Toll-Like Receptors-2 and -4 in Graves’ Disease—Key Players or Bystanders?
Abstract
1. Introduction
2. Results
2.1. Comparison of the Percentage Values of CD4+/TLR-2+ T Cells in GD Patients before Treatment, after Treatment and in the Control Group
2.2. Evaluation of TLR-2 Expression Level on CD4+ T Cells in GD Patients before Treatment, after Treatment and in the Control Group
2.3. Comparison of the Percentage Values of CD8+/TLR-2+ T Cells in Patients with GD before Treatment, after Treatment and in the Control Group
2.4. Evaluation of TLR-2 Expression Level on CD8+ T Cells in GD Patients before Treatment, after Treatment and in the Control Group
2.5. Comparison of the Percentage Values of CD19+/TLR-2+ B Cells in Patients with GD before Treatment, after Treatment and in the Control Group
2.6. Evaluation of TLR-2 Expression Levels on CD19+ B Cells in GD Patients before Treatment, after Treatment and in the Control Group
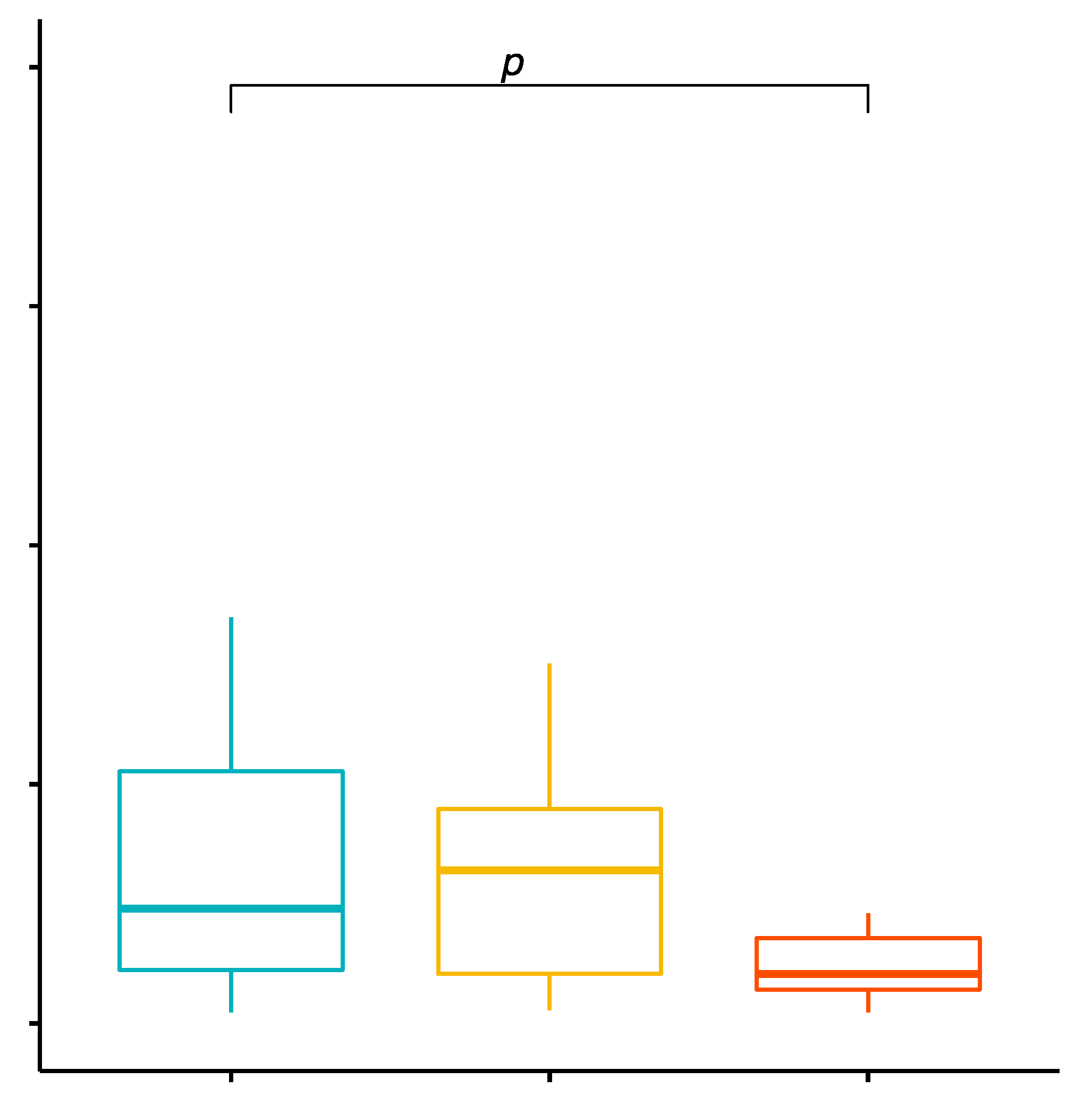
2.7. Comparison of the Percentage Values of CD4+/TLR-4+ T Cells in Patients with GD before Treatment, after Treatment and in the Control Group
2.8. Evaluation of TLR-4 Expression Level on CD4+ T Cells in GD Patients before Treatment, after Treatment and with the Control Group
2.9. Evaluation of TLR-4 Expression Level on CD8+ T Cells in GD Patients before Treatment, after treaTment and in the Control Group
2.10. Comparison of the Percentage Values of CD19+/TLR-4+ B Cells in Patients with GD before Treatment, after Treatment and in the Control Group
2.11. Evaluation of TLR-4 Expression Level on CD19+ B Cells in GD Patients before Treatment, after Treatment and in the Control Group
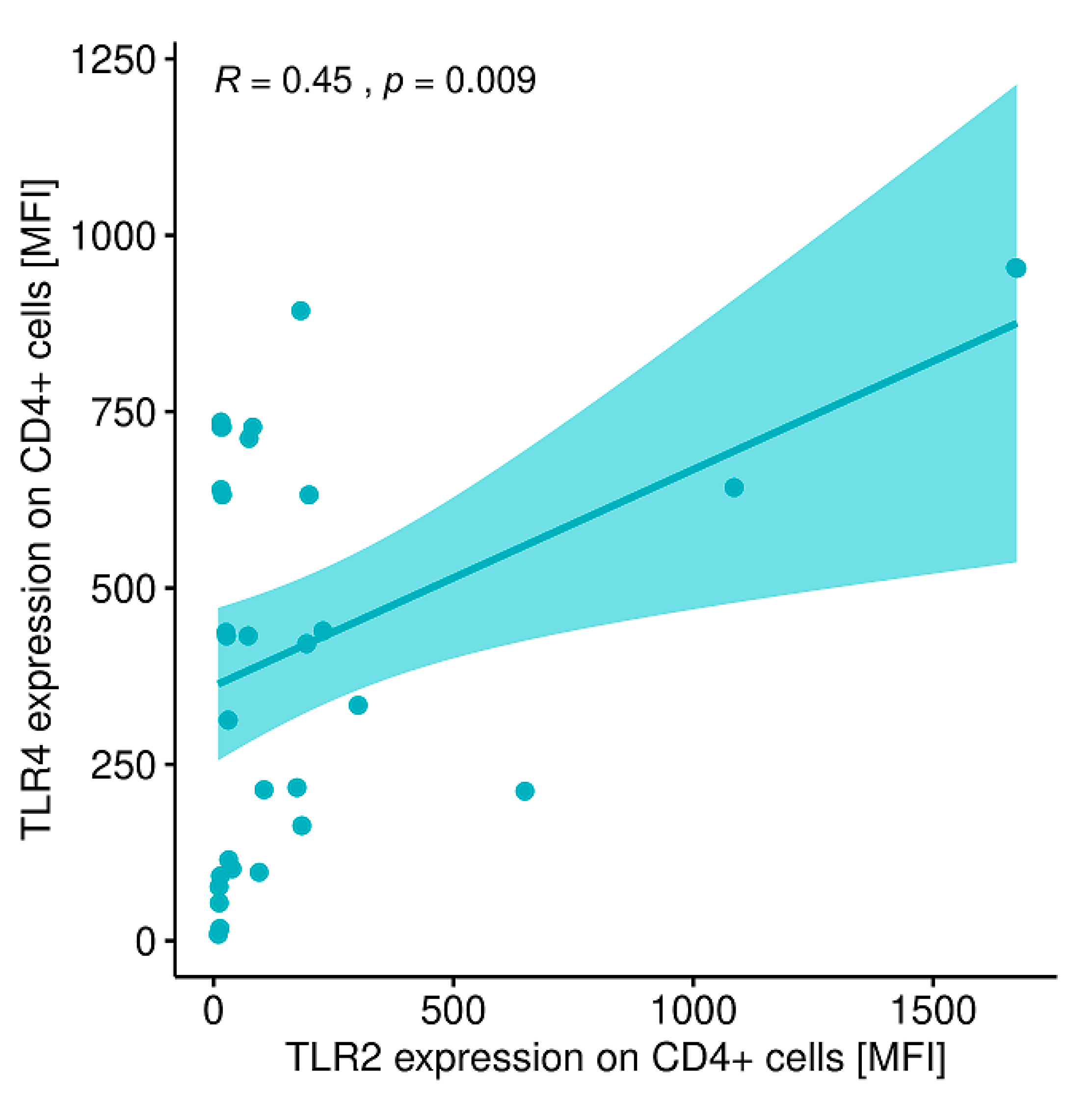
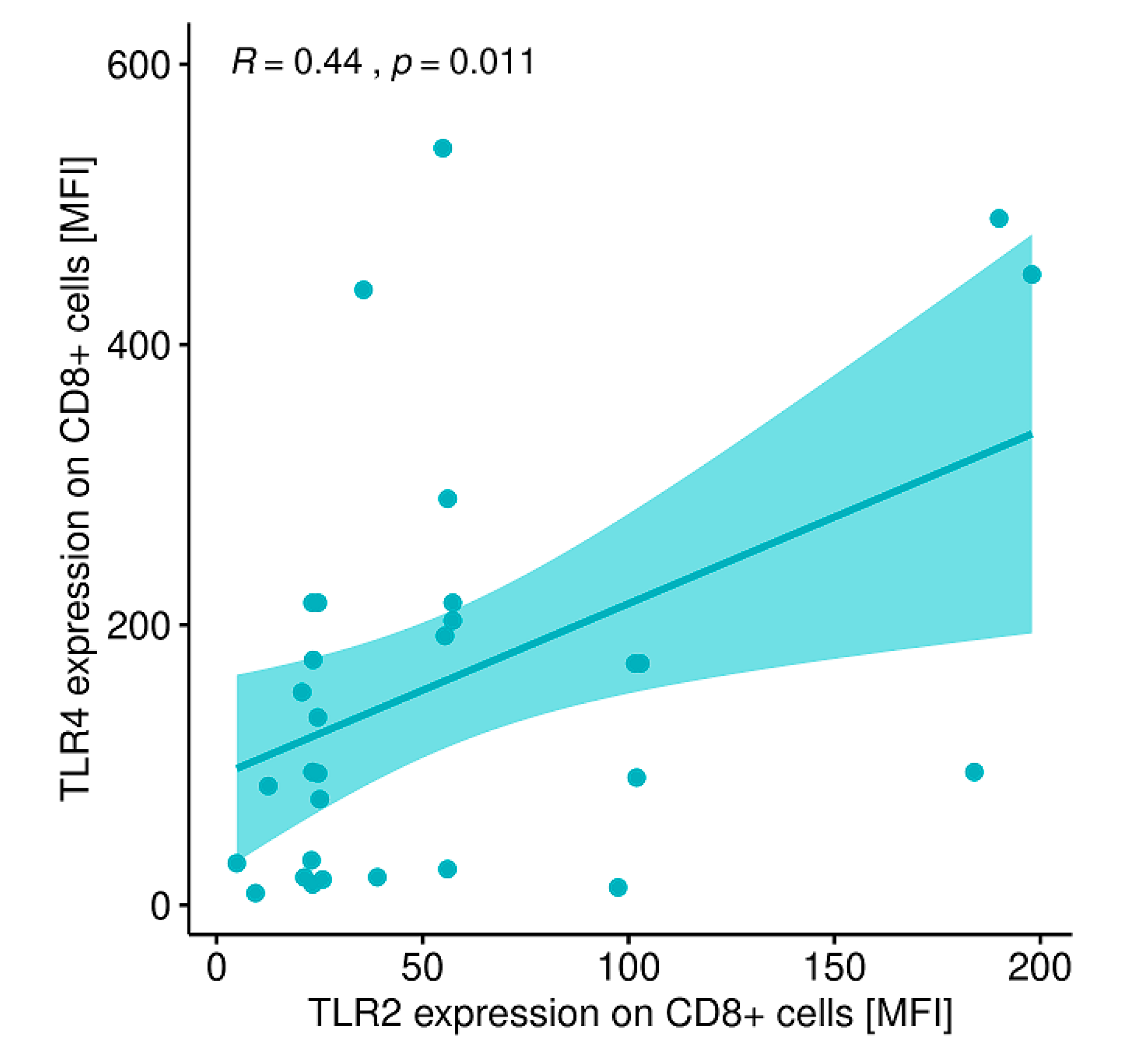
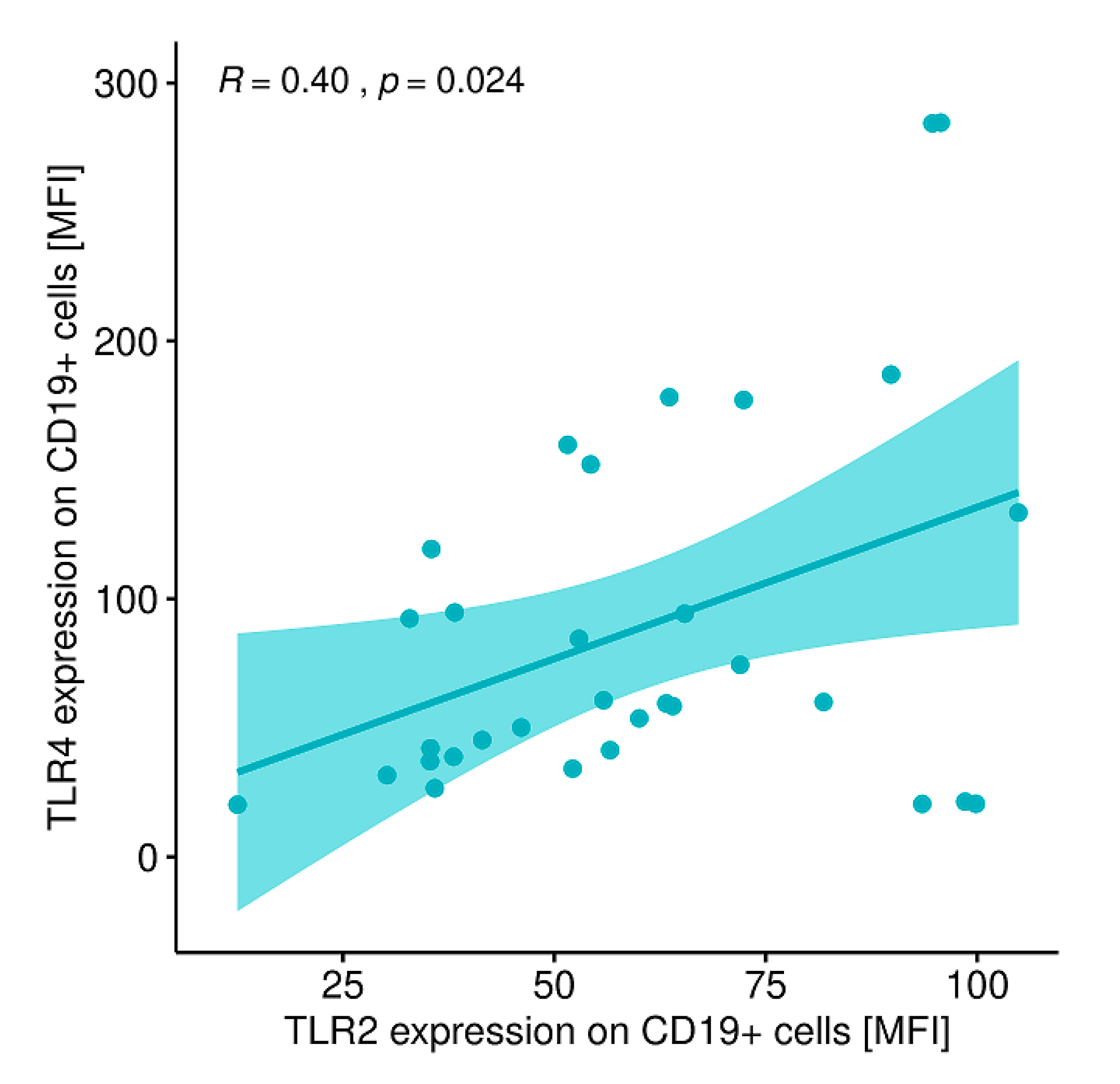
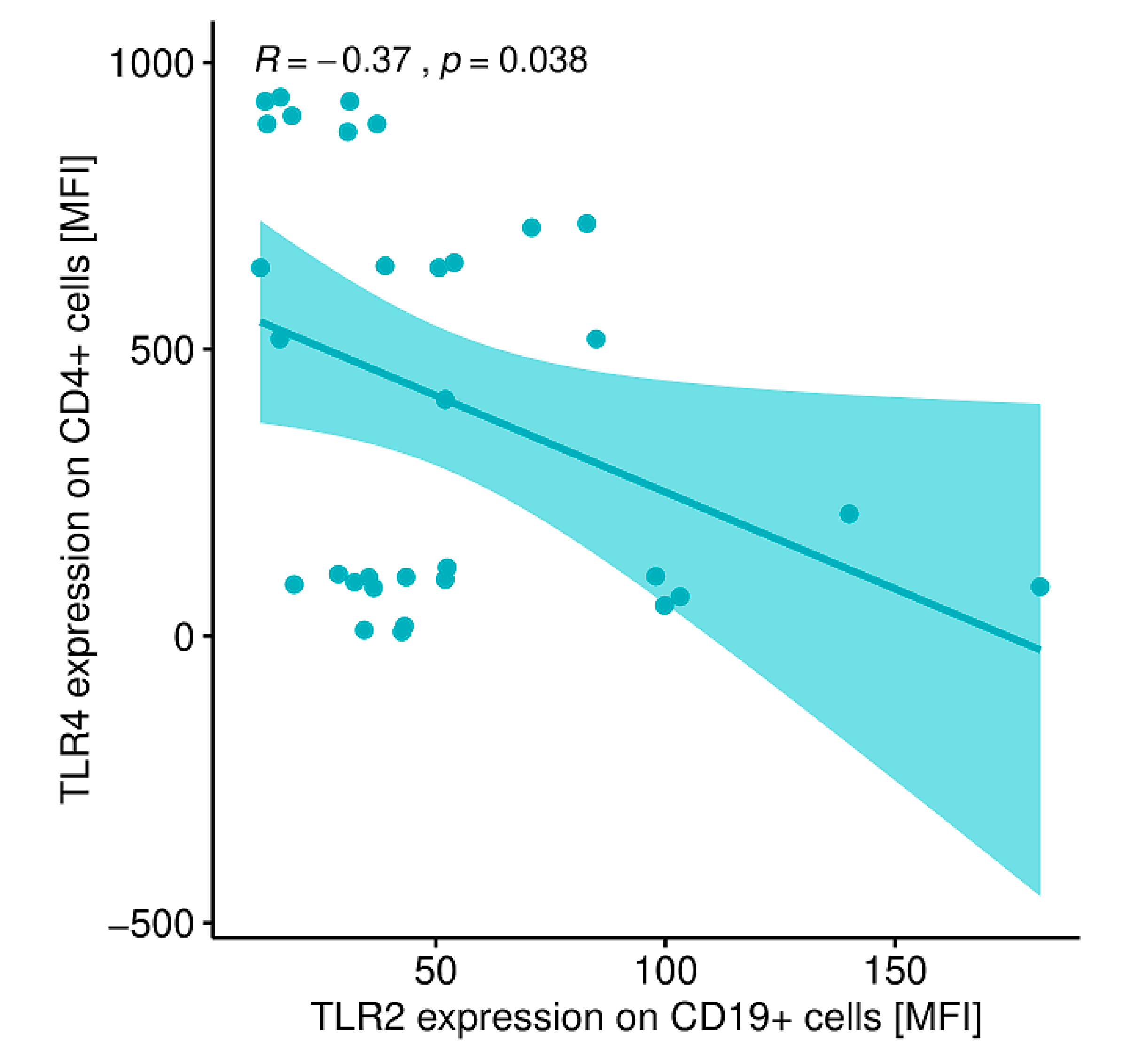
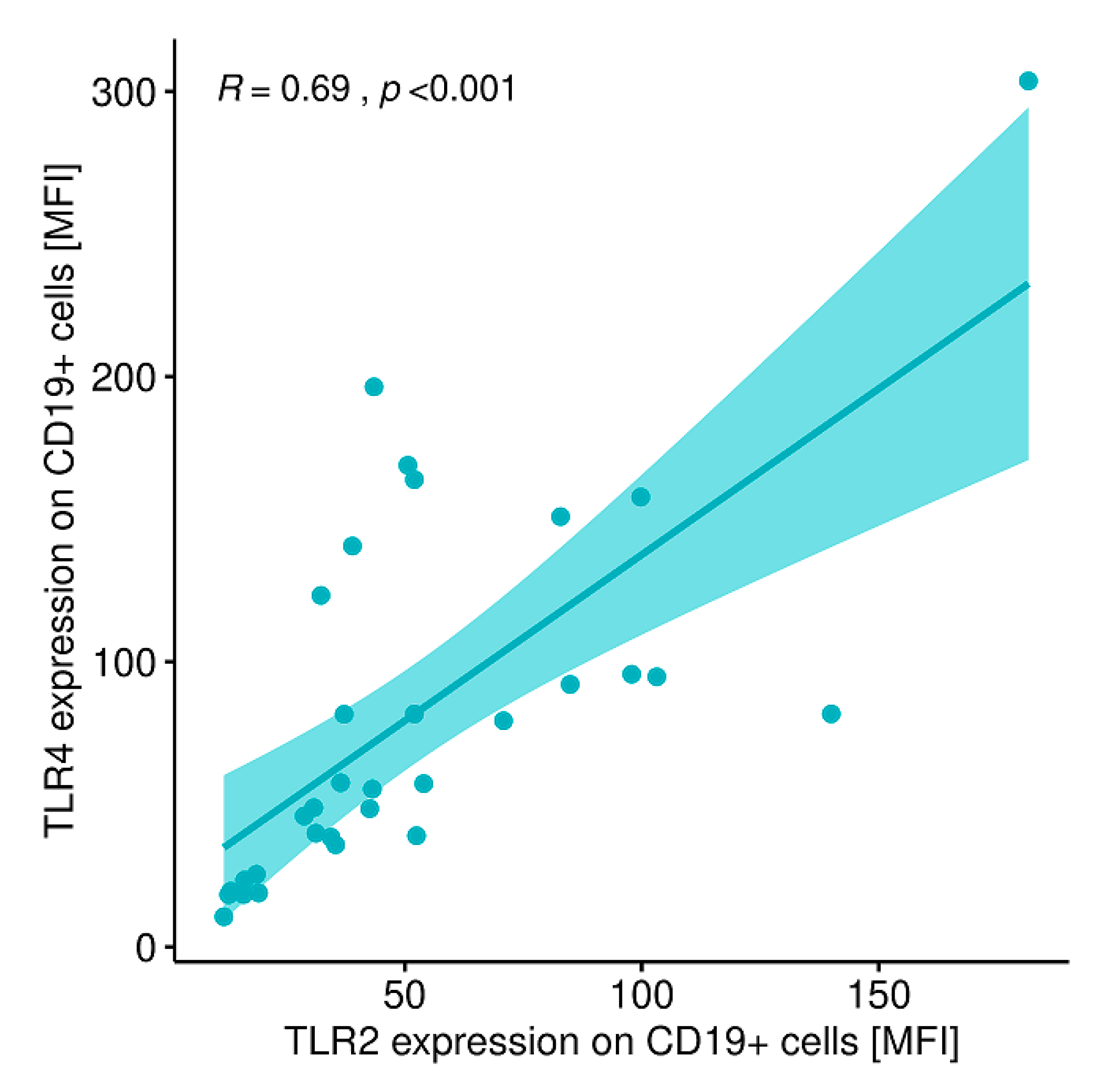
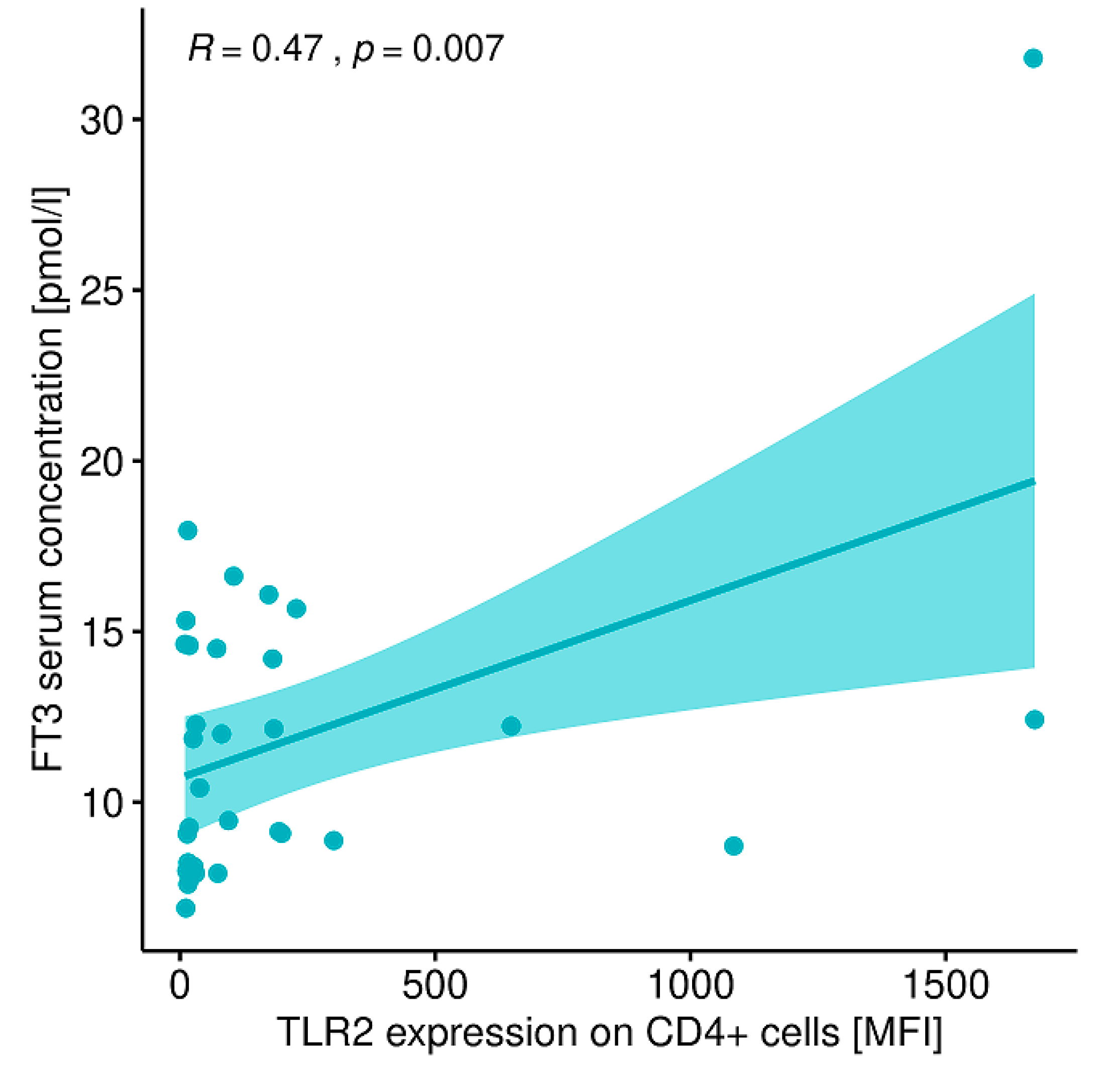
2.12. Evaluation of Correlations between the Expression of the Analyzed TLRs on Particular Cell Populations and Clinical Parameters in Patients with GD Prior to the Implementation of Antithyroid Therapy, after Obtaining Euthyroidism and in the Control Group
2.13. Evaluation of the Sum Activity of Peripheral Deiodinases (SPINA-GD), Thyroid’s Secretory Capacity (SPINA-GT) and the Jostel’s THSK Index (JTI)
3. Discussion
4. Materials and Methods
4.1. Characteristics of Study and Control Groups
