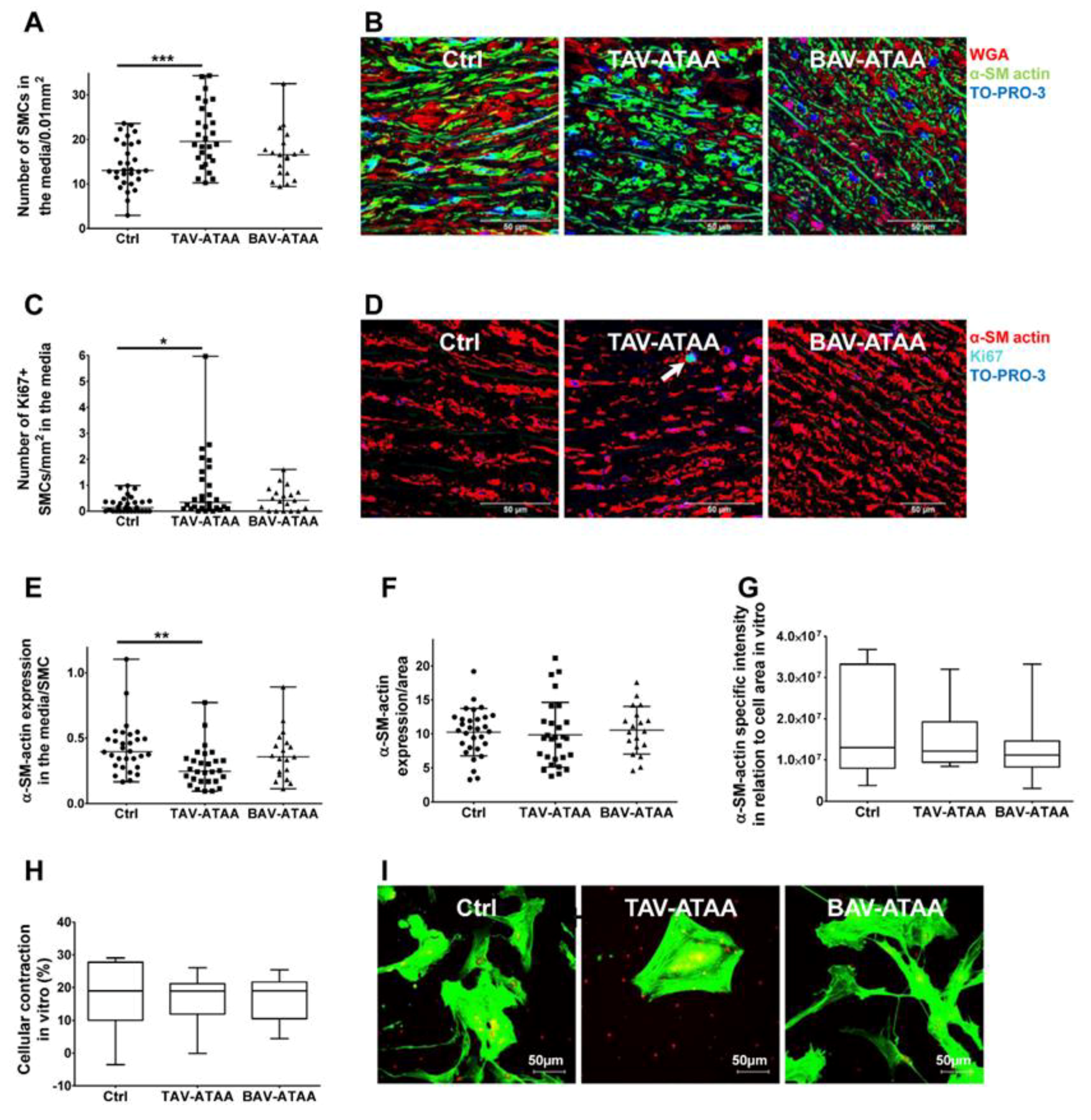
2.6. Increased Smooth Muscle Cell Density and Reduced Cellular Expression of Alpha Smooth Muscle Actin in TAV-ATAAs
3. Discussion
4. Material and Methods
4.1. Study Population
4.2. Histological Stainings and Analyses
4.3. Auto-Fluorescence Based Analyses and Quantifications
4.4. Immunofluorescence Based Stainings and Quantifications
4.5. Isolation of Primary Smooth Muscle Cells
4.6. Cell Contraction Assay
4.7. Immunofluorescence-Based Staining of Primary Smooth Muscle Cells
4.8. Western Blot Analyses
4.9. Statistical Analyses
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isselbacher, E.M. Thoracic and abdominal aortic aneurysms. Circulation 2005, 111, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A. Diseases of the aorta: Aneurysm of the ascending aorta. Heart 2010, 96, 979–985. [Google Scholar] [CrossRef] [PubMed]
- El-Hamamsy, I.; Yacoub, M.H. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat. Rev. Cardiol. 2009, 6, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Booher, A.M.; Eagle, K.A. Diagnosis and management issues in thoracic aortic aneurysm. Am. Heart J. 2011, 162, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Blunder, S.; Messner, B.; Aschacher, T.; Zeller, I.; Turkcan, A.; Wiedemann, D.; Andreas, M.; Bluschke, G.; Laufer, G.; Schachner, T.; et al. Characteristics of TAV- and BAV-associated thoracic aortic aneurysms--smooth muscle cell biology, expression profiling, and histological analyses. Atherosclerosis 2012, 220, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Fedak, P.W.; Verma, S.; David, T.E.; Leask, R.L.; Weisel, R.D.; Butany, J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002, 106, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Nakatani, S.; Stugaard, M.; Tsujita-Kuroda, Y.; Bando, K.; Kobayashi, J.; Yamagishi, M.; Kitakaze, M.; Kitamura, S.; Miyatake, K. Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: Comparison with tricuspid aortic valve. Circulation 2003, 108, II291-4. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Pasic, M.; Meyer, R.; Goetze, N.; Bauer, U.; Siniawski, H.; Hetzer, R. Morphometric analysis of aortic media in patients with bicuspid and tricuspid aortic valve. Ann. Thorac. Surg. 2002, 74, 58–62. [Google Scholar] [CrossRef]
- Matthias Bechtel, J.F.; Noack, F.; Sayk, F.; Erasmi, A.W.; Bartels, C.; Sievers, H.H. Histopathological grading of ascending aortic aneurysm: Comparison of patients with bicuspid versus tricuspid aortic valve. J. Heart Valve Dis. 2003, 12, 54–59. [Google Scholar]
- Collins, M.J.; Dev, V.; Strauss, B.H.; Fedak, P.W.; Butany, J. Variation in the histopathological features of patients with ascending aortic aneurysms: A study of 111 surgically excised cases. J. Clin. Pathol. 2008, 61, 519–523. [Google Scholar] [CrossRef]
- Tang, P.C.; Coady, M.A.; Lovoulos, C.; Dardik, A.; Aslan, M.; Elefteriades, J.A.; Tellides, G. Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation 2005, 112, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Tsamis, A.; Pal, S.; Phillippi, J.A.; Gleason, T.G.; Maiti, S.; Vorp, D.A. Effect of aneurysm on biomechanical properties of "radially-oriented" collagen fibers in human ascending thoracic aortic media. J. Biomech. 2014, 47, 3820–3824. [Google Scholar] [CrossRef] [PubMed]
