Long and Very-Long-Chain Ceramides Correlate with A More Aggressive Behavior in Skull Base Chordoma Patients
Abstract
1. Introduction
2. Results
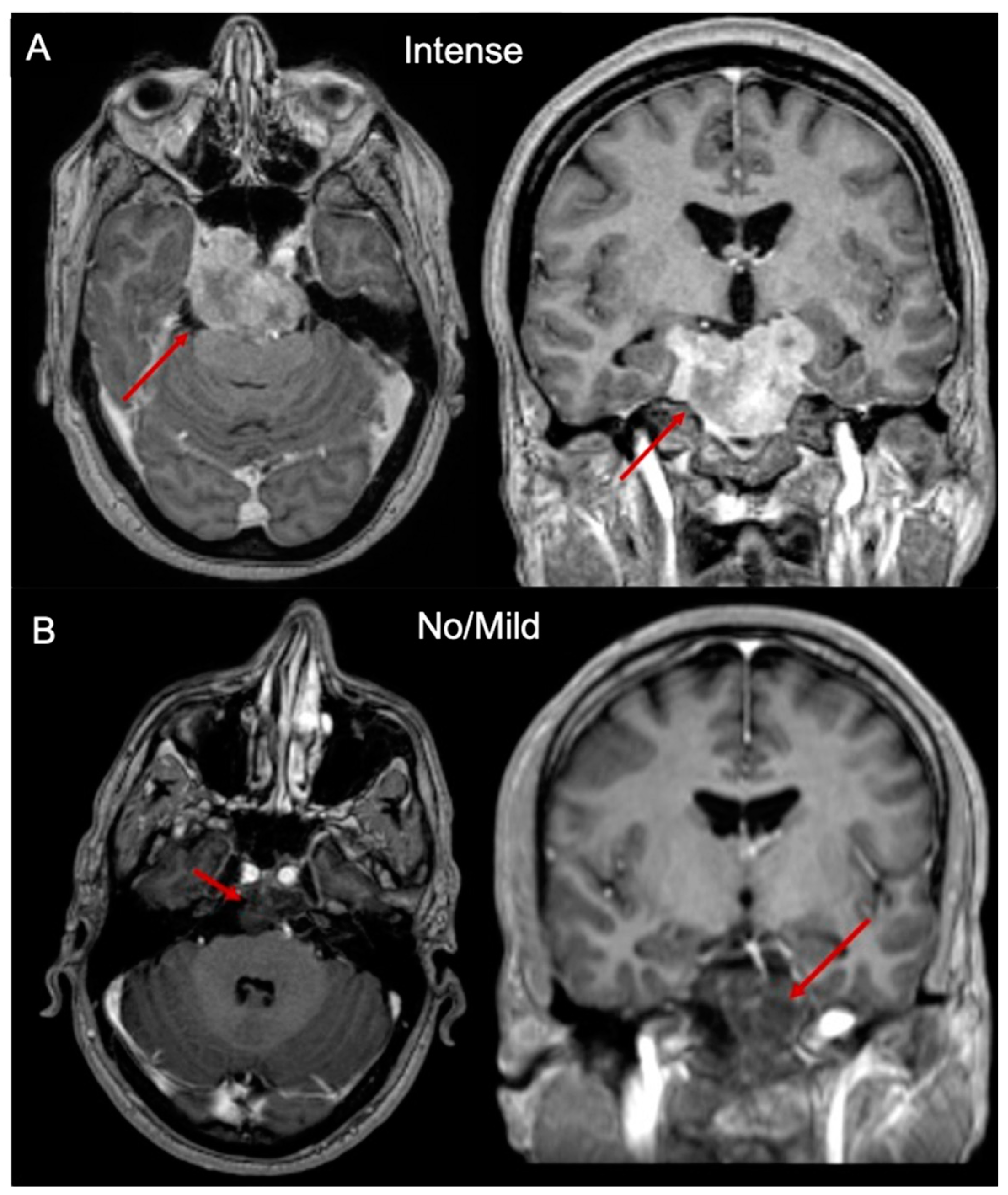
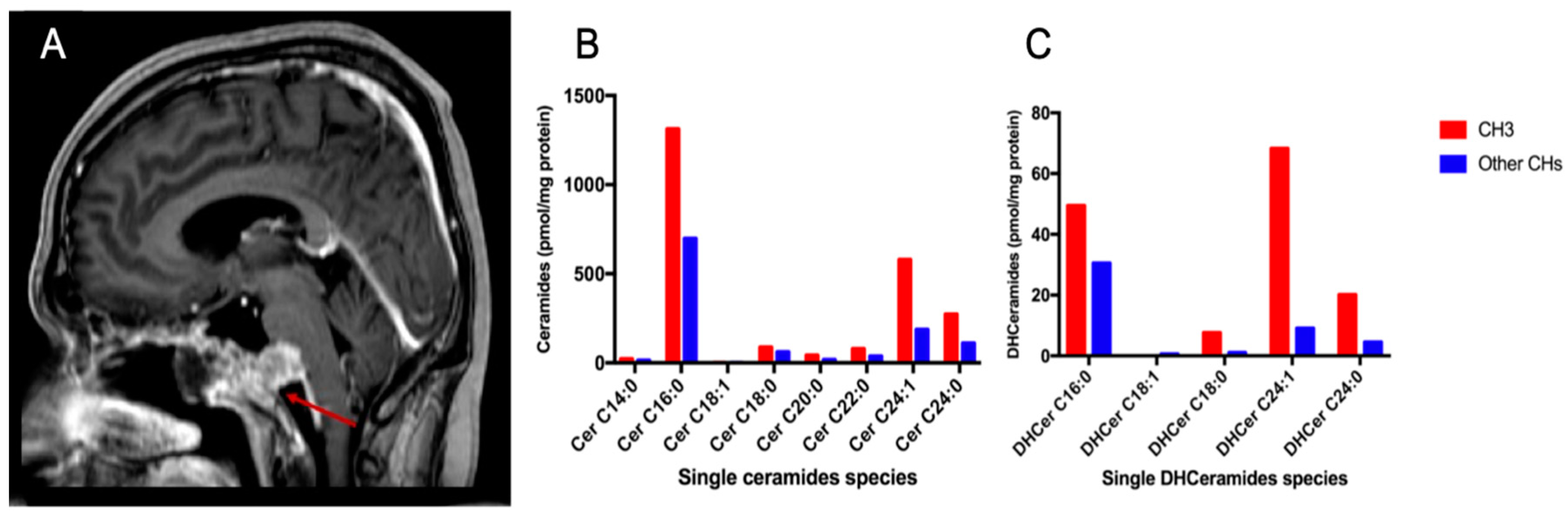
2.1. Clinical and Radiological Features
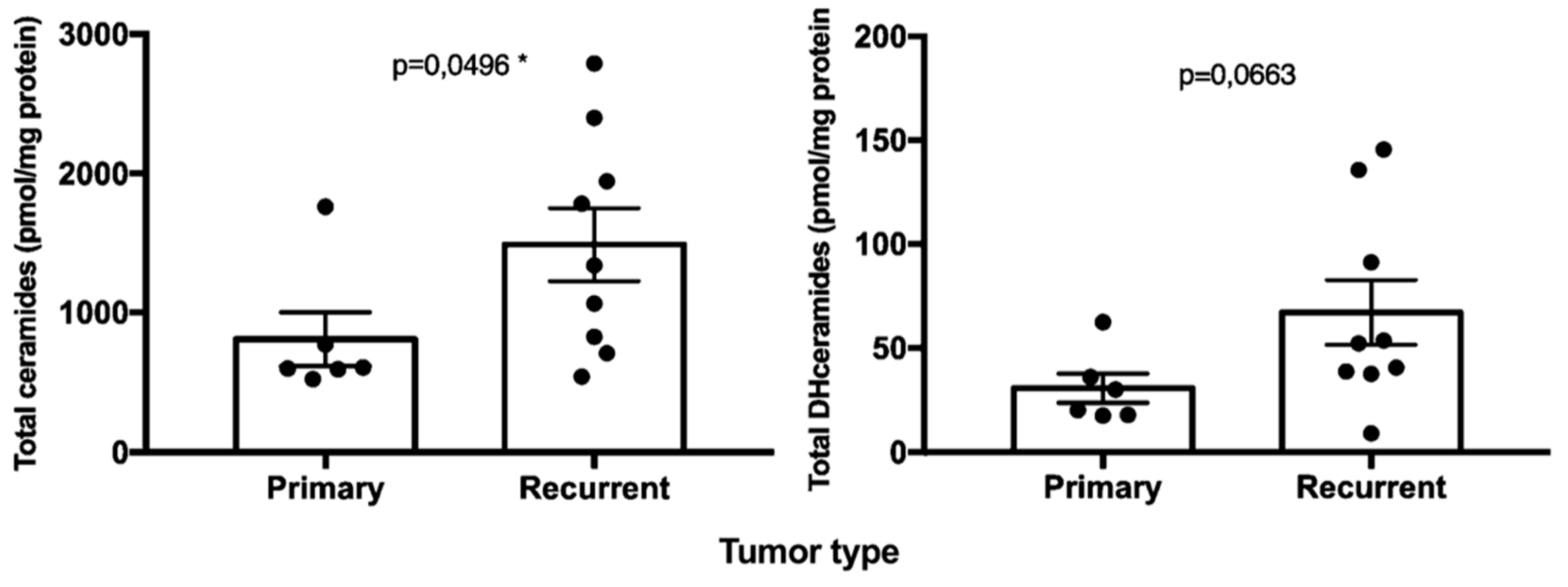
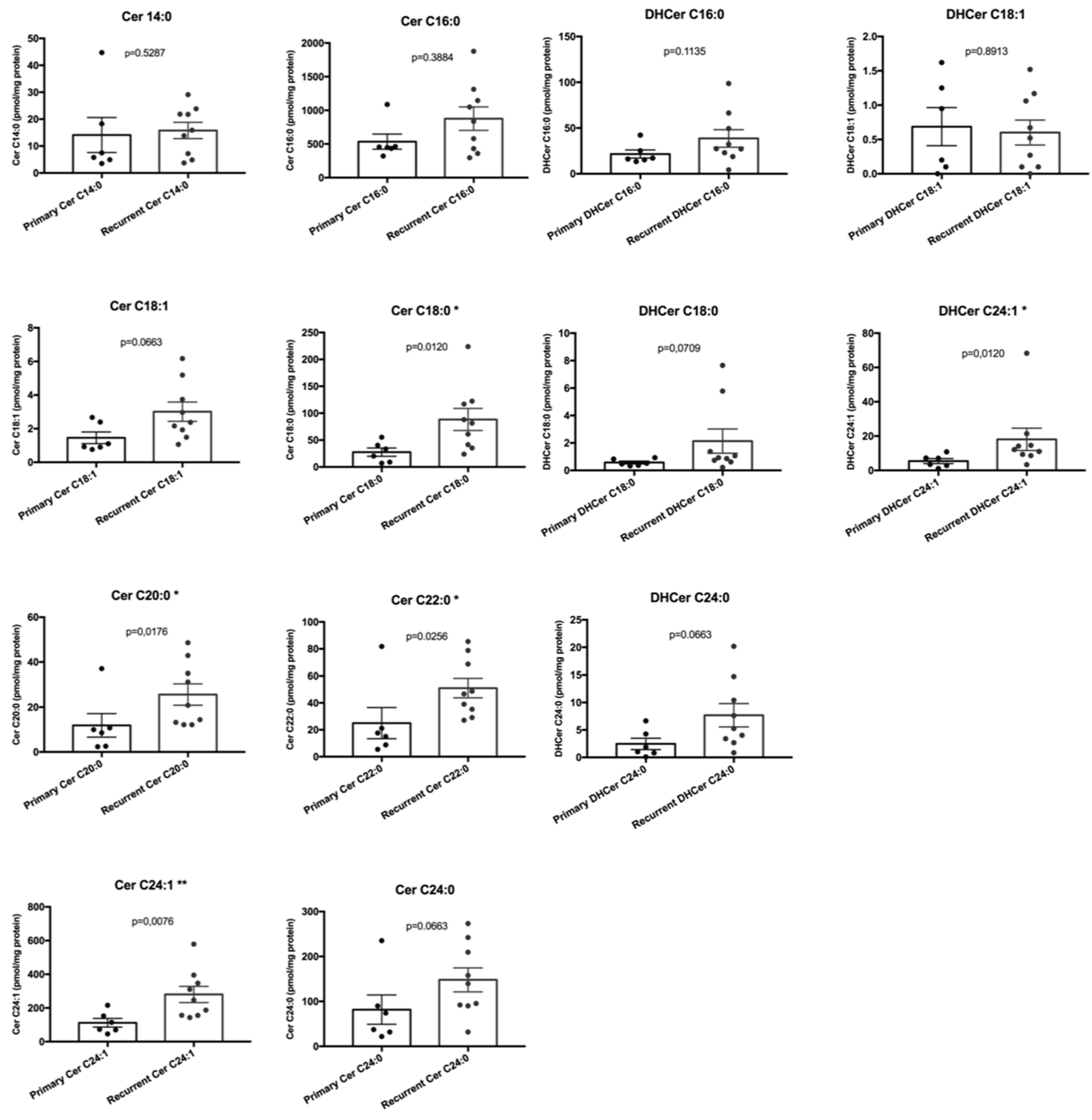
2.2. Sphingolipids Characterization and Tumor Type
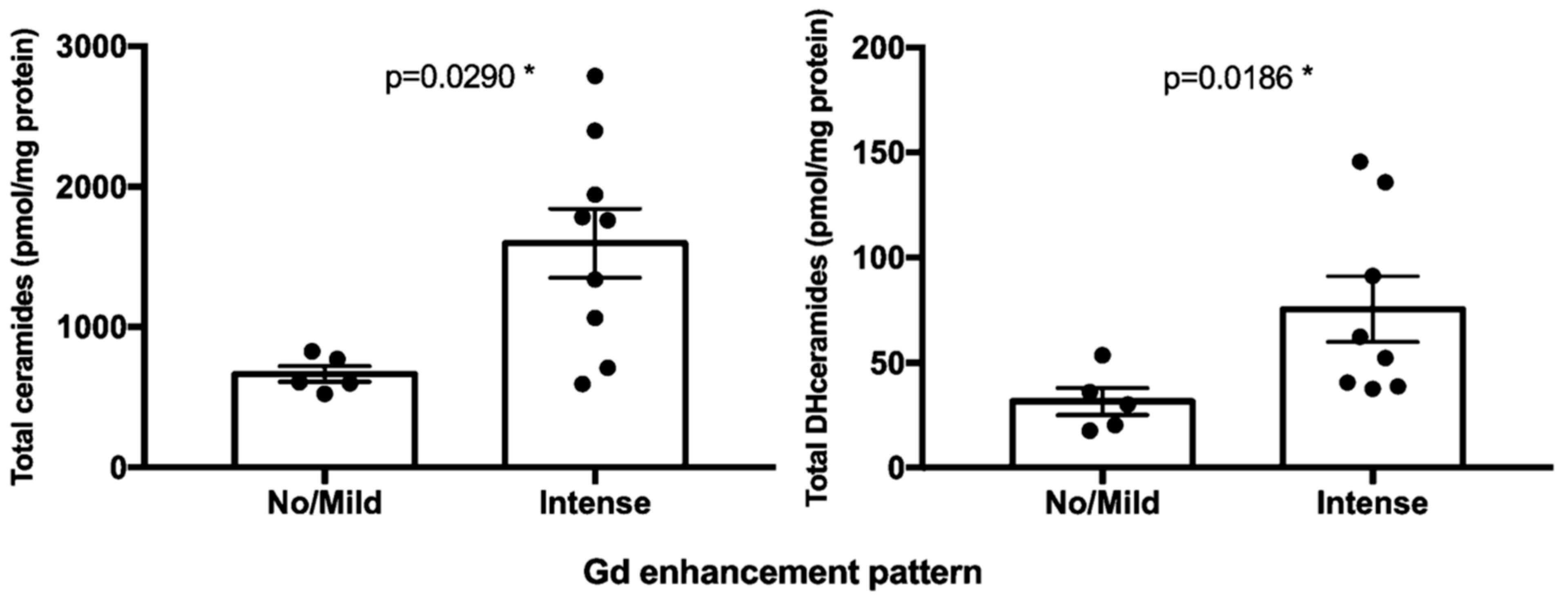
2.3. Sphingolipids Characterization and MR Contrast Enhancement Pattern
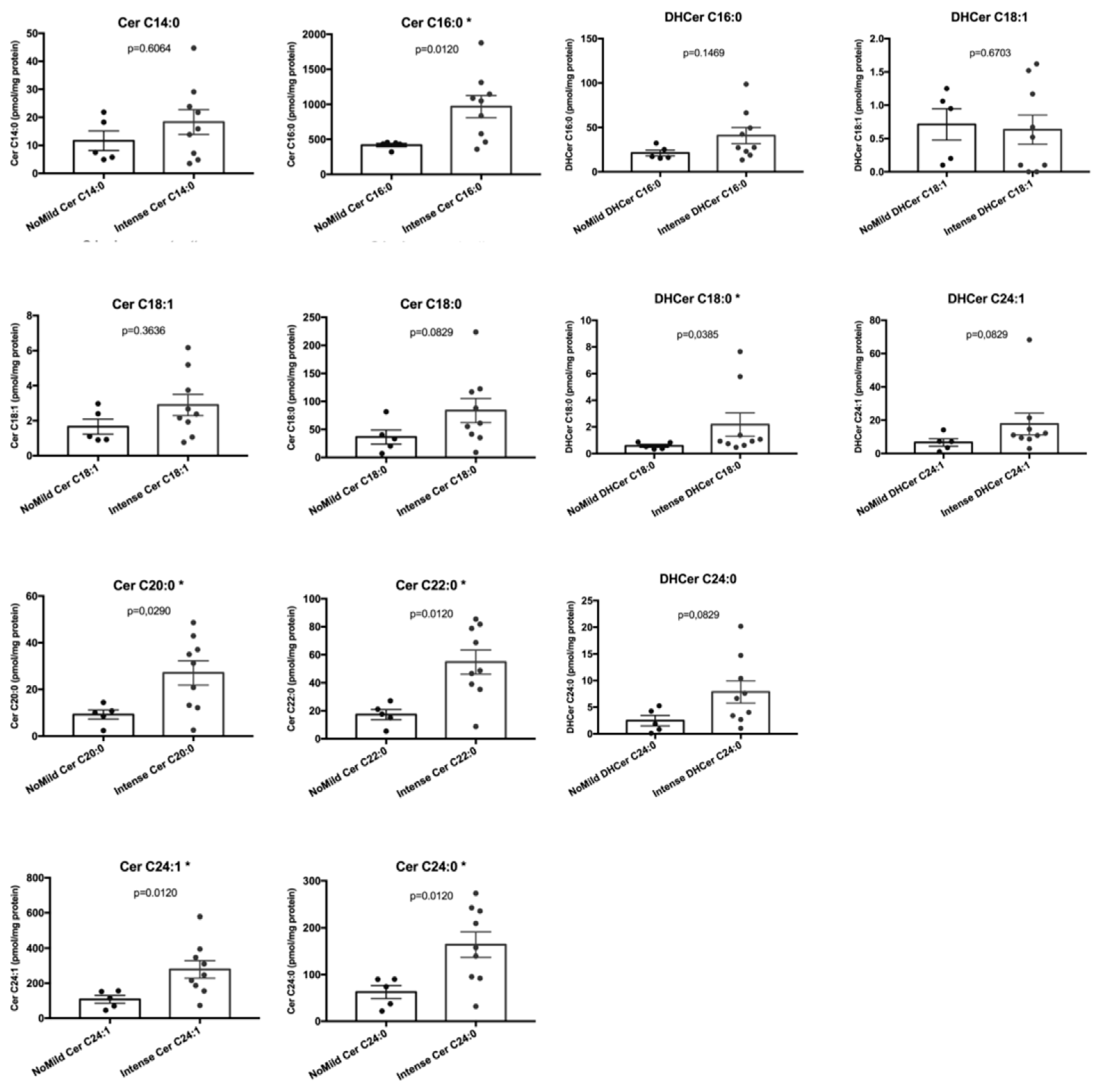
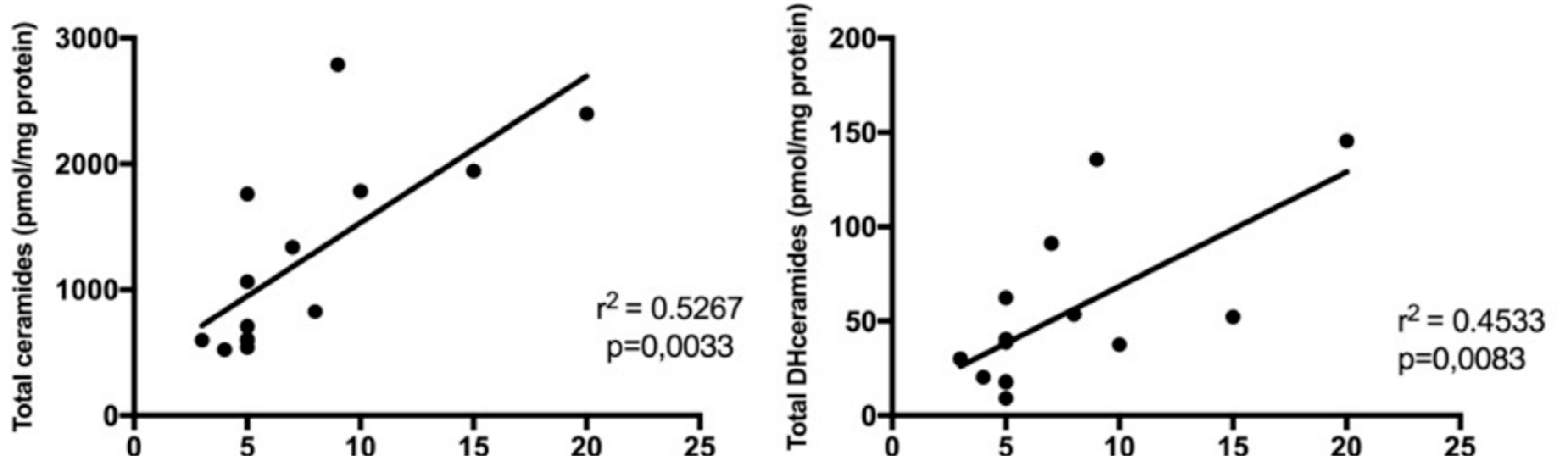
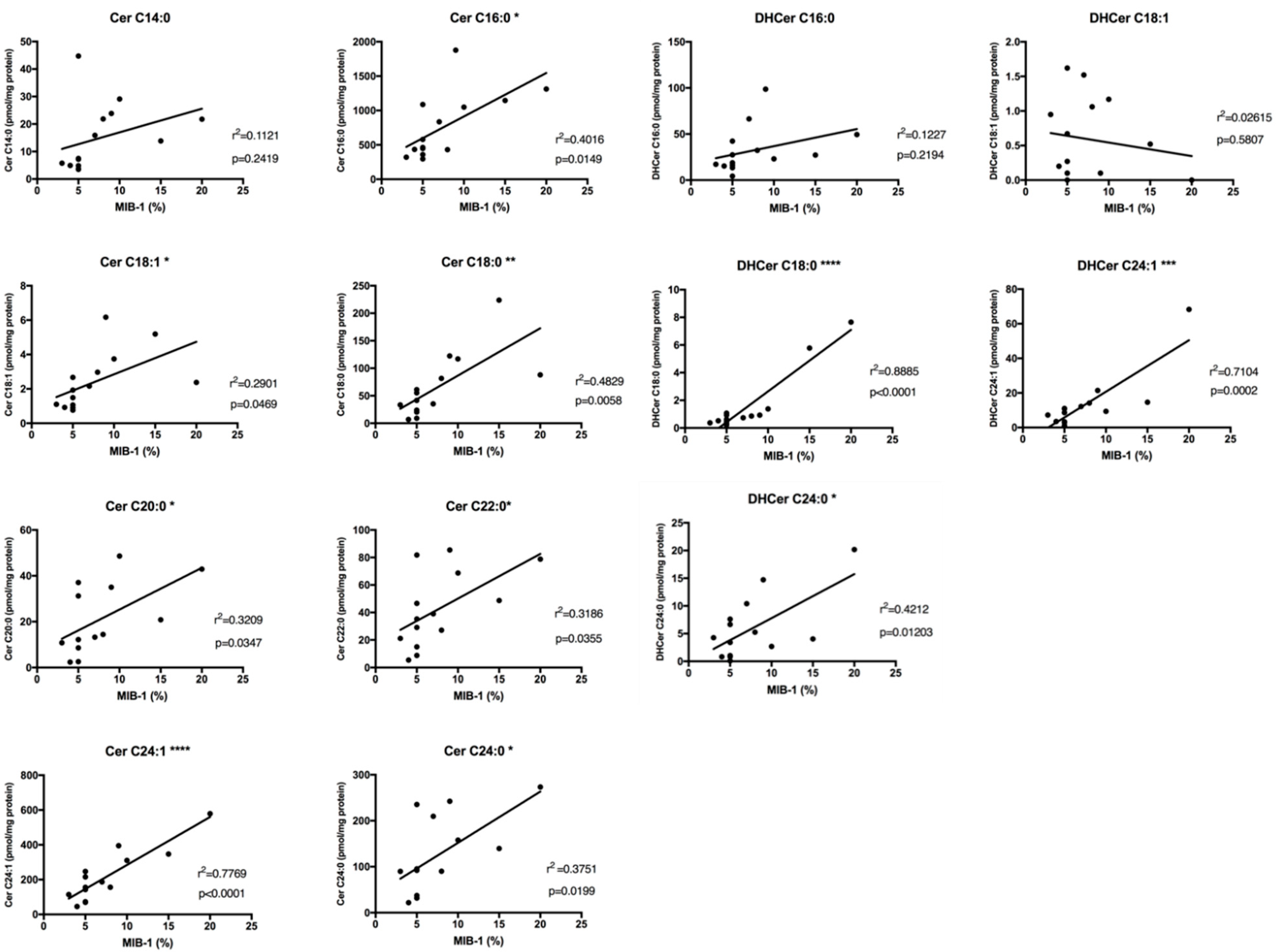
2.4. Sphingolipids Characterization and Proliferative Index
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Sphingolipids Analysis with LC-MS/MS
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Classification Of Tumours Of Soft Tissue; World Health Organization: Geneva, Switzerland, 2013; Volume 46, pp. 10–12. [Google Scholar]
- Colia, V.; Stacchiotti, S. Medical treatment of advanced chordomas. Eur. J. Cancer 2017, 83, 220–228. [Google Scholar] [CrossRef]
- Casali, P.G.; Stacchiotti, S.; Sangalli, C.; Olmi, P.; Gronchi, A. Chordoma. Curr. Opin. Oncol. 2007, 19, 367–370. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Sommer, J. Chordoma Global Consensus Group Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015, 16, e71–e83. [Google Scholar] [CrossRef]
- Di Maio, S.; Rostomily, R.; Sekhar, L.N. Current surgical outcomes for cranial base chordomas: Cohort study of 95 patients. Neurosurgery 2012, 70, 1355–1360; discussion 1360. [Google Scholar] [CrossRef]
- Chibbaro, S.; Cornelius, J.F.; Froelich, S.; Tigan, L.; Kehrli, P.; Debry, C.; Romano, A.; Herman, P.; George, B.; Bresson, D. Endoscopic endonasal approach in the management of skull base chordomas—Clinical experience on a large series, technique, outcome, and pitfalls. Neurosurg. Rev. 2014, 37, 217–224. [Google Scholar] [CrossRef]
- Ferroli, P.; Broggi, M.; Schiavolin, S.; Acerbi, F.; Bettamio, V.; Caldiroli, D.; Cusin, A.; La Corte, E.; Leonardi, M.; Raggi, A.; et al. Predicting functional impairment in brain tumor surgery: The Big Five and the Milan Complexity Scale. Neurosurg. Focus 2015, 39, E14. [Google Scholar] [CrossRef]