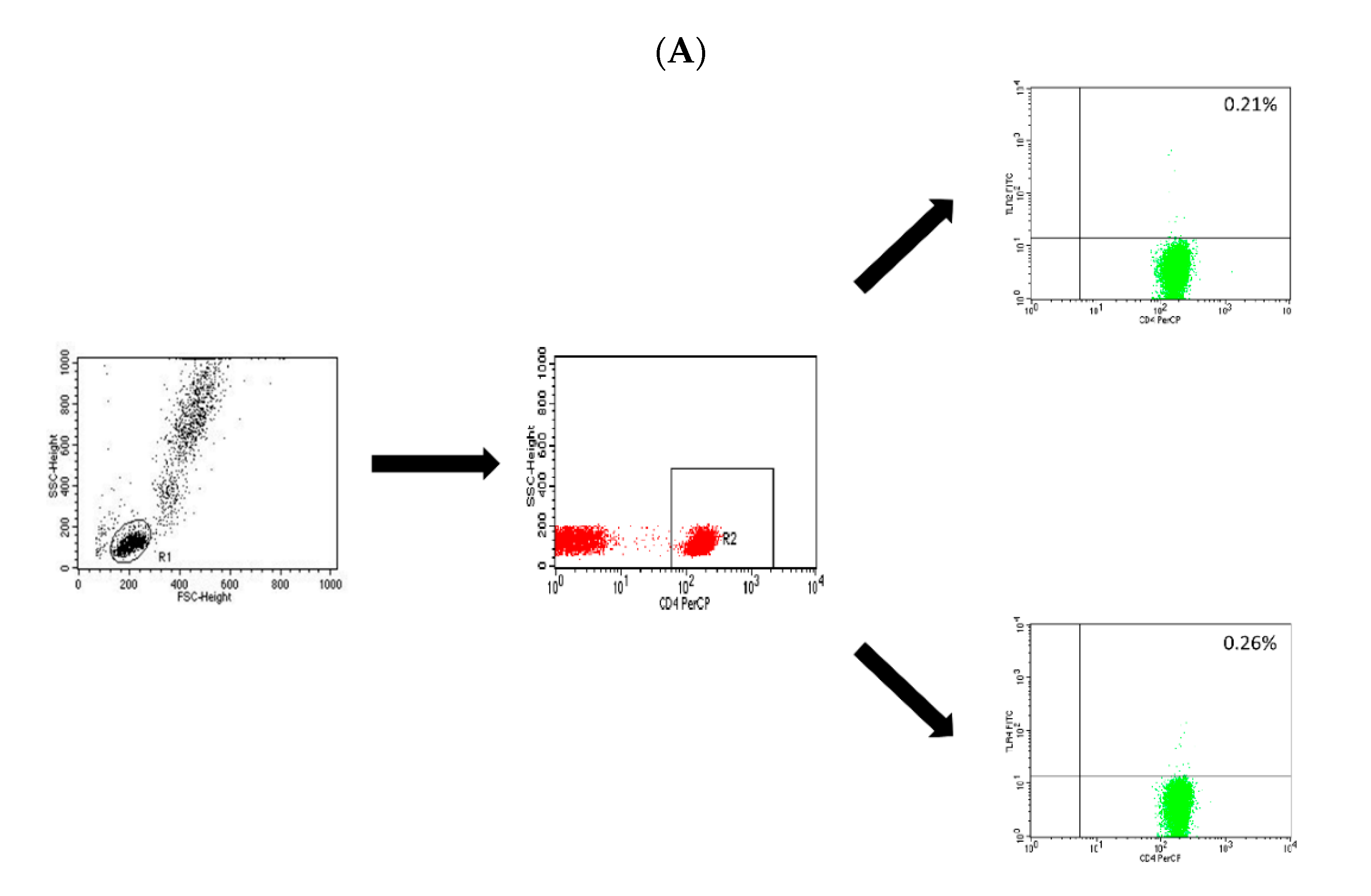
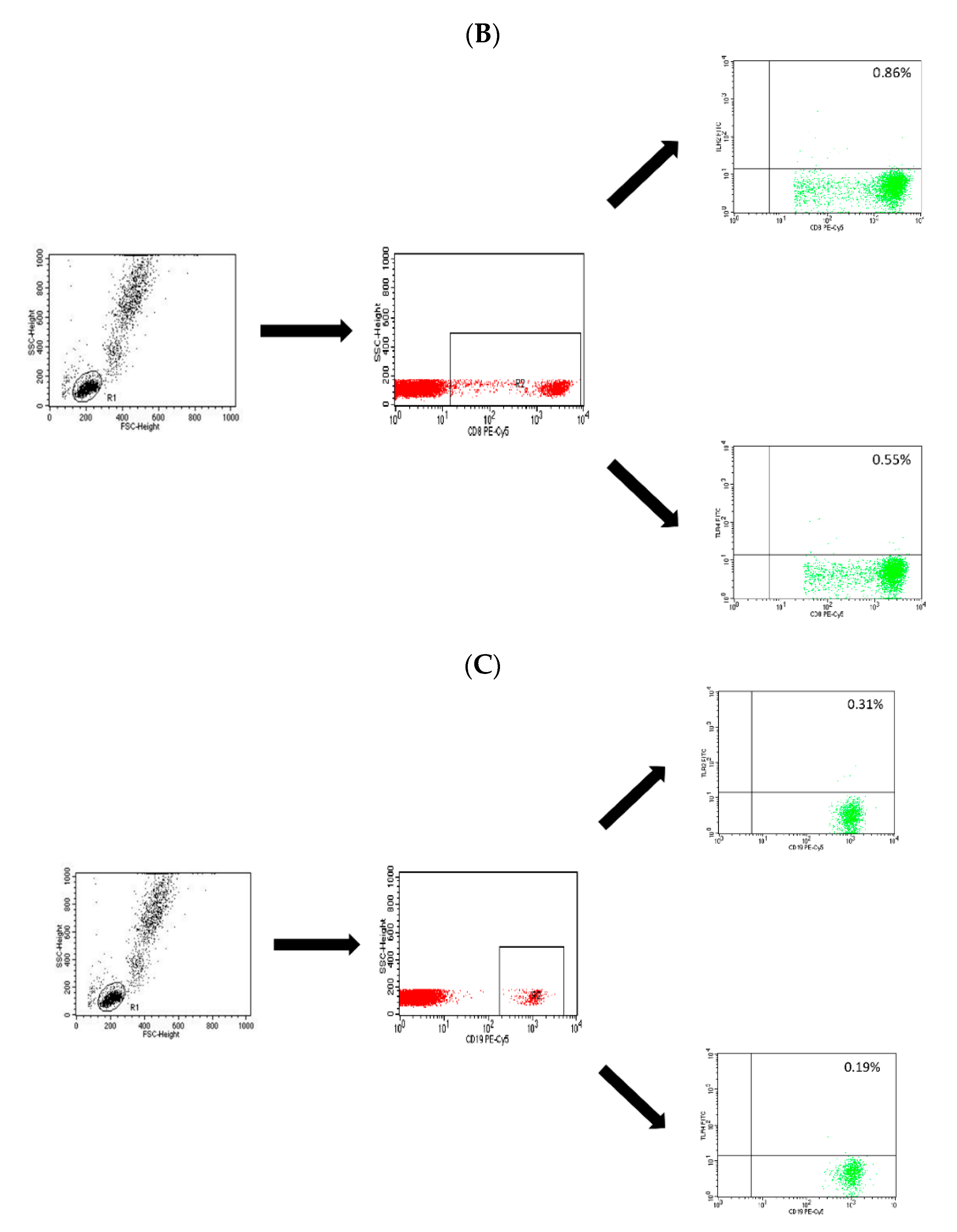
4.2. Assessment of the T CD4+, T CD8+ and B CD19+ Lymphocytes with the Expression Level of TLR-2 and TLR-4 Receptors
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GD | Graves’ disease |
| TLR | Toll-like receptor |
| AITD | autoimmune thyroid diseases |
| Anti-TG | anti-thyroglobulin antibodies |
| Anti-TPO | anti-thyroperoxidase antibodies |
| DAMP | damage-associated molecular patterns |
| FT3 | free triiodothyronine |
| FT4 | free thyroxine |
| IFN-gamma | interferon gamma |
| IL | interleukin |
| JTI | Jostel’s TSHI index |
| MFI | mean fluorescence intensity |
| PAMP | pathogen-associated molecular patterns |
| RA | rheumatoid arthritis |
| SLE | systemic lupus erythematosus |
| SPINA | structure parameter inference approach |
| SPINA-GT | thyroid’s secretory capacity |
| SPINA-GD | sum activity of peripheral deiodinases |
| TGF-beta | tumor growth factor beta |
| Th | T helper |
| TNF-alpha | Tumor necrosis factor alpha |
| TRAb | anti-TSH receptor antibody |
| TSH | thyroid stimulating hormone |
References
- Grimm, D. Cell and Molecular Biology of Thyroid Disorders. Int. J. Mol. Sci. 2019, 20, 2895. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Nagy, L.I.; Berkó, A.; Hoffmann, A.; Fehér, L.Z.; Bagyánszki, M.; Kari, B.; Balog, J.A.; Hackler, L., Jr.; Kanizsai, I.; et al. The Anti-Inflammatory Role of Mannich Curcuminoids; Special Focus on Colitis. Molecules 2019, 24, 1546. [Google Scholar] [CrossRef] [PubMed]
- Stasiołek, M.; Śliwka, P.W.; Stasiak, M.; Krawczyk-Rusiecka, K.; Skowrońska-Jóźwiak, E.; Adamczewski, Z.; Lewiński, A. Differences of the Structure of Immune Regulatory Cell Populations between Cellular Material from Sonographically Detected Focal Thyroid Lesions and Peripheral Blood in Humans. Int. J. Mol. Sci. 2019, 20, 918. [Google Scholar]
- Stasiak, M.; Michalak, R.; Stasiak, B.; Lewinski, A. Clinical characteristics of subacute thyroiditis is different than it used to be—Current state based on 15 years own material. Neuro Endocrinol. Lett. 2019, 39, 489–495. [Google Scholar] [PubMed]
- Marinò, M.; Latrofa, F.; Menconi, F.; Chiovato, L.; Vitti, P. Role of genetic and non-genetic factors in the etiology of Graves’ disease. J. Endocrinol. Investig. 2015, 38, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, K.; Watanabe, M.; Matsuzuka, F.; Miyauchi, A.; Iwatani, Y. Increase of serum interleukin-10 in intractable Graves’ disease. Thyroid 2004, 14, 201–205. [Google Scholar] [CrossRef] [PubMed]
