Effect of Central Injection of Neostigmine on the Bacterial Endotoxin Induced Suppression of GnRH/LH Secretion in Ewes during the Follicular Phase of the Estrous Cycle
Abstract
:1. Introduction
2. Results
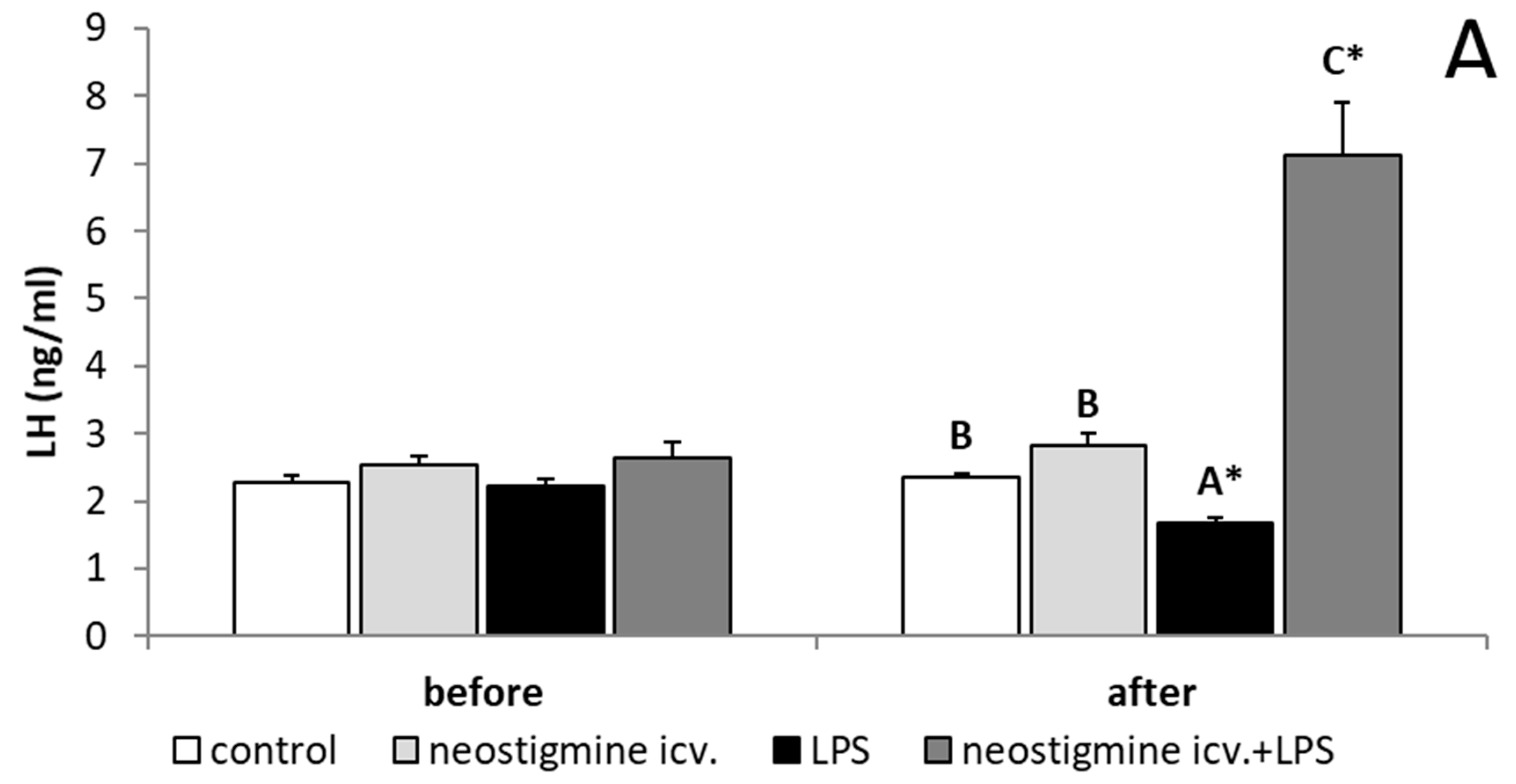
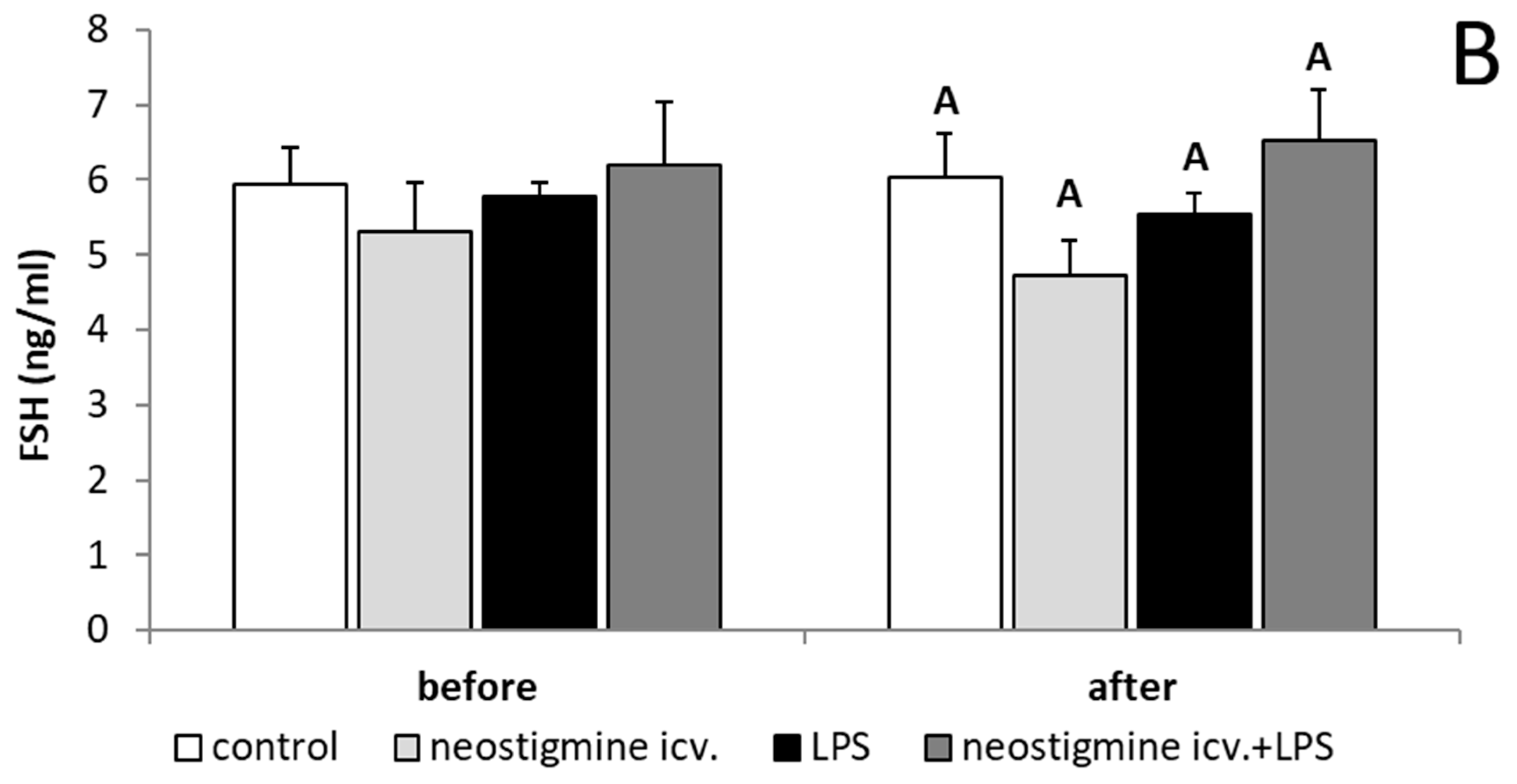
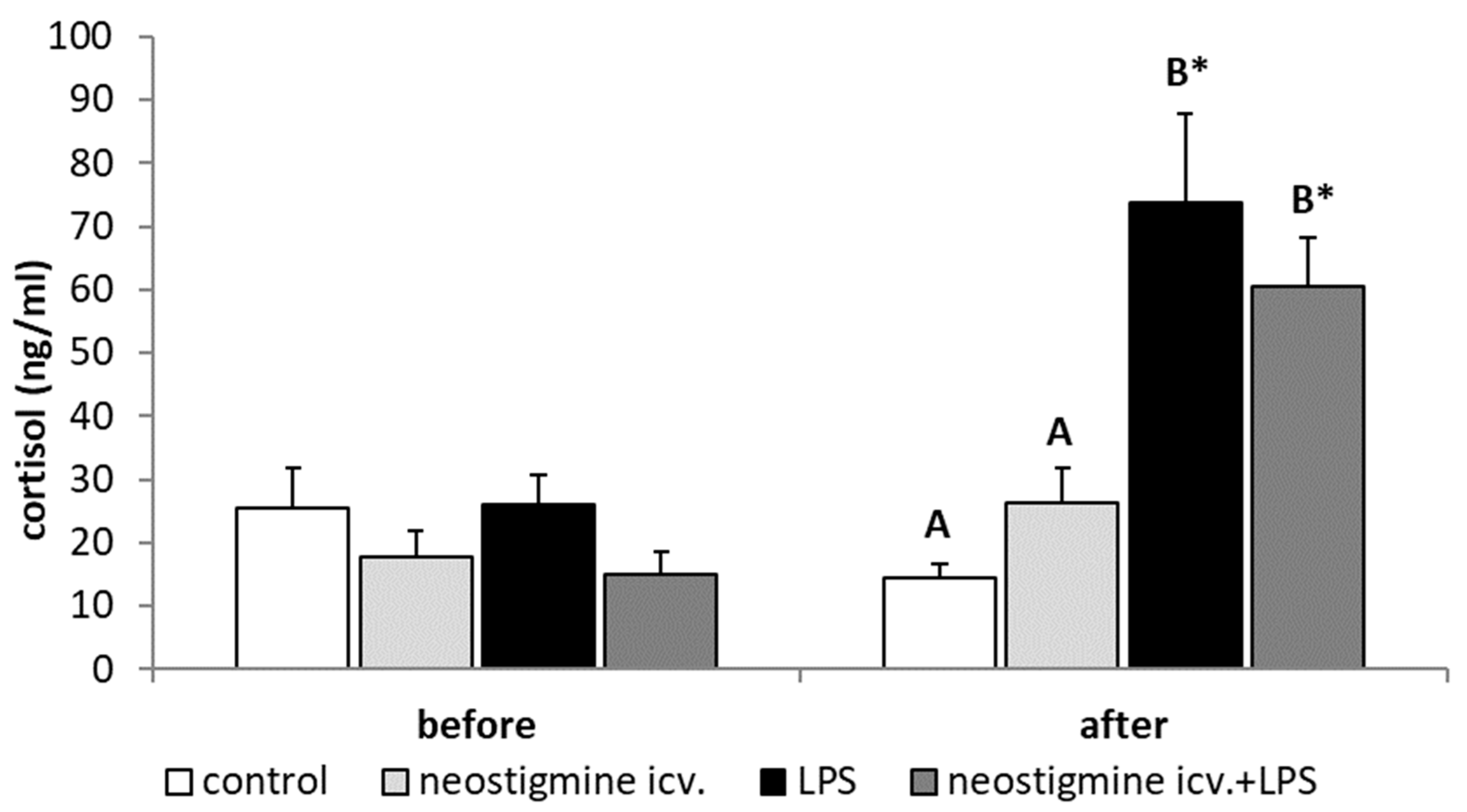
2.1. Effect of Central Injection of Neostigmine and LPS Administration on LH, FSH, and Cortisol Releases
2.2. Effect of Central Injection of Neostigmine and LPS Administration on GnRHR Expression in the AP
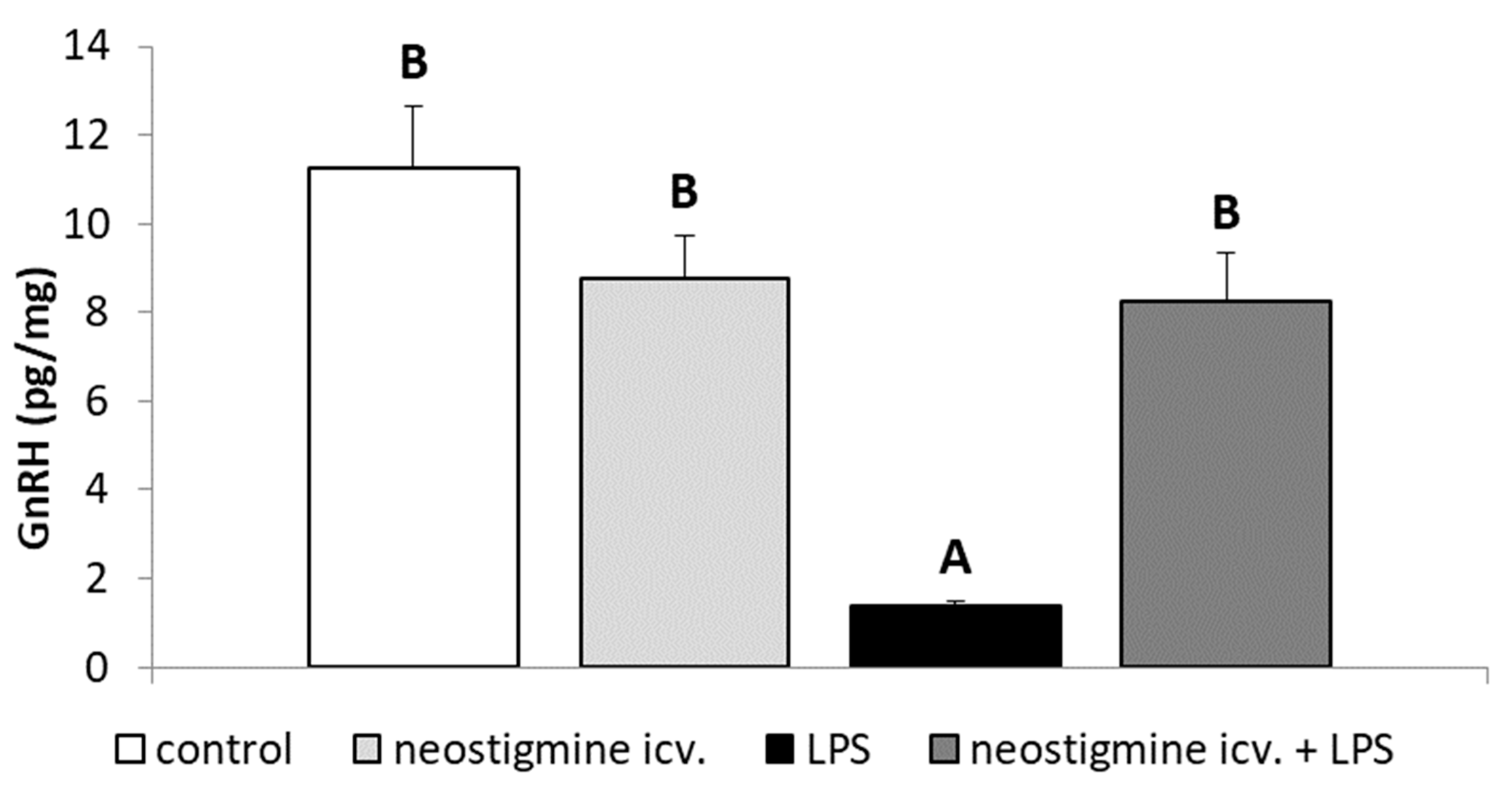
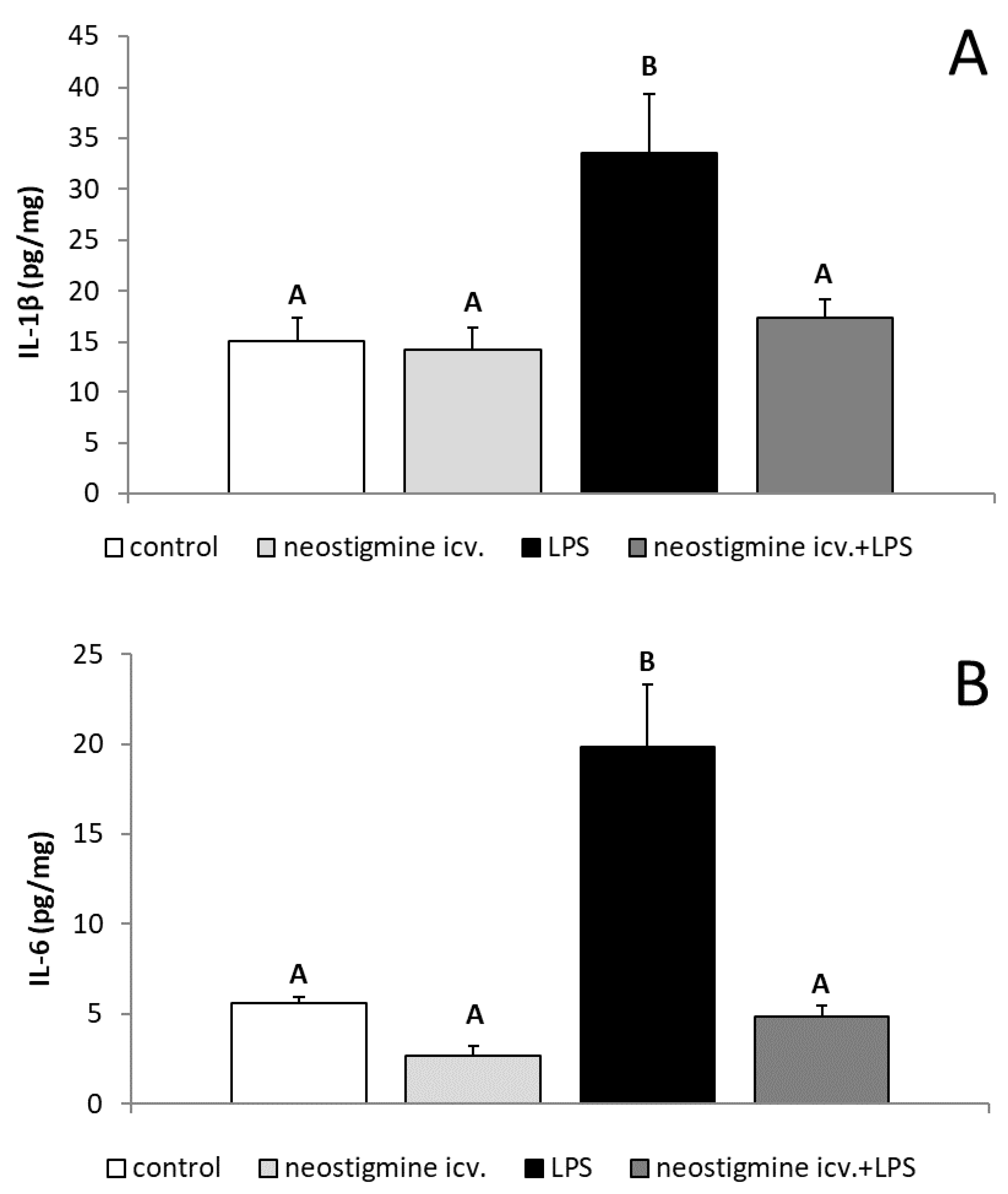
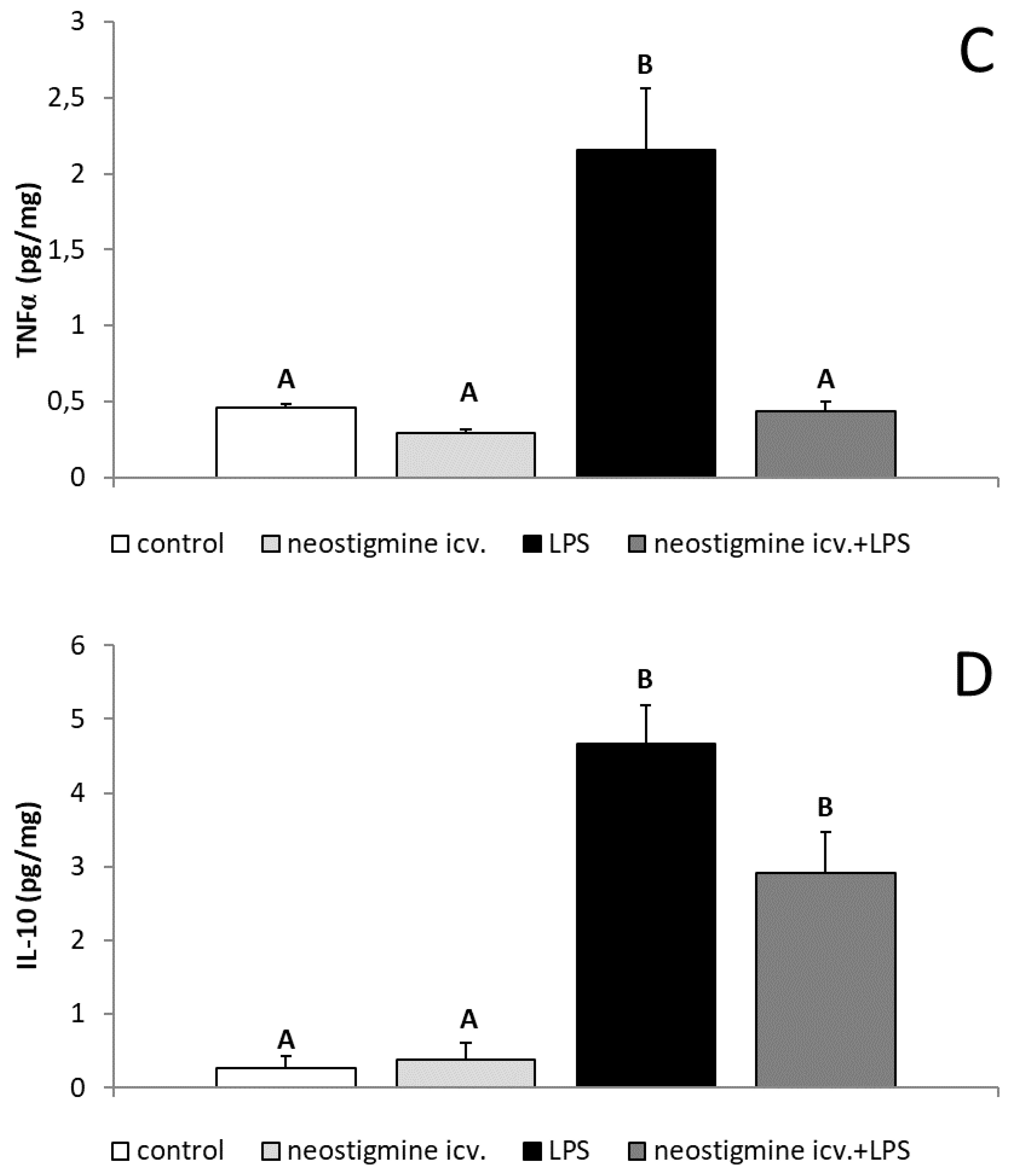
2.3. Effect of Central Injection of Neostigmine and LPS Administration on GnRH, IL-1β, IL-6, TNFα, and IL-10 Contents in the POA
2.4. Effect of Central Injection of Neostigmine and LPS Administration on the Gene Expression of GnRH and Neuronal Acetylcholine Receptor Subunit Alpha-7 (CHRNA7) in the Hypothalamus
2.5. Effect of Central injection of Neostigmine and LPS Administration on the Gene Expression of GnRHR, LHβ, FSHβ, and CHRNA7 in the AP
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Procedures
4.2. Assays
4.2.1. Radioimmunoassay of Hormones
4.2.2. ELISA Assay for GnRH and Inflammatory Cytokines
4.2.3. Determining the Relative Gene Expression
4.2.4. Western Blot Assay for GnRHR Expression in the AP
4.3. Statistical Analysis of Data
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ulevitch, R.J.; Tobias, P.S. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 1999, 11, 19–22. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Shai, Y. Lipopolysaccharide (endotoxin)-host defense antibacterial peptides interactions: Role in bacterial resistance and prevention of sepsis. Biochim. Biophys. Acta 2006, 1758, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska-Zaremba, D.; Herman, A.P.; Haziak, K. How does bacterial endotoxin influence gonadoliberin/gonadotropins secretion and action? J. Anim. Feed Sci. 2016, 25, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haziak, K.; Herman, A.P.; Tomaszewska-Zaremba, D. Effects of central injection of anti-LPS antibody and blockade of TLR4 on GnRH/LH secretion during immunological stress in anestrous ewes. Med. Inflamm. 2014, 2014, 867170. [Google Scholar] [CrossRef] [PubMed]
- Haziak, K.; Herman, A.P.; Tomaszewska-Zaremba, D. The effect of LPS on LH release and gene expression of LH-β, GnRH-R and TLR4 in the anterior pituitary of follicular phase ewes—An in vitro study. J. Anim. Feed Sci. 2013, 22, 97–105. [Google Scholar] [CrossRef]
- Król, K.; Tomaszewska-Zaremba, D.; Herman, A.P. Photoperiod-dependent effect of inflammation on nocturnal gene expression of proinflammatory cytokines and their receptors in pars tuberalis of ewe. J. Anim. Feed Sci. 2016, 25, 3–11. [Google Scholar] [Green Version]
