Extracellular Vesicles Containing MicroRNA-92a-3p Facilitate Partial Endothelial-Mesenchymal Transition and Angiogenesis in Endothelial Cells
Abstract
1. Introduction
2. Results
2.1. Colon Cancer Cells Actively Secrete MiR-92a-3p via EVs
2.2. EVs and MiR-92a-3p Promote Proliferation, Migration, and Tube Formation in HUVECs
2.3. MiR-92a-3p Upregulates Cell-Cycle- and Mitosis-Related Genes and Downregulates Adhesion-Related Genes in HUVECs
2.4. CLDN11 is a Novel Target Gene of MiR-92a-3p
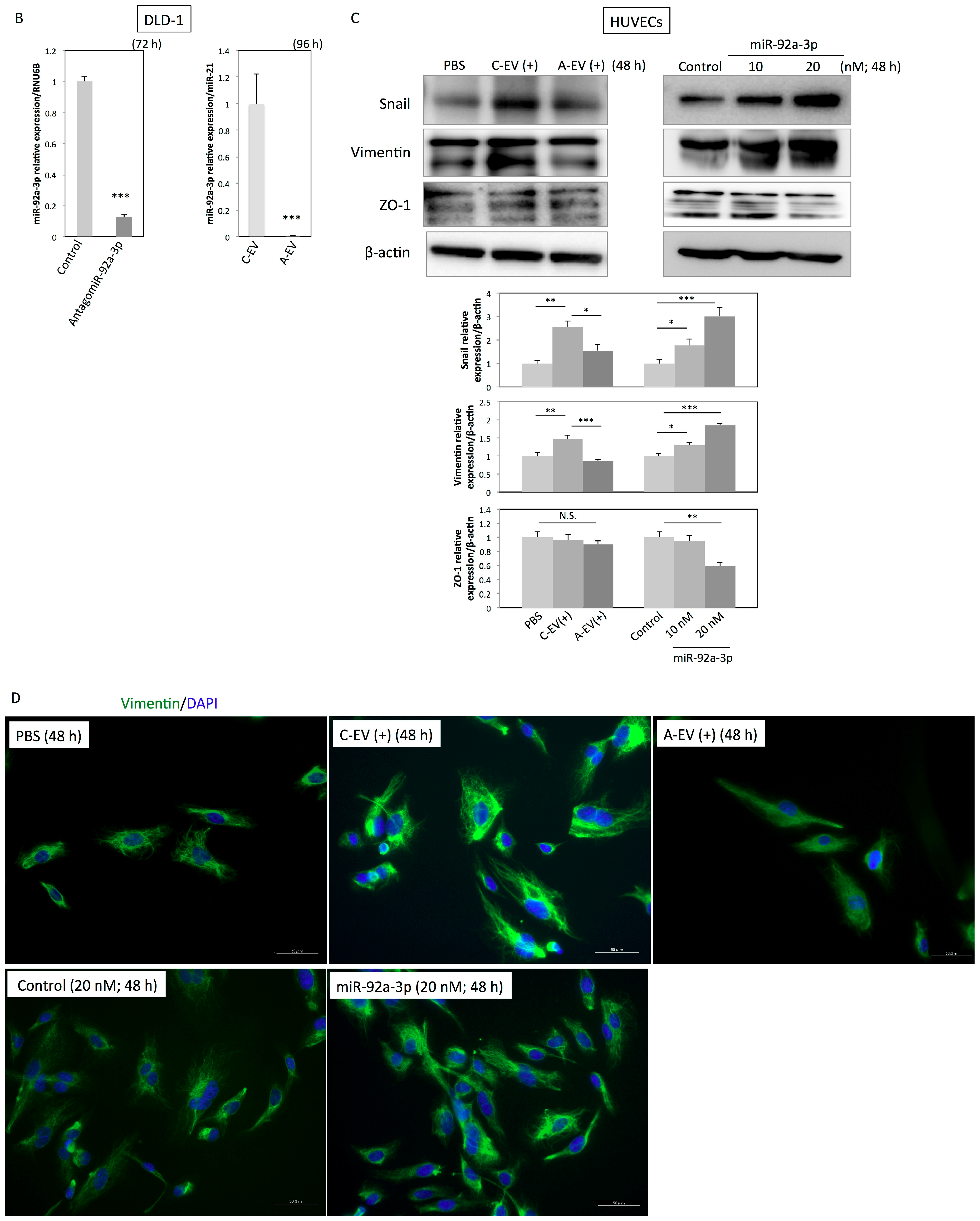
2.5. MiR-92a-3p Induces Partial Endothelial-to-Mesenchymal Transition (EndoMT) in HUVECs
3. Discussion
- accelerating the cell cycle, including mitosis, which leads to endothelial cell proliferation;
- loosening intercellular adhesions, which promotes migration;
- promoting tube formation through direct regulation of miR-92a-3p target genes, including a newly identified target gene (CLDN11), and indirectly regulating their related genes.