- McIver, B.; Morris, J.C. The pathogenesis of Graves’ disease. Endocrinol. Metab. Clin. N. Am. 1998, 27, 73–89. [Google Scholar] [CrossRef]
- Perricone, C.; Shoenfeld, Y. The forefront of autoimmunity. Best Pract. Res. Clin. Rheumatol. 2018, 32, 487–488. [Google Scholar] [CrossRef]
- Toubi, E.; Shoenfeld, Y. Toll-like receptors and their role in the development of autoimmune diseases. Autoimmunity 2004, 37, 183–188. [Google Scholar] [CrossRef]
- Kessel, A.; Peri, R.; Haj, T.; Snir, A.; Slobodin, G.; Sabo, E.; Rosner, I.; Shoenfeld, Y.; Toubi, E. IVIg attenuates TLR-9 activation in B cells from SLE patients. J. Clin. Immunol. 2011, 31, 30–38. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Seidi, K.; Majidinia, M.; Karimian, A.; Yousefi, B.; Nabavi, S.M.; Astani, A.; Berindan-Neagoe, I.; Gulei, D.; Fallarino, F.; et al. Toll-like receptors as novel therapeutic targets for herpes simplex virus infection. Rev. Med. Virol. 2019, 29, 2048. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Gargaro, M.; Mondanell, G.; Downer, E.J.; Hossain, M.J.; Gran, B. Delineating the Role of Toll-Like Receptors in the Neuro-Inflammation Model EAE. Methods Mol. Biol. 2016, 1390, 383–411. [Google Scholar] [PubMed]
- Iwamura, C.; Nakayama, T. Toll-like receptors in the respiratory system: Their roles in inflammation. Curr. Allergy Asthma Rep. 2008, 8, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sandor, F.; Buc, M. Toll-like receptors. II. Distribution and pathways involved in TLR signalling. Folia Biol. 2005, 51, 188–197. [Google Scholar]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Cao, X. Regulation of Toll-like receptor signalling pathways in innate immune responses. Ann. N.Y. Acad. Sci. 2013, 1283, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, H.; Zhao, M.; Lu, Q. TLR2- and TLR-4 in autoimmune diseases: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 47, 136–147. [Google Scholar] [CrossRef]
- Theofilopouluos, A.N.; Gonzalez-Quintal, R.; Lawson, B.R.; Koh, Y.T.; Stern, M.E.; Kono, D.H.; Beutler, B.; Baccala, R. Sensors of innate immune system: Their link to rheumatic diseases. Nat. Rev. Rheumatol. 2010, 6, 146–156. [Google Scholar] [CrossRef]
