Design, Synthesis, and Anti-Bacterial Evaluation of Triazolyl-Pterostilbene Derivatives
Abstract
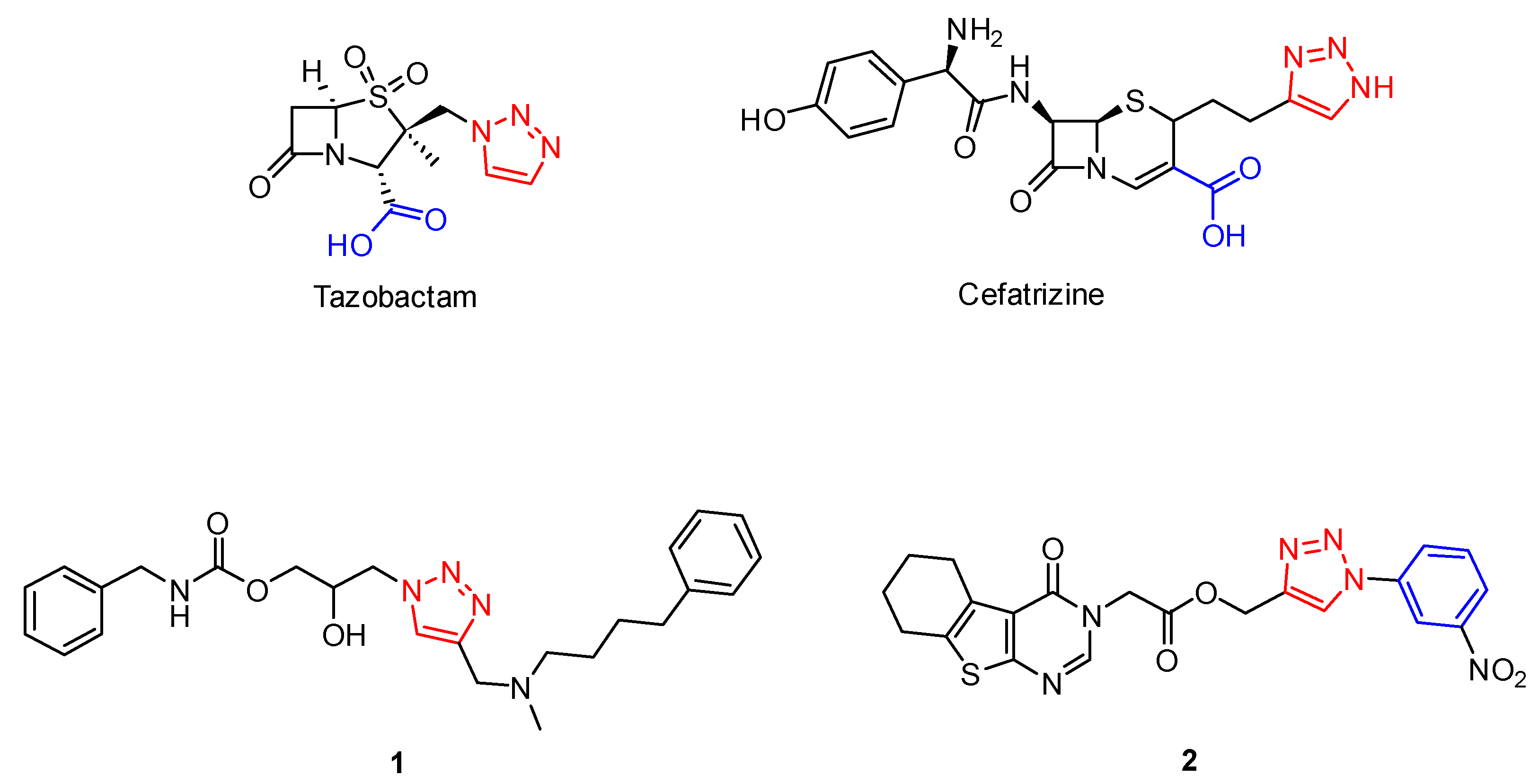
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Anti-MRSA and VISA Activity of 4d, 5d, 7c, 7d, and 7e
2.2.2. Structure-Activity Relationship
2.2.3. Compounds 4d, 7d, and 7e Treatment did not Affect Bacterial Cell Wall and Cell Membrane
2.2.4. Antibacterial Mechanism of Compounds 4d, 7d, and 7e as DNA Polymerase Inhibitor
2.3. Molecular Docking Study
3. Conclusions
4. Materials and Methods
4.1. Chemistry Section
4.1.1. (E)-1,3-Dimethoxy-5-(4-(prop-2-yn-1-yloxy)styryl)benzene (3)
4.1.2. The Synthesis of Azide Derivatives 14–17 Series
4.1.3. General Procedure for the Synthesis of Compound 4–7 Series
4.2. Biological Activity
4.2.1. Bacterial Strains and Culture Conditions
4.2.2. Agar Diffusion Assay
4.2.3. Minimum Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) Determination
4.2.4. Cell Viability Assay
4.2.5. Bacterial Viability Determined by Fluorescence Microscopy
4.2.6. Anti-Taq DNA Polymerase Activity
4.3. Molecular Docking Study
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domalaon, R.; Idowu, T.; Zhanel, G.G.; Schweizer, F. Antibiotic hybrids: The next generation of agents and adjuvants against Gram-negative pathogens? Clin. Microbiol. Rev. 2018, 31, e00077-17. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Pasberg-Gauhl, C. A need for new generation antibiotics against MRSA resistant bacteria. Drug Discov. Today Technol. 2014, 11, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Barman, T.K.; Kumar, M.; Mathur, T.; Chaira, T.; Ramkumar, G.; Kalia, V.; Rao, M.; Pandya, M.; Yadav, A.S.; Das, B.; et al. In vitro and in vivo activities of a Bi-Aryl oxazolidinone, RBx 11760, against gram-positive bacteria. Antimicrob. Agents Chemother. 2016, 60, 7134–7145. [Google Scholar] [PubMed]
- Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/biggest_threats.html (accessed on 13 September 2019).
- Boswihi, S.S.; Udo, E.E. Methicillin-resistant staphylococcus aureus: An update on the epidemiology, treatment options and infection control. Curr. Med. Res. Pract. 2018, 8, 18–24. [Google Scholar] [CrossRef]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Tang, K.W.; Lin, C.H.; Alalaiwe, A.; Tseng, C.H.; Fang, J.Y. Discovery of furanoquinone derivatives as a novel class of DNA polymerase and gyrase inhibitors for MRSA eradication in cutaneous infection. Front. Microbiol. 