4. Materials and Methods
4.1. Cell Culture
4.2. Isolation of Colon Cancer Cell-Derived EVs
4.3. Transmission Electron Microscopy (TEM)
4.4. Nanoparticle Tracking Analysis (NTA)
4.5. Transfection with MiR-92a-3p, Antisense Inhibitor for MiR-92a-3p (AntagomiR-92a-3p), or Short-Interfering RNA for CLDN11
4.6. RNA Isolation and Quantitative Real-Time PCR
4.7. DNA Microarray Analysis and Data Mining
4.8. Antibodies
4.9. Western Blotting
4.10. Wound Healing Assay
4.11. Tube Formation Assay
4.12. Immunocytochemistry
4.13. Luciferase Assay
4.14. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microrna targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zeng, Z.; Hou, Y.; Yuan, T.; Gao, C.; Jia, W.; Yi, X.; Liu, M. Microrna-92a as a potential biomarker in diagnosis of colorectal cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e88745. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Akao, Y. Extracellular vesicles in cancer. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 23, pp. 187–204. [Google Scholar]
- Yamada, N.; Nakagawa, Y.; Tsujimura, N.; Kumazaki, M.; Noguchi, S.; Mori, T.; Hirata, I.; Maruo, K.; Akao, Y. Role of intracellular and extracellular microrna-92a in colorectal cancer. Transl. Oncol. 2013, 6, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Zhang, G.J.; Li, L.F.; Yang, G.D.; Xia, S.S.; Wang, R.; Leng, Z.W.; Liu, Z.L.; Tian, H.P.; He, Y.; Meng, C.Y.; et al. Mir-92a promotes stem cell-like properties by activating wnt/beta-catenin signaling in colorectal cancer. Oncotarget 2017, 8, 101760–101770. [Google Scholar] [PubMed]
