Involvement of Dual Strands of miR-143 (miR-143-5p and miR-143-3p) and Their Target Oncogenes in the Molecular Pathogenesis of Lung Adenocarcinoma
Abstract
1. Introduction
2. Results
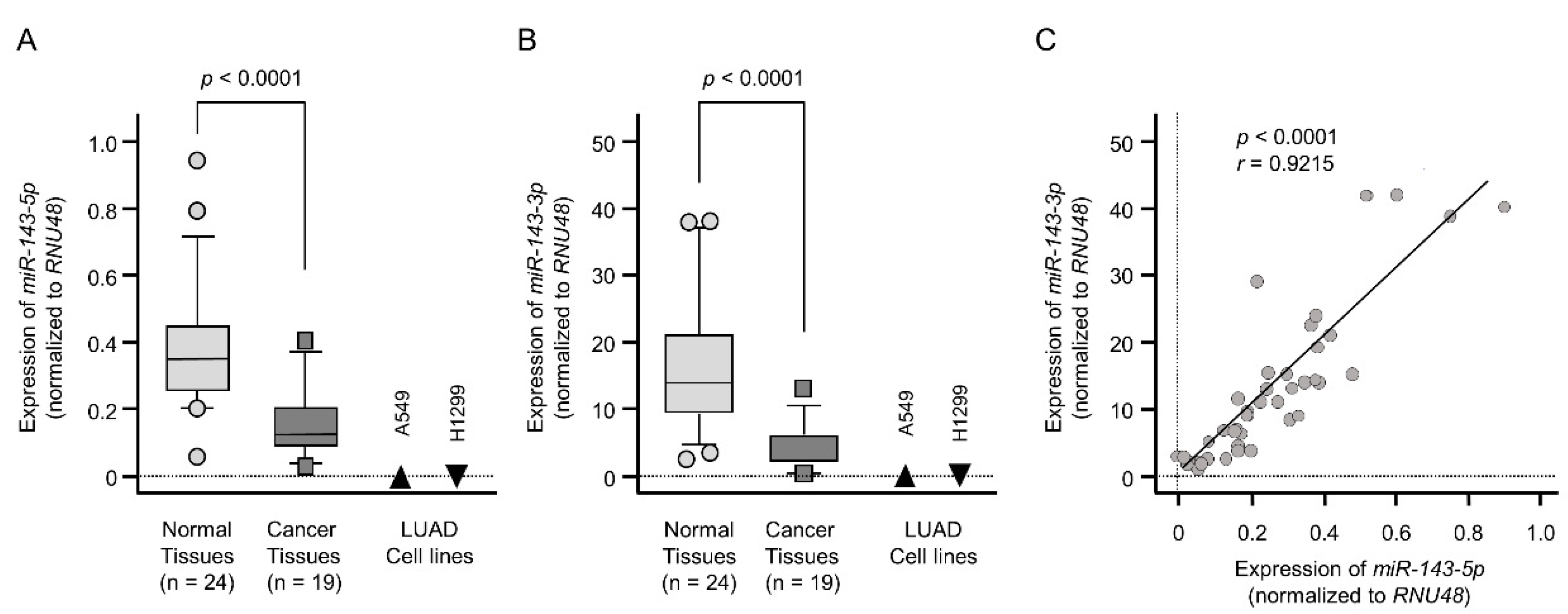
2.1. Downregulation of the miR-143 Duplex (miR-143-5p and miR-143-3p) in LUAD Clinical Specimens and Cell Lines
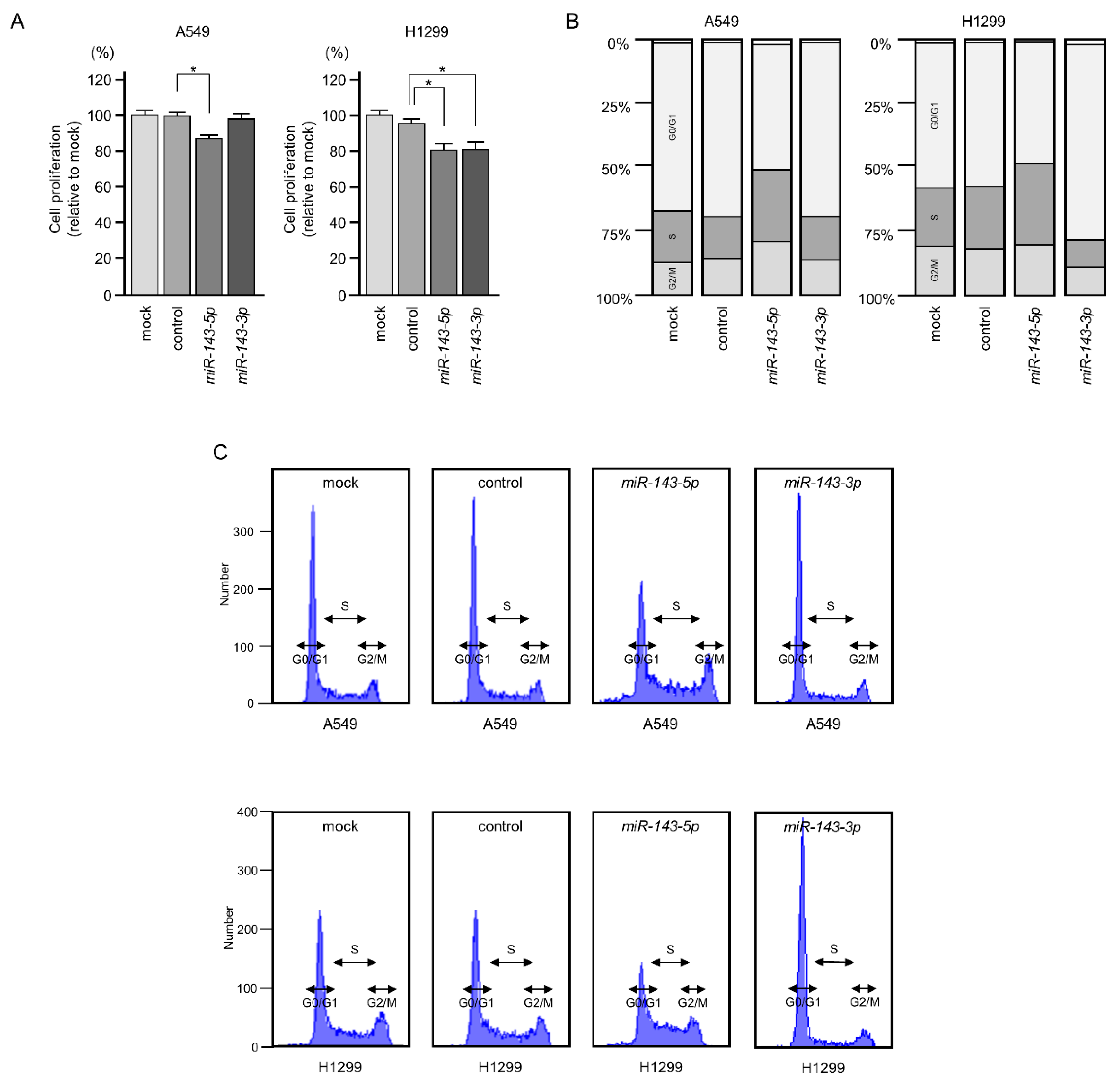
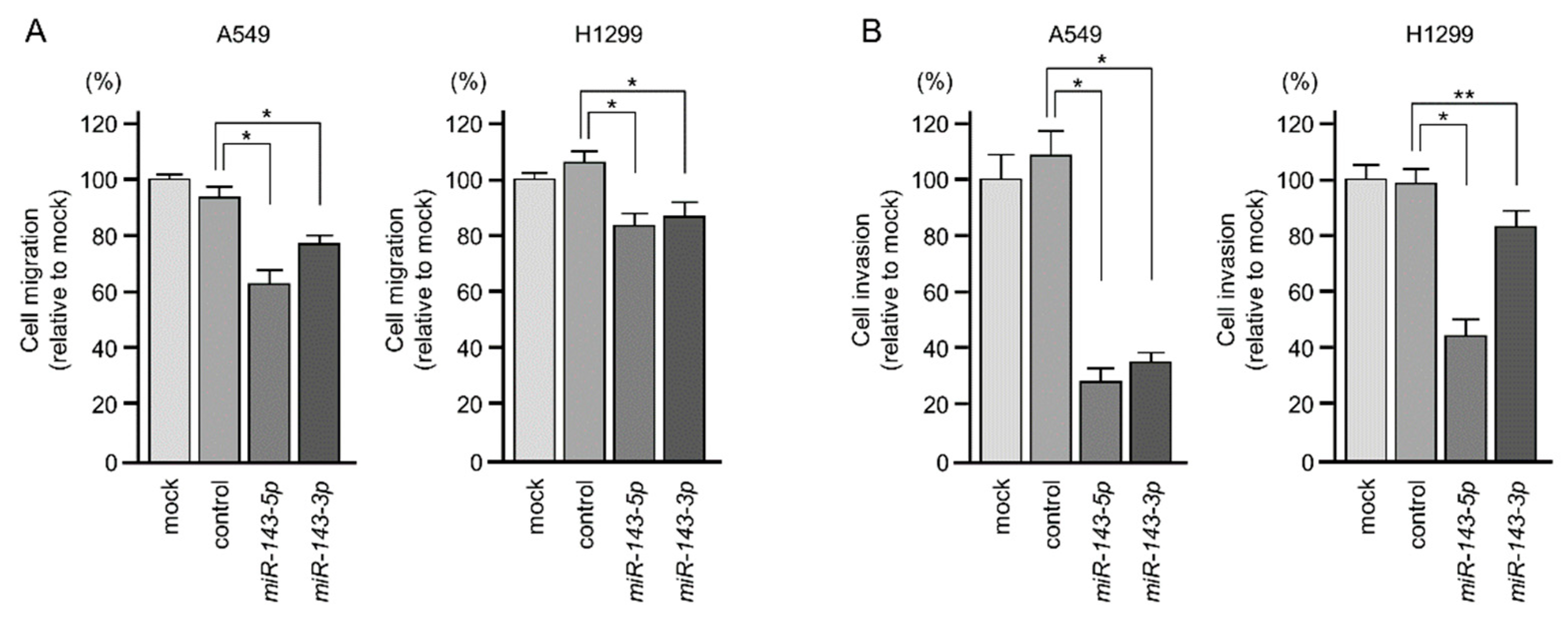
2.2. Ectopic Expression of miR-143-5p and miR-143-3p Blocked LUAD Aggressiveness
2.3. Incorporation of miR-143-5p into the RISC in LUAD Cells
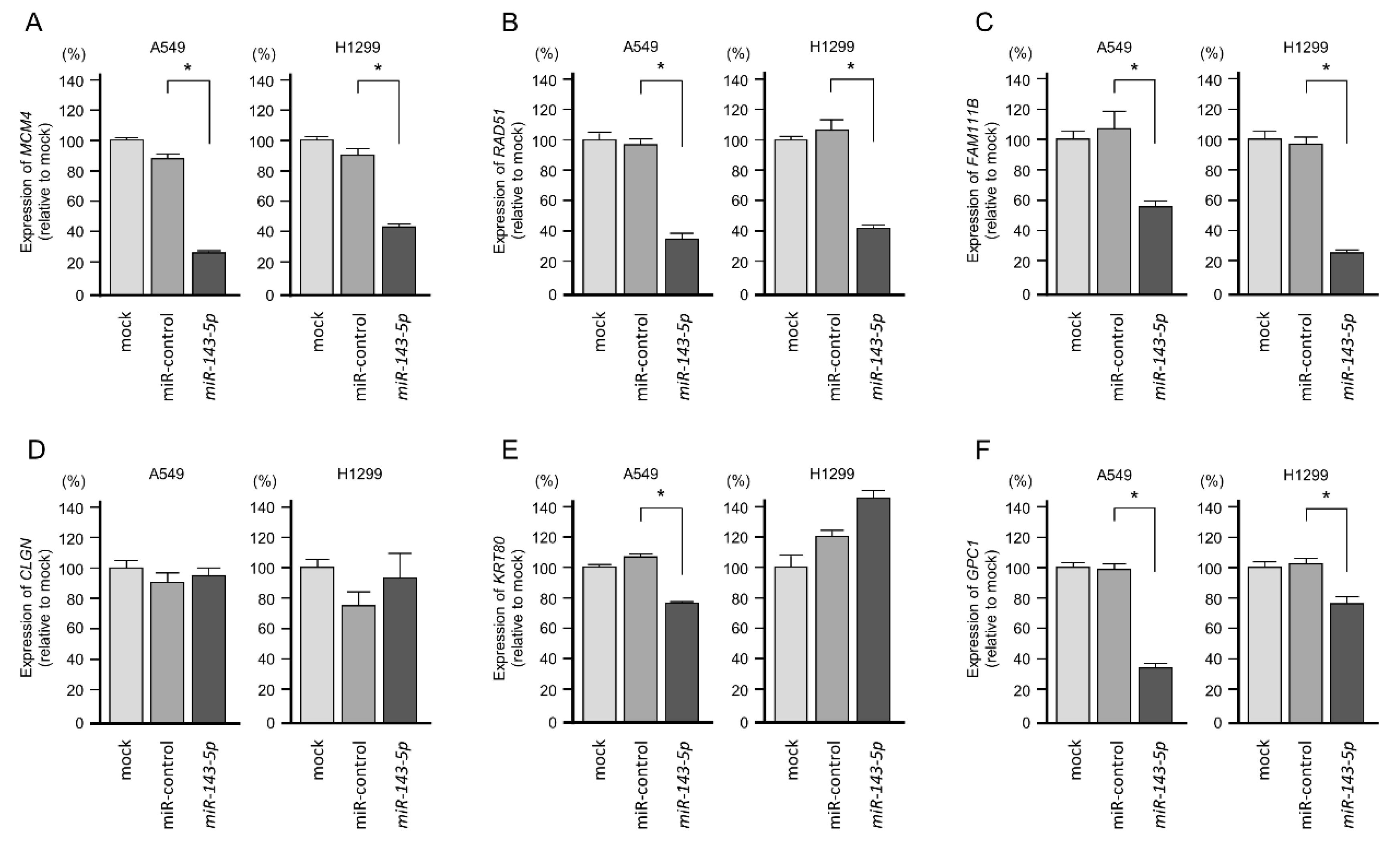
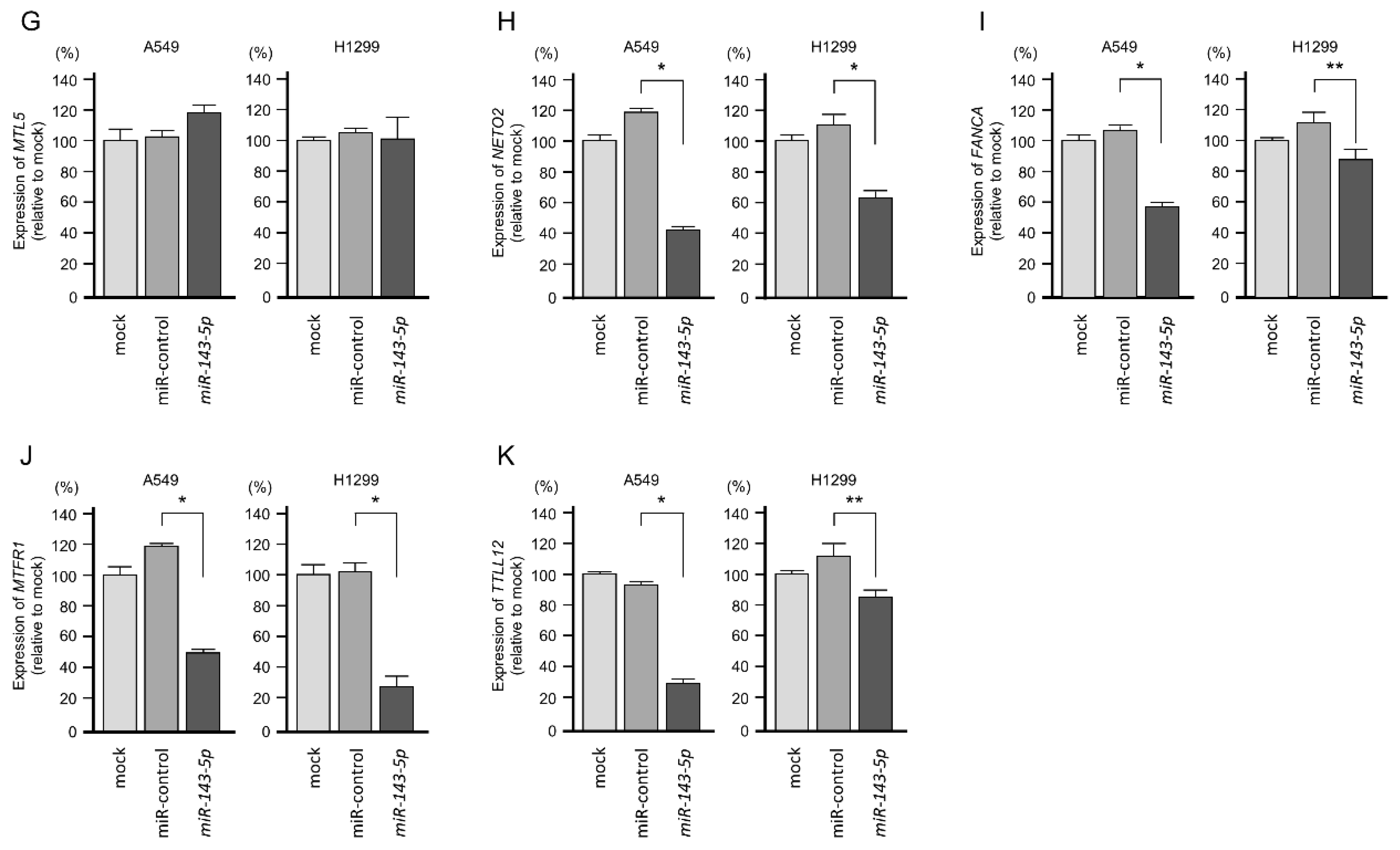
2.4. Screening of miR-143-5p-Controlled Oncogenes in LUAD Cells
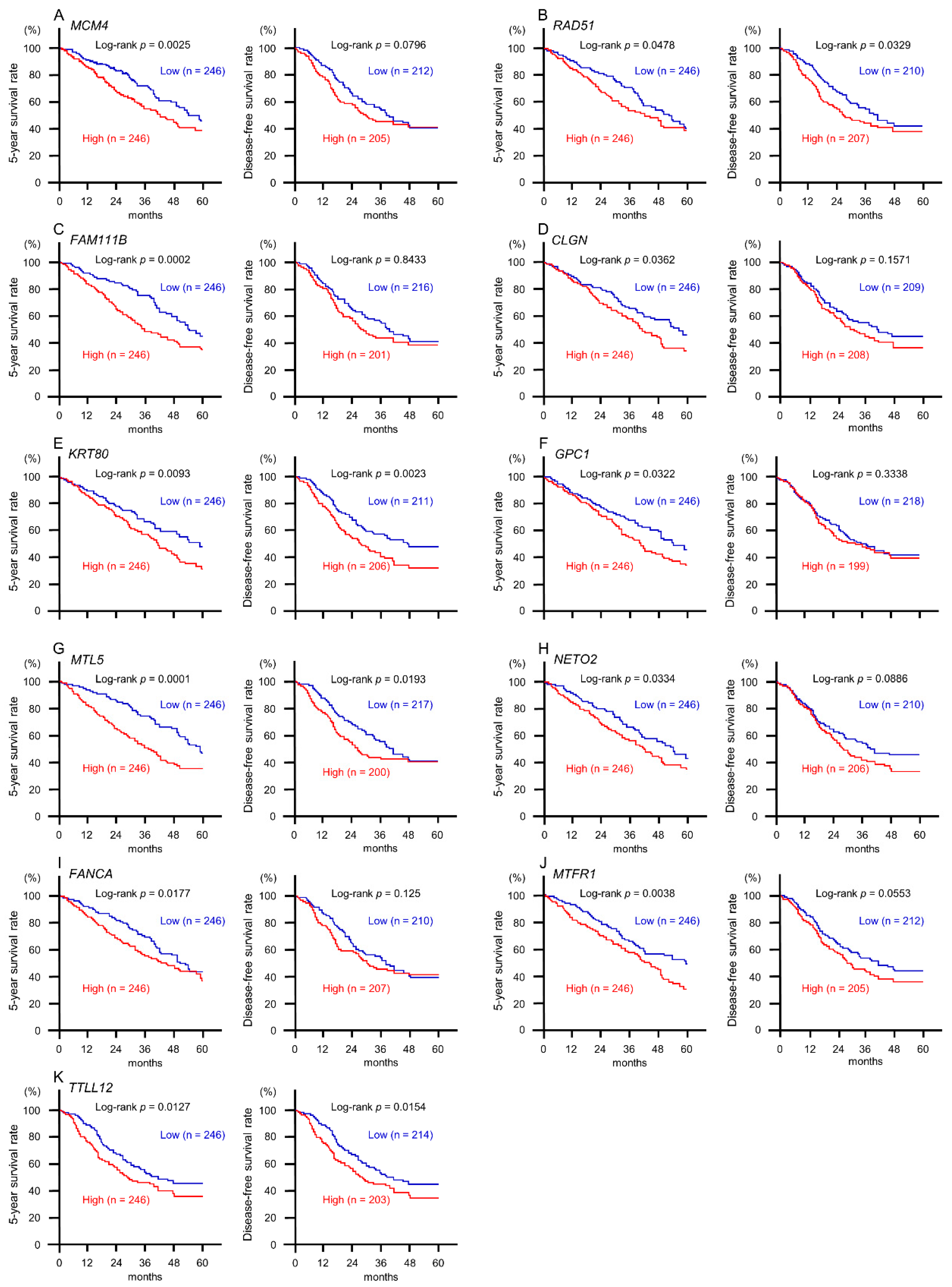
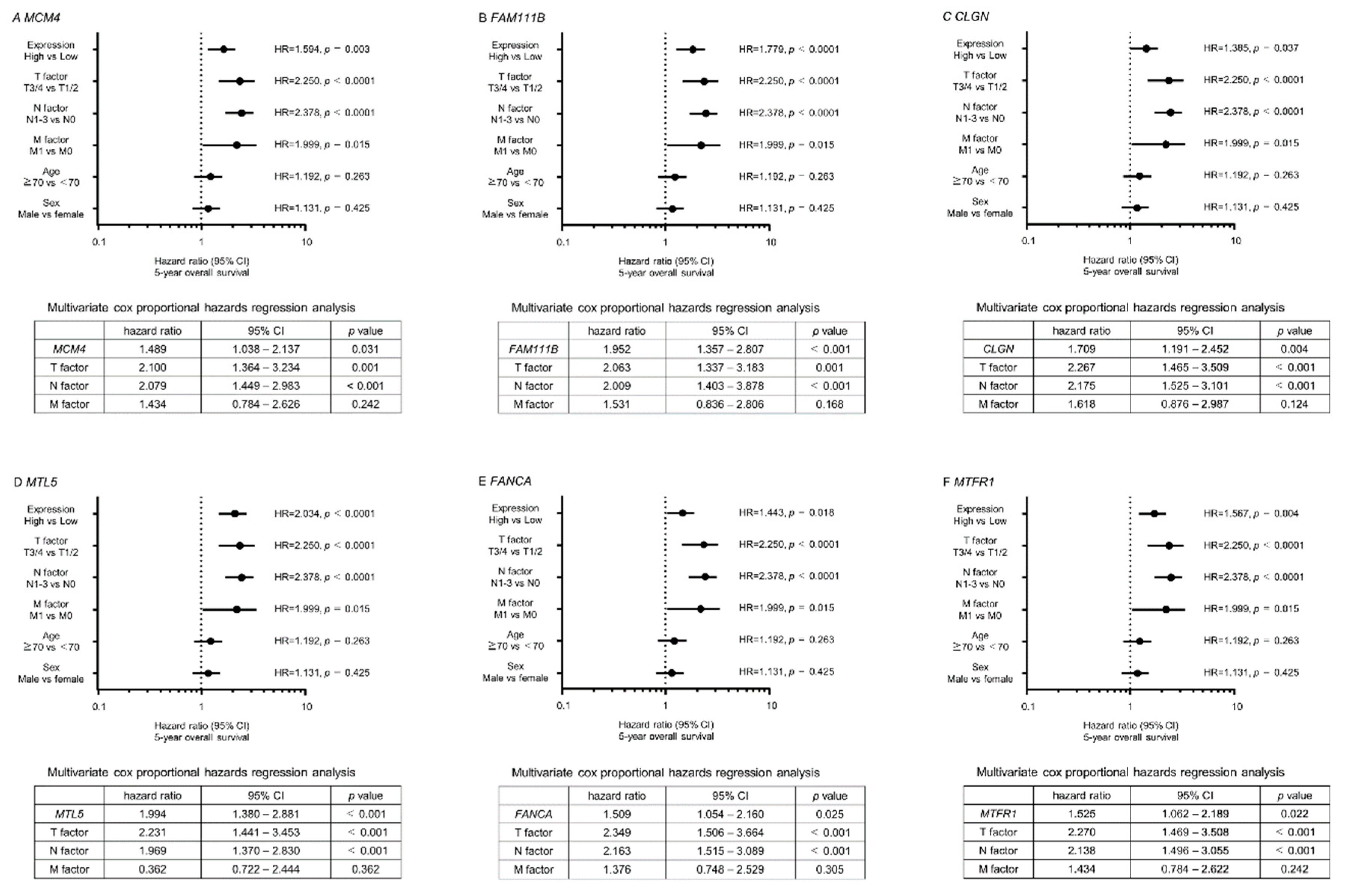
2.5. Clinical Significance of miR-143-5p Targets in LUAD Pathogenesis
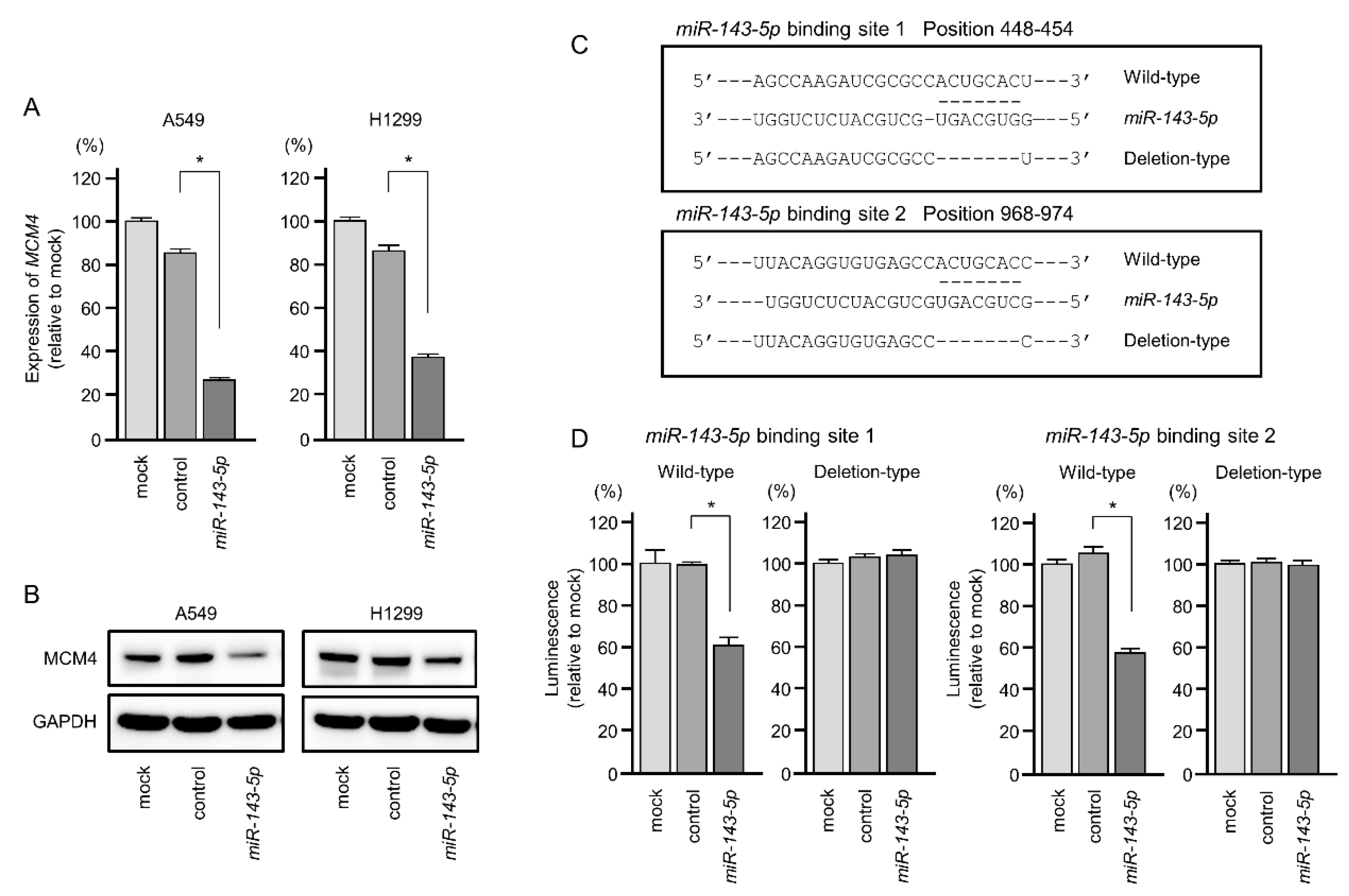
2.6. Direct Regulation of MCM4 Expression by miR-143-5p in LUAD Cells
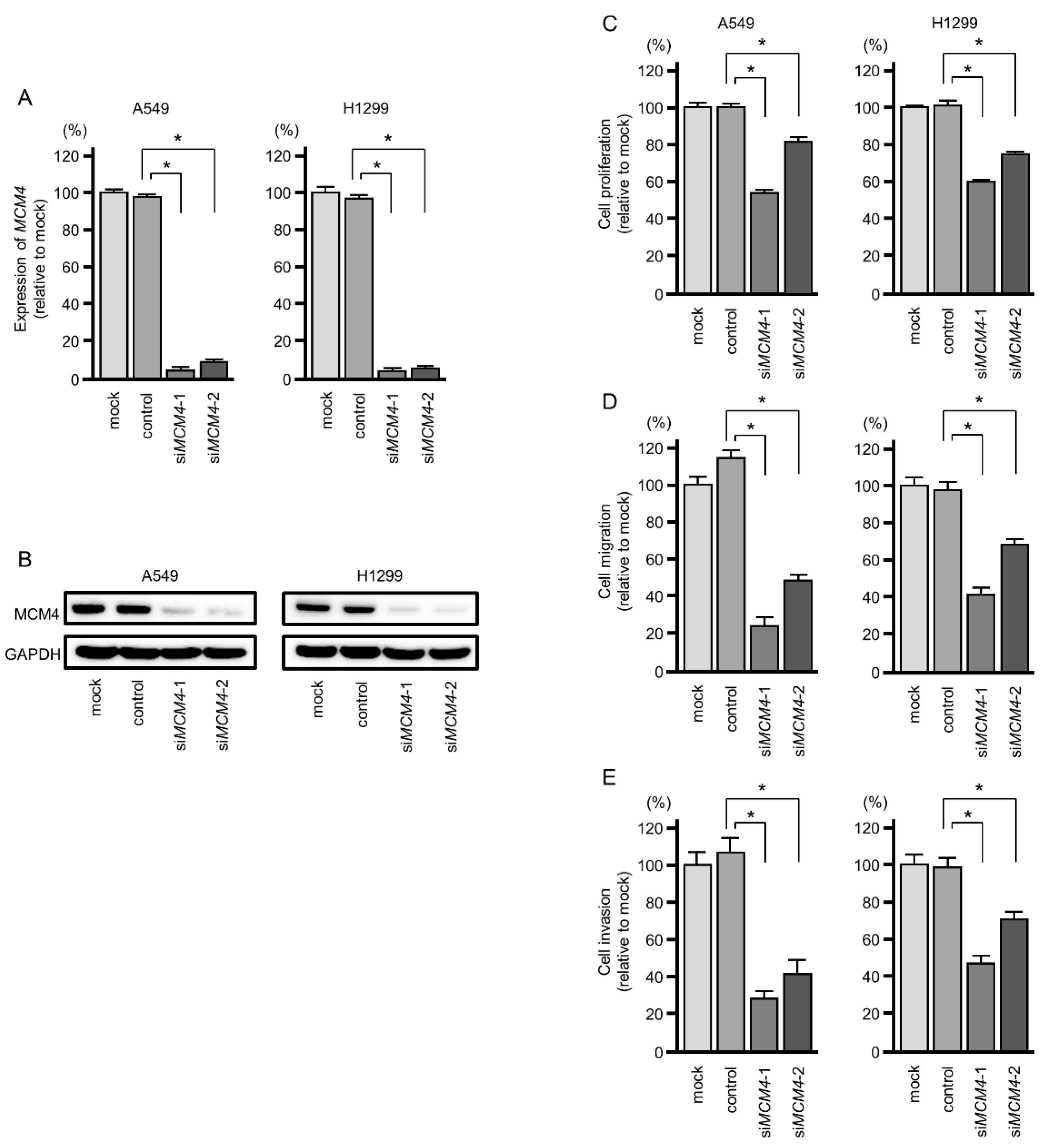
2.7. Effects of MCM4 Knockdown on LUAD Cells Malignant Phenotypes
2.8. Aberrant Expression of MCM4 in LUAD Clinical Specimens by Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Clinical Human LUAD Specimens and LUAD Cell Lines
4.2. RNA Preparation and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.3. Transfection of miRNAs, siRNAs, and Plasmid Vectors into LUAD Cells
4.4. Functional Assays for LUAD Cells (i.e., Cell Proliferation, Migration, Invasion, and Cell Cycle Assays)
4.5. Measurement of Amount of miR-143-5p Incorporated into the RISC
4.6. Identification of Oncogenes Regulated by miR-143-5p and miR-143-3p in LUAD Cells
4.7. Plasmid Construction and Dual-Luciferase Reporter Assay
4.8. Clinical Data Analyses of miRNAs and Target Genes in LUAD Specimens
4.9. Western Blotting and Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D. Pathology of lung cancer. Clin. Chest Med. 2011, 32, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Alkan, A.; Koksoy, E.B.; Utkan, G. First-line crizotinib in ALK-positive lung cancer. N. Engl. J. Med. 2015, 372, 781–782. [Google Scholar] [PubMed]