2019, 10, 1197. [Google Scholar] [CrossRef]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013, 575482. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharm. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Tseng, C.H.; Wang, P.W.; Lu, P.L.; Weng, Y.H.; Yen, F.L.; Fang, J.Y. Pterostilbene, a methoxylated resveratrol derivative, efficiently eradicates planktonic, biofilm, and intracellular MRSA by topical application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef] [PubMed]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, A.; Aprile, S.; Mercalli, V.; Grosso, E.D.; Grosa, G.; Sorba, G.; Tron, G.C. Are 1,4- and 1,5-disubstituted 1,2,3-triazoles good pharmacophoric groups? Chem. Med. Chem. 2014, 9, 2497–2508. [Google Scholar] [CrossRef] [PubMed]
- Bezouska, K. Design, functional evaluation and biomedical applications of carbohydrate dendrimers (glycodendrimers). Rev. Mol. Biotechnol. 2002, 90, 269–290. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, D.; Agarwal, D.; Gupta, R.D.; Tilak, R.; Awasthi, S.K.; Agarwal, A. Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2016, 113, 34–49. [Google Scholar] [CrossRef]
- Acquaah-Harrison, G.; Zhou, S.; Hines, J.V.; Bergmeier, S.C. A library of 1,4-disubstituted 1,2,3-triazole analogs of oxazolidinone RNA-binding agents. J. Comb. Chem. 2010, 12, 491–496. [Google Scholar] [CrossRef]
- Aruna-Kumari, M.; Triloknadh, S.; Harikrishna, N.; Vijjulatha, M.; Venkata-Rao, C. Synthesis, antibacterial activity, and docking studies of 1,2,3-triazole-tagged thieno[2,3-d]pyrimidinone derivatives. J. Heterocycl. Chem. 2017, 54, 3672–3681. [Google Scholar] [CrossRef]
- Liang, S.; Li, H.; Shen, L.; Li, H.; Mao, Z.; Li, H. Measurement and correlation of the solubility of (1-benzyl-1H-1, 2, 3-triazole-4-yl) methanol in water and alcohols at temperatures from 292.15 K to 310.15 K. Thermochim. Acta 2016, 630, 1–10. [Google Scholar] [CrossRef]
- Kuleshov, K.; Borovkov, K.Y.; Rodin, O.; Perevalov, V. Synthesis of novel 5-piperidyl-substituted 7-hydroxy-3H-1, 2, 3-triazolo [4–d] pyrimidines. Chem. Heterocycl. Compd. 2006, 42, 246–260. [Google Scholar] [CrossRef]
- Patonay, T.; Juhász-Tóth, E.; Bényei, A. Base-induced coupling of α-azido ketones with aldehydes—An easy and efficient eoute to trifunctionalized synthons 2-azido-3-hydroxy ketones, 2-acylaziridines, and 2-acylspiroaziridines. Eur. J. Org. Chem. 2002, 2002, 285–295. [Google Scholar] [CrossRef]
