Exogenous Promoter Triggers APETALA3 Silencing through RNA-Directed DNA Methylation Pathway in Arabidopsis
Abstract
1. Introduction
2. Results
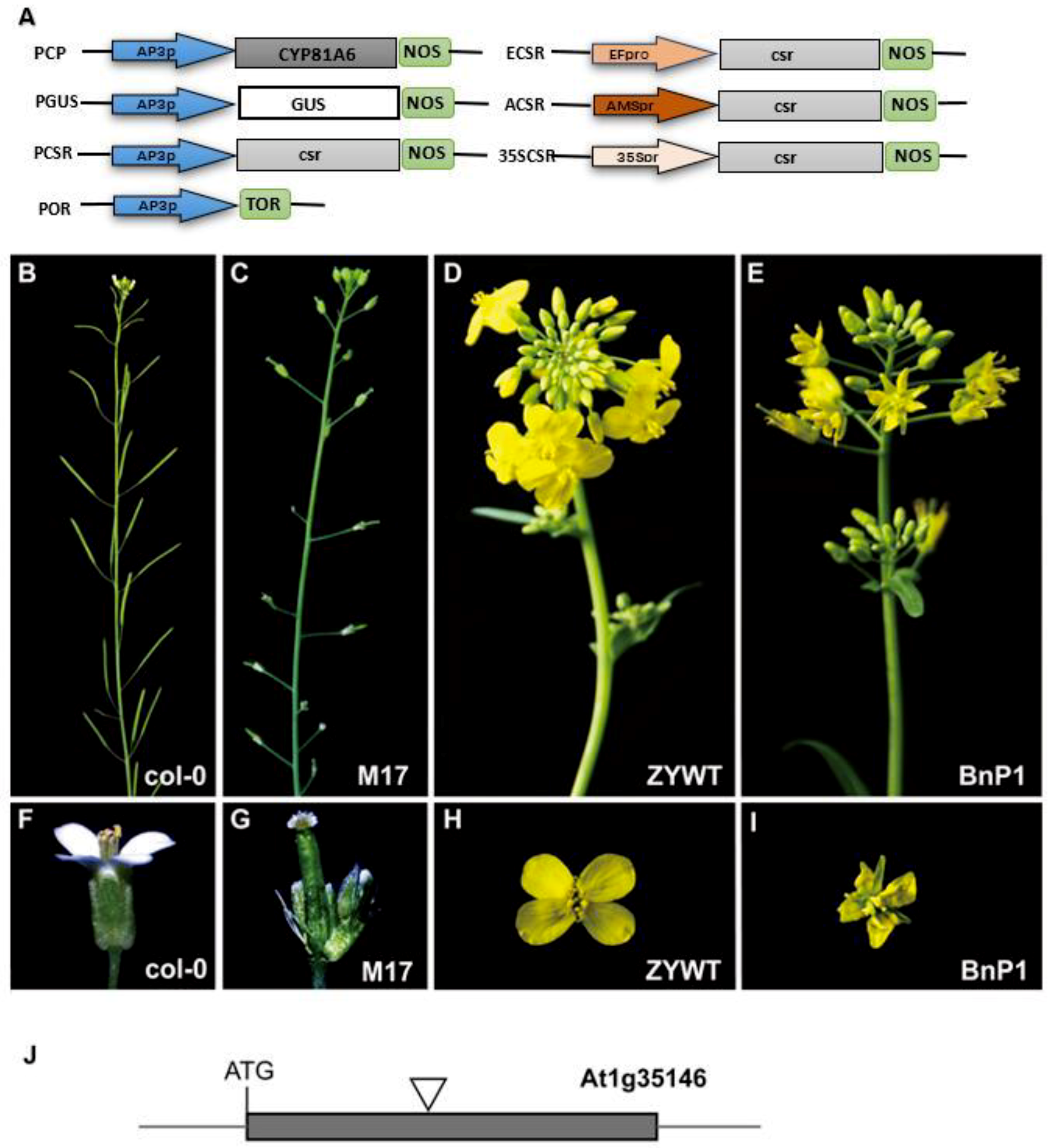
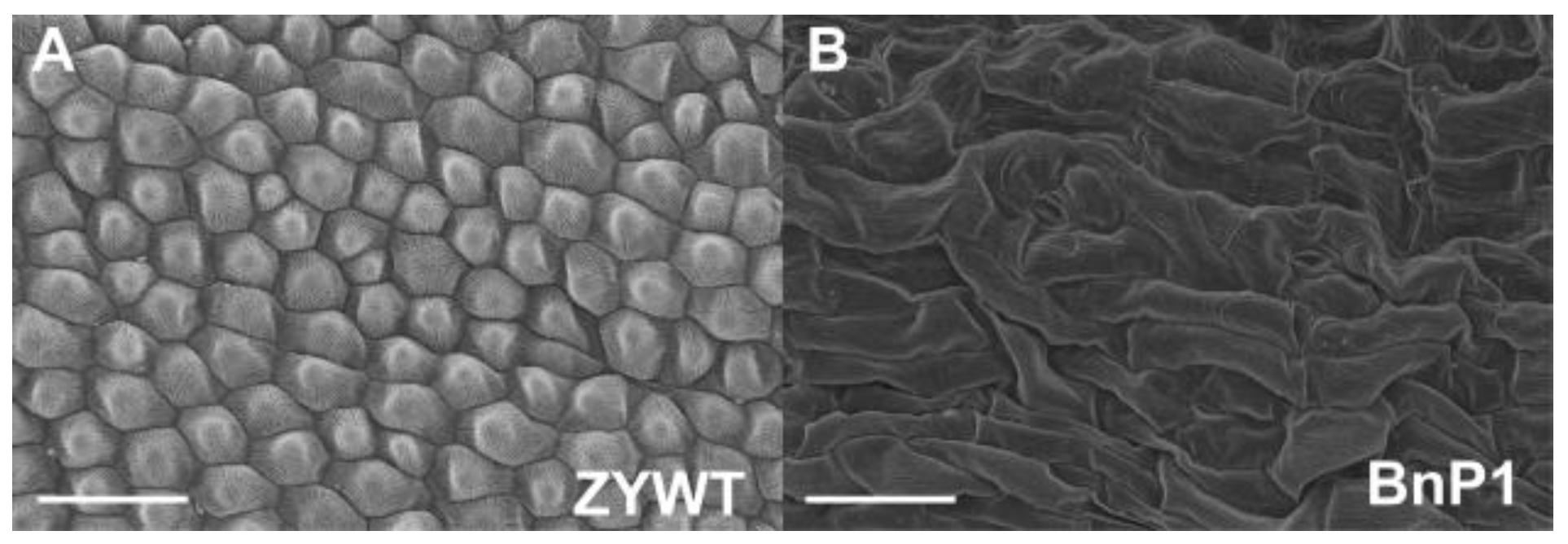
2.1. Defective Phenotypes Observed in the Transgenic Lines
2.2. Exogenous AP3 Promoter was Responsible for the ap3-Like Phenotypes
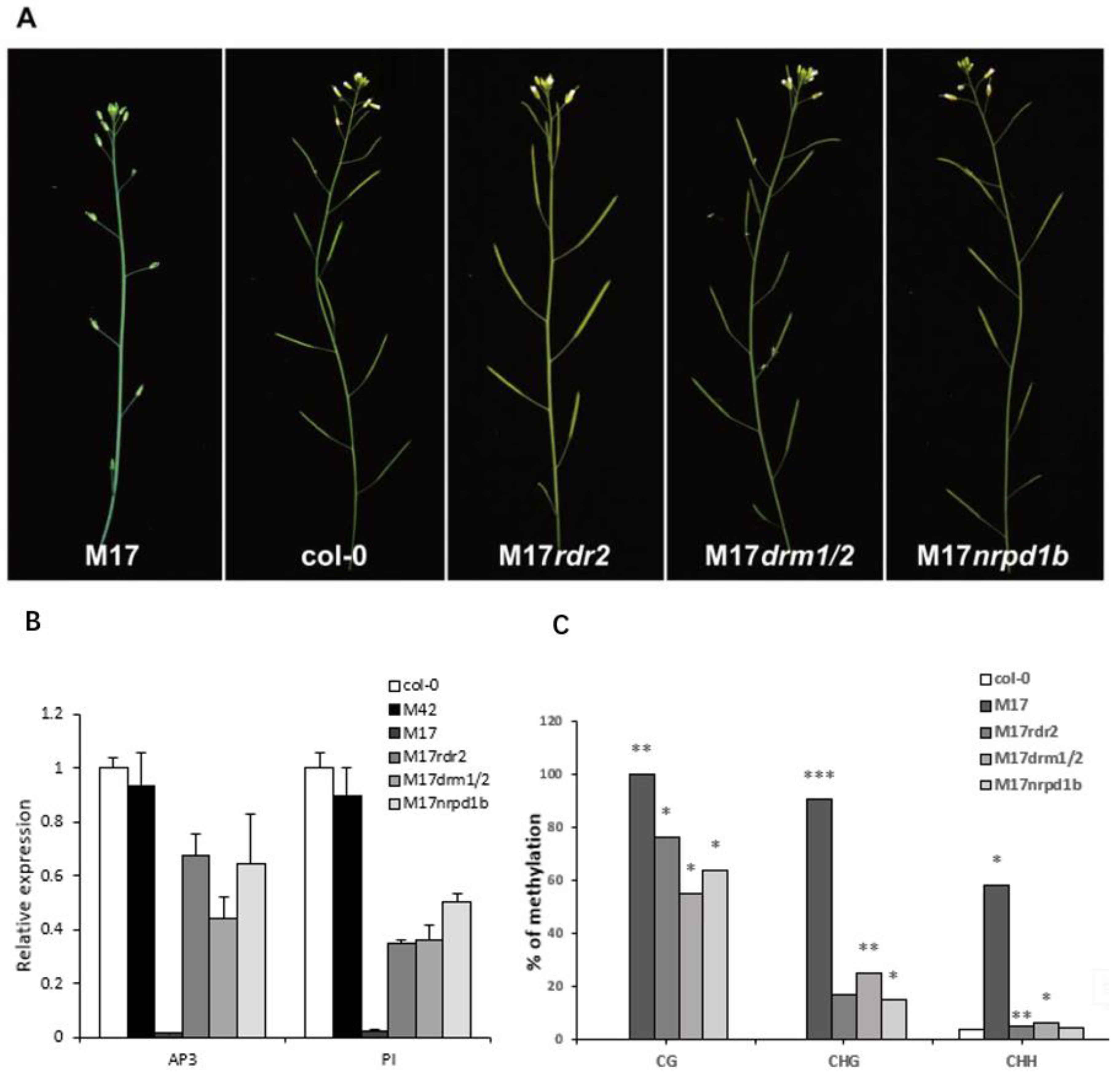
2.3. ap3-Like Phenotypes were Correlated with the DNA Hypermethylation of AP3 Promoter
3. Discussion
3.1. Exogenous AP3 Promoter may Trigger the Hypermethylation of AP3 Promoter Mediated by RdDM in Transgenic Arabidopsis Plants
3.2. The ap3-like Phenotypes was Inherited
3.3. The Characteristic of AP3 Promoter Methylation in Plants with ap3-Like Phenotypes
3.4. An Objective View on Transgenes Silencing
4. Materials and Methods
4.1. Plant Materials
4.2. Vector Construction and Expression of csr or CYP81A6 in Arabidiopsis and Rapeseed
4.3. Scanning Electron Micrograph
4.4. RNA Isolation and Real-Time RT-PCR
4.5. DNA Methylation Analysis
4.6. Transgenic and SALK Lines Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsunaga, W.; Shimura, H.; Shirakawa, S.; Isoda, R.; Inukai, T.; Matsumura, T.; Masuta, C. Transcriptional silencing of 35S driven-transgene is differentially determined depending on promoter methylation heterogeneity at specific cytosines in both plus- and minus-sense strands. BMC Plant Biol. 2019, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Mlotshwa, S.; Pruss, G.J.; Gao, Z.; Mgutshini, N.L.; Li, J.; Chen, X.; Bowman, L.H.; Vance, V. Transcriptional silencing induced by Arabidopsis T-DNA mutants is associated with 35S promoter siRNAs and requires genes involved in siRNA-mediated chromatin silencing. Plant J. 2010, 64, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Daxinger, L.; Hunter, B.; Sheikh, M.; Jauvion, V.; Gasciolli, V.; Vaucheret, H.; Matzke, M.; Furner, I. Unexpected silencing effects from T-DNA tags in Arabidopsis. Trends Plant Sci. 2008, 13, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell 1990, 2, 279. [Google Scholar] [CrossRef] [PubMed]
- Vaucheret, H.; Béclin, C.; Fagard, M. Post-transcriptional gene silencing in plants. J. Cell Sci. 2001, 114, 3083–3091. [Google Scholar] [PubMed]