- Kumar, V.; Torben, W.; Kenway, C.S.; Schiro, F.R.; Mohan, M. Longitudinal examination of the intestinal lamina propria cellular compartment of simian immunodeficiency virus-infected rhesus macaques provides broader and deeper insights into the link between aberrant microrna expression and persistent immune activation. J. Virol. 2016, 90, 5003–5019. [Google Scholar] [PubMed]
- Ohyagi-Hara, C.; Sawada, K.; Kamiura, S.; Tomita, Y.; Isobe, A.; Hashimoto, K.; Kinose, Y.; Mabuchi, S.; Hisamatsu, T.; Takahashi, T.; et al. Mir-92a inhibits peritoneal dissemination of ovarian cancer cells by inhibiting integrin alpha5 expression. Am. J. Pathol. 2013, 182, 1876–1889. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Popper, P.; Micevych, P.E.; Farber, D.B. Isolation and characterization of a novel oligodendrocyte-specific protein. Neurology 1996, 47, 772–778. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Micevych, P.E.; Chen, K. Oligodendrocyte-specific protein (osp) is a major component of cns myelin. J. Neurosci. Res. 1997, 50, 713–720. [Google Scholar] [CrossRef]
- Morita, K.; Sasaki, H.; Fujimoto, K.; Furuse, M.; Tsukita, S. Claudin-11/osp-based tight junctions of myelin sheaths in brain and sertoli cells in testis. J. Cell Biol. 1999, 145, 579–588. [Google Scholar] [CrossRef]
- Gow, A.; Southwood, C.M.; Li, J.S.; Pariali, M.; Riordan, G.P.; Brodie, S.E.; Danias, J.; Bronstein, J.M.; Kachar, B.; Lazzarini, R.A. Cns myelin and sertoli cell tight junction strands are absent in osp/claudin-11 null mice. Cell 1999, 99, 649–659. [Google Scholar] [CrossRef]
- Wessells, H.; Sullivan, C.J.; Tsubota, Y.; Engel, K.L.; Kim, B.; Olson, N.E.; Thorner, D.; Chitaley, K. Transcriptional profiling of human cavernosal endothelial cells reveals distinctive cell adhesion phenotype and role for claudin 11 in vascular barrier function. Physiol. Genom. 