- Shi, F.; Waldo, J.P.; Chen, Y.; Larock, R.C. Benzyne click chemistry: Synthesis of benzotriazoles from benzynes and azides. Org. Lett. 2008, 10, 2409–2412. [Google Scholar] [CrossRef]
- Zhou, S.; Liao, H.; Liu, M.; Feng, G.; Fu, B.; Li, R.; Cheng, M.; Zhao, Y.; Gong, P. Discovery and biological evaluation of novel 6,7-disubstituted-4-(2-fluorophenoxy)quinoline derivatives possessing 1,2,3-triazole-4-carboxamide moiety as c-Met kinase inhibitors. Bioorg. Med. Chem. 2014, 22, 6438–6452. [Google Scholar] [CrossRef]
- Locatelli, G.A.; Savio, M.; Forti, L.; Shevelev, I.; Ramadan, K.; Stivala, L.A.; Vannini, V.; Hübscher, U.; Spadari, S.; Maga, G. Inhibition of mammalian DNA polymerases by resveratrol: Mechanism and structural determinants. Biochem. J. 2005, 389, 259–268. [Google Scholar] [CrossRef]
- Mangiaterra, G.; Laudadio, E.; Cometti, M.; Mobbili, G.; Minnelli, C.; Massaccesi, L.; Citterio, B.; Biavasco, F.; Galeazzi, R. Inhibitors of multidrug efflux pumps of Pseudomonas aeruginosa from natural sources: An in silico high-throughput virtual screening and in vitro validation. Med. Chem. Res. 2017, 26, 414–430. [Google Scholar] [CrossRef]
- Hoopman, T.C.; Liu, W.; Joslin, S.N.; Pybus, C.; Brautigam, C.A.; Hansen, E.J. Identification of gene products involved in the oxidative stress response of Moraxella catarrhalis. Infect Immun. 2011, 79, 745–755. [Google Scholar] [CrossRef]
- Alalaiwe, A.; Wang, P.W.; Lu, P.L.; Chen, Y.P.; Fang, J.Y.; Yang, S.C. Synergistic anti-MRSA activity of cationic nanostructured lipid carriers in combination with oxacillin for cutaneous application. Front. Microbiol. 2018, 9, 1493. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 15–16. [Google Scholar] [CrossRef]
- Manteca, A.; Fernández, M.; Sánchez, J. A death round affecting a young compartmentalized mycelium precedes aerial mycelium dismantling in confluent surface cultures of Streptomyces antibioticus. Microbiology 2005, 151, 3689–3697. [Google Scholar] [CrossRef]
- Yang, S.C.; Huang, T.H.; Chiu, C.H.; Chou, W.L.; Alalaiwe, A.; Yeh, Y.C.; Su, K.W.; Fang, J.Y. The atopic dermatitis-like lesion and the associated MRSA infection and barrier dysfunction can be alleviated by 2,4-dimethoxy-6-methylbenzene-1,3-diol from Antrodia camphorata. J. Derm. Sci. 2018, 92, 188–196. [Google Scholar] [CrossRef]
- Sánchez-Linares, I.; Pérez-Sánchez, H.; Cecilia, J.M.; García, J.M. High-throughput parallel blind virtual screening using BINDSURF. BMC Bioinform. 2012, 13 (Suppl. 14), S13. [Google Scholar]