- Jahangiri, A.; Chin, A.T.; Wagner, J.R.; Kunwar, S.; Ames, C.; Chou, D.; Barani, I.; Parsa, A.T.; McDermott, M.W.; Benet, A.; et al. Factors predicting recurrence after resection of clival chordoma using variable surgical approaches and radiation modalities. Neurosurgery 2015, 76, 179–185. [Google Scholar] [CrossRef]
- La Corte, E.; Aldana, P.R.; Ferroli, P.; Greenfield, J.P.; Härtl, R.; Anand, V.K.; Schwartz, T.H. The rhinopalatine line as a reliable predictor of the inferior extent of endonasal odontoidectomies. Neurosurg. Focus 2015, 38, E16. [Google Scholar] [CrossRef]
- La Corte, E.; Broggi, M.; Bosio, L.; Danesi, G.; Ferroli, P. Tailored surgical strategy in clival chordomas: An extraordinary selection bias that limits approach comparison. J. Neurosurg. Sci. 2018, 62, 519–521. [Google Scholar]
- La Corte, E.; Aldana, P.R.; Schiariti, M.; Maccari, A.; Ferroli, P. Endoscopic approaches to the craniovertebral junction. Acta Neurochir. (Wien) 2014, 156, 293–295. [Google Scholar] [CrossRef]
- La Corte, E.; Aldana, P.R. Endoscopic approach to the upper cervical spine and clivus: An anatomical study of the upper limits of the transoral corridor. Acta Neurochir. (Wien) 2017, 159, 633–639. [Google Scholar] [CrossRef]
- Tamborini, E.; Miselli, F.; Negri, T.; Lagonigro, M.S.; Staurengo, S.; Dagrada, G.P.; Stacchiotti, S.; Pastore, E.; Gronchi, A.; Perrone, F.; et al. Molecular and biochemical analyses of platelet-derived growth factor receptor (PDGFR) B, PDGFRA, and KIT receptors in chordomas. Clin. Cancer Res. 2006, 12, 6920–6928. [Google Scholar] [CrossRef]
- Di Maio, S.; Yip, S.; Al Zhrani, G.A.; Alotaibi, F.E.; Al Turki, A.; Kong, E.; Rostomily, R.C. Novel targeted therapies in chordoma: An update. Ther. Clin. Risk Manag. 2015, 11, 873–883. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Tamborini, E.; Lo Vullo, S.; Bozzi, F.; Messina, A.; Morosi, C.; Casale, A.; Crippa, F.; Conca, E.; Negri, T.; et al. Phase II study on lapatinib in advanced EGFR-positive chordoma. Ann. Oncol. 2013, 24, 1931–1936. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Marrari, A.; Tamborini, E.; Palassini, E.; Virdis, E.; Messina, A.; Crippa, F.; Morosi, C.; Gronchi, A.; Pilotti, S.; et al. Response to imatinib plus sirolimus in advanced chordoma. Ann. Oncol. 