- Wassenegger, M. The role of the RNAi machinery in heterochromatin formation. Cell 2005, 122, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Matzke, M.; Matzke, A.J.; Kooter, J.M. RNA: Guiding gene silencing. Science 2001, 293, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Liu, J.; Kiba, T.; Woo, J.; Ojo, T.; Hafner, M.; Tuschl, T.; Chua, N.H.; Wang, X.J. Deep sequencing of small RNAs specifically associated with Arabidopsis AGO1 and AGO4 uncovers new AGO functions. Plant J. 2011, 67, 292–304. [Google Scholar] [CrossRef]
- Wakasa, Y.; Kawakatsu, T.; Harada, T.; Takaiwa, F. Transgene-independent heredity of RdDM-mediated transcriptional gene silencing of endogenous genes in rice. Plant Biotechnol. J. 2018, 16, 2007–2015. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.-K. RNA-directed DNA methylation. Curr. Opin. Plant Biol. 2011, 14, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, M.M. RNA-directed DNA methylation: Mechanisms and functions. Plant Signal. Behav. 2010, 5, 806. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.T.; O’Leary, M.; Won, S.Y.; Li, S.; Arroyo, L.; Liu, X.; Defries, A.; Zheng, B.; Cutler, S.R.; Chen, X. Generation of a luciferase-based reporter for CHH and CG DNA methylation in Arabidopsis thaliana. Silence 2013, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Aufsatz, W.; Zilberman, D.; Mette, M.F.; Huang, M.S.; Matzke, M.; Jacobsen, S.E. Role of the DRM and CMT3 Methyltransferases in RNA-Directed DNA Methylation. Curr. Biol. 2003, 13, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jacobsen, S.E. Role of the Arabidopsis DRM Methyltransferases in De Novo DNA Methylation and Gene Silencing. Curr. Biol. 2002, 12, 1138–1144. [Google Scholar] [CrossRef]
- Moritoh, S.; Eun, C.H.; Ono, A.; Asao, H.; Okano, Y.; Yamaguchi, K.; Shimatani, Z.; Koizumi, A.; Terada, R. Targeted disruption of an orthologue of DOMAINS REARRANGED METHYLASE 2, OsDRM2, impairs the growth of rice plants by abnormal DNA methylation. Plant J. 2012, 71, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Lahmy, S.; Bies-Etheve, N.; Lagrange, T. Plant-specific multisubunit RNA polymerase in gene silencing. Epigenetics 2010, 5, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, Z.; Li, S.; Yu, B.; Liu, J.-Y.; Chen, X. Intergenic transcription by RNA polymerase II coordinates Pol IV and Pol V in siRNA-directed transcriptional gene silencing in Arabidopsis. Genes Dev. 2009, 23, 2850–2860. [Google Scholar] [CrossRef]