- Corte, A.D.; Quarto, C.; Bancone, C.; Castaldo, C.; Di Meglio, F.; Nurzynska, D.; de Santo, L.S.; de Feo, M.; Scardone, M.; Montagnani, S.; et al. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: Focus on cell-matrix signaling. J. Thorac. Cardiovasc. Surg. 2008, 135, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.X.; Bielenberg, K.; Schneider, A.; Haussler, A.; Keyser, A.; Birnbaum, D. Ascending aortic aneurysm associated with bicuspid and tricuspid aortic valve: Involvement and clinical relevance of smooth muscle cell apoptosis and expression of cell death-initiating proteins. Eur. J. Cardio-Thorac. Surg. 2003, 23, 537–543. [Google Scholar] [CrossRef]
- Albini, P.T.; Segura, A.M.; Liu, G.; Minard, C.G.; Coselli, J.S.; Milewicz, D.M.; Shen, Y.H.; LeMaire, S.A. Advanced atherosclerosis is associated with increased medial degeneration in sporadic ascending aortic aneurysms. Atherosclerosis 2014, 232, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Pisano, C.; Candore, G.; Maresi, E.; Codispoti, M.; Ruvolo, G. Focus on the unique mechanisms involved in thoracic aortic aneurysm formation in bicuspid aortic valve versus tricuspid aortic valve patients: Clinical implications of a pilot study. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2013, 43, e180-6. [Google Scholar] [CrossRef] [PubMed]
- Folkersen, L.; Wagsater, D.; Paloschi, V.; Jackson, V.; Petrini, J.; Kurtovic, S.; Maleki, S.; Eriksson, M.J.; Caidahl, K.; Hamsten, A.; et al. Unraveling divergent gene expression profiles in bicuspid and tricuspid aortic valve patients with thoracic aortic dilatation: The ASAP study. Mol. Med. 2011, 17, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Kjellqvist, S.; Maleki, S.; Olsson, T.; Chwastyniak, M.; Branca, R.M.; Lehtio, J.; Pinet, F.; Franco-Cereceda, A.; Eriksson, P. A combined proteomic and transcriptomic approach shows diverging molecular mechanisms in thoracic aortic aneurysm development in patients with tricuspid- and bicuspid aortic valve. Mol. Cell. Proteom. Mcp 2013, 12, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Bjorck, H.M.; Paloschi, V.; Kjellqvist, S.; Folkersen, L.; Jackson, V.; Franco-Cereceda, A.; Eriksson, P. Aneurysm Development in Patients With Bicuspid Aortic Valve (BAV): Possible Connection to Repair Deficiency? Aorta 2013, 1, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Paloschi, V.; Gadin, J.R.; Khan, S.; Bjorck, H.M.; Du, L.; Maleki, S.; Roy, J.; Lindeman, J.H.; Mohamed, S.A.; Tsuda, T.; et al. Aneurysm development in patients with a bicuspid aortic valve is not associated with transforming growth factor-beta activation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.A.; Green, B.R.; Eskay, M.A.; Kotlarczyk, M.P.; Hill, M.R.; Robertson, A.M.; Watkins, S.C.; Vorp, D.A.; Gleason, T.G. Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J. Thorac. Cardiovasc. Surg. 2014, 147, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Messner, B.; Bernhard, D. Bicuspid aortic valve-associated aortopathy: Where do we stand? J. Mol. Cell Cardiol. 