2009, 20, 1886–1894. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Longhi, A.; Ferraresi, V.; Grignani, G.; Comandone, A.; Stupp, R.; Bertuzzi, A.; Tamborini, E.; Pilotti, S.; Messina, A.; et al. Phase II study of imatinib in advanced chordoma. J. Clin. Oncol. 2012, 30, 914–920. [Google Scholar] [CrossRef]
- Hindi, N.; Casali, P.G.; Morosi, C.; Messina, A.; Palassini, E.; Pilotti, S.; Tamborini, E.; Radaelli, S.; Gronchi, A.; Stacchiotti, S. Imatinib in advanced chordoma: A retrospective case series analysis. Eur. J. Cancer 2015, 51, 2609–2614. [Google Scholar] [CrossRef]
- La Corte, E. Clinical and Molecular Bio-Markers In Skull Base Chordomas. Ph.D. Thesis, University of Milan, Milano, Italy, January 2019. [Google Scholar]
- Truman, J.-P.; García-Barros, M.; Obeid, L.M.; Hannun, Y.A. Evolving concepts in cancer therapy through targeting sphingolipid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 1174–1188. [Google Scholar] [CrossRef]
- Henry, B.; Möller, C.; Dimanche-Boitrel, M.-T.; Gulbins, E.; Becker, K.A. Targeting the ceramide system in cancer. Cancer Lett. 2013, 332, 286–294. [Google Scholar] [CrossRef]
- Schenck, M.; Carpinteiro, A.; Grassmé, H.; Lang, F.; Gulbins, E. Ceramide: Physiological and pathophysiological aspects. Arch. Biochem. Biophys. 2007, 462, 171–175. [Google Scholar] [CrossRef]
- Hartmann, D.; Lucks, J.; Fuchs, S.; Schiffmann, S.; Schreiber, Y.; Ferreirós, N.; Merkens, J.; Marschalek, R.; Geisslinger, G.; Grösch, S. Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int. J. Biochem. Cell Biol. 2012, 44, 620–628. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, L.; Tian, Y.; Zhang, Z.; Dong, W.; Wang, X.; Zhang, X.; Cao, C. C6 ceramide dramatically enhances docetaxel-induced growth inhibition and apoptosis in cultured breast cancer cells: A mechanism study. Exp. Cell Res. 2015, 332, 47–59. [Google Scholar] [CrossRef]
- Scarlatti, F.; Sala, G.; Ricci, C.; Maioli, C.; Milani, F.; Minella, M.; Botturi, M.; Ghidoni, R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007, 253, 124–130. [Google Scholar] [CrossRef]