- Komatsuda, A.; Wakui, H.; Iwamoto, K.; Ozawa, M.; Togashi, M.; Masai, R.; Maki, N.; Hatakeyama, T.; Sawada, K. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin. Exp. Immunol. 2008, 152, 482–487. [Google Scholar] [CrossRef]
- Kirchner, M.; Sonnenschein, A.; Schoofs, S.; Schmidtke, P.; Umlauf, V.N.; Mannhardt-Laakmann, W. Surface expression and genotypes of Toll-like receptors 2 and 4 in patients with juvenile idiopathic arthritis and systemic lupus erythematosus. Pediatr. Rheumatol. Online J. 2013, 11, 9. [Google Scholar] [CrossRef]
- Traks, T.; Keermann, M.; Karelson, M.; Rätsep, R.; Reimann, E.; Silm, H.; Vasar, E.; Kõks, S.; Kingo, K. Polymorphisms in Toll-like receptor genes are associated with vitiligo. Front. Genet. 2015, 6, 278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hari, A.; Flach, T.L.; Shi, Y.; Mydlarski, P.R. Toll-like receptors: Role in dermatological disease. Mediators Inflamm. 2010, 2010, 437246. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S. Toll-like receptors in skin. Adv. Dermatol. 2008, 24, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Zhang, S.; Zuo, F.; Kang, K.; Guan, M.; Xiang, L. Cultured human melanocytes express functional toll-like receptors 2-4, 7 and 9. J. Dermatol. Sci. 2009, 56, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Park, T.J.; Jin, S.H. Imiquimod, a Toll-like receptor 7 agonist, inhibits melanogenesis and proliferation of human melanocytes. J. Invest. Dermatol. 2009, 129, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Devarai, S.; Dasu, M.R.; Rockwood, J.; Winter, W.; Griffen, S.C.; Jialal, L. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: Further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2008, 93, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Nakashima, K.; Tamai, M.; Nakamura, H.; Iwanaga, N.; Fujikawa, K.; Aramaki, T.; Arima, K.; Iwamoto, N.; Ichinose, K.; et al. Toll-like receptor in salivary glands from patients with Sjogren’s syndrome: Functional analysis by human salivary gland cell line. J. Rheumatol. 2007, 34, 1019–1026. [Google Scholar] [PubMed]
- Merrill, S.J.; Mu, Y. Thyroid autoimmunity as a window to autoimmunity: An explanation for sex differences in the prevalence of thyroid autoimmunity. J. Theor. Biol. 2015, 375, 95–100. [Google Scholar] [CrossRef]
