Nonalcoholic Fatty Liver Disease (NAFLD), But not Its Susceptibility Gene Variants, Influences the Decrease of Kidney Function in Overweight/Obese Children
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Population
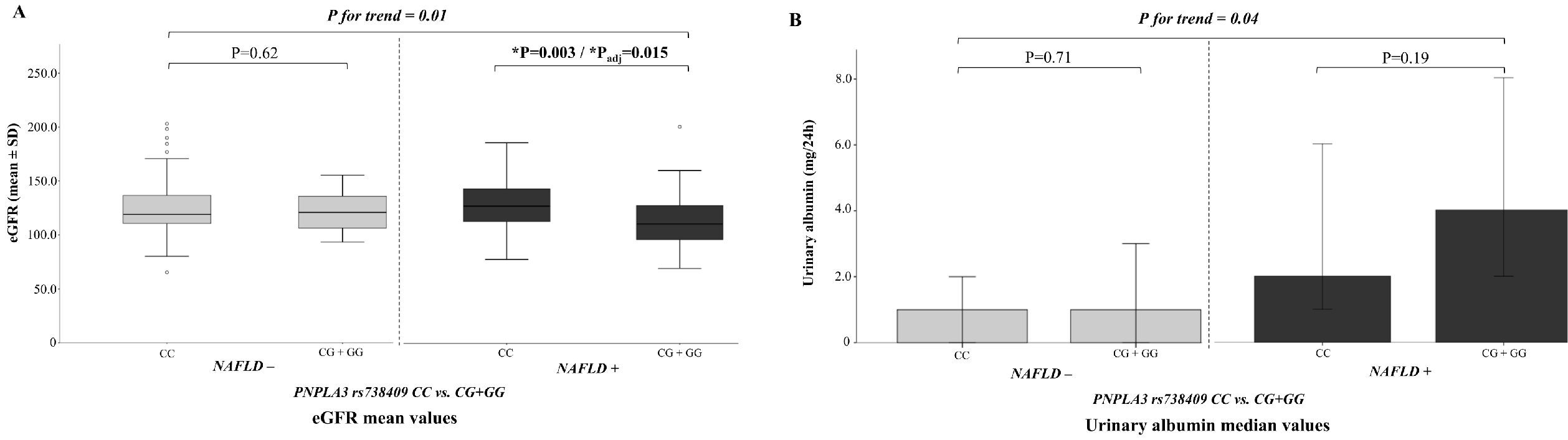
2.2. Kidney Function According to Nonalcoholic Fatty Liver disease (NAFLD) Status
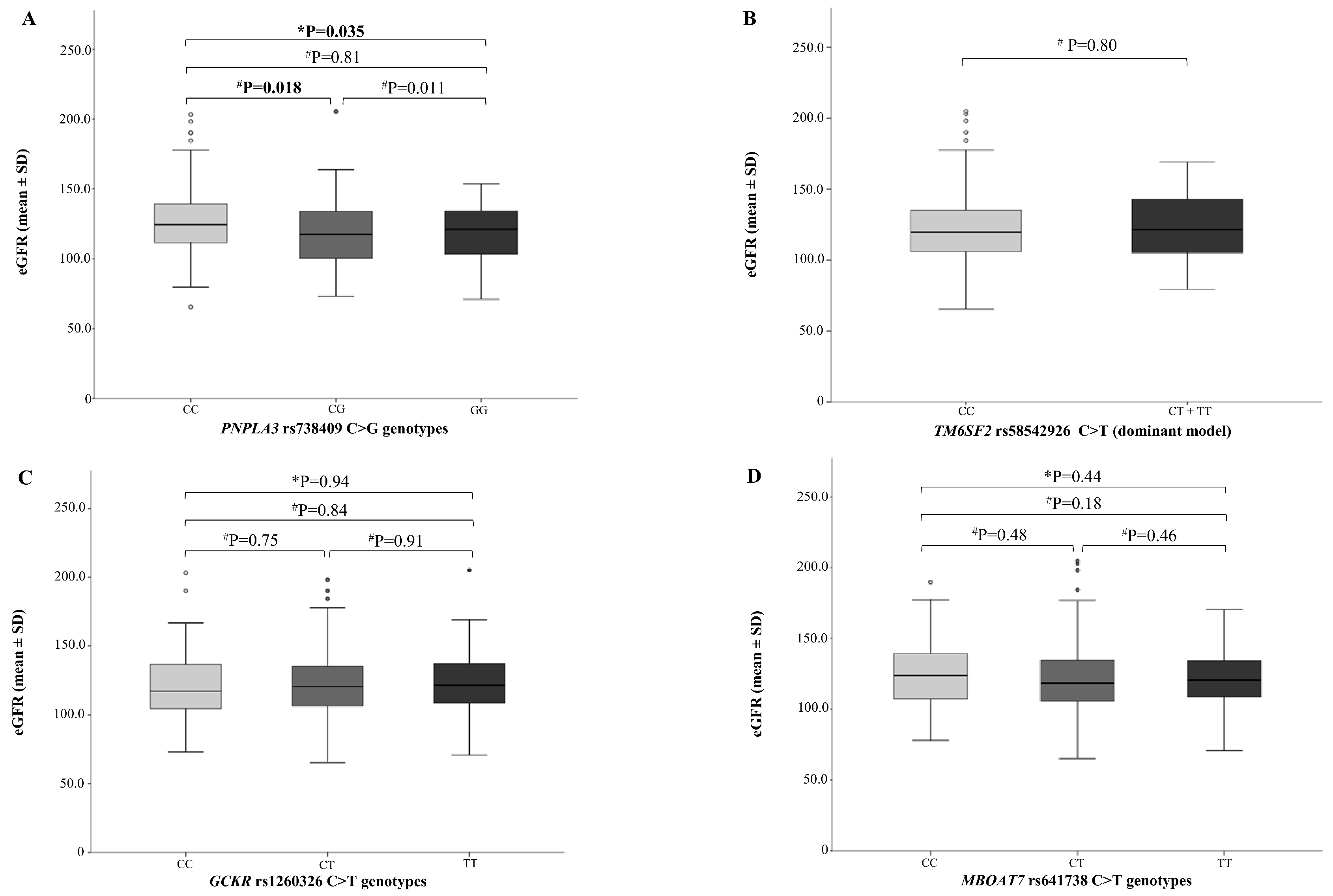
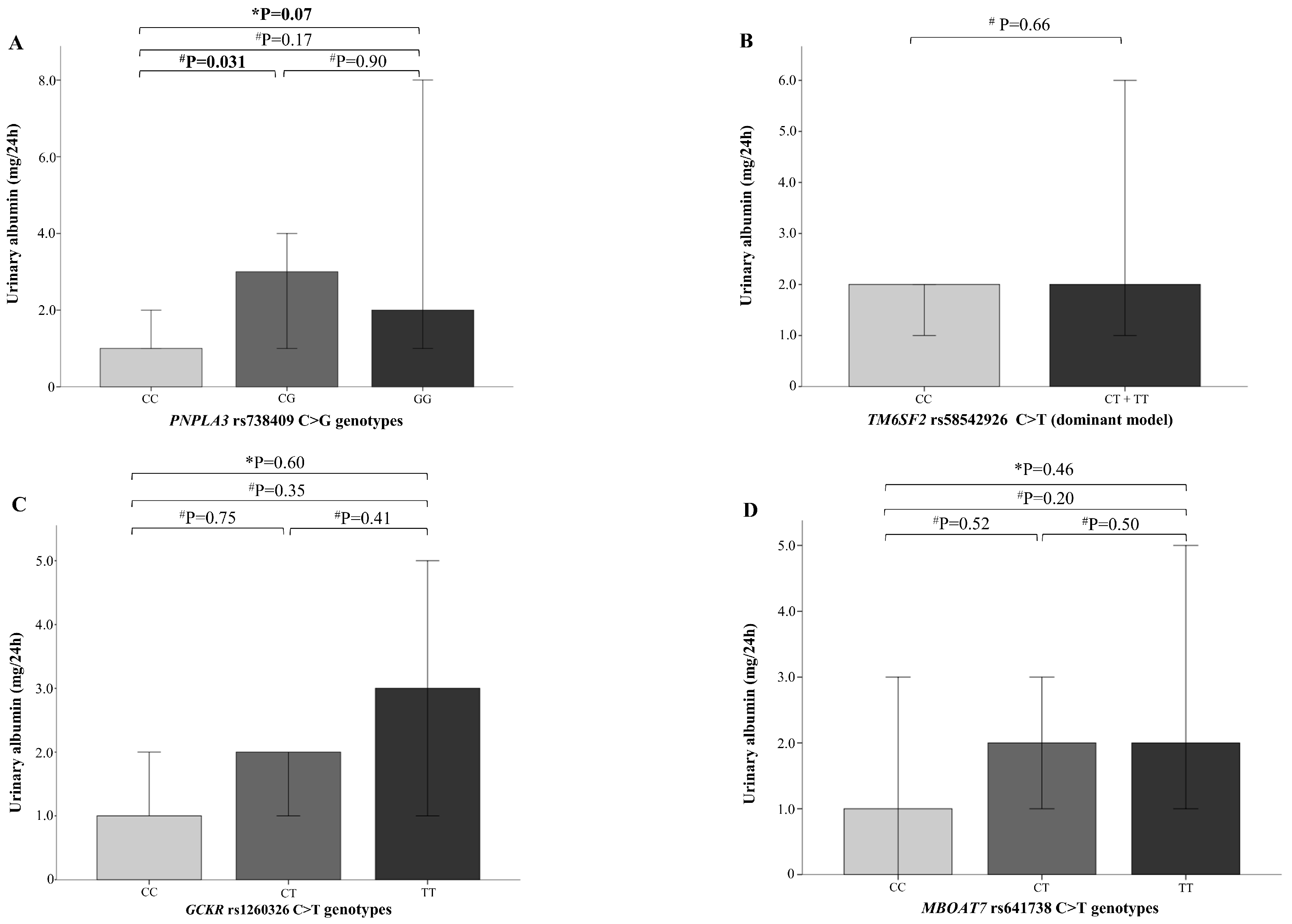
2.3. NAFLD Genetic Variants and Kidney Function
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Laboratory Measurements
4.3. Determination of Estimated Glomerular Filtration Rate (eGFR)
4.4. Abdominal Magnetic Resonance Imaging (MRI).
4.5. Genetic analysis.
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.; Marengo, A.; Mezzabotta, L.; Bugianesi, E. Sytemic complications of nonalcoholic fatty liver disease: When the liver is not an innocent bystander. Semin. Liver Dis. 2015, 35, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Chonchol, M.B.; Byrne, C.D. CKD and nonalcoholic fatty liver disease. Am. J. Kidney Dis. 2014, 64, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Li, A.A.; Ahmed, A.; Kim, D. Extrahepatic manifestations of nonalcoholic fatty liver disease. Gut Liver 2019, (in press). [CrossRef] [PubMed]
- Dongiovanni, P.; Stender, S.; Pietrelli, A.; Mancina, R.M.; Cespiati, A.; Petta, S.; Pelusi, S.; Pingitore, P.; Badiali, S.; Maggioni, M.; et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J. Intern. Med. 2018, 283, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Valenti, L.V.C.; Baselli, G.A. Genetics of nonalcoholic fatty liver disease: A 2018 update. Curr. Pharm. Des. 2018, 24, 4566–4573. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Speliotes, E.K.; Yerges-Armstrong, L.M.; Wu, J.; Hernaez, R.; Kim, L.J.; Palmer, C.D.; Gudnason, V.; Eiriksdottir, G.; Garcia, M.E.; Launer, L.J.; et al. GOLD consortium. genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011, 7, e1001324. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 variant rs641738 increases risk of non-alcoholic fatty liver disease in individuals of European descent. Gastroenterology 2016, 150, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Oniki, K.; Saruwatari, J.; Izuka, T.; Kajiwara, A.; Morita, K.; Sakata, M.; Otake, K.; Ogata, Y.; Nakagawa, K. Influence of the PNPLA3 rs738409 polymorphism on non-alcoholic fatty liver disease and renal function among normal weight subjects. PLoS ONE 2015, 10, e0132640. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Gambino, R. PNPLA3 rs738409 and TM6SF2 rs58542926 gene variants affect renal disease and function in nonalcoholic fatty liver disease. Hepatology 2015, 62, 658–659. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Alisi, A.; Mosca, A.; Panera, N.; Byrne, C.D.; Nobili, V. Relationship between PNPLA3 rs738409 polymorphism and decreased kidney function in children with NAFLD. Hepatology 2019, 70, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Marzuillo, P.; Di Sessa, A.; Guarino, S.; Capalbo, D.; Umano, G.R.; Pedullà, M.; La Manna, A.; Cirillo, G.; Miraglia Del Giudice, E. Nonalcoholic fatty liver disease and eGFR levels could be linked by the PNPLA3 I148M polymorphism in children with obesity. Pediatr. Obes. 2019, e12539. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Belardinilli, F.; Bailetti, D.; Sponziello, M.; D’Erasmo, L.; Polimeni, L.; Baratta, F.; Pastori, D.; Ceci, F.; Montali, A.; et al. Evaluation of polygenic determinants of non-alcoholic fatty liver disease (NAFLD) by a candidate genes resequencing strategy. Sci. Rep. 2018, 8, 3702. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Pacifico, L.; Chiesa, C.; Perla, F.M.; Ceci, F.; Angeloni, A.; D’Erasmo, L.; Di Martino, M.; Arca, M. Genetic and metabolic predictors of hepatic fat content in a cohort of Italian children with obesity. Pediatr. Res. 2019, 85, 671–677. [Google Scholar] [CrossRef]