- Bernhart, E.; Damm, S.; Wintersperger, A.; Nusshold, C.; Brunner, A.M.; Plastira, I.; Rechberger, G.; Reicher, H.; Wadsack, C.; Zimmer, A.; et al. Interference with distinct steps of sphingolipid synthesis and signaling attenuates proliferation of U87MG glioma cells. Biochem. Pharmacol. 2015, 96, 119–130. [Google Scholar] [CrossRef]
- Hartmann, D.; Wegner, M.-S.; Wanger, R.A.; Ferreirós, N.; Schreiber, Y.; Lucks, J.; Schiffmann, S.; Geisslinger, G.; Grösch, S. The equilibrium between long and very long chain ceramides is important for the fate of the cell and can be influenced by co-expression of CerS. Int. J. Biochem. Cell Biol. 2013, 45, 1195–1203. [Google Scholar] [CrossRef]
- Kolb, D.; Pritz, E.; Steinecker-Frohnwieser, B.; Lohberger, B.; Deutsch, A.; Kroneis, T.; El-Heliebi, A.; Dohr, G.; Meditz, K.; Wagner, K.; et al. Extended Ultrastructural Characterization of Chordoma Cells: The Link to New Therapeutic Options. PLoS ONE 2014, 9, e114251. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef]
- Csordás, G.; Renken, C.; Várnai, P.; Walter, L.; Weaver, D.; Buttle, K.F.; Balla, T.; Mannella, C.A.; Hajnóczky, G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006, 174, 915–921. [Google Scholar] [CrossRef]
- Hu, L.; Wang, H.; Huang, L.; Zhao, Y.; Wang, J. Crosstalk between autophagy and intracellular radiation response (Review). Int. J. Oncol. 2016, 49, 2217–2226. [Google Scholar] [CrossRef]
- Aureli, M.; Bassi, R.; Prinetti, A.; Chiricozzi, E.; Pappalardi, B.; Chigorno, V.; Di Muzio, N.; Loberto, N.; Sonnino, S. Ionizing radiations increase the activity of the cell surface glycohydrolases and the plasma membrane ceramide content. Glycoconj. J. 2012, 29, 585–597. [Google Scholar] [CrossRef]
- Aureli, M.; Murdica, V.; Loberto, N.; Samarani, M.; Prinetti, A.; Bassi, R.; Sonnino, S. Exploring the link between ceramide and ionizing radiation. Glycoconj. J. 2014, 31, 449–459. [Google Scholar] [CrossRef]
- Ma, J.P.; Tian, K.B.; Wang, L.; Wang, K.; Li, D.; Yang, Y.; Du, J.; Ru, X.J.; Zhang, L.W.; Wu, Z.; et al. Proposal and Validation of a Basic Progression Scoring System for Patients with Skull Base Chordoma. World Neurosurg. 2016, 91, 409–418. [Google Scholar] [CrossRef]