- Henderson, I.R.; Jacobsen, S.E. Epigenetic inheritance in plants. Nature 2007, 447, 418–424. [Google Scholar] [CrossRef]
- Haag, J.R.; Pikaard, C.S. Multisubunit RNA polymerases IV and V: Purveyors of non-coding RNA for plant gene silencing. Nat. Rev. Mol. Cell Biol. 2011, 12, 483–492. [Google Scholar] [CrossRef]
- Huang, C.-F.; Zhu, J.-K. RNA Splicing Factors and RNA-Directed DNA Methylation. Biology 2014, 3, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Law, J.A.; Ausin, I.; Johnson, L.M.; Vashisht, A.A.; Zhu, J.-K.; Wohlschlegel, J.A.; Jacobsen, S.E. A Protein Complex Required for Polymerase V Transcripts and RNA-Directed DNA Methylation in Arabidopsis. Curr. Biol. 2010, 20, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Gurazada, S.; Zhai, J.; Li, S.; Simon, S.A.; Matzke, M.A.; Chen, X.; Meyers, B.C. RNA polymerase V-dependent small RNAs in Arabidopsis originate from small, intergenic loci including most SINE repeats. Epigenetics. 2012, 7, 781–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, Q.; Rowley, M.J.; Böhmdorfer, G.; Sandhu, D.; Gregory, B.D.; Wierzbicki, A.T. RNA polymerase V targets transcriptional silencing components to promoters of protein-coding genes. Plant J. 2013, 73, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.-K. Seeing the forest for the trees: A wide perspective on RNA-directed DNA methylation. Genes Dev. 2012, 26, 1769–1773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krizek, B.A.; Fletcher, J.C. Molecular mechanisms of flower development: An armchair guide. Nat. Rev. Genet. 2005, 6, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Theissen, G.; Melzer, R. Molecular mechanisms underlying origin and diversification of the angiosperm flower. Ann. Bot. 2007, 100, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.E.; O’Maoileidigh, D.S.; Rae, L.; Kwasniewska, K.; Raganelli, A.; Hanczaryk, K.; Lohan, A.J.; Loftus, B.; Graciet, E.; Wellmer, F. Molecular basis for the specification of floral organs by APETALA3 and PISTILLATA. Proc. Natl. Acad. Sci. USA 2012, 109, 13452–13457. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.A.; Day, C.D.; Zondlo, S.C.; Thackeray, A.G.; Irish, V.F. Discrete spatial and temporal cis-acting elements regulate transcription of the Arabidopsis floral homeotic gene APETALA3. Development 1998, 125, 1711–1721. [Google Scholar] [PubMed]
- Pan, G.; Zhang, X.; Liu, K.; Zhang, J.; Wu, X.; Zhu, J.; Tu, J. Map-based cloning of a novel rice cytochrome P450 gene CYP81A6 that confers resistance to two different classes of herbicides. Plant Mol. Biol. 2006, 61, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Haughn, G.W.; Smith, J.; Mazur, B.; Somerville, C. Transformation with a mutant Arabidopsis acetolactate synthase gene renders tobacco resistant to sulfonylurea herbicides. Mol. Gen. Genet. 1988, 211, 266–271. [Google Scholar] [CrossRef]
- Li, H.; Freeling, M.; Lisch, D. Epigenetic reprogramming during vegetative phase change in maize. Proc. Natl. Acad. Sci. USA 2010, 107, 22184. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, M.; Damaris, R.N.; Nyong’a, T.M.; Cao, D.; Ou, K.; Yang, P. Genome-Wide DNA Methylation Profiling in the Lotus (Nelumbo nucifera) Flower Showing its Contribution to the Stamen Petaloid. Plants (Basel) 2019, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qin, Q.; Sun, F.; Wang, Y.; Xu, D.; Li, Z.; Fu, B. Genome-Wide Differences in DNA Methylation Changes in Two Contrasting Rice Genotypes in Response to Drought Conditions. Front. Plant Sci. 2016, 7, 1675. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Meyerowitz, E.M. Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 1994, 8, 1548–1560. [Google Scholar] [CrossRef]
- Peragine, A.; Yoshikawa, M.; Wu, G.; Albrecht, H.L.; Poethig, R.S. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev. 2004, 18, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.A.; Meyers, B.C. Small RNA-mediated epigenetic modifications in plants. Curr. Opin. Plant Biol. 2011, 14, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, O.; Bender, J. RNA-directed DNA methylation. J. Cell Sci. 2004, 117, 4881–4888. [Google Scholar] [CrossRef]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Baulcombe, D.C. Unwinding RNA silencing. Science 2000, 290, 1108–1109. [Google Scholar] [CrossRef]