- Joo, J.W.; Hong, M.H.; Shim, H.S. Clinical characteristics of T790M-positive lung adenocarcinoma after resistance to epidermal growth factor receptor-tyrosine kinase inhibitors with an emphasis on brain metastasis and survival. Lung Cancer 2018, 121, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Baer, C.; Claus, R.; Plass, C. Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res. 2013, 73, 473–477. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Nelson, K.M.; Weiss, G.J. MicroRNAs and cancer: Past, present, and potential future. Mol. Cancer Ther. 2008, 7, 3655–3660. [Google Scholar] [CrossRef]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kojima, S.; Kurozumi, A.; Kato, M.; Okato, A.; Matsushita, R.; Ichikawa, T.; Seki, N. Regulation of E3 ubiquitin ligase-1 (WWP1) by microRNA-452 inhibits cancer cell migration and invasion in prostate cancer. Br. J. Cancer 2016, 114, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Toda, H.; Kurozumi, S.; Kijima, Y.; Idichi, T.; Shinden, Y.; Yamada, Y.; Arai, T.; Maemura, K.; Fujii, T.; Horiguchi, J.; et al. Molecular pathogenesis of triple-negative breast cancer based on microRNA expression signatures: Antitumor miR-204-5p targets AP1S3. J. Hum. Genet. 2018, 63, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Fukuhisa, H.; Seki, N.; Idichi, T.; Kurahara, H.; Yamada, Y.; Toda, H.; Kita, Y.; Kawasaki, Y.; Tanoue, K.; Mataki, Y.; et al. Gene regulation by antitumor miR-130b-5p in pancreatic ductal adenocarcinoma: The clinical significance of oncogenic EPS8. J. Hum. Genet. 2019, 64, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Yamada, Y.; Arai, T.; Okato, A.; Idichi, T.; Kato, M.; Koshizuka, K.; Ichikawa, T.; Seki, N. Dual strands of the miR-223 duplex (miR-223-5p and miR-223-3p) inhibit cancer cell aggressiveness: Targeted genes are involved in bladder cancer pathogenesis. J. Hum. Genet. 2018, 63, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Chendrimada, T.P.; Cooch, N.; Shiekhattar, R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005, 123, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Mah, S.M.; Buske, C.; Humphries, R.K.; Kuchenbauer, F. miRNA*: A passenger stranded in RNA-induced silencing complex? Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Seki, N.; Mizuno, K.; Misono, S.; Yamada, Y.; Kikkawa, N.; Sanada, H.; Kumamoto, T.; Suetsugu, T.; Inoue, H. Involvement of dual-strand of the miR-144 duplex and their targets in the pathogenesis of lung squamous cell carcinoma. Cancer Sci. 2019, 110, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Mataki, H.; Seki, N.; Mizuno, K.; Nohata, N.; Kamikawaji, K.; Kumamoto, T.; Koshizuka, K.; Goto, Y.; Inoue, H. Dual-strand tumor-suppressor microRNA-145 (miR-145-5p and miR-145-3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget 2016, 7, 72084–72098. [Google Scholar] [CrossRef] [PubMed]
- Misono, S.; Seki, N.; Mizuno, K.; Yamada, Y.; Uchida, A.; Arai, T.; Kumamoto, T.; Sanada, H.; Suetsugu, T.; Inoue, H. Dual strands of the miR-145 duplex (miR-145-5p and miR-145-3p) regulate oncogenes in lung adenocarcinoma pathogenesis. J. Hum. Genet. 2018, 63, 1015–1028. [Google Scholar] [CrossRef]
- Misono, S.; Seki, N.; Mizuno, K.; Yamada, Y.; Uchida, A.; Sanada, H.; Moriya, S.; Kikkawa, N.; Kumamoto, T.; Suetsugu, T.; et al. Molecular Pathogenesis of Gene Regulation by the miR-150 Duplex: miR-150-3p Regulates TNS4 in Lung Adenocarcinoma. Cancers (Basel) 2019, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Piper-Hunter, M.G.; Crawford, M.; Nuovo, G.J.; Marsh, C.B.; Otterson, G.A.; Nana-Sinkam, S.P. MicroRNAs in the pathogenesis of Lung Cancer. J. Thorac. Oncol. 2009, 4, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiong, D.; Yang, H.; Ye, L.; Mei, S.; Wu, J.; Chen, S.; Shang, X.; Wang, K.; Huang, L. Long noncoding RNA OPA-interacting protein 5 antisense transcript 1 upregulated SMAD3 expression to contribute to metastasis of cervical cancer by sponging miR-143-3p. J. Cell. Physiol. 2019, 234, 5264–5275. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Yang, Y.; Kong, F.; Kong, Q.; Shan, C. MiR-143-3p inhibits the proliferation, cell migration and invasion of human breast cancer cells by modulating the expression of MAPK7. Biochimie 2018, 147, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Tang, M.; Xu, X.; Jiang, B.; Wei, Y.; Qian, H.; Lu, X. MiR-143-3p suppresses cell proliferation, migration, and invasion by targeting Melanoma-Associated Antigen A9 in laryngeal squamous cell carcinoma. J. Cell. Biochem. 2019, 120, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Enokida, H.; Yoshino, H.; Itesako, T.; Chiyomaru, T.; Kinoshita, T.; Fuse, M.; Nishikawa, R.; Goto, Y.; Naya, Y.; et al. The tumor-suppressive microRNA-143/145 cluster inhibits cell migration and invasion by targeting GOLM1 in prostate cancer. J. Hum. Genet. 2014, 59, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Enokida, H.; Itesako, T.; Kojima, S.; Kinoshita, T.; Tatarano, S.; Chiyomaru, T.; Nakagawa, M.; Seki, N. Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in renal cell carcinoma. Cancer Sci. 2013, 104, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X. MYBL2 Is Targeted by miR-143-3p and Regulates Breast Cancer Cell Proliferation and Apoptosis. Oncol. Res. 2018, 26, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kurozumi, A.; Arai, T.; Nohata, N.; Kojima, S.; Okato, A.; Kato, M.; Yamazaki, K.; Ishida, Y.; Naya, Y.; et al. Impact of novel miR-145-3p regulatory networks on survival in patients with castration-resistant prostate cancer. Br. J. Cancer 2017, 117, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Koshizuka, K.; Hanazawa, T.; Kikkawa, N.; Okato, A.; Idichi, T.; Arai, T.; Sugawara, S.; Katada, K.; Okamoto, Y.; et al. Passenger strand of miR-145-3p acts as a tumor-suppressor by targeting MYO1B in head and neck squamous cell carcinoma. Int. J. Oncol. 2018, 52, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Shimonosono, M.; Idichi, T.; Seki, N.; Yamada, Y.; Arai, T.; Arigami, T.; Sasaki, K.; Omoto, I.; Uchikado, Y.; Kita, Y.; et al. Molecular pathogenesis of esophageal squamous cell carcinoma: Identification of the antitumor effects of miR1453p on gene regulation. Int. J. Oncol. 