- Zhai, Y.; Bai, J.; Wang, S.; Du, J.; Wang, J.; Li, C.; Gui, S.; Zhang, Y. Differences in Dural Penetration of Clival Chordomas Are Associated with Different Prognosis and Expression of Platelet-Derived Growth Factor Receptor-β. World Neurosurg. 2017, 98, 288–295. [Google Scholar] [CrossRef]
- Tian, K.; Wang, L.; Ma, J.; Wang, K.; Li, D.; Du, J.; Jia, G.; Wu, Z.; Zhang, J. MR Imaging Grading System for Skull Base Chordoma. AJNR. Am. J. Neuroradiol. 2017, 38, 1206–1211. [Google Scholar] [CrossRef]
- Sims, K.; Haynes, C.A.; Kelly, S.; Allegood, J.C.; Wang, E.; Momin, A.; Leipelt, M.; Reichart, D.; Glass, C.K.; Sullards, M.C.; et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem. 2010, 285, 38568–38579. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, J.; McCoy, D.M.; Mallampalli, R.K. LASS5 is the predominant ceramide synthase isoform involved in de novo sphingolipid synthesis in lung epithelia. J. Lipid Res. 2005, 46, 1229–1238. [Google Scholar] [CrossRef]
- Devlin, C.M.; Lahm, T.; Hubbard, W.C.; Van Demark, M.; Wang, K.C.; Wu, X.; Bielawska, A.; Obeid, L.M.; Ivan, M.; Petrache, I. Dihydroceramide-based Response to Hypoxia. J. Biol. Chem. 2011, 286, 38069–38078. [Google Scholar] [CrossRef]
- Levine, B. Autophagy and cancer. Nature 2007, 446, 745–747. [Google Scholar] [CrossRef]
- Bedia, C.; Levade, T.; Codogno, P. Regulation of Autophagy by Sphingolipids. Anticancer Agents Med. Chem. 2011, 11, 844–853. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Qin, X.; Hou, W.; Dong, H.; Yao, L.; Xiong, L. The pleiotropic roles of sphingolipid signaling in autophagy. Cell Death Dis. 2014, 5, e1245. [Google Scholar] [CrossRef]
- Morad, S.A.F.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Alva, A.; Su, H.; Dutt, P.; Freundt, E.; Welsh, S.; Baehrecke, E.H.; Lenardo, M.J. Regulation of an ATG7-beclin 1 Program of Autophagic Cell Death by Caspase-8. Science (80-). 2004, 304, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 Links the Autophagy Pathway and the Ubiqutin-Proteasome System Upon Ubiquitinated Protein Degradation. Cell. Mol. Biol. Lett. 2016, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Kozutsumi, Y.; Nakamura, S.; Fujita, T.; Kawasaki, T. Serine Palmitoyltransferase Is the Primary Target of a Sphingosine-like Immunosuppressant, ISP-1/Myriocin. Biochem. Biophys. Res. Commun. 1995, 211, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Mandala, S.M.; Harris, G.H. [35] Isolation and characterization of novel inhibitors of sphingolipid synthesis: Australifungin, viridiofungins, rustmicin, and khafrefungin. Methods Enzymol. 2000, 311, 335–348. [Google Scholar] [PubMed]