2009, 39, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Tam, L.C.; Reina-Torres, E.; Sherwood, J.M.; Cassidy, P.S.; Crosbie, D.E.; Lutjen-Drecoll, E.; Flugel-Koch, C.; Perkumas, K.; Humphries, M.M.; Kiang, A.S.; et al. Enhancement of outflow facility in the murine eye by targeting selected tight-junctions of schlemm’s canal endothelia. Sci. Rep. 2017, 7, 40717. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, C.; Ni, S.; Wang, S.; Ni, C.; Yang, P.; Ye, M. Methylated claudin-11 associated with metastasis and poor survival of colorectal cancer. Oncotarget 2017, 8, 96249–96262. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Mori, Y.; Cheng, Y.; Jin, Z.; Olaru, A.V.; Hamilton, J.P.; David, S.; Selaru, F.M.; Yang, J.; Abraham, J.M.; et al. Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS ONE 2009, 4, e8002. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Cao, B.; Lin, L.; Zhou, C.; Ye, D.; Qiu, S.; Li, Q.; Cui, X. The clinical signification of claudin-11 promoter hypermethylation for laryngeal squamous cell carcinoma. Med. Sci. Monit. 2017, 23, 3635–3640. [Google Scholar] [CrossRef] [PubMed]
- Awsare, N.S.; Martin, T.A.; Haynes, M.D.; Matthews, P.N.; Jiang, W.G. Claudin-11 decreases the invasiveness of bladder cancer cells. Oncol. Rep. 2011, 25, 1503–1509. [Google Scholar] [PubMed]
- Frid, M.G.; Kale, V.A.; Stenmark, K.R. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: In vitro analysis. Circ. Res. 2002, 90, 1189–1196. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Pechoux, C.; Bogaard, H.J.; Dorfmuller, P.; Remy, S.; Lecerf, F.; Plante, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef]