- Tsagarakis, S.; Kontogeorgos, G.; Kovacs, K. The role of cytokines in the normal and neoplastic pituitary. Crit. Rev. Oncol. Hematol. 1998, 28, 73–90. [Google Scholar] [CrossRef]
- Takao, T.; Culp, S.G.; De Souza, E.B. Reciprocal modulation of interleukin-1 beta (IL-1 beta) and IL-1 receptors by lipopolysaccharide (endotoxin) treatment in the mouse brain-endocrine- immune axis. Endocrinology 1993, 132, 1497–1504. [Google Scholar] [CrossRef]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Dobek, E.; Herman, A.; Tomaszewska-Zaremba, D. LPS-induced inflammation potentiates the IL-1β-mediated reduction of LH secretion from the anterior pituitary explants. Clin. Dev. Immunol. 2013, 2013, 926937. [Google Scholar] [CrossRef]
- Braden, T.D.; Fry, C.; Sartin, J.L. Effects of interleukins on secretion of luteinizing hormone from ovine pituitary cells. Am. J. Vet. Res. 1998, 59, 1488–1493. [Google Scholar] [PubMed]
- Russell, S.H.; Small, C.J.; Stanley, S.A.; Franks, S.; Ghatei, M.A.; Bloom, S.R. The in vitro role of tumour necrosis factor-alpha and interleukin-6 in the hypothalamic-pituitary gonadal axis. J. Neuroendocrinol. 2001, 13, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Skipor, J.; Kowalewska, M.; Szczepkowska, A.; Majewska, A.; Misztal, T.; Jalynski, M.; Herman, A.P.; Zabek, K. Plasma and cerebrospinal fluid interleukin-1β during lipopolysaccharide-induced systemic inflammation in ewes implanted or not with slow-release melatonin. J. Anim. Sci. Biotechnol. 2017, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Watanobe, H.; Hayakawa, Y. Hypothalamic interleukin-1β and tumor necrosis factor-α, but not interleukin-6, mediate the endotoxin-induced suppression of the reproductive axis in rats. Endocrinology 2003, 144, 4868–4875. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Stone, K.P.; Hsuchou, H.; Manda, V.K.; Zhang, Y.; Kastin, A.J. Cytokine signaling modulates blood-brain barrier function. Curr. Pharm. Des. 2011, 17, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, M.; Herman, A.P.; Szczepkowska, A.; Skipor, J. The effect of melatonin from slow-release implants on basic and TLR-4-mediated gene expression of inflammatory cytokines and their receptors in the choroid plexus in ewes. Res. Vet. Sci. 2017, 113, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Capriglione, S.; Peterlunger, I.; La Rosa, V.L.; Vitagliano, A.; Noventa, M.; Valenti, G.; Sapia, F.; Angioli, R.; Lopez, S.; et al. The role of oxidative stress and membrane transport systems during endometriosis: A fresh look at a busy corner. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kyama, C.M.; Overbergh, L.; Debrock, S.; Valckx, D.; Vander Perre, S.; Meuleman, C.; Mihalyi, A.; Mwenda, J.M.; Mathieu, C.; D’Hooghe, T.M. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during menstrual phase in women with endometriosis. Fertil. Steril. 2006, 85, 1667–1675. [Google Scholar] [CrossRef]