- Ikushiro, H.; Hayashi, H.; Kagamiyama, H. Reactions of Serine Palmitoyltransferase with Serine and Molecular Mechanisms of the Actions of Serine Derivatives as Inhibitors. Biochemistry 2004, 43, 1082–1092. [Google Scholar] [CrossRef]
- Wadsworth, J.M.; Clarke, D.J.; McMahon, S.A.; Lowther, J.P.; Beattie, A.E.; Langridge-Smith, P.R.R.; Broughton, H.B.; Dunn, T.M.; Naismith, J.H.; Campopiano, D.J. The Chemical Basis of Serine Palmitoyltransferase Inhibition by Myriocin. J. Am. Chem. Soc. 2013, 135, 14276–14285. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Holland, W.L.; Bikman, B.T.; Wang, L.-P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid–induced ceramide biosynthesis in mice. J. Clin. Invest. 2011, 121, 1858–1870. [Google Scholar] [CrossRef]
- Posse de Chaves, E.I. Sphingolipids in apoptosis, survival and regeneration in the nervous system. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1995–2015. [Google Scholar] [CrossRef] [PubMed]
- Caretti, A.; Bragonzi, A.; Facchini, M.; De Fino, I.; Riva, C.; Gasco, P.; Musicanti, C.; Casas, J.; Fabriàs, G.; Ghidoni, R.; et al. Anti-inflammatory action of lipid nanocarrier-delivered myriocin: Therapeutic potential in cystic fibrosis. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Tuson, M.; Marfany, G.; Gonzàlez-Duarte, R. Mutation of CERKL, a novel human ceramide kinase gene, causes autosomal recessive retinitis pigmentosa (RP26). Am. J. Hum. Genet. 2004, 74, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Caretti, A.; Torelli, R.; Perdoni, F.; Falleni, M.; Tosi, D.; Zulueta, A.; Casas, J.; Sanguinetti, M.; Ghidoni, R.; Borghi, E.; et al. Inhibition of ceramide de novo synthesis by myriocin produces the double effect of reducing pathological inflammation and exerting antifungal activity against A. fumigatus airways infection. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Caretti, A.; Vasso, M.; Bonezzi, F.T.; Gallina, A.; Trinchera, M.; Rossi, A.; Adami, R.; Casas, J.; Falleni, M.; Tosi, D.; et al. Myriocin treatment of CF lung infection and inflammation: Complex analyses for enigmatic lipids. Naunyn. Schmiedebergs. Arch. Pharmacol. 