2019, 54, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Cheng, B.; Li, P.Y.; Huang, H.J.; Zhao, Q.; Dan, Z.L.; Tian, D.A.; Zhang, P. MicroRNA-143 suppresses gastric cancer cell growth and induces apoptosis by targeting COX-2. World J. Gastroenterol. 2013, 19, 7758–7765. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhan, M.; Chen, W.; Xu, S.; Long, M.; Shen, H.; Shi, Y.; Liu, Q.; Mohan, M.; Wang, J. MiR-143-5p Deficiency Triggers EMT and Metastasis by Targeting HIF-1alpha in Gallbladder Cancer. Cell. Physiol. Biochem. 2017, 42, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, X.; Hu, Y.; Ying, F.; Zou, R.; Lin, F.; Shi, Z.; Zhu, X.; Yan, X.; Li, S.; et al. LncRNA-TCONS_00026907 is involved in the progression and prognosis of cervical cancer through inhibiting miR-143-5p. Cancer Med 2017, 6, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Gao, H.; Liu, K.; Gao, B.; Ren, H.; Li, Z.; Liu, F. The lncRNA ZEB2-AS1 is upregulated in gastric cancer and affects cell proliferation and invasion via miR-143-5p/HIF-1alpha axis. Onco Targets Ther. 2019, 12, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.O.; Zhou, W.Y.; Chang, X.Y.; Zhang, M.M.; Zhang, Y.; Yang, X.H. Long non-coding RNA LINC01207 silencing suppresses AGR2 expression to facilitate autophagy and apoptosis of pancreatic cancer cells by sponging miR-143-5p. Mol. Cell. Endocrinol. 2019, 493, 110424. [Google Scholar] [CrossRef] [PubMed]
- Grzegrzolka, J.; Gomulkiewicz, A.; Olbromski, M.; Glatzel-Plucinska, N.; Piotrowska, A.; Ratajczak-Wielgomas, K.; Rzechonek, A.; Podhorska-Okolow, M.; Krawczuk, Z.; Dziegiel, P. Expression of tesmin (MTL5) in nonsmall cell lung cancer: A preliminary study. Oncol. Rep. 2019, 42, 253–262. [Google Scholar] [PubMed]
- Sun, H.; Liu, K.; Huang, J.; Sun, Q.; Shao, C.; Luo, J.; Xu, L.; Shen, Y.; Ren, B. FAM111B, a direct target of p53, promotes the malignant process of lung adenocarcinoma. Onco Targets Ther. 2019, 12, 2829–2842. [Google Scholar] [CrossRef] [PubMed]