2019, 133, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Achneck, H.; Modi, B.; Shaw, C.; Rizzo, J.; Albornoz, G.; Fusco, D.; Elefteriades, J. Ascending thoracic aneurysms are associated with decreased systemic atherosclerosis. Chest 2005, 128, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Agmon, Y.; Khandheria, B.K.; Meissner, I.; Schwartz, G.L.; Sicks, J.D.; Fought, A.J.; O’Fallon, W.M.; Wiebers, D.O.; Tajik, A.J. Is aortic dilatation an atherosclerosis-related process? Clinical, laboratory, and transesophageal echocardiographic correlates of thoracic aortic dimensions in the population with implications for thoracic aortic aneurysm formation. J. Am. Coll. Cardiol. 2003, 42, 1076–1083. [Google Scholar] [CrossRef]
- Jones, J.A.; Beck, C.; Barbour, J.R.; Zavadzkas, J.A.; Mukherjee, R.; Spinale, F.G.; Ikonomidis, J.S. Alterations in aortic cellular constituents during thoracic aortic aneurysm development: Myofibroblast-mediated vascular remodeling. Am. J. Pathol. 2009, 175, 1746–1756. [Google Scholar] [CrossRef]
- Guo, D.C.; Papke, C.L.; Tran-Fadulu, V.; Regalado, E.S.; Avidan, N.; Johnson, R.J.; Kim, D.H.; Pannu, H.; Willing, M.C.; Sparks, E.; et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am. J. Hum. Genet. 2009, 84, 617–627. [Google Scholar] [CrossRef] [PubMed]
- De Sa, M.; Moshkovitz, Y.; Butany, J.; David, T.E. Histologic abnormalities of the ascending aorta and pulmonary trunk in patients with bicuspid aortic valve disease: Clinical relevance to the ross procedure. J. Thorac. Cardiovasc. Surg. 1999, 118, 588–594. [Google Scholar] [CrossRef]
- De Figueiredo Borges, L.; Jaldin, R.G.; Dias, R.R.; Stolf, N.A.; Michel, J.B.; Gutierrez, P.S. Collagen is reduced and disrupted in human aneurysms and dissections of ascending aorta. Hum. Pathol. 2008, 39, 437–443. [Google Scholar] [CrossRef]
- Demer, L.L.; Tintut, Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef]
- Zhu, D.; Mackenzie, N.C.; Farquharson, C.; Macrae, V.E. Mechanisms and clinical consequences of vascular calcification. Front. Endocrinol (Lausanne) 2012, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M. Calcification of bicuspid aortic valves. Heart 2002, 88, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. J. Int. Soc. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Speer, M.Y.; Li, X.; Rajachar, R.M.; Yang, H. Regulation of vascular calcification: Roles of phosphate and osteopontin. Circ. Res. 2005, 96, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; McKee, M.D.; Steitz, S.; Giachelli, C.M. Calcification of vascular smooth muscle cell cultures: Inhibition by osteopontin. Circ. Res. 1999, 84, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.Y.; McKee, M.D.; Guldberg, R.E.; Liaw, L.; Yang, H.Y.; Tung, E.; Karsenty, G.; Giachelli, C.M. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: Evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J. Exp. Med. 2002, 196, 1047–1055. [Google Scholar] [CrossRef]
- Speer, M.Y.; Chien, Y.C.; Quan, M.; Yang, H.Y.; Vali, H.; McKee, M.D.; Giachelli, C.M. Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc. Res. 2005, 66, 324–333. [Google Scholar] [CrossRef]
