Xanthine Oxidase Inhibitor Febuxostat Exerts an Anti-Inflammatory Action and Protects against Diabetic Nephropathy Development in KK-Ay Obese Diabetic Mice
Abstract
1. Introduction
2. Results
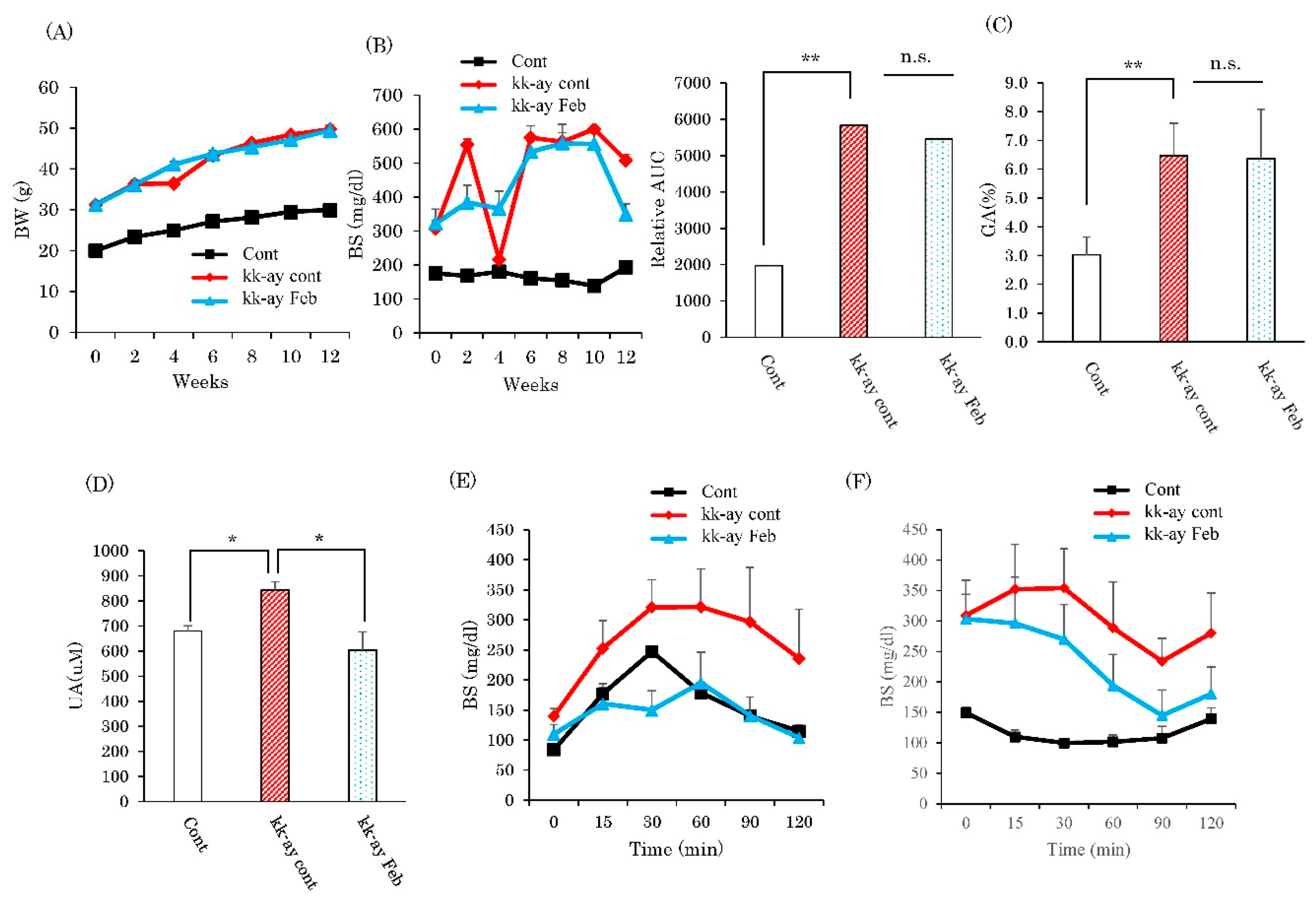
2.1. Effects of XO Inhibitor Febuxostat on Glycemic Control and Serum Uric Acid Levels
2.2. Effects of Febuxostat Administration on Glomerular Sclerosis
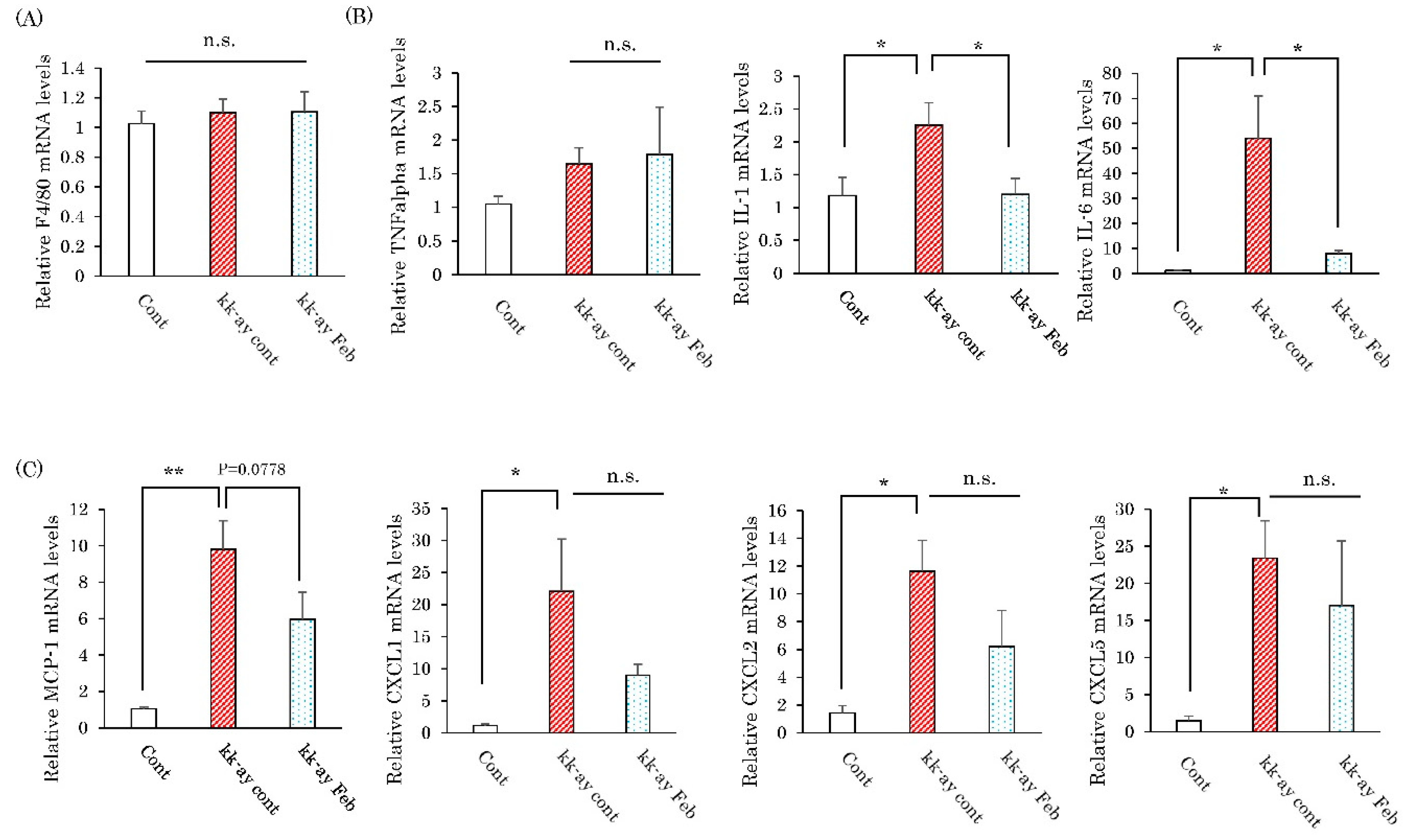
2.3. Effects of Febuxostat on the Renal Expression Levels of Inflammatory Cytokines
2.4. Effects of Febuxostat on Fibrosis-Related Collagen Gene Expressions
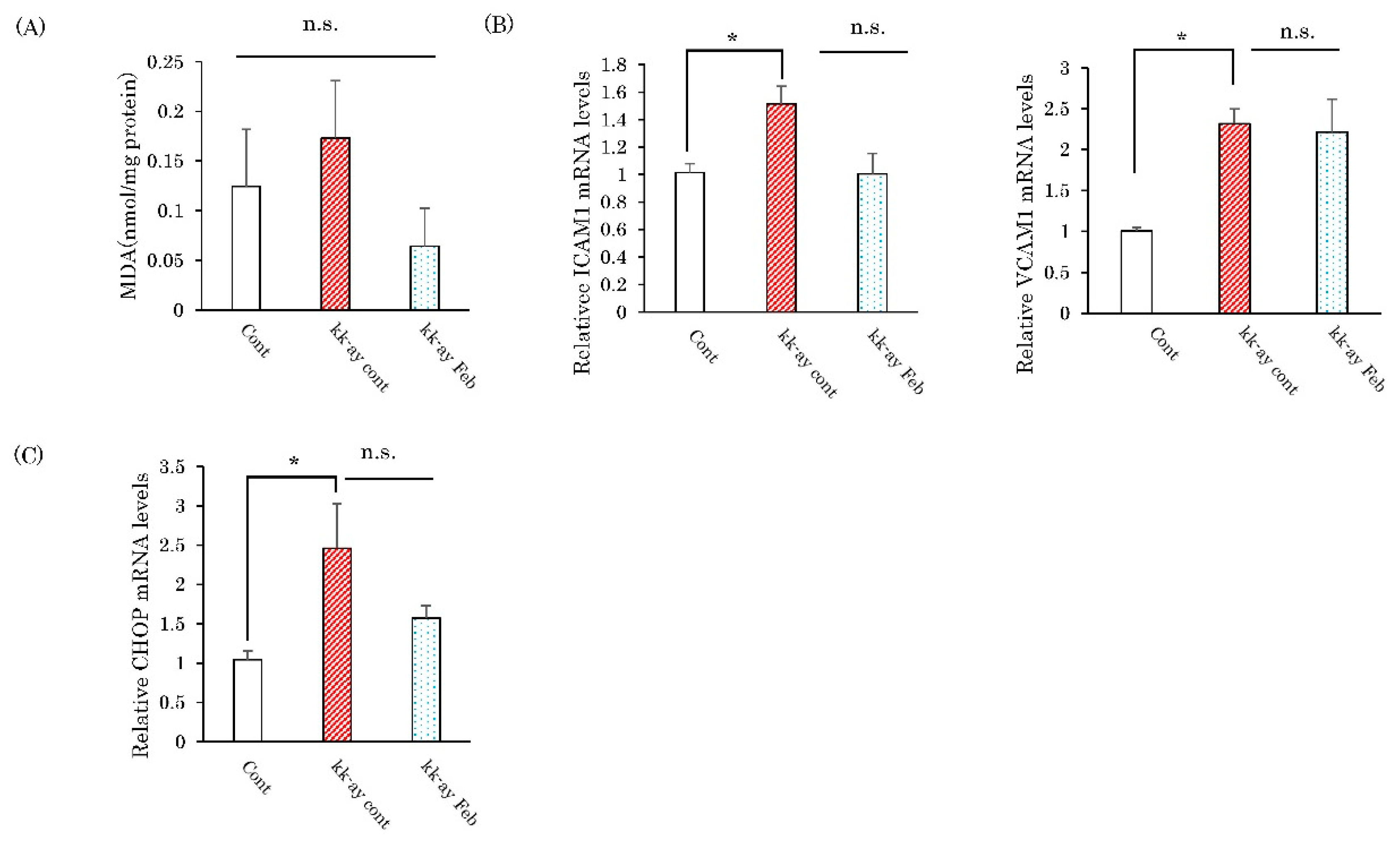
2.5. Effects of Febuxostat on Markers of Oxidative Stress and the Endoplasmic Reticulum
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Metabolic Analysis
4.3. Histological Study
4.4. Malondialdehyde (MDA) assay
4.5. Quantitative Real-Time RT-PCR
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease. Int J. Mol. Sci. 2019. [Google Scholar] [CrossRef]
- Vejakama, P.; Ingsathit, A.; McKay, G.J.; Maxwell, A.P.; McEvoy, M.; Attia, J.; Thakkinstian, A. Treatment effects of renin-angiotensin aldosterone system blockade on kidney failure and mortality in chronic kidney disease patients. BMC Nephrol. 2017, 18, 342. [Google Scholar] [CrossRef]
- Riccio, E.; Di Nuzzi, A.; Pisani, A. Nutritional treatment in chronic kidney disease: The concept of nephroprotection. Clin. Exp. Nephrol. 2015, 19, 161–167. [Google Scholar] [CrossRef]
- Tanaka, K.; Hara, S.; Hattori, M.; Sakai, K.; Onishi, Y.; Yoshida, Y.; Kawazu, S.; Kushiyama, A. Role of elevated serum uric acid levels at the onset of overt nephropathy in the risk for renal function decline in patients with type 2 diabetes. J. Diabetes Investig. 2015, 6, 98–104. [Google Scholar] [CrossRef]