- Wu, H.; Liao, W.; Li, Q.; Long, H.; Yin, H.; Zhao, M.; Chan, V.; Lau, C.S.; Lu, Q. Pathogenic role of tissue-resident memory T cells in autoimmune diseases. Autoimmun. Rev. 2018, 17, 906–911. [Google Scholar] [CrossRef]
- Baldini, E.; Odorisio, T.; Tuccilli, C.; Persechino, S.; Sorrenti, S.; Catania, A.; Pironi, D.; Carbotta, G.; Giacomelli, L.; Arcieri, S.; et al. Thyroid diseases and skin autoimmunity. Rev. Endocr. Metab. Disord. 2018, 19, 311–323. [Google Scholar] [CrossRef]
- Baldini, E.; Odorisio, T.; Sorrenti, S.; Catania, A.; Tartaglia, F.; Carbotta, G.; Pironi, D.; Rendina, R.; D’Armiento, E.; Persechino, S.; et al. Vitiligo and Autoimmune Thyroid Disorders. Front. Endocrinol. (Lausanne) 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Senese, R.; Cioffi, F.; De Matteis, R.; Petito, G.; de Lange, P.; Silvestri, E.; Lombardi, A.; Moreno, M.; Goglia, F.; Lanni, A. 3,5 Diiodo-l-Thyronine (T₂) Promotes the Browning of White Adipose Tissue in High-Fat Diet-Induced Overweight Male Rats Housed at Thermoneutrality. Cells 2019, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Giacco, A.; Delli Paoli, G.; Senese, R.; Cioffi, F.; Silvestri, E.; Moreno, M.; Ruoppolo, M.; Caterino, M.; Costanzo, M.; Lombardi, A.; et al. The saturation degree of fatty acids and their derived acylcarnitines determines the direct effect of metabolically active thyroid hormones on insulin sensitivity in skeletal muscle cells. FASEB J. 2019, 33, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Mazzi, V.; Antonelli, A.; Fallahi, P. Chemokines in hyperthyroidism. J. Clin. Transl. Endocrinol. 2019, 16, 100196. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Paparo, S.R.; Antonelli, A. Thyroid autoimmune disorders and cancer. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Patrizio, A.; Galdiero, M.R.; Baldini, E.; Ulisse, S.; Marone, G.; et al. Autoimmune Endocrine Dysfunctions Associated with Cancer Immunotherapies. Int. J. Mol. Sci. 2019, 20, 2560. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.; Sniecikowska, B.; Zygmunt, A.; Brzezinski, J.; Dedecjus, M.; Lewinski, A. Postoperative hypoparathyroidism in patients after total thyroidectomy - retrospective analysis. Neuro Endocrinol. Lett. 2017, 38, 488–494. [Google Scholar] [PubMed]
- Wojciechowska-Durczynska, K.; Lewinski, A. Search for relevant indications for selenium supplementation in thyroid diseases. Neuro Endocrinol. Lett. 2017, 38, 237–241. [Google Scholar]