- Rego, N.; Koes, D. 3Dmol.js: Molecular visualization with WebGL. Bioinformatics 2015, 31, 1322–1324. [Google Scholar] [CrossRef]







| Compounds | Strains | Inhibition Zone (mm) | ||||
|---|---|---|---|---|---|---|
| 500 (μg/mL) | 2500 (μg/mL) | 1250 (μg/mL) | 625 (μg/mL) | 312 (μg/mL) | ||
| 4d | MRSA | 12.18 ± 1.36 | 10.15 ± 0.26 | 10.59 ± 0.60 | 11.24 ± 0.55 | 11.27 ± 0.34 |
| VISA | 11.64 ± 0.68 | 10.23 ± 0.13 | 11.83 ± 1.25 | 14.28 ± 0.14 | 12.98 ± 0.50 | |
| 5d | MRSA | 10.46 ± 0.97 | 9.79 ± 0.28 | 10.91 ± 0.47 | 10.41 ± 0.33 | 8.29 ± 0.18 |
| VISA | 13.55 ± 1.20 | 13.68 ± 3.37 | 16.68 ± 1.06 | 15.10 ± 0.30 | 11.91 ± 0.24 | |
| 7c | MRSA | 13.06 ± 1.28 | 8.78 ± 1.82 | 7.90 ± 1.43 | 6.11 ± 0.47 | 0.57 ± 0.98 |
| VISA | 15.75 ± 0.78 | 10.85 ± 1.13 | 8.36 ± 0.06 | 2.76 ± 4.79 | 0 | |
| 7d | MRSA | 21.43 ± 0.16 | 19.79 ± 0.11 | 15.295 ± 0.16 | 11.16 ± 0.17 | 9.06 ± 0.01 |
| VISA | 16.94 ± 0.94 | 14.04 ± 0.70 | 11.78 ± 2.39 | 6.25 ± 5.55 | 0 | |
| 7e | MRSA | 20.88 ± 0.04 | 19.59 ± 0.34 | 14.96 ± 0.17 | 10.11 ± 0.04 | 8.72 ± 0.08 |
| VISA | 17.19 ± 0.99 | 14.89 ± 0.75 | 11.75 ± 1.51 | 6.93 ± 6.46 | 0 | |
| Compounds | MIC (μg/mL) | MBC (μg/mL) | ||
|---|---|---|---|---|
| MRSA | VISA | MRSA | VISA | |
| 4d | 1.2–2.4 | N/D | 19.5–39 | >1250 |
| 5d | 312.5–625 | N/D | 625 | >1250 |
| 7c | 625 | 156 | 625 | 1250 |
| 7d | 78.1 | 156 | >1250 | 1250 |
| 7e | 78.1 | 156 | >1250 | 1250 |
| Pterostilbene | 41–161.5 | 20 | 41–161.5 | 20–40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.-W.; Yang, S.-C.; Tseng, C.-H. Design, Synthesis, and Anti-Bacterial Evaluation of Triazolyl-Pterostilbene Derivatives. Int. J. Mol. Sci. 2019, 20, 4564. https://doi.org/10.3390/ijms20184564
Tang K-W, Yang S-C, Tseng C-H. Design, Synthesis, and Anti-Bacterial Evaluation of Triazolyl-Pterostilbene Derivatives. International Journal of Molecular Sciences. 2019; 20(18):4564. https://doi.org/10.3390/ijms20184564
Chicago/Turabian StyleTang, Kai-Wei, Shih-Chun Yang, and Chih-Hua Tseng. 2019. "Design, Synthesis, and Anti-Bacterial Evaluation of Triazolyl-Pterostilbene Derivatives" International Journal of Molecular Sciences 20, no. 18: 4564. https://doi.org/10.3390/ijms20184564
APA StyleTang, K.-W., Yang, S.-C., & Tseng, C.-H. (2019). Design, Synthesis, and Anti-Bacterial Evaluation of Triazolyl-Pterostilbene Derivatives. International Journal of Molecular Sciences, 20(18), 4564. https://doi.org/10.3390/ijms20184564