- Shichiri, M.; Iwamoto, H.; Marumo, F. Diabetic hypouricemia as an indicator of clinical nephropathy. Am. J. Nephrol. 1990, 10, 115–122. [Google Scholar] [CrossRef]
- Ficociello, L.H.; Rosolowsky, E.T.; Niewczas, M.A.; Maselli, N.J.; Weinberg, J.M.; Aschengrau, A.; Eckfeldt, J.H.; Stanton, R.C.; Galecki, A.T.; Doria, A.; et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: Results of a 6-year follow-up. Diabetes Care 2010, 33, 1337–1343. [Google Scholar] [CrossRef]
- Kushiyama, A.; Tanaka, K.; Hara, S.; Kawazu, S. Linking uric acid metabolism to diabetic complications. World J. Diabetes 2014, 5, 787–795. [Google Scholar] [CrossRef]
- Pisano, A.; Cernaro, V.; Gembillo, G.; D′Arrigo, G.; Buemi, M.; Bolignano, D. Xanthine Oxidase Inhibitors for Improving Renal Function in Chronic Kidney Disease Patients: An Updated Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2017. [Google Scholar] [CrossRef]
- Siu, Y.P.; Leung, K.T.; Tong, M.K.; Kwan, T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006, 47, 51–59. [Google Scholar] [CrossRef]
- Beddhu, S.; Filipowicz, R.; Wang, B.; Wei, G.; Chen, X.; Roy, A.C.; DuVall, S.L.; Farrukh, H.; Habib, A.N.; Bjordahl, T.; et al. A Randomized Controlled Trial of the Effects of Febuxostat Therapy on Adipokines and Markers of Kidney Fibrosis in Asymptomatic Hyperuricemic Patients With Diabetic Nephropathy. Can. J. Kidney Health Dis. 2016, 3, 2054358116675343. [Google Scholar] [CrossRef]
- Mukri, M.N.A.; Kong, W.Y.; Mustafar, R.; Shaharir, S.S.; Shah, S.A.; Abdul Gafor, A.H.; Mohd, R.; Abdul Cader, R.; Kamaruzaman, L. Role of febuxostat in retarding progression of diabetic kidney disease with asymptomatic hyperuricemia: A 6-months open-label, randomized controlled trial. Excli. J. 2018, 17, 563–575. [Google Scholar]