- Santosa, V.; Martha, S.; Hirose, N.; Tanaka, K. The fission yeast minichromosome maintenance (MCM)-binding protein (MCM-BP), Mcb1, regulates MCM function during prereplicative complex formation in DNA replication. J. Biol. Chem. 2013, 288, 6864–6880. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, B.S.; Jiang, Y.Y.; Cao, J.; Hao, J.J.; Zhang, Y.; Xu, X.; Cai, Y.; Wang, M.R. MCMs expression in lung cancer: Implication of prognostic significance. J. Cancer 2017, 8, 3641–3647. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Shan, C.; Li, Y.; Li, F. Minichromosome maintenance protein 2 correlates with the malignant status and regulates proliferation and cell cycle in lung squamous cell carcinoma. Onco Targets Ther. 2018, 11, 5025–5034. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Ma, Y.; Zhang, M.; Liu, X.; Luo, Y.; Wang, C.; Zhang, H.; Zhang, W.; Han, Y. RACK1 promotes lung cancer cell growth via an MCM7/RACK1/ Akt signaling complex. Oncotarget 2017, 8, 40501–40513. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xiong, A.; Zhang, Z.; Li, S.; Huang, H.; Yu, T.T.; Cao, X.; Cheng, S.Y. MicroRNA-31 suppresses medulloblastoma cell growth by inhibiting DNA replication through minichromosome maintenance 2. Oncotarget 2014, 5, 4821–4833. [Google Scholar] [CrossRef] [PubMed]
- Afanasyeva, E.A.; Mestdagh, P.; Kumps, C.; Vandesompele, J.; Ehemann, V.; Theissen, J.; Fischer, M.; Zapatka, M.; Brors, B.; Savelyeva, L.; et al. MicroRNA miR-885-5p targets CDK2 and MCM5, activates p53 and inhibits proliferation and survival. Cell Death Differ. 2011, 18, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Li, Y.; Li, Q. MiR-362-3p functions as a tumor suppressor through targeting MCM5 in cervical adenocarcinoma. Biosci. Rep. 2018, 38, BSR20180668. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Smolka, M.B.; Schimenti, J.C. Chronic DNA Replication Stress Reduces Replicative Lifespan of Cells by TRP53-Dependent, microRNA-Assisted MCM2-7 Downregulation. PLoS Genet. 2016, 12, 1005787. [Google Scholar] [CrossRef]
- Uchida, A.; Seki, N.; Mizuno, K.; Yamada, Y.; Misono, S.; Sanada, H.; Kikkawa, N.; Kumamoto, T.; Suetsugu, T.; Inoue, H. Regulation of KIF2A by Antitumor miR-451a Inhibits Cancer Cell Aggressiveness Features in Lung Squamous Cell Carcinoma. Cancers (Basel) 2019, 11, 258. [Google Scholar] [CrossRef]