- Vespasiani-Gentilucci, U.; Gallo, P.; Dell’Unto, C.; Volpentesta, M.; Antonelli-Incalzi, R.; Picardi, A. Promoting genetics in non-alcoholic fatty liver disease: Combined risk score through polymorphisms and clinical variables. World. J. Gastroenterol. 2018, 24, 4835–4845. [Google Scholar] [CrossRef]
- Pacifico, L.; Bonci, E.; Andreoli, G.M.; Di Martino, M.; Gallozzi, A.; De Luca, E.; Chiesa, C. The Impact of nonalcoholic fatty liver disease on renal function in children with overweight/obesity. Int. J. Mol. Sci. 2016, 17, 1218. [Google Scholar] [CrossRef] [PubMed]
- Koulouridis, E.; Georgalidis, K.; Kostimpa, I.; Koulouridis, I.; Krokida, A.; Houliara, D. Metabolic syndrome risk factors and estimated glomerular filtration rate among children and adolescents. Pediatr. Nephrol. 2010, 25, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Franchini, S.; Savino, A.; Marcovecchio, M.L.; Tumini, S.; Chiarelli, F.; Mohn, A. The effect of obesity and type 1 diabetes on renal function in children and adolescents. Pediatr. Diabetes 2015, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Cohney, S.; De Michieli, F.; Pinach, S.; Saba, F.; Gambino, R. Fatty liver and chronic kidney disease: novel mechanistic insight and therapeutic opportunities. Diabetes Care 2016, 39, 1830–1845. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, J.F.; Chan, M.K.; El-Nahas, M.; Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 1982, 2, 1309–1311. [Google Scholar] [CrossRef]