- Kojima, S.; Matsui, K.; Hiramitsu, S.; Hisatome, I.; Waki, M.; Uchiyama, K.; Yokota, N.; Tokutake, E.; Wakasa, Y.; Jinnouchi, H.; et al. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur. Heart J. 2019, 40, 1778–1786. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Y.; Zhang, Q.Y.; Wang, F.M.; Kong, L.D. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS ONE 2012, 7, e38285. [Google Scholar] [CrossRef]
- Komers, R.; Xu, B.; Schneider, J.; Oyama, T.T. Effects of xanthine oxidase inhibition with febuxostat on the development of nephropathy in experimental type 2 diabetes. Br. J. Pharm. 2016, 173, 2573–2588. [Google Scholar] [CrossRef]
- Kosugi, T.; Nakayama, T.; Heinig, M.; Zhang, L.; Yuzawa, Y.; Sanchez-Lozada, L.G.; Roncal, C.; Johnson, R.J.; Nakagawa, T. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am. J. Physiol. Ren. Physiol. 2009, 297, F481–488. [Google Scholar] [CrossRef]
- Iwatsuka, H.; Shino, A.; Suzuoki, Z. General survey of diabetic features of yellow KK mice. Endocrinol Jpn. 1970, 17, 23–35. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Remuzzi, G. Nephropathy of type-2 diabetes mellitus. J. Am. Soc. Nephrol. 1998, 9, 2157–2169. [Google Scholar]
- Bo, S.; Cavallo-Perin, P.; Gentile, L.; Repetti, E.; Pagano, G. Hypouricemia and hyperuricemia in type 2 diabetes: Two different phenotypes. Eur. J. Clin. Invest. 2001, 31, 318–321. [Google Scholar] [CrossRef]
- Rosolowsky, E.T.; Ficociello, L.H.; Maselli, N.J.; Niewczas, M.A.; Binns, A.L.; Roshan, B.; Warram, J.H.; Krolewski, A.S. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin. J. Am. Soc. Nephrol. 2008, 3, 706–713. [Google Scholar] [CrossRef]