| A. Characteristics of Patients with LUAD | ||
|---|---|---|
| Patients with Lung Cancer | n | (%) |
| Total number | 19 | |
| Median age (range) | 73 (57–86) | |
| Sex | ||
| Male | 10 | 52.6 |
| Female | 9 | 47.4 |
| Pathological stage | ||
| IA | 1 | 5.3 |
| IB | 4 | 21.0 |
| IIA | 9 | 47.4 |
| IIB | 1 | 5.3 |
| IIIA | 4 | 21.0 |
| B. Characteristics of controls | ||
| Controls | n | (%) |
| Total number | 24 | |
| Median age (range) | 72.5 (50–88) | |
| Sex | ||
| Male | 21 | 87.5 |
| Female | 3 | 12.5 |
| Entrez Gene | Gene Symbol | Gene Name | Total Sites | GSE19188 FC (log2) | A549 miR-143-5p Transfectant FC (log2) | TCGA OncoLnc 5-Year OS p-Value |
|---|---|---|---|---|---|---|
| 4173 | MCM4 | minichromosome maintenance complex component 4 | 4 | 3.1253 | −1.0075 | 0.0025 |
| 8091 | HMGA2 | high mobility group AT-hook 2 | 3 | 2.8253 | −2.5651 | 0.1389 |
| 5888 | RAD51 | RAD51 recombinase | 3 | 2.0850 | −1.0812 | 0.0478 |
| 374393 | FAM111B | family with sequence similarity 111, member B | 2 | 2.0754 | −1.8781 | 0.0003 |
| 1047 | CLGN | calmegin | 1 | 1.8038 | −1.1616 | 0.0362 |
| 51155 | HN1 | hematological and neurological expressed 1 | 1 | 1.6878 | −3.2334 | 0.4579 |
| 222962 | SLC29A4 | solute carrier family 29 (equilibrative nucleoside transporter), member 4 | 3 | 1.6166 | −1.0253 | 0.9072 |
| 144501 | KRT80 | keratin 80 | 1 | 1.4711 | −1.4127 | 0.0093 |
| 2817 | GPC1 | glypican 1 | 1 | 1.4008 | −1.1491 | 0.0322 |
| 9633 | MTL5 | metallothionein-like 5, testis-specific (tesmin) | 1 | 1.4001 | −1.0459 | 0.0001 |
| 81831 | NETO2 | neuropilin (NRP) and tolloid (TLL)-like 2 | 1 | 1.3829 | −2.5034 | 0.0344 |
| 2175 | FANCA | Fanconi anemia, complementation group A | 5 | 1.3767 | −1.1330 | 0.0218 |
| 771 | CA12 | carbonic anhydrase XII | 2 | 1.2698 | −1.5449 | 0.2834 |
| 84524 | ZC3H8 | zinc finger CCCH-type containing 8 | 3 | 1.2393 | −1.1569 | 0.9583 |
| 6929 | TCF3 | transcription factor 3 | 2 | 1.2092 | −1.3071 | 0.1287 |
| 9650 | MTFR1 | mitochondrial fission regulator 1 | 1 | 1.2079 | −1.4214 | 0.0038 |
| 29993 | PACSIN1 | protein kinase C and casein kinase substrate in neurons 1 | 1 | 1.1587 | −2.3003 | 0.3508 |