- De Vries, A.P.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; D’Agati, V.D.; Lamb, H.J.; Pongrac Barlovic, D.; Hojs, R.; et al. ERA-EDTA working group diabesity. fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Romeo, S.; Valenti, L. Genetic factors in the pathogenesis of nonalcoholic fatty liver and steatohepatitis. BioMed Res. Int. 2015, 2015, 460190. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Chronic kidney disease epidemiology collaboration using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Yeckel, C.W.; Weiss, R.; Dziura, J.; Taksali, S.E.; Dufour, S.; Burgert, T.S.; Tamborlane, W.V.; Caprio, S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J. Clin. Endocrinol. Metab. 2004, 89, 1096–1101. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Martino, M.D.; Catalano, C.; Panebianco, V.; Bezzi, M.; Anania, C.; Chiesa, C. T1-weighted dual echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J. Gastroenterol. 2011, 17, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Di Martino, M.; Anania, C.; Andreoli, G.M.; Bezzi, M.; Catalano, C.; Chiesa, C. Pancreatic fat and β-cell function in overweight/obese children with nonalcoholic fatty liver disease. World J. Gastroenterol. 2015, 21, 4688–4695. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Brancorsini, M.; Di Costanzo, A.; D’Erasmo, L.; Baratta, F.; Loffredo, L.; Pastori, D.; Pignatelli, P.; Violi, F.; et al. Non-alcoholic fatty liver disease, metabolic syndrome and patatin-like phospholipase domain-containing protein 3 gene variants. Eur. J. Intern. Med. 2014, 25, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; D’Erasmo, L.; Polimeni, L.; Baratta, F.; Coletta, P.; Di Martino, M.; Loffredo, L.; Perri, L.; Ceci, F.; Montali, A.; et al. Non-alcoholic fatty liver disease and subclinical atherosclerosis: A comparison of metabolically-versus genetically-driven excess fat hepatic storage. Atherosclerosis 2017, 257, 232–239. [Google Scholar] [CrossRef] [PubMed]
| NAFLD (n = 105) | Without NAFLD (n = 125) | p Value | |
|---|---|---|---|