- Tseng, C.H. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int. 2005, 68, 796–801. [Google Scholar] [CrossRef]
- Kushiyama, A.; Okubo, H.; Sakoda, H.; Kikuchi, T.; Fujishiro, M.; Sato, H.; Kushiyama, S.; Iwashita, M.; Nishimura, F.; Fukushima, T.; et al. Xanthine oxidoreductase is involved in macrophage foam cell formation and atherosclerosis development. Arter. Thromb. Vasc. Biol. 2012, 32, 291–298. [Google Scholar] [CrossRef]
- Nakatsu, Y.; Seno, Y.; Kushiyama, A.; Sakoda, H.; Fujishiro, M.; Katasako, A.; Mori, K.; Matsunaga, Y.; Fukushima, T.; Kanaoka, R.; et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G42–51. [Google Scholar] [CrossRef]
- Nomura, J.; Busso, N.; Ives, A.; Matsui, C.; Tsujimoto, S.; Shirakura, T.; Tamura, M.; Kobayashi, T.; So, A.; Yamanaka, Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci. Rep. 2014, 4, 4554. [Google Scholar] [CrossRef]
- Kondo, M.; Imanishi, M.; Fukushima, K.; Ikuto, R.; Murai, Y.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Goda, M.; Zamami, Y.; Takechi, K.; et al. Xanthine Oxidase Inhibition by Febuxostat in Macrophages Suppresses Angiotensin II-Induced Aortic Fibrosis. Am. J. Hypertens 2019, (3), 249–256. [Google Scholar] [CrossRef]
- Yisireyili, M.; Hayashi, M.; Wu, H.; Uchida, Y.; Yamamoto, K.; Kikuchi, R.; Shoaib Hamrah, M.; Nakayama, T.; Wu Cheng, X.; Matsushita, T.; et al. Xanthine oxidase inhibition by febuxostat attenuates stress-induced hyperuricemia, glucose dysmetabolism, and prothrombotic state in mice. Sci. Rep. 2017, 7, 1266. [Google Scholar] [CrossRef]
- Inoue, M.K.; Yamamotoya, T.; Nakatsu, Y.; Ueda, K.; Inoue, Y.; Matsunaga, Y.; Sakoda, H.; Fujishiro, M.; Ono, H.; Morii, K.; et al. The Xanthine Oxidase Inhibitor Febuxostat Suppresses the Progression of IgA Nephropathy, Possibly via Its Anti-Inflammatory and Anti-Fibrotic Effects in the gddY Mouse Model. Int. J. Mol. Sci. 2018. [Google Scholar] [CrossRef]