- Lewandowski, K.; Dąbrowska, K.; Makarewicz, J.; Lewiński, A. Pendulum swings from hypo- to hyperthyroidism: Thyrotoxicosis after severe hypothyroidism following neck irradiation in a patient with a history of Hodgkin’s lymphoma. Thyroid Res. 2016, 9, 1. [Google Scholar] [CrossRef][Green Version]
- Ferrari, S.M.; Ragusa, F.; Paparo, S.R.; Nasini, F.; Nardi, M.; Franceschini, S.S.; Fallahi, P.; Antonelli, A. Differential modulation of CXCL8 versus CXCL10, by cytokines, PPAR-gamma, or PPAR-alpha agonists, in primary cells from Graves’ disease and ophthalmopathy. Autoimmun. Rev. 2019, 18, 673–678. [Google Scholar] [CrossRef]
- McGrogan, A.; Seaman, H.E.; Wright, J.W.; de Vries, C.S. The incidence of autoimmune thyroid disease: A systematic review of the literature. Clin. Endocrinol. 2008, 69, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, M.; Cojocaru, I.M.; Siloşi, I.; Rogoz, S. Role of leptin in autoimmune diseases. Maedica (Buchar) 2013, 8, 68–74. [Google Scholar] [PubMed]
- Akeno, N.; Smith, E.; Stefan, M.; Huber, A.; Zhang, W.; Keddache, M.; Tomer, Y. IFN-α mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. J. Immunol. 2011, 186, 4693–4706. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, C.; Wang, X.; Liu, X.; Han, C.; Jin, T.; Liu, S.; Zhang, X.; Zhang, H.; He, X.; et al. Increased Toll-Like Receptors Activity and TLR Ligands in Patients with Autoimmune Thyroid Diseases. Front. Immunol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, S.; Arias-Santiago, S.; Perandrés-López, R.; Castellote, L.; Zumaquero, E.; Navarro, P.; Buendía-Eisman, A.; Ruiz, J.C.; Orgaz-Molina, J.; Sancho, J.; et al. Increased gene expression of Toll-like receptor 4 on peripheral blood mononuclear cells in patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 242–250. [Google Scholar] [CrossRef] [PubMed]
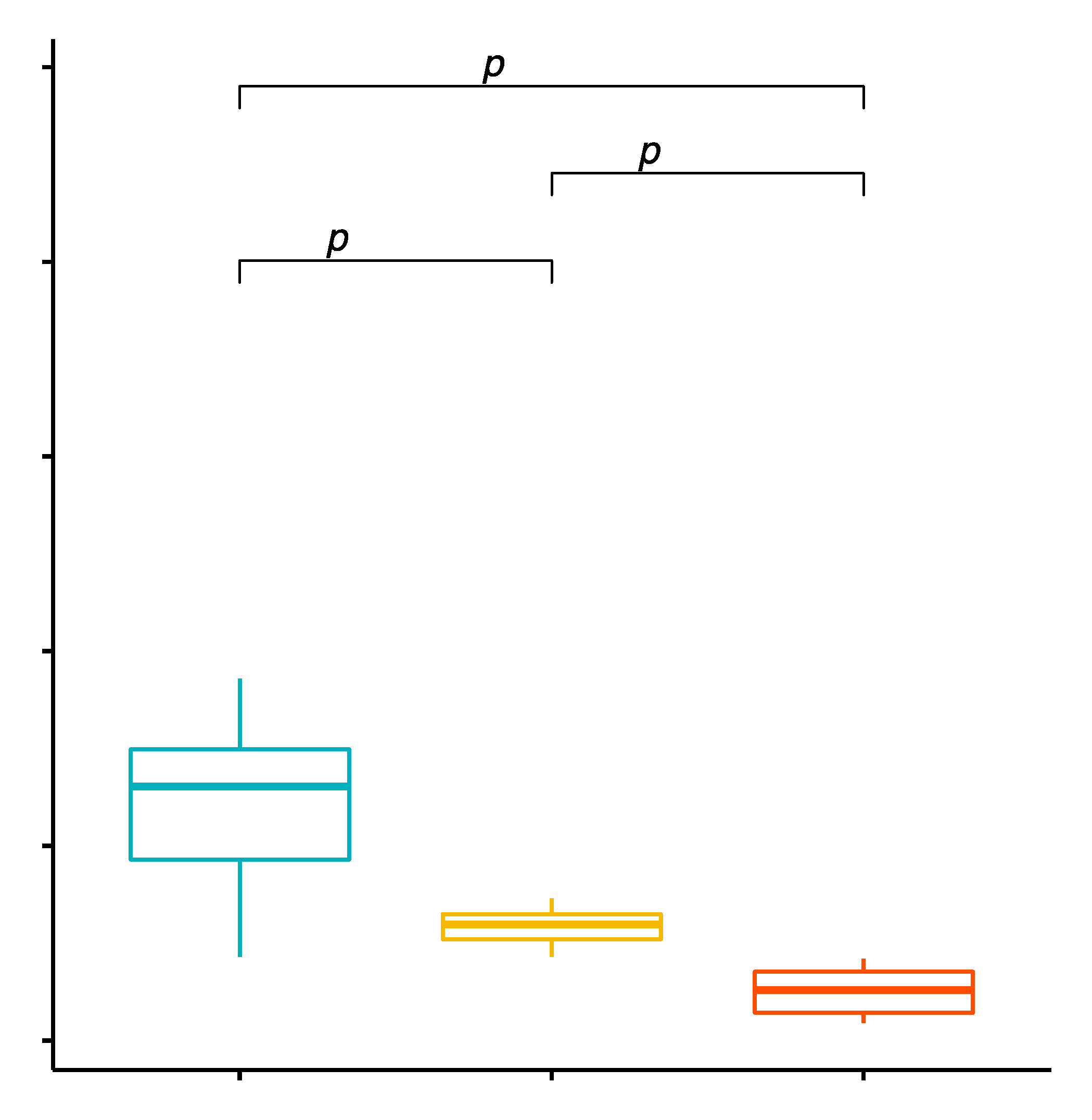
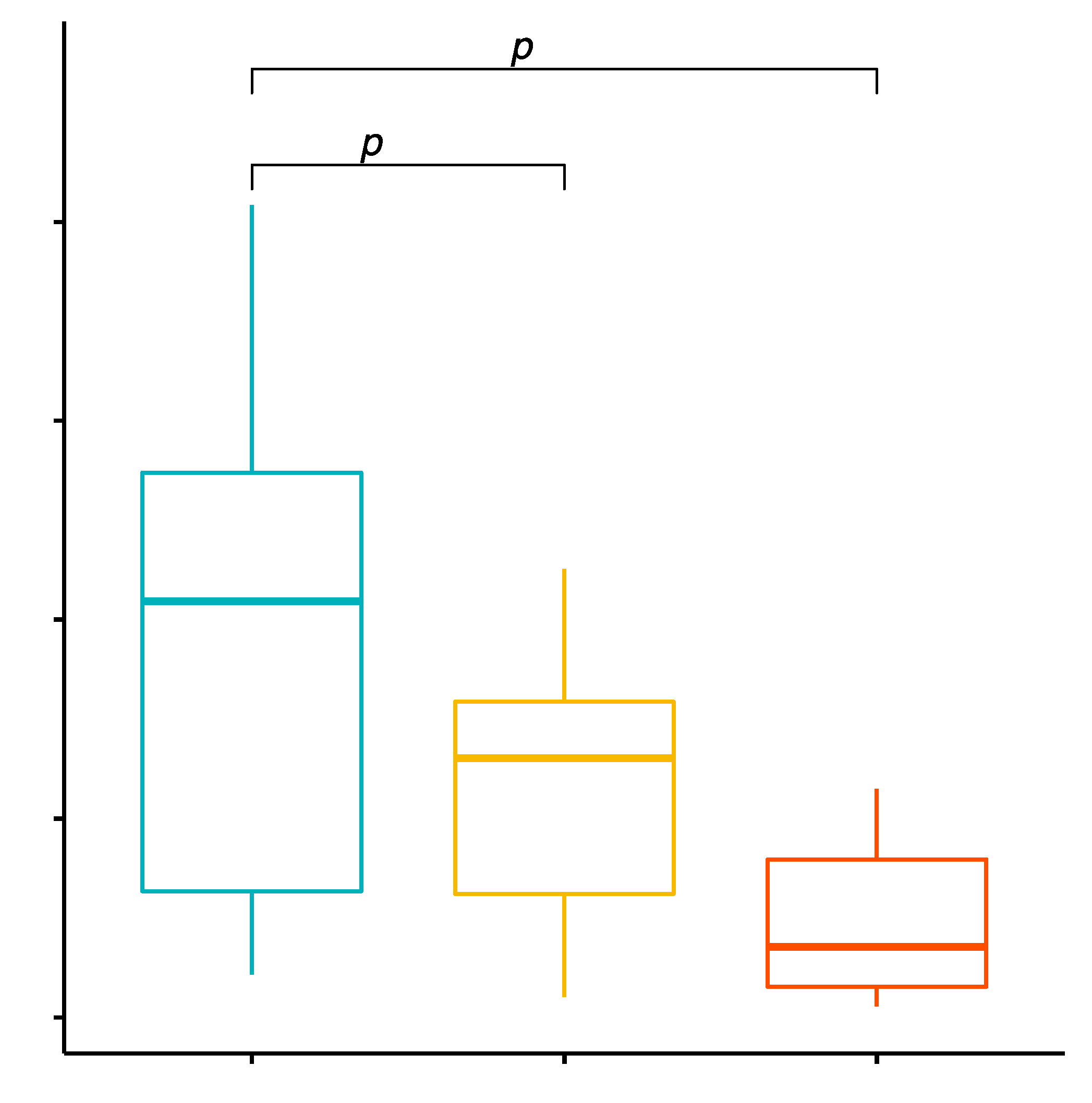
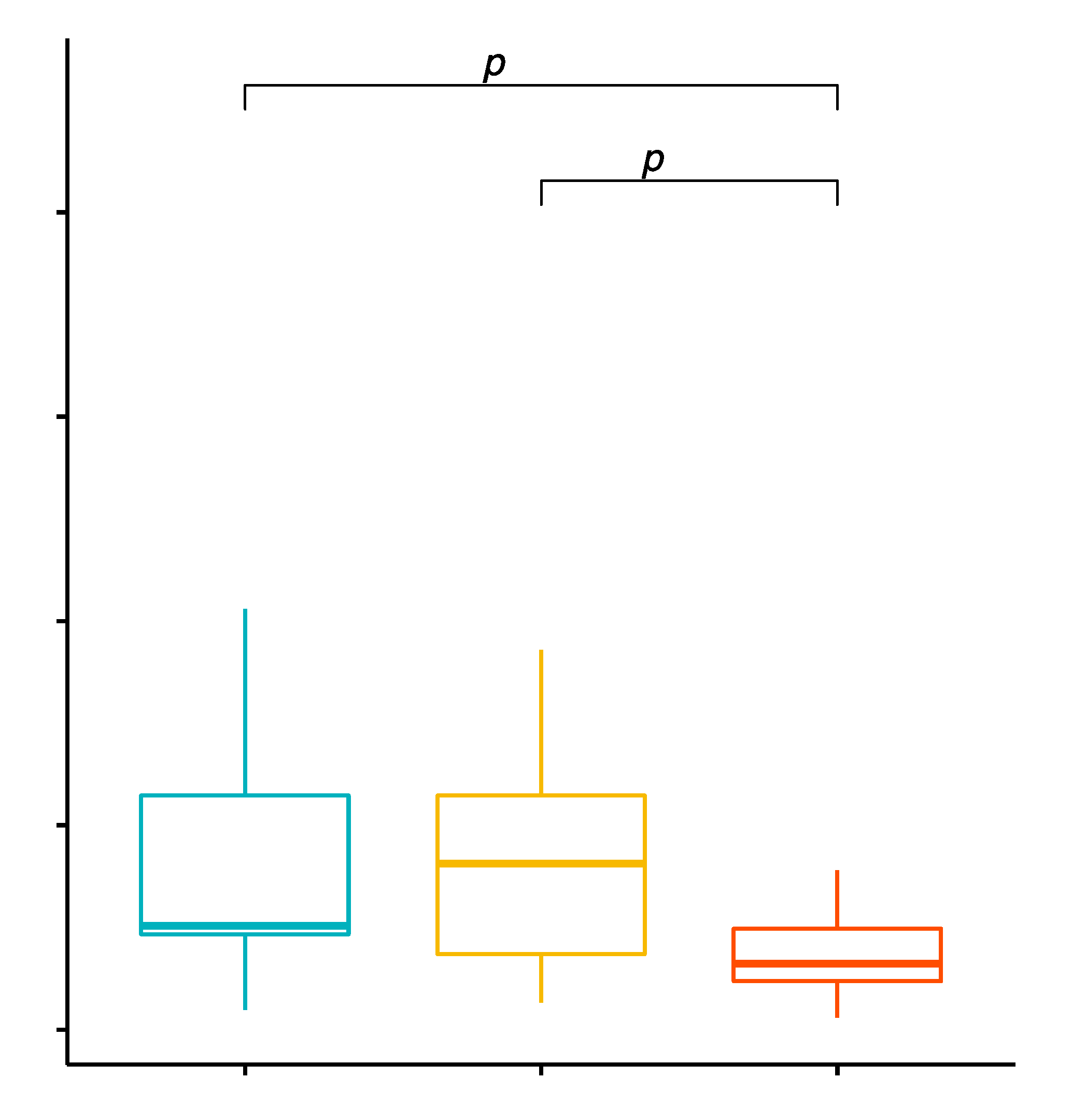
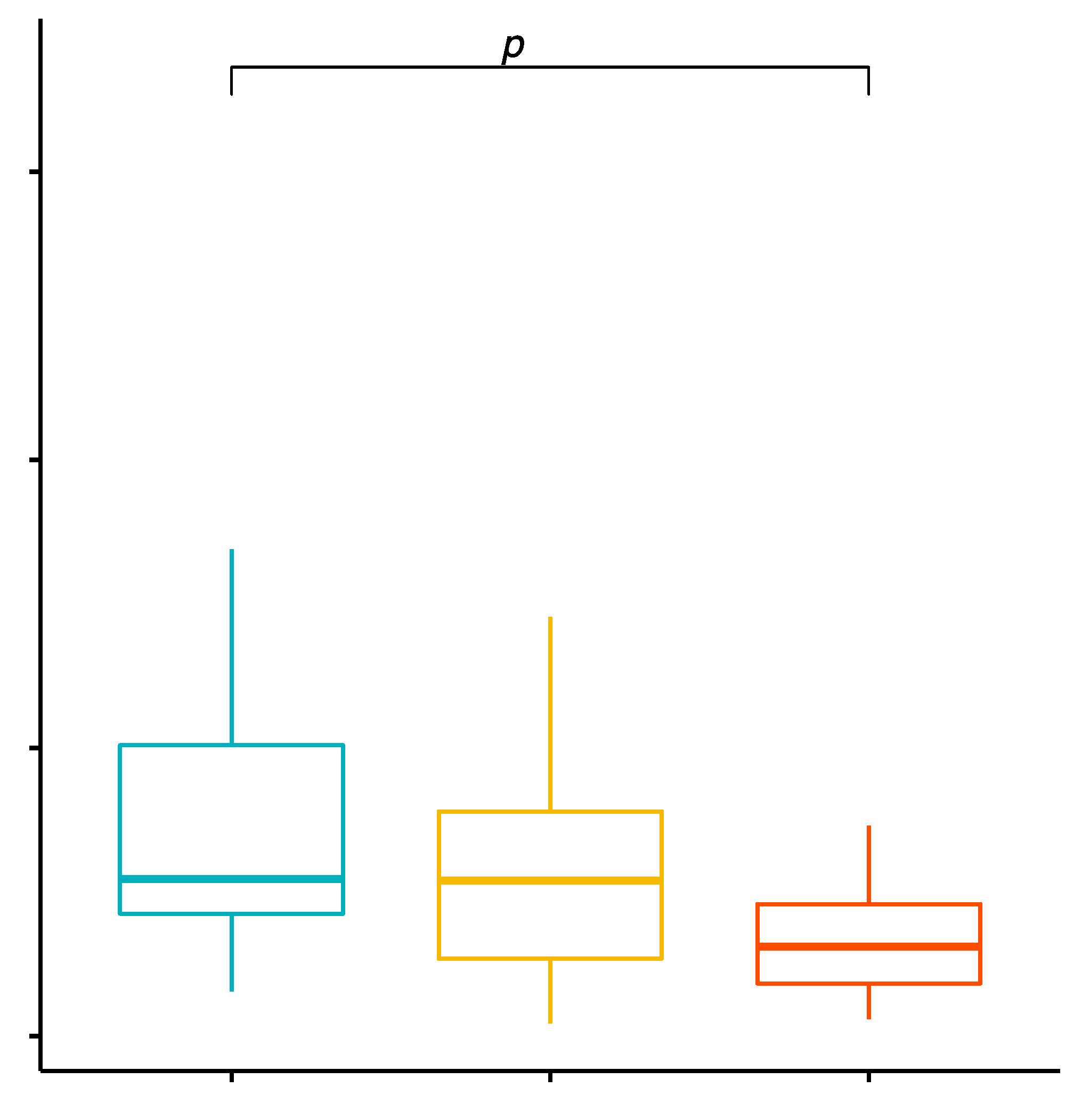
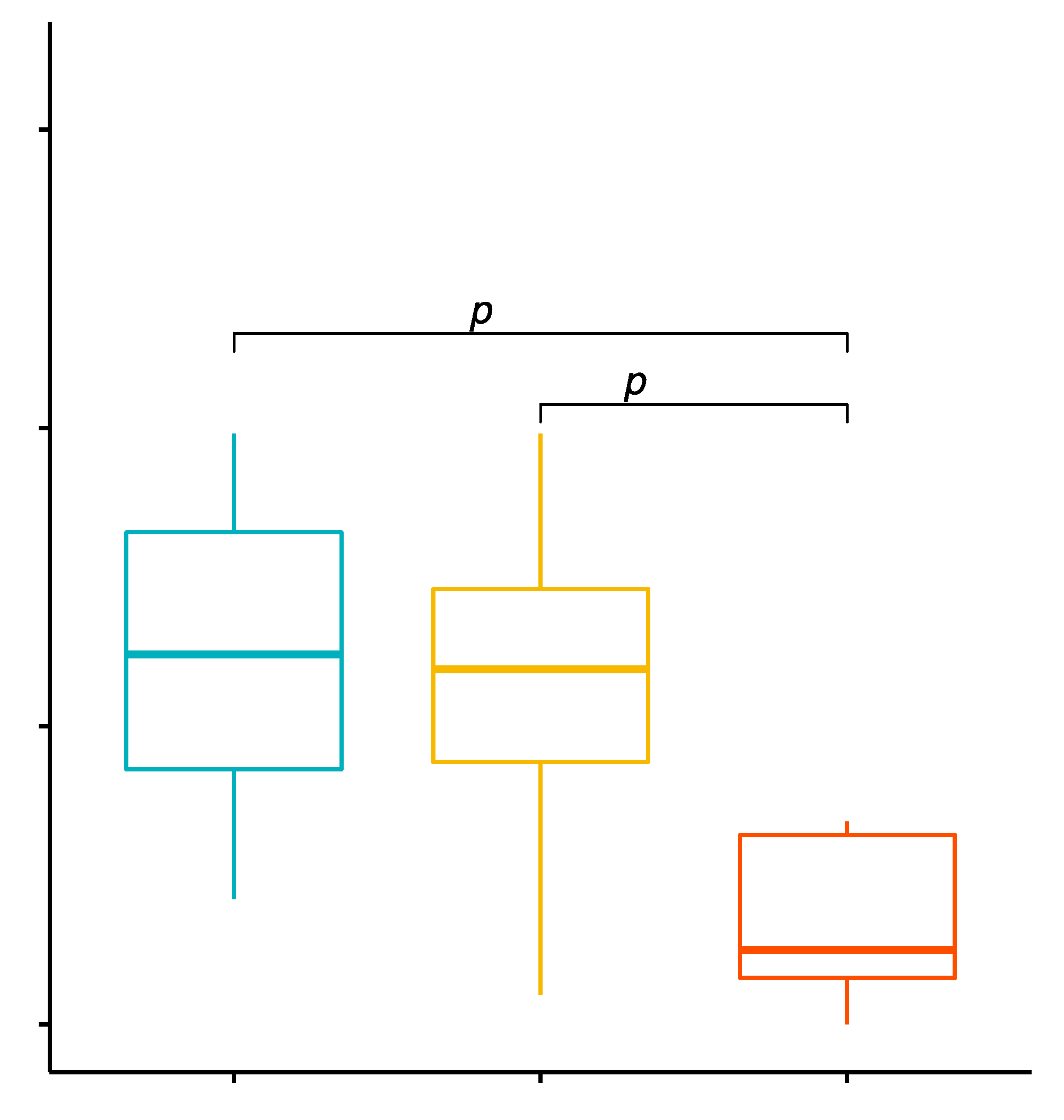
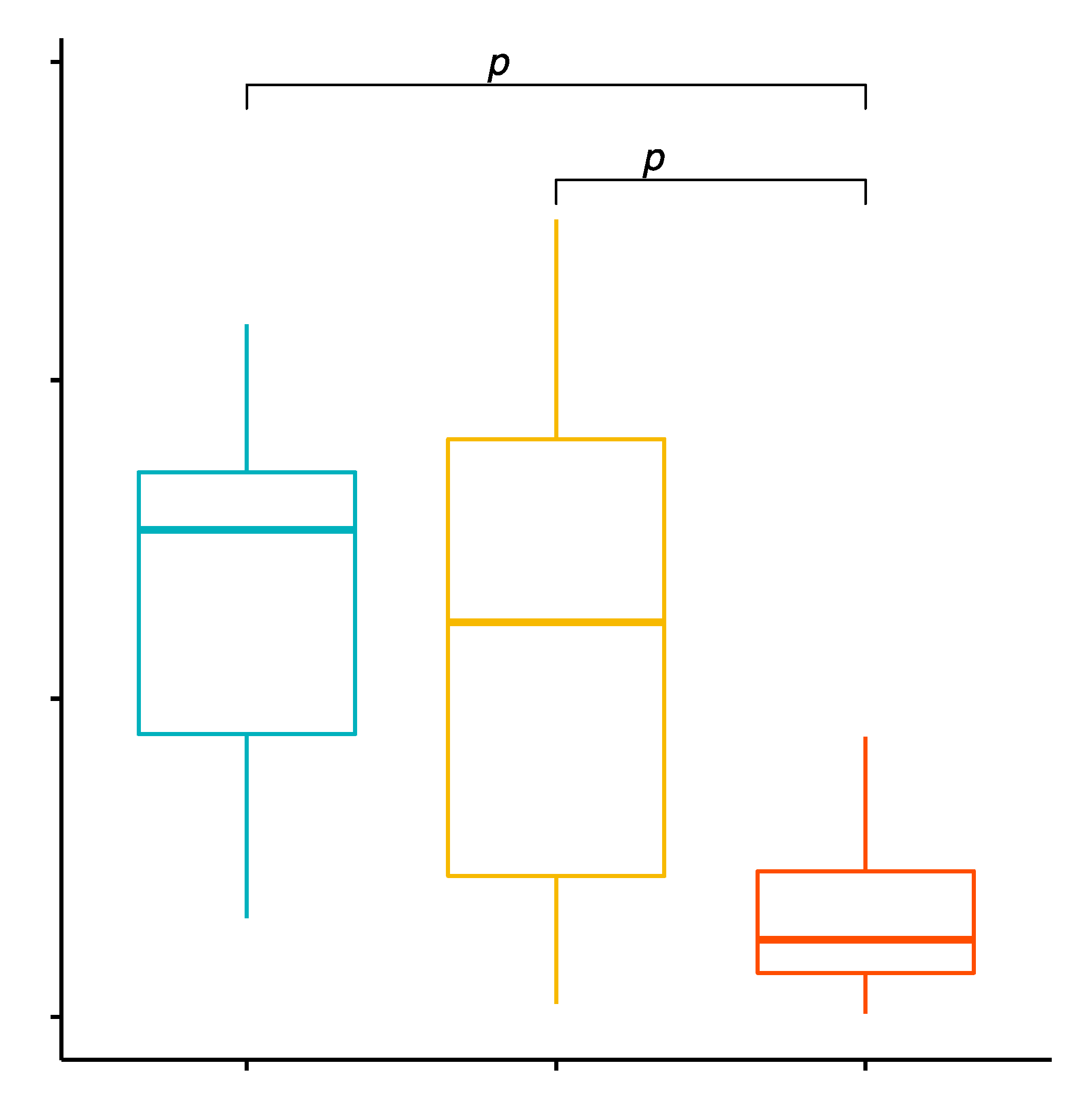
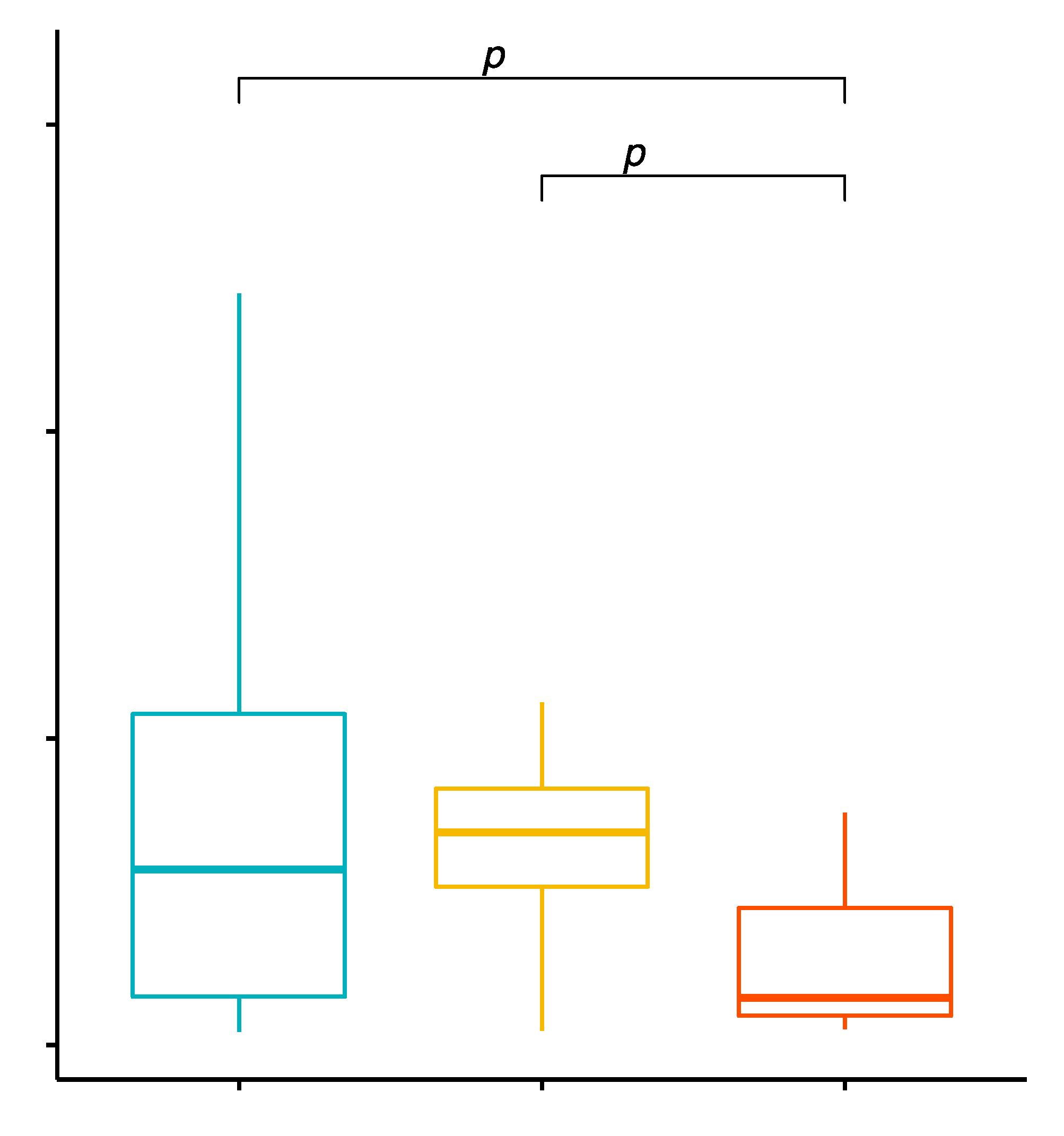


| Age (years) | Mean ± SD | 41.00 ± 16.21 |
| Median | 36.00 | |
| Min. | 22.00 | |
| Max. | 95.00 | |
| Signs and symptoms duration from the time of diagnosis until the implementation of thyrostatic therapy (months) | Mean ± SD | 2.34 ± 2.01 |
| Median | 2.00 | |
| Min. | 0.00 | |