| Age, years | 11.0 ± 2.8 | 9.6 ± 2.7 | <0.001 |
| Male sex, % | 64.8 | 50.4 | 0.028 |
| BMI-SD score | 2.0 ± 0.46 | 2.0 ± 0.49 | 0.86 |
| Waist circumference, cm | 90.6 ± 11.3 | 83.9 ± 10.9 | <0.001 |
| Systolic BP, mmHg | 116 (110–120) | 110 (105–118) | 0.007 |
| Diastolic BP, mmHg | 66.5 (60.2–72.5) | 65.0 (60–70) | 0.05 |
| Total cholesterol, mg/dL | 156.7 ± 32.7 | 163.6 ± 31.05 | 0.10 |
| HDL-C, mg/dL | 47.9 ± 11.2 | 52.1 ± 13.5 | 0.013 |
| Triglycerides, mg/dL | 77 (55–119) | 70 (50–96) | 0.10 |
| AST, U/L | 24 (20–30) | 23 (20–26) | 0.10 |
| ALT, U/L | 27 (18–36) | 17 (15–22) | <0.001 |
| Glucose, mg/dL | 83 (78.1–86.6) | 83 (77.0–86.0) | 0.95 |
| Insulin, µU/mL | 14.05 (9.7–20.7) | 10.6 (7.5–14.4) | <0.001 |
| HOMA-IR | 2.7 (1.9–4.3) | 2.1 (1.5–2.9) | 0.002 |
| HbA1c, % | 5.1 (4.8–5.3) | 5.2 (4.8–5.3) | 0.69 |
| Hepatic fat fraction, % | 10.0 (7.0–19.5) | 1.0 (0–2.0) | <0.001 |
| MetS, % | 26.7 | 9.6 | 0.001 |
| eGFR, mL/min/1.73 m2 | 120.4 ± 25.4 | 123.9 ± 23.2 | 0.27 |
| Urinary albumin, mg/24 h * | 3 (1–11) | 1 (0–3) | <0.001 |
| eGFR < 90 mL/min/1.73 m2, n (%) | 14 (13.3) | 2 (1.6) | <0.001 |
| Microalbuminuria, n (%) * | 9 (8.6) | 3 (3.4) | 0.025 |
| eGFR < 90 mL/min/1.73 m2 and/or microalbuminuria, n (%) | 22 (21) | 5 (4.0) | <0.001 |
| PNPLA3 rs738409 | <0.001 | ||
| C/C | 32 (30.5) | 76 (60.8) | |
| C/G | 55 (52.4) | 45 (36.0) | |
| G/G | 18 (17.1) | 4 (3.2) | |
| TM6SF2 rs58542926 | 0.039 | ||
| C/C | 84 (80.0) | 114 (91.2) | |
| C/T | 20 (19.0) | 11 (8.8) | |
| T/T | 1 (1.0) | - | |
| GCKR rs1260326 | 0.28 | ||
| C/C | 19 (18.1) | 28 (22.4) | |
| C/T | 53 (50.5) | 69 (55.2) | |
| T/T | 33 (31.4) | 28 (22.4) | |
| MBOAT7 rs641738 | 0.12 | ||
| C/C | 36 (34.3) | 31 (24.8) | |
| C/T | 40 (38.1) | 64 (51.2) | |
| T/T | 29 (27.6) | 30 (24.0) |
| Odds Ratio (95% CI) | Adjusted p Value | |
|---|---|---|
| Age, years | 1.1 (0.8–1.5) | 0.65 |