- Suzuki, H.; Suzuki, Y.; Novak, J.; Tomino, Y. Development of Animal Models of Human IgA Nephropathy. Drug Discov. Today Dis. Models 2014, 11, 5–11. [Google Scholar] [CrossRef]
- Tsirmoula, S.; Lamprou, M.; Hatziapostolou, M.; Kieffer, N.; Papadimitriou, E. Pleiotrophin-induced endothelial cell migration is regulated by xanthine oxidase-mediated generation of reactive oxygen species. Microvasc. Res. 2015, 98, 74–81. [Google Scholar] [CrossRef][Green Version]
- Oh, S.H.; Choi, S.Y.; Choi, H.J.; Ryu, H.M.; Kim, Y.J.; Jung, H.Y.; Cho, J.H.; Kim, C.D.; Park, S.H.; Kwon, T.H.; et al. The emerging role of xanthine oxidase inhibition for suppression of breast cancer cell migration and metastasis associated with hypercholesterolemia. FASEB J. 2019, 33, 7301–7314. [Google Scholar] [CrossRef]
- Mizuno, K.; Okamoto, H.; Horio, T. Inhibitory influences of xanthine oxidase inhibitor and angiotensin I-converting enzyme inhibitor on multinucleated giant cell formation from monocytes by downregulation of adhesion molecules and purinergic receptors. Br. J. Derm. 2004, 150, 205–210. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Tapia, E.; Soto, V.; Avila-Casado, C.; Franco, M.; Wessale, J.L.; Zhao, L.; Johnson, R.J. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008, 108, 69–78. [Google Scholar] [CrossRef]
- Choudhary, N.; Ahlawat, R.S. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: New evidence linking inflammation, glycemic control, and microalbuminuria. Iran. J. Kidney Dis. 2008, 2, 72–79. [Google Scholar]