2017, 390, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.M.; Signorelli, P.; Rizzo, J.; Ghilardi, C.; Antognetti, J.; Caretti, A.; Lazarević, J.S.; Strettoi, E.; Novelli, E.; Ghidoni, R.; et al. Determination of the serine palmitoyl transferase inhibitor myriocin by electrospray and Q-trap mass spectrometry. Biomed. Chromatogr. 2017, 31, e4026. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Bresson, D.; Bouazza, S.; Froelich, S.; Mandonnet, E.; Hamdi, S.; Orabi, M.; Polivka, M.; Cazorla, A.; Adle-Biassette, H.; et al. [Chordoma]. Neurochirurgie 2014, 60, 63–140. [Google Scholar] [CrossRef] [PubMed]
- Pamir, M.N.; Özduman, K. Analysis of radiological features relative to histopathology in 42 skull-base chordomas and chondrosarcomas. Eur. J. Radiol. 2006, 58, 461–470. [Google Scholar] [CrossRef]
- Santegoeds, R.G.C.; Temel, Y.; Beckervordersandforth, J.C.; Van Overbeeke, J.J.; Hoeberigs, C.M. State-of-the-Art Imaging in Human Chordoma of the Skull Base. Curr. Radiol. Rep. 2018, 6, 16. [Google Scholar] [CrossRef]
- Mesicek, J.; Lee, H.; Feldman, T.; Jiang, X.; Skobeleva, A.; Berdyshev, E.V.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell. Signal. 2010, 22, 1300–1307. [Google Scholar] [CrossRef]
- Jiang, W.; Ogretmen, B. Autophagy paradox and ceramide. Biochim. Biophys. Acta 2014, 1841, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Kawaguchi, T.; Tsuchida, J.; Gabriel, E.; Qi, Q.; Yan, L.; Wakai, T.; Takabe, K.; Nagahashi, M. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 2018, 9, 19874–19890. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H., Jr.; Sullards, M.C.; Allegood, J.C.; Kelly, S.; Wang, E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005, 36, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Dei Cas, M.; Cianciolo, S.; Fidilio, A.; Lazzara, F.; Paroni, R.; Pignatello, R.; Strettoi, E.; Ghidoni, R.; Drago, F.; et al. Novel ophthalmic formulation of myriocin: Implications in retinitis pigmentosa. Drug Deliv. 2019, 26, 237–243. [Google Scholar] [CrossRef] [PubMed]
| Variable. | r | p-value | |
|---|---|---|---|