- Kyama, C.M.; Overbergh, L.; Mihalyi, A.; Meuleman, C.; Mwenda, J.M.; Mathieu, C.; D’Hooghe, T.M. Endometrial and peritoneal expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertil. Steril. 2008, 89, 301–310. [Google Scholar] [CrossRef]
- Reyes-Muñoz, E.; Sathyapalan, T.; Rossetti, P.; Shah, M.; Long, M.; Buscema, M.; Valenti, G.; La Rosa, V.L.; Cianci, S.; Vitale, S.G. Polycystic ovary syndrome: Implication for drug metabolism on assisted reproductive techniques-A literature review. Adv. Ther. 2018, 11, 1805–1815. [Google Scholar]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Tomaszewska-Zaremba, D.; Kowalewska, M.; Szczepkowska, A.; Oleszkiewicz, M.; Krawczyńska, A.; Wójcik, M.; Antushevich, H.; Skipor, J. Neostigmine attenuates proinflammatory cytokine expression in preoptic area but not choroid plexus during lipopolysaccharide-induced systemic inflammation. Med. Inflamm. 2018, 2018, 9150207. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. Peripheral injection of SB203580 inhibits the inflammatory-dependent synthesis of proinflammatory cytokines in the hypothalamus. BioMed Res. Internat. 2014, 2014, 475152. [Google Scholar] [CrossRef]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Haziak, K.; Antushevitch, H.; Herman, A.; Tomaszewska-Zaremba, D. Inhibition of acetylcholinesterase activity by rivastigmine decreases lipopolysaccharide-induced IL-1β expression in the hypothalamus of ewes. Domest. Anim. Endocrinol. 2013, 44, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Misztal, T.; Herman, A.; Tomaszewska-Zaremba, D. Expression of interleukin (IL)-1β and IL-1 receptors genes in the hypothalamus of anoestrous ewes after lipopolysaccharide treatment. Reprod. Domest. Anim. 2010, 45, e426–e433. [Google Scholar] [CrossRef] [PubMed]
- Layé, S.; Gheusi, G.; Cremona, S.; Combe, C.; Kelley, K.; Dantzer, R.; Parnet, P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R93–R98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, S.J.; Rothwell, N.J. Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci. 1995, 18, 83–88. [Google Scholar] [CrossRef]
- Herman, A.P.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Wojtulewicz, K.; Antushevich, H.; Herman, A.; Paczesna, K.; Romanowicz, K.; Tomaszewska-Zaremba, D. Peripheral inhibitor of AChE, Neostigmine, prevents the inflammatory dependent suppression of GnRH/LH secretion during the follicular phase of the estrous cycle. Biomed. Res. Int. 2017, 2017, 6823209. [Google Scholar] [CrossRef] [PubMed]