- Morii, T.; Fujita, H.; Narita, T.; Shimotomai, T.; Fujishima, H.; Yoshioka, N.; Imai, H.; Kakei, M.; Ito, S. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J. Diabetes Complicat. 2003, 17, 11–15. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Allopurinol protects human glomerular endothelial cells from high glucose-induced reactive oxygen species generation, p53 overexpression and endothelial dysfunction. Int. Urol. Nephrol. 2018, 50, 179–186. [Google Scholar] [CrossRef]
- Dong, G.; Ren, M.; Wang, X.; Jiang, H.; Yin, X.; Wang, S.; Wang, X.; Feng, H. Allopurinol reduces severity of delayed neurologic sequelae in experimental carbon monoxide toxicity in rats. Neurotoxicology 2015, 48, 171–179. [Google Scholar] [CrossRef]
- Yang, C.C.; Ma, M.C.; Chien, C.T.; Wu, M.S.; Sun, W.K.; Chen, C.F. Hypoxic preconditioning attenuates lipopolysaccharide-induced oxidative stress in rat kidneys. J. Physiol. 2007, 582, 407–419. [Google Scholar] [CrossRef]
- Szalay, C.I.; Erdelyi, K.; Kokeny, G.; Lajtar, E.; Godo, M.; Revesz, C.; Kaucsar, T.; Kiss, N.; Sarkozy, M.; Csont, T.; et al. Oxidative/Nitrative Stress and Inflammation Drive Progression of Doxorubicin-Induced Renal Fibrosis in Rats as Revealed by Comparing a Normal and a Fibrosis-Resistant Rat Strain. PLoS ONE 2015, 10, e0127090. [Google Scholar] [CrossRef]
- Afroz, T.; Sagar, R.; Reddy, S.; Gandhe, S.; Rajaram, K.G. Clinical and histological correlation of diabetic nephropathy. Saudi J. Kidney Dis. Transpl. 2017, 28, 836–841. [Google Scholar]
- Lassila, M.; Seah, K.K.; Allen, T.J.; Thallas, V.; Thomas, M.C.; Candido, R.; Burns, W.C.; Forbes, J.M.; Calkin, A.C.; Cooper, M.E.; et al. Accelerated nephropathy in diabetic apolipoprotein e-knockout mouse: Role of advanced glycation end products. J. Am. Soc. Nephrol. 