- Van der Krol, A.R.; Mur, L.A.; Beld, M.; Mol, J.; Stuitje, A.R. Flavonoid genes in petunia: Addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell Online 1990, 2, 291–299. [Google Scholar]
- Smith, C.; Watson, C.; Bird, C.; Ray, J.; Schuch, W.; Grierson, D. Expression of a truncated tomato polygalacturonase gene inhibits expression of the endogenous gene in transgenic plants. Mol. Gen. Genet. 1990, 224, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Elmayan, T.; Vaucheret, H. Expression of single copies of a strongly expressed 35S transgene can be silenced post-transcriptionally. Plant J. 1996, 9, 787–797. [Google Scholar] [CrossRef]
- Wu, L.; Mao, L.; Qi, Y. Roles of DICER-LIKE and ARGONAUTE Proteins in TAS-Derived Small Interfering RNA-Triggered DNA Methylation. Plant Physiol. 2012, 160, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, A.; Kallenbach, M.; Baldwin, I.T. Progressive 35S promoter methylation increases rapidly during vegetative development in transgenic Nicotiana attenuata plants. BMC Plant Biol. 2013, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brunner, A.M.; Shevchenko, O.; Meilan, R.; Ma, C.; Skinner, J.S.; Strauss, S.H. Efficient and stable transgene suppression via RNAi in field-grown poplars. Transgenic Res. 2008, 17, 679–694. [Google Scholar] [CrossRef]
- Scheid, O.M.; Paszkowski, J.; Potrykus, I. Reversible inactivation of a transgene in Arabidopsis thaliana. Mol. Gen. Genet. 1991, 228, 104–112. [Google Scholar] [CrossRef]
- Otagaki, S.; Kawai, M.; Masuta, C.; Kanazawa, A. Size and positional effects of promoter RNA segments on virus-induced RNA-directed DNA methylation and transcriptional gene silencing. Epigenetics 2011, 6, 681–691. [Google Scholar] [CrossRef]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.-L.; Chen, H.; Henderson, I.R.; Shinn, P.; Pellegrini, M.; Jacobsen, S.E. Genome-wide High-Resolution Mapping and Functional Analysis of DNA Methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef]
- Kooter, J.M.; Matzke, M.A.; Meyer, P. Listening to the silent genes: Transgene silencing, gene regulation and pathogen control. Trends Plant Sci. 1999, 4, 340–347. [Google Scholar] [CrossRef]