- Hassanain, M.; Bhatt, S.; Zalcman, S.; Siegel, A. Potentiating role of interleukin-1beta (IL-1beta) and IL-1beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res. 2005, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Utsuyama, M.; Hirokawa, K. Differential expression of various cytokine receptors in the brain after stimulation with LPS in young and old mice. Exp. Gerontol. 2002, 37, 411–420. [Google Scholar] [CrossRef]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Haziak, K.; Romanowicz, K.; Misztal, T.; Antushevich, H.; Herman, A.; Tomaszewska–Zaremba, D. The effect of rivastigmine on the LPS-induced suppression of GnRH/LH secretion during the follicular phase of the estrous cycle in ewes. Anim. Reprod. Sci. 2013, 138, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.B.; Cheng, Z.; Vargas, J.D.; Birnbaum, H.A.; Hoffmann, A. Network dynamics determine the autocrine and paracrine signaling functions of TNF. Genes Dev. 2014, 28, 120–2133. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front Immunol. 2014, 5, 491. [Google Scholar]
- Yeo, T.T.S.; Gore, A.C.; Jakubowski, M.; Dong, K.; Blum, M.; Roberts, J.L. Characterization of gonadotropin-releasing hormone gene transcripts in a mouse hypothalamic neuronal GT1 cell line. Mol. Brain Res. 1996, 42, 255–262. [Google Scholar] [CrossRef]
- Jakubowski, M.; Roberts, J.L. Processing of gonadotropin-releasing hormone gene transcripts in the rat brain. J. Biol. Chem. 1994, 269, 4078–4083. [Google Scholar] [PubMed]
- Haziak, K.; Herman, A.P.; Wojtulewicz, K.; Pawlina, B.; Paczesna, K.; Bochenek, J.; Tomaszewska-Zaremba, D. Effect of CD14/TLR4 antagonist on GnRH/LH secretion in ewe during central inflammation induced by intracerebroventricular administration of LPS. J. Anim. Sci. Biotechnol. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.F.; Bowen, J.M.; Krasa, H.B.; Thrun, L.A.; Viguié, C.; Karsch, F.J. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: A simultaneous view from hypophyseal portal and peripheral blood. Endocrinology 1997, 138, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Debus, N.; Breen, K.M.; Barrell, G.K.; Billings, H.J.; Brown, M.; Young, E.A.; Karsch, F.J. Does cortisol mediate endotoxin-induced inhibition of pulsatile luteinizing hormone and gonadotropin-releasing hormone secretion? Endocrinology 2002, 143, 3748–3758. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.F.; Brown, M.E.; Krasa, H.B.; Thrun, L.A.; Viguié, C.; Karsch, F.J. Systemic challenge with endotoxin stimulates corticotropin-releasing hormone and arginine vasopressin secretion into hypophyseal portal blood: Coincidence with gonadotropin-releasing hormone suppression. Endocrinology 1998, 139, 4175–4181. [Google Scholar] [CrossRef]