| Cer14:0 | 0,3349 | 0,2419 | ns |
| Cer16:0 | 0,6338 | 0,0149 | * |
| Cer18:1 | 0,5386 | 0,0469 | * |
| Cer18:0 | 0,6949 | 0,0058 | ** |
| Cer20:0 | 0,5665 | 0,0347 | * |
| Cer22:0 | 0,5645 | 0,0355 | * |
| Cer24:1 | 0,8814 | ≤ 0,0001 | **** |
| Cer24:0 | 0,6125 | 0,0199 | * |
| DHCer16:0 | 0,3503 | 0,2194 | ns |
| DHCer18:1 | −0,1617 | 0,5807 | ns |
| DHCer18:0 | 0,9426 | ≤ 0,0001 | **** |
| DHCer24:1 | 0,8429 | 0,0002 | *** |
| DHCer24:0 | 0,6490 | 0,0120 | * |
| Variable | B Value (SE) | Beta | p-value | Tolerance | VIF |
|---|---|---|---|---|---|
| Constant | 2.97 (0.73) | 0.002 | |||
| Cer C24:1 | 0.011 (0.004) | 0.348 | 0.025 | 0.351 | 2.85 |
| DHCer C18:0 | 1.40 (0.28) | 0.663 | ≤ 0.0001 | 0.351 | 2.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Corte, E.; Dei Cas, M.; Raggi, A.; Patanè, M.; Broggi, M.; Schiavolin, S.; Calatozzolo, C.; Pollo, B.; Pipolo, C.; Bruzzone, M.G.; et al. Long and Very-Long-Chain Ceramides Correlate with A More Aggressive Behavior in Skull Base Chordoma Patients. Int. J. Mol. Sci. 2019, 20, 4480. https://doi.org/10.3390/ijms20184480
La Corte E, Dei Cas M, Raggi A, Patanè M, Broggi M, Schiavolin S, Calatozzolo C, Pollo B, Pipolo C, Bruzzone MG, et al. Long and Very-Long-Chain Ceramides Correlate with A More Aggressive Behavior in Skull Base Chordoma Patients. International Journal of Molecular Sciences. 2019; 20(18):4480. https://doi.org/10.3390/ijms20184480
Chicago/Turabian StyleLa Corte, Emanuele, Michele Dei Cas, Alberto Raggi, Monica Patanè, Morgan Broggi, Silvia Schiavolin, Chiara Calatozzolo, Bianca Pollo, Carlotta Pipolo, Maria Grazia Bruzzone, and et al. 2019. "Long and Very-Long-Chain Ceramides Correlate with A More Aggressive Behavior in Skull Base Chordoma Patients" International Journal of Molecular Sciences 20, no. 18: 4480. https://doi.org/10.3390/ijms20184480
APA StyleLa Corte, E., Dei Cas, M., Raggi, A., Patanè, M., Broggi, M., Schiavolin, S., Calatozzolo, C., Pollo, B., Pipolo, C., Bruzzone, M. G., Campisi, G., Paroni, R., Ghidoni, R., & Ferroli, P. (2019). Long and Very-Long-Chain Ceramides Correlate with A More Aggressive Behavior in Skull Base Chordoma Patients. International Journal of Molecular Sciences, 20(18), 4480. https://doi.org/10.3390/ijms20184480