- Meyer, P.; Saedler, H. Homology-dependent gene silencing in plants. Annu. Rev. Plant Biol. 1996, 47, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.; Zhou, Z.; Xia, S.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Tu, J.; Fu, T. BnaC. Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. Plant J. 2011, 68, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Zeng, F.; Lei, S.; Chen, Y.; Yao, X.; Zhu, Y.; Wen, J.; Shen, J.; Ma, C.; Tu, J. Two duplicate CYP704B1-homologous genes BnMs1 and BnMs2 are required for pollen exine formation and tapetal development in Brassica napus. Plant J. 2010, 63, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Frommer, M.; McDonald, L.E.; Millar, D.S.; Collis, C.M.; Watt, F.; Grigg, G.W.; Molloy, P.L.; CL, P. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA 1992, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Lye, K.-W.; Barton, M.K. MicroRNA binding sites in Arabidopsis class III HD-ZIP mRNAs are required for methylation of the template chromosome. Dev. Cell 2004, 7, 653–662. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Liu, J.; Chu, L.; Jing, X.; Wang, H.; Guo, J.; Yi, B. Exogenous Promoter Triggers APETALA3 Silencing through RNA-Directed DNA Methylation Pathway in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 4478. https://doi.org/10.3390/ijms20184478
Wang B, Liu J, Chu L, Jing X, Wang H, Guo J, Yi B. Exogenous Promoter Triggers APETALA3 Silencing through RNA-Directed DNA Methylation Pathway in Arabidopsis. International Journal of Molecular Sciences. 2019; 20(18):4478. https://doi.org/10.3390/ijms20184478
Chicago/Turabian StyleWang, Benqi, Jie Liu, Lei Chu, Xue Jing, Huadong Wang, Jian Guo, and Bin Yi. 2019. "Exogenous Promoter Triggers APETALA3 Silencing through RNA-Directed DNA Methylation Pathway in Arabidopsis" International Journal of Molecular Sciences 20, no. 18: 4478. https://doi.org/10.3390/ijms20184478
APA StyleWang, B., Liu, J., Chu, L., Jing, X., Wang, H., Guo, J., & Yi, B. (2019). Exogenous Promoter Triggers APETALA3 Silencing through RNA-Directed DNA Methylation Pathway in Arabidopsis. International Journal of Molecular Sciences, 20(18), 4478. https://doi.org/10.3390/ijms20184478