- de Jonge, W.J.; Ulloa, L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 2007, 151, 915–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamano, R.; Takahashi, H.K.; Iwagaki, H.; Yoshino, T.; Nishibori, M.; Tanaka, N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock 2006, 26, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Gallowitsch-Puerta, M.; Tracey, K.J. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann. N. Y. Acad. Sci. 2005, 1062, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Foucault-Fruchard, L.; Doméné, A.; Page, G.; Windsor, M.; Emond, P.; Rodrigues, N.; Dollé, F.; Damont, A.; Buron, F.; Routier, S.; et al. Neuroprotective effect of the alpha 7 nicotinic receptor agonist PHA 543613 in an in vivo excitotoxic adult rat model. Neuroscience 2017, 356, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Rivier, C.; Vale, W. Cytokines act within the brain to inhibit luteinizing hormone secretion and ovulation in the rat. Endocrinology 1990, 127, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska-Zaremba, D.; Herman, A.P.; Misztal, T. Does central IL-1β affect GnRH secretion in the hypothalamus of anoestrous ewes via different regulatory pathways? J. Anim. Feed Sci. 2013, 22, 5–12. [Google Scholar] [CrossRef]
- Fiorindo, R.P.; Martini, L. Evidence for a cholinergic component in the neuroendocrine control of luteinizing hormone (LH) secretion. Neuroendocrinology 1975, 18, 322–332. [Google Scholar] [CrossRef]
- Richardson, S.B.; Prasad, J.A.; Hollander, C.S. Acetylcholine, melatonin, and potassium depolarization stimulate release of luteinizing hormone-releasing hormone from rat hypothalamus in vitro. Proc. Natl. Acad. Sci. USA 1982, 79, 2686–2689. [Google Scholar] [CrossRef]
- Krsmanovic, L.Z.; Mores, N.; Navarro, C.E.; Saeed, S.A.; Arora, K.K.; Catt, K.J. Muscarinic regulation of intracellular signaling and neurosecretion in gonadotropin-releasing hormone neurons. Endocrinology 1998, 139, 4037–4043. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Arredondo, J.; Skok, M.; Grando, S.A. Auto/paracrine control of inflammatory cytokines by acetylcholine in macrophage-like U937 cells through nicotinic receptors. Int. Immunopharmacol. 2010, 10, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turzillo, A.M.; Nolan, T.E.; Nett, T.M. Regulation of gonadotropin-releasing hormone (GnRH) receptor gene expression in sheep: Interaction of GnRH and estradiol. Endocrinology 1998, 139, 4890–4894. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Conn, P.M. Transcriptional activation of gonadotropin-releasing hormone (GnRH) receptor gene by GnRH: Involvement of multiple signal transduction pathways. Endocrinology 1999, 140, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowska, M.; Łapot, M.; Malewski, T.; Mateusiak, K.; Misztal, T.; Przekop, F. Effects of corticotropin-releasing hormone and its antagonist on the gene expression of gonadotrophin-releasing hormone (GnRH) and GnRH receptor in the hypothalamus and anterior pituitary gland of follicular phase ewes. Reprod. Fert. Develop. 2011, 23, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kim, S.R.; Leonhardt, S.; Jarry, H.; Wuttke, W.; Kim, K. Effect of interleukin-1β on gonadotropin-releasing hormone (GnRH) and GnRH receptor gene expression in castrated male rats. J. Neuroendocrinol. 2000, 12, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Zemkova, H.; Kucka, M.; Bjelobaba, I.; Tomić, M.; Stojilkovic, S.S. Multiple cholinergic signaling pathways in pituitary gonadotrophs. Endocrinology 2013, 154, 421–433. [Google Scholar] [CrossRef]
- Saunders, A.; Granger, A.J.; Sabatini, B.L. Corelease of acetylcholine and GABA from cholinergic forebrain neurons. eLife 2015, 4, e06412. [Google Scholar] [CrossRef]
- Mitchell, R.; Grieve, G.; Dow, R.; Fink, G. Endogenous GABA receptor ligands in hypophysial portal blood. Neuroendocrinology 1983, 37, 169–176. [Google Scholar] [CrossRef]
- Virmani, M.A.; Stojilković, S.S.; Catt, K.J. Stimulation of luteinizing hormone release by γ-aminobutyric acid (GABA) agonists: Mediation by GABAA-type receptors and activation of chloride and voltage-sensitive calcium channels. Endocrinology 1990, 126, 2499–2505. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Persani, L. Premature ovarian failure. Orphanet. J. Rare. Dis. 2006, 1, 9. [Google Scholar] [CrossRef]
- Laganà, A.S.; Rossetti, P.; Sapia, F.; Chiofalo, B.; Buscema, M.; Valenti, G.; Rapisarda, A.M.C.; Vitale, S.G. Evidence-based and patient-oriented inositol treatment in polycystic ovary syndrome: Changing the perspective of the disease. Int. J. Endocrinol. Metab. 2017, 15, e43695. [Google Scholar]
- Russel, A. Body condition scoring of sheep. In Sheep and Goat Practice; Boden, E., Ed.; Bailliere Tindall: Philadelphia, PA, USA, 1991. [Google Scholar]
- Roś, R. Nutrient Requirements for Cattle and Sheep in the Traditional System; Instytut Zootechniki: Krakow, Poland, 1993. [Google Scholar]
- Herman, A.P.; Misztal, T.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central injection of exogenous IL-1β in the control activities of hypothalamic–pituitary–gonadal axis in anestrous ewes. Reprod. Domest. Animal. 2012, 47, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Krawczyńska, A.; Antushevich, H.; Bochenek, J.; Wojtulewicz, K.; Pawlina, B.; Herman, A.P.; Zięba, D.A. Photoperiodic conditions as a factor modulating leptin influence on pro-inflammatory cytokines and their receptors gene expression in ewe’s aorta. J. Anim. Feed Sci. 2019, 28, 128–137. [Google Scholar] [CrossRef]