2004, 15, 2125–2138. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, Y.; Yamamotoya, T.; Nakatsu, Y.; Ueda, K.; Matsunaga, Y.; Inoue, M.-K.; Sakoda, H.; Fujishiro, M.; Ono, H.; Kikuchi, T.; et al. Xanthine Oxidase Inhibitor Febuxostat Exerts an Anti-Inflammatory Action and Protects against Diabetic Nephropathy Development in KK-Ay Obese Diabetic Mice. Int. J. Mol. Sci. 2019, 20, 4680. https://doi.org/10.3390/ijms20194680
Mizuno Y, Yamamotoya T, Nakatsu Y, Ueda K, Matsunaga Y, Inoue M-K, Sakoda H, Fujishiro M, Ono H, Kikuchi T, et al. Xanthine Oxidase Inhibitor Febuxostat Exerts an Anti-Inflammatory Action and Protects against Diabetic Nephropathy Development in KK-Ay Obese Diabetic Mice. International Journal of Molecular Sciences. 2019; 20(19):4680. https://doi.org/10.3390/ijms20194680
Chicago/Turabian StyleMizuno, Yu, Takeshi Yamamotoya, Yusuke Nakatsu, Koji Ueda, Yasuka Matsunaga, Masa-Ki Inoue, Hideyuki Sakoda, Midori Fujishiro, Hiraku Ono, Takako Kikuchi, and et al. 2019. "Xanthine Oxidase Inhibitor Febuxostat Exerts an Anti-Inflammatory Action and Protects against Diabetic Nephropathy Development in KK-Ay Obese Diabetic Mice" International Journal of Molecular Sciences 20, no. 19: 4680. https://doi.org/10.3390/ijms20194680
APA StyleMizuno, Y., Yamamotoya, T., Nakatsu, Y., Ueda, K., Matsunaga, Y., Inoue, M.-K., Sakoda, H., Fujishiro, M., Ono, H., Kikuchi, T., Takahashi, M., Morii, K., Sasaki, K., Masaki, T., Asano, T., & Kushiyama, A. (2019). Xanthine Oxidase Inhibitor Febuxostat Exerts an Anti-Inflammatory Action and Protects against Diabetic Nephropathy Development in KK-Ay Obese Diabetic Mice. International Journal of Molecular Sciences, 20(19), 4680. https://doi.org/10.3390/ijms20194680