- Roberts, W.C.; Vowelsm, T.J.; Kitchens, B.L.; Ko, J.M.; Filardo, G.; Henry, A.C.; Hamman, B.L.; Matter, G.J.; Hebeler, R.F., Jr. Aortic medial elastic fiber loss in acute ascending aortic dissection. Am. J. Cardiol. 2011, 108, 1639–1644. [Google Scholar] [CrossRef]
- Vogel, B.; Siebert, H.; Hofmann, U.; Frantz, S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX 2015, 2, 124–134. [Google Scholar] [CrossRef]
- Wittmann, F.; Turkcan, A.; Baranyi, U.; Eichmair, E.; Laufer, G.; Bernhard, D.; Messner, B. To Be Or Not to Be: The “Smoker’s Paradox” - An in-Vitro Study. Cell. Physiol. Biochem. 2018, 48, 1638–1651. [Google Scholar] [CrossRef]



| p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | T | B | C vs. T | C vs. B | T vs. B | |||
| Number | n = 30 | n = 28 | n = 19 | |||||
| Age (years) | 54.4 ± 12.8 | 65.7 ± 11.3 | 52.8 ± 14 | 0.002 | ns | 0.002 | ||
| Gender (male) | 66.7% | 57.1% | 94.7% | ns | 0.037 | 0.004 | ||
| Internal aortic diameter (mm) | nd | 55.8 ± 3.2 | 53.3 ± 3.6 | nd | nd | 0.033 | ||
| Current smoker | 6.7% | 7.1% | 0% | ns | ns | ns | ||
| CHD | 50% | 25% | 15.8% | 0.062 | 0.018 | ns | ||
| Hypertension | 46.7% | 75% | 52.6% | ns | ns | ns | ||
| Hyperlipidemia | 30% | 35.7% | 31.6% | ns | ns | ns | ||
| Diabetes | 36.7% | 3.6% | 10.5% | 0.003 | 0.053 | ns | ||
| Aortic stenosis | 0% | 15.4% | 73.3% | 0.037 | <0.001 | <0.001 | ||
| Aortic Regurgitation | p-Value | |||||||
| No | Minimal | Mild | Moderate | Severe | C vs. T | C vs. B | T vs. B | |
| C | 18 | 5 | 2 | 1 | 0 | <0.001 | <0.001 | ns |
| T | 4 | 3 | 4 | 7 | 8 | |||
| B | 1 | 2 | 1 | 7 | 4 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stern, C.; Scharinger, B.; Tuerkcan, A.; Nebert, C.; Mimler, T.; Baranyi, U.; Doppler, C.; Aschacher, T.; Andreas, M.; Stelzmueller, M.-E.; et al. Strong Signs for a Weak Wall in Tricuspid Aortic Valve Associated Aneurysms and a Role for Osteopontin in Bicuspid Aortic Valve Associated Aneurysms. Int. J. Mol. Sci. 2019, 20, 4782. https://doi.org/10.3390/ijms20194782
Stern C, Scharinger B, Tuerkcan A, Nebert C, Mimler T, Baranyi U, Doppler C, Aschacher T, Andreas M, Stelzmueller M-E, et al. Strong Signs for a Weak Wall in Tricuspid Aortic Valve Associated Aneurysms and a Role for Osteopontin in Bicuspid Aortic Valve Associated Aneurysms. International Journal of Molecular Sciences. 2019; 20(19):4782. https://doi.org/10.3390/ijms20194782
Chicago/Turabian StyleStern, Christian, Bernhard Scharinger, Adrian Tuerkcan, Clemens Nebert, Teresa Mimler, Ulrike Baranyi, Christian Doppler, Thomas Aschacher, Martin Andreas, Marie-Elisabeth Stelzmueller, and et al. 2019. "Strong Signs for a Weak Wall in Tricuspid Aortic Valve Associated Aneurysms and a Role for Osteopontin in Bicuspid Aortic Valve Associated Aneurysms" International Journal of Molecular Sciences 20, no. 19: 4782. https://doi.org/10.3390/ijms20194782
APA StyleStern, C., Scharinger, B., Tuerkcan, A., Nebert, C., Mimler, T., Baranyi, U., Doppler, C., Aschacher, T., Andreas, M., Stelzmueller, M.-E., Ehrlich, M., Graf, A., Laufer, G., Bernhard, D., & Messner, B. (2019). Strong Signs for a Weak Wall in Tricuspid Aortic Valve Associated Aneurysms and a Role for Osteopontin in Bicuspid Aortic Valve Associated Aneurysms. International Journal of Molecular Sciences, 20(19), 4782. https://doi.org/10.3390/ijms20194782