- Welento, J.; Szteyn, S.; Milartz, Z. Observations on the stereotaxic configuration of the hypothalamus nuclei in the sheep. Anat. Anz. 1969, 124, 1–27. [Google Scholar] [PubMed]
- Stupnicki, R.; Madej, A. Radioimmunoassay of LH in blood plasma of farm animals. Endokrynologie 1976, 68, 6–13. [Google Scholar]
- L’Hermite, M.; Niswender, G.D.; Reichert, L.E.; Midgley, A.R. Serum follicle stimulating hormone in sheep as measured by radioimmunoassay. Biol. Reprod. 1972, 6, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kokot, F.; Stupnicki, R. Metody Radioimmunologiczne i Radiokompetyncyjne Stosowane w Klinice, 2nd ed.; PZWL: Warsaw, Poland, 1985. [Google Scholar]
- Rasmussen, R. Quantification on the LightCycler. In Rapid Cycle Real-Time PCR Methods and Applications; Meuer, S., Wittwer, C., Nakagawara, K., Eds.; Springer: Berlin, Germany, 2001; pp. 21–34. [Google Scholar]

| Structure | GnRH Relative Gene Expression | |||
|---|---|---|---|---|
| Control | Neostigmine icv. | LPS | Neostigmine icv. + LPS | |
| POA | 1 ± 0.2 A | 0.9 ± 0.2 A | 0.6 ± 0.2 A | 0.7 ± 0.2 A |
| AHA | 1 ± 0.1 A | 0,9 ± 0.1 A | 1 ± 0.1 A | 1 ± 0.2 A |
| MBH | 1 ± 0.1 A | 0.9 ± 0.2 A | 1 ± 0.1 A | 1.2 ± 0.2 A |
| ME | 1 ± 0.1 B | 1 ± 0.1 B | 0.5 ± 0.2 A | 1.7 ± 0.2 C |
| Structure | CHRNA7 Relative Gene Expression | |||
|---|---|---|---|---|
| Control | Neostigmine icv. | LPS | Neostigmine icv. + LPS | |
| POA | 1 ± 0.2 A | 1.7 ± 0.2 B | 1.3 ± 0.1 A | 1.7 ± 0.1 B |
| AHA | 1 ± 0.1 A | 1.3 ± 0.1 B | 0.9 ± 0.1 A | 1.3 ± 0.1 B |
| MBH | 1 ± 0.1 A | 1.4 ± 0.1 B | 0.9 ± 0.1 A | 1.3 ± 0.1 B |
| ME | 1 ± 0.1 A | 2.2 ± 0.3 B | 1 ± 0.1 A | 1.8 ± 0.2 B |
| Gene | Anterior Pituitary | |||
|---|---|---|---|---|
| Control | Neostigmine icv. | LPS | Neostigmine icv. + LPS | |
| GnRHR | 1 ± 0.1 BC | 1.2 ± 0.1 C | 0.5 ± 0.1 A | 0.7 ± 0.1 AB |
| LHβ | 1 ± 0.1 B | 1.3 ± 0.2 B | 0.5 ± 0.1 A | 1 ± 0.1 B |
| FSHβ | 1 ± 0.3 A | 1 ± 0.3 A | 1.1 ± 0.2 A | 0.8 ± 0.2 A |
| CHRNA7 | 1 ± 0.3 A | 6.8 ± 1.8 C | 2.4 ± 0.6 B | 4.8 ± 0.8 C |
| Group No. | Group Name | No. of Animals | Experimental Treatment I (icv.) | Dose (mg/Animal) | Experimental Treatment II (iv.) | Dose (ng/kg) |
|---|---|---|---|---|---|---|
| 1 | Control | 6 | Ringer’s solution | 0 | NaCl | 0 |
| 2 | Neostigmine-treated | 6 | Neostigmine | 1 | NaCl | 0 |
| 3 | LPS-treated | 6 | Ringer’s solution | 0 | LPS | 400 |
| 4 | Neostigmine- + LPS-treated | 6 | Neostigmine | 1 | LPS | 400 |
| Total number of animals | 24 | |||||
| GenBank Acc. No. | Gene | Amplicon Size (bp) | Forward/Reverse | Sequence 5′→3′ | Reference |
|---|---|---|---|---|---|
| NM_001034034 | GAPDH glyceraldehyde-3-phosphate dehydrogenase | 134 | forward | AGAAGGCTGGGGCTCACT | [24] |