- Rodriguez, M.I.; Peralta-Leal, A.; O’Valle, F.; Rodriguez-Vargas, J.M.; Gonzalez-Flores, A.; Majuelos-Melguizo, J.; Lopez, L.; Serrano, S.; de Herreros, A.G.; Rodriguez-Manzaneque, J.C.; et al. Parp-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet. 2013, 9, e1003531. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chang, J.S.; Hsiao, J.R.; Yen, Y.C.; Jiang, S.S.; Liu, S.H.; Chen, Y.L.; Shen, Y.Y.; Chang, J.Y.; Chen, Y.W. Tumour cell-derived wnt5b modulates in vitro lymphangiogenesis via induction of partial endothelial-mesenchymal transition of lymphatic endothelial cells. Oncogene 2017, 36, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.S.; Chen, W.S.; Chen, L.L.; Chen, C.C.; Hsu, Y.T.; Chua, K.V.; Wang, H.D.; Huang, T.S. Osteopontin-integrin engagement induces hif-1alpha-tcf12-mediated endothelial-mesenchymal transition to exacerbate colorectal cancer. Oncotarget 2018, 9, 4998–5015. [Google Scholar] [CrossRef] [PubMed]
- Welch-Reardon, K.M.; Ehsan, S.M.; Wang, K.; Wu, N.; Newman, A.C.; Romero-Lopez, M.; Fong, A.H.; George, S.C.; Edwards, R.A.; Hughes, C.C. Angiogenic sprouting is regulated by endothelial cell expression of slug. J. Cell Sci. 2014, 127, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.M.; Serbanovic-Canic, J.; Feng, S.; Souilhol, C.; Xing, R.; Hsiao, S.; Mammoto, A.; Chen, J.; Ariaans, M.; Francis, S.E.; et al. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor snail. Sci. Rep. 2017, 7, 3375. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Kuranaga, Y.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Akao, Y. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of t cells into tumor-growth supporting cells with transforming growth factor-beta1-mediated suppression. Oncotarget 2016, 7, 27033–27043. [Google Scholar] [CrossRef] [PubMed]
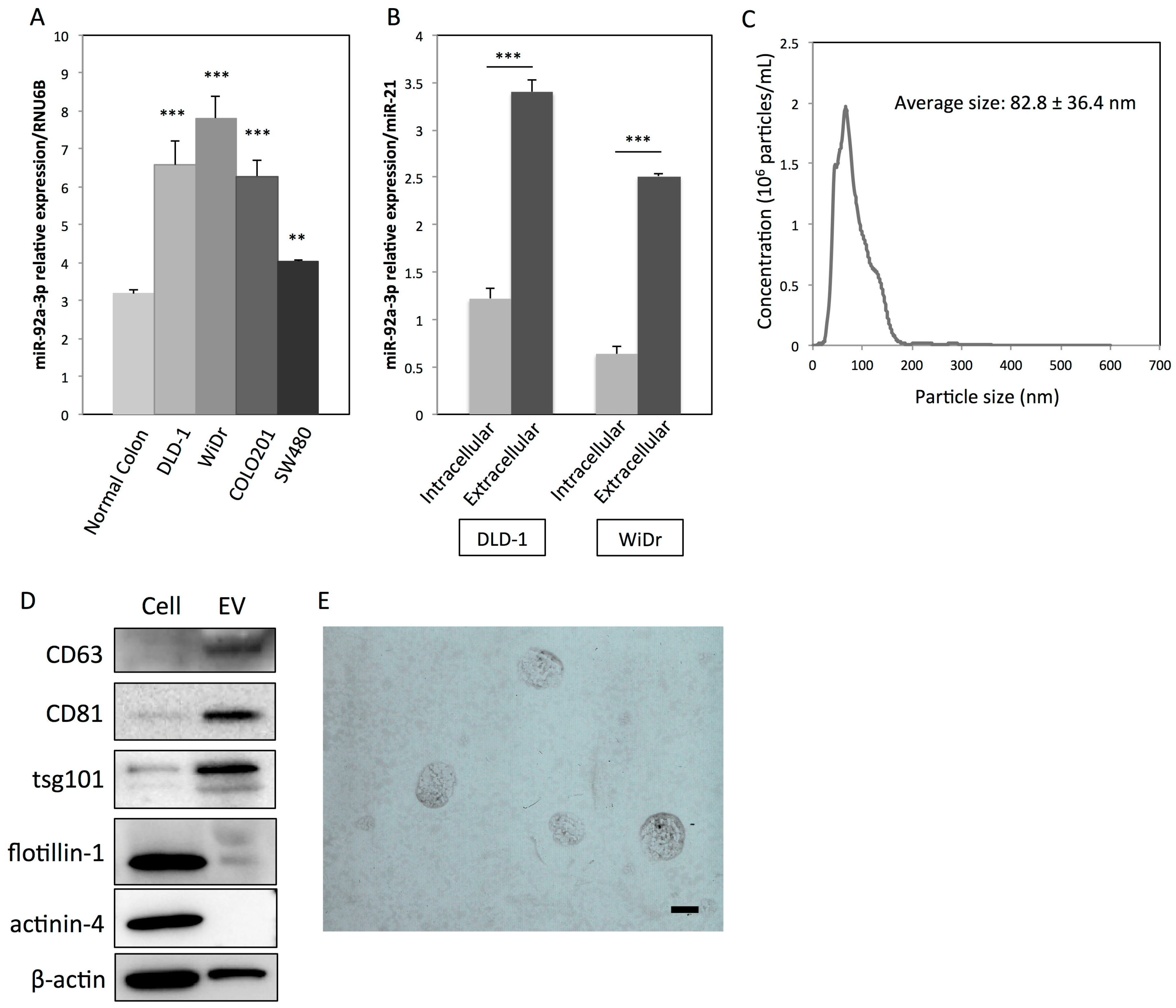

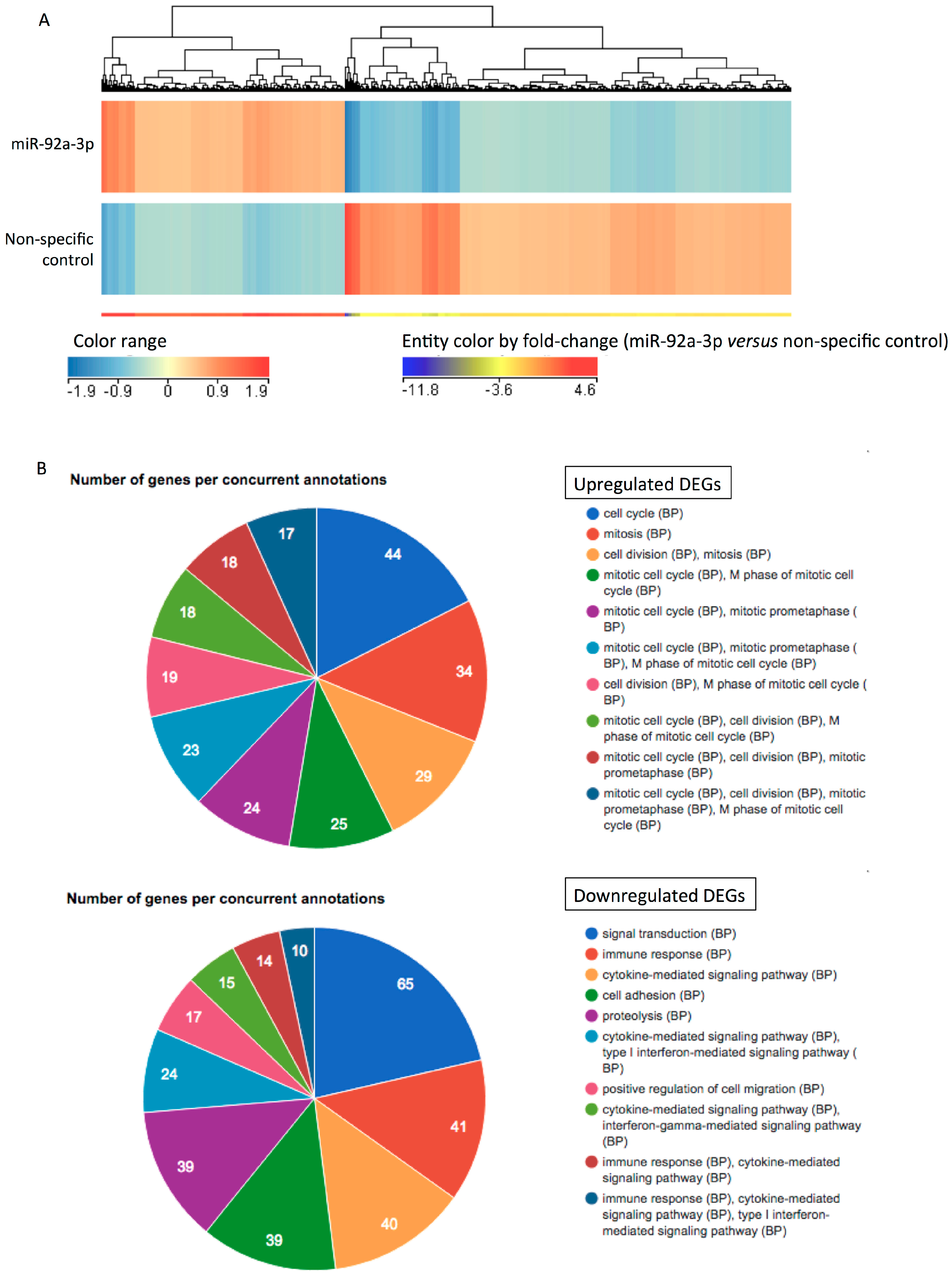