| Max. | 8.00 | |
| TRAb (U/l) | Mean ± SD | 12.17 ± 9.69 |
| Median | 9.75 | |
| Min. | 1.70 | |
| Max. | 39.40 | |
| Anti-TPO antibodies (U/mL) | Mean ± SD | 1871.47 ± 2691.30 |
| Median | 1237.50 | |
| Min. | 13.70 | |
| Max. | 14753.00 | |
| Anti-TG antibodies (U/mL) | Mean ± SD | 250.43 ± 325.95 |
| Median | 129.00 | |
| Min. | 10.00 | |
| Max. | 1360.00 | |
| Thyroid gland volume (mL) | Mean ± SD | 21.20 ± 7.34 |
| Median | 20.35 | |
| Min. | 13.30 | |
| Max. | 47.60 | |
| Methimazole daily dosage (mg) | Mean ± SD | 34.53 ± 7.66 |
| Median | 40.00 | |
| Min. | 20.00 | |
| Max. | 45.00 | |
| Time of thyrostatic therapy until receiving euthyroidism (methimazole treatment; days) | Mean ± SD | 45.75 ± 9.38 |
| Median | 43.00 | |
| Min. | 31.00 | |
| Max. | 74.00 | |
| Relapse of hyperthyroidism | Yes—number of patients (%) | 10 (31.25%) |
| No—number of patients (%) | 22 (68.75%) | |
| Time elapsed between receiving euthyroidism and relapse (months) | Mean ± SD | 22.50 ± 11.00 |
| Median | 24.50 | |
| Min. | 6.00 | |
| Max. | 35.00 |
| GD Patients before Treatment | GD Patients after Receiving Euthyroidism | Control Group | GD Patients before Treatment vs. GD Patients after Receiving Euthyroidism | GD Patients before Treatment vs. Control Group | GD Patients after Receiving Euthyroidism vs. Control Group | ||
|---|---|---|---|---|---|---|---|
| TSH concentration in the peripheral blood (mU/L) | Mean ± SD | 0.01 ± 0.02 | 0.15 ± 0.34 | 2.71 ± 0.81 | NS | p < 0.0001 | p < 0.0001 |
| Median | 0.01 | 0.02 | 2.71 | ||||
| Min. | 0.0010 | 0.0020 | 0.92 | ||||
| Max. | 0.09 | 1.33 | 3.98 | ||||
| FT3 concentration in the peripheral blood (pmol/L) | Mean ± SD | 11.90 ± 4.82 | 5.42 ± 1.24 | 4.97 ± 0.74 | p < 0.0001 | p < 0.0001 | NS |
| Median | 11.14 | 4.89 | 4.88 | ||||
| Min. | 6.90 | 3.78 | 3.60 | ||||
| Max. | 31.79 | 7.66 | 6.40 | ||||
| FT4 concentration in the peripheral blood (pmol/L) | Mean ± SD | 35.01 ± 15.26 | 16.93 ± 2.78 | 17.27 ± 1.99 | p < 0.0001 | p < 0.0001 | NS |
| Median | 30.95 | 16.34 | 17.04 | ||||
| Min. | 19.05 | 12.23 | 14.00 | ||||
| Max. | 82.22 | 22.39 | 21.47 | ||||
| Lymphocytosis (103/mm3) | Mean ± SD | 1.85 ± 0.45 | 2.58 ± 0.43 | 2.35 ± 0.59 | NS | NS | NS |
| Median | 1.75 | 2.57 | 2.37 | ||||
| Min. | 1.22 | 1.75 | 1.39 | ||||
| Max. | 2.87 | 3.34 | 3.38 | ||||
| T CD3+/CD4+ lymphocytes (%) | Mean ± SD | 41.96 ± 1.15 | 41.56 ± 1.27 | 42.5 ± 1.33 | NS | NS | NS |