| Male gender | 1.2 (0.4–3.6) | 0.70 |
| Pubertal status | 1.6 (0.9–3.0) | 0.09 |
| Waist Circumference | 1.0 (0.9–1.05) | 0.92 |
| Diastolic BP | 1.0 (0.9–1.08) | 0.74 |
| NAFLD | 4.7 (1.5–14.8) | 0.007 |
| PNPLA3 (dominant model) | 0.9 (0.4–3.2) | 0.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Costanzo, A.; Pacifico, L.; D’Erasmo, L.; Polito, L.; Di Martino, M.; Perla, F.M.; Iezzi, L.; Chiesa, C.; Arca, M. Nonalcoholic Fatty Liver Disease (NAFLD), But not Its Susceptibility Gene Variants, Influences the Decrease of Kidney Function in Overweight/Obese Children. Int. J. Mol. Sci. 2019, 20, 4444. https://doi.org/10.3390/ijms20184444
Di Costanzo A, Pacifico L, D’Erasmo L, Polito L, Di Martino M, Perla FM, Iezzi L, Chiesa C, Arca M. Nonalcoholic Fatty Liver Disease (NAFLD), But not Its Susceptibility Gene Variants, Influences the Decrease of Kidney Function in Overweight/Obese Children. International Journal of Molecular Sciences. 2019; 20(18):4444. https://doi.org/10.3390/ijms20184444
Chicago/Turabian StyleDi Costanzo, Alessia, Lucia Pacifico, Laura D’Erasmo, Luca Polito, Michele Di Martino, Francesco Massimo Perla, Ludovica Iezzi, Claudio Chiesa, and Marcello Arca. 2019. "Nonalcoholic Fatty Liver Disease (NAFLD), But not Its Susceptibility Gene Variants, Influences the Decrease of Kidney Function in Overweight/Obese Children" International Journal of Molecular Sciences 20, no. 18: 4444. https://doi.org/10.3390/ijms20184444
APA StyleDi Costanzo, A., Pacifico, L., D’Erasmo, L., Polito, L., Di Martino, M., Perla, F. M., Iezzi, L., Chiesa, C., & Arca, M. (2019). Nonalcoholic Fatty Liver Disease (NAFLD), But not Its Susceptibility Gene Variants, Influences the Decrease of Kidney Function in Overweight/Obese Children. International Journal of Molecular Sciences, 20(18), 4444. https://doi.org/10.3390/ijms20184444