| reverse | GGCATTGCTGACAATCTTGA | ||||
| U39357 | ACTB beta actin | 168 | forward | CTTCCTTCCTGGGCATGG | [24] |
| reverse | GGGCAGTGATCTCTTTCTGC | ||||
| BC108088.1 | HDAC1 histone deacetylase1 | 115 | forward | CTGGGGACCTACGGGATATT | [24] |
| reverse | GACATGACCGGCTTGAAAAT | ||||
| NM_001009397 | GnRHR gonadotropin-releasing hormone receptor | 150 | forward | TCTTTGCTGGACCACAGTTAT | [32] |
| reverse | GGCAGCTGAAGGTGAAAAAG | ||||
| U02517 | GnRH gonadotropin-releasing hormone | 123 | forward | GCCCTGGAGGAAAGAGAAAT | [32] |
| reverse | GAGGAGAATGGGACTGGTGA | ||||
| X52488 | LHB luteinizing hormone beta-subunit | 184 | forward | AGATGCTCCAGGGACTGCT | [32] |
| reverse | TGCTTCATGCTGAGGCAGTA | ||||
| X15493 | FSHB follicle stimulating hormone beta-subunit | 131 | forward | TATTGCTACACCCGGGACTT | [32] |
| reverse | TACAGGGAGTCTGCATGGTG | ||||
| BC_149340 | CHRNA7 neuronal acetylcholine receptor subunit alpha-7 | 114 | forward | TGGAAGCCAGACATTCTCCT | [25] |
| reverse | GATGCCTGGAGGGAGGTACT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, A.P.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Wojtulewicz, K.; Pawlina, B.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. Effect of Central Injection of Neostigmine on the Bacterial Endotoxin Induced Suppression of GnRH/LH Secretion in Ewes during the Follicular Phase of the Estrous Cycle. Int. J. Mol. Sci. 2019, 20, 4598. https://doi.org/10.3390/ijms20184598
Herman AP, Skipor J, Krawczyńska A, Bochenek J, Wojtulewicz K, Pawlina B, Antushevich H, Herman A, Tomaszewska-Zaremba D. Effect of Central Injection of Neostigmine on the Bacterial Endotoxin Induced Suppression of GnRH/LH Secretion in Ewes during the Follicular Phase of the Estrous Cycle. International Journal of Molecular Sciences. 2019; 20(18):4598. https://doi.org/10.3390/ijms20184598
Chicago/Turabian StyleHerman, Andrzej Przemysław, Janina Skipor, Agata Krawczyńska, Joanna Bochenek, Karolina Wojtulewicz, Bartosz Pawlina, Hanna Antushevich, Anna Herman, and Dorota Tomaszewska-Zaremba. 2019. "Effect of Central Injection of Neostigmine on the Bacterial Endotoxin Induced Suppression of GnRH/LH Secretion in Ewes during the Follicular Phase of the Estrous Cycle" International Journal of Molecular Sciences 20, no. 18: 4598. https://doi.org/10.3390/ijms20184598
APA StyleHerman, A. P., Skipor, J., Krawczyńska, A., Bochenek, J., Wojtulewicz, K., Pawlina, B., Antushevich, H., Herman, A., & Tomaszewska-Zaremba, D. (2019). Effect of Central Injection of Neostigmine on the Bacterial Endotoxin Induced Suppression of GnRH/LH Secretion in Ewes during the Follicular Phase of the Estrous Cycle. International Journal of Molecular Sciences, 20(18), 4598. https://doi.org/10.3390/ijms20184598