| Median | 42.47 | 41.19 | 42.45 | ||||
| Min. | 40.63 | 40.54 | 41.06 | ||||
| Max. | 43.99 | 44.19 | 44.13 | ||||
| T CD3+/CD8+ lymphocytes (%) | Mean ± SD | 30.79 ± 1.07 | 29.42 ± 0.46 | 30.58 ± 1.23 | NS | NS | NS |
| Median | 30.61 | 29.47 | 30.29 | ||||
| Min. | 29.35 | 28.9 | 29.45 | ||||
| Max. | 33.08 | 30.09 | 33.17 | ||||
| B CD19+ lymphocytes (%) | Mean ± SD | 11.17 ± 1.02 | 10.26 ± 2.61 | 10.59 ± 1.89 | NS | NS | NS |
| Median | 11.61 | 10.31 | 10.5 | ||||
| Min. | 9.46 | 6.04 | 7.62 | ||||
| Max. | 12.23 | 14.52 | 13.9 | ||||
| SPINA-GD | Mean ± SD | 33.91 ± 12.85 | 30.49 ± 8.58 | 26.75 ± 3.61 | NS | p = 0.007 | NS |
| Median | 33.09 | 30.13 | 27.01 | ||||
| Min. | 12.79 | 16.85 | 19.61 | ||||
| Max. | 80.66 | 45.64 | 33.31 | ||||
| SPINA-GT | Mean ± SD | 2336.39 ± 2665.83 | 428.71 ± 589.93 | 2.778 ± 0.55 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Median | 893.37 | 183.838 | 2.58 | ||||
| Min. | 59.71 | 3.27 | 2.11 | ||||
| Max. | 8388.75 | 2242.32 | 4.48 | ||||
| JTI | Mean ± SD | −0.34 ± 2.11 | −1.40 ± 1.74 | 3.27 ± 0.53 | p = 0.020 | p < 0.0001 | p < 0.0001 |
| Median | −1.00 | −1.89 | 3.37 | ||||
| Min. | −2.96 | −3.52 | 1.91 | ||||
| Max. | 5.76 | 2.66 | 4.24 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polak, A.; Grywalska, E.; Klatka, J.; Roliński, J.; Matyjaszek-Matuszek, B.; Klatka, M. Toll-Like Receptors-2 and -4 in Graves’ Disease—Key Players or Bystanders? Int. J. Mol. Sci. 2019, 20, 4732. https://doi.org/10.3390/ijms20194732
Polak A, Grywalska E, Klatka J, Roliński J, Matyjaszek-Matuszek B, Klatka M. Toll-Like Receptors-2 and -4 in Graves’ Disease—Key Players or Bystanders? International Journal of Molecular Sciences. 2019; 20(19):4732. https://doi.org/10.3390/ijms20194732
Chicago/Turabian StylePolak, Agnieszka, Ewelina Grywalska, Janusz Klatka, Jacek Roliński, Beata Matyjaszek-Matuszek, and Maria Klatka. 2019. "Toll-Like Receptors-2 and -4 in Graves’ Disease—Key Players or Bystanders?" International Journal of Molecular Sciences 20, no. 19: 4732. https://doi.org/10.3390/ijms20194732
APA StylePolak, A., Grywalska, E., Klatka, J., Roliński, J., Matyjaszek-Matuszek, B., & Klatka, M. (2019). Toll-Like Receptors-2 and -4 in Graves’ Disease—Key Players or Bystanders? International Journal of Molecular Sciences, 20(19), 4732. https://doi.org/10.3390/ijms20194732