| 23097 | CDK19 | cyclin-dependent kinase 19 | 1 | 1.1498 | −1.4269 | 0.8145 |
| 1869 | E2F1 | E2F transcription factor 1 | 1 | 1.1114 | −1.1265 | 0.38 |
| 10160 | FARP1 | FERM, RhoGEF (ARHGEF) and pleckstrin domain protein 1 (chondrocyte-derived) | 1 | 1.0674 | −1.0327 | 0.0699 |
| 23170 | TTLL12 | tubulin tyrosine ligase-like family, member 12 | 4 | 1.0180 | −1.1575 | 0.0127 |
| 84419 | C15orf48 | chromosome 15 open reading frame 48 | 2 | 1.0106 | −1.0710 | 0.134 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanada, H.; Seki, N.; Mizuno, K.; Misono, S.; Uchida, A.; Yamada, Y.; Moriya, S.; Kikkawa, N.; Machida, K.; Kumamoto, T.; et al. Involvement of Dual Strands of miR-143 (miR-143-5p and miR-143-3p) and Their Target Oncogenes in the Molecular Pathogenesis of Lung Adenocarcinoma. Int. J. Mol. Sci. 2019, 20, 4482. https://doi.org/10.3390/ijms20184482
Sanada H, Seki N, Mizuno K, Misono S, Uchida A, Yamada Y, Moriya S, Kikkawa N, Machida K, Kumamoto T, et al. Involvement of Dual Strands of miR-143 (miR-143-5p and miR-143-3p) and Their Target Oncogenes in the Molecular Pathogenesis of Lung Adenocarcinoma. International Journal of Molecular Sciences. 2019; 20(18):4482. https://doi.org/10.3390/ijms20184482
Chicago/Turabian StyleSanada, Hiroki, Naohiko Seki, Keiko Mizuno, Shunsuke Misono, Akifumi Uchida, Yasutaka Yamada, Shogo Moriya, Naoko Kikkawa, Kentaro Machida, Tomohiro Kumamoto, and et al. 2019. "Involvement of Dual Strands of miR-143 (miR-143-5p and miR-143-3p) and Their Target Oncogenes in the Molecular Pathogenesis of Lung Adenocarcinoma" International Journal of Molecular Sciences 20, no. 18: 4482. https://doi.org/10.3390/ijms20184482
APA StyleSanada, H., Seki, N., Mizuno, K., Misono, S., Uchida, A., Yamada, Y., Moriya, S., Kikkawa, N., Machida, K., Kumamoto, T., Suetsugu, T., & Inoue, H. (2019). Involvement of Dual Strands of miR-143 (miR-143-5p and miR-143-3p) and Their Target Oncogenes in the Molecular Pathogenesis of Lung Adenocarcinoma. International Journal of Molecular Sciences, 20(18), 4482. https://doi.org/10.3390/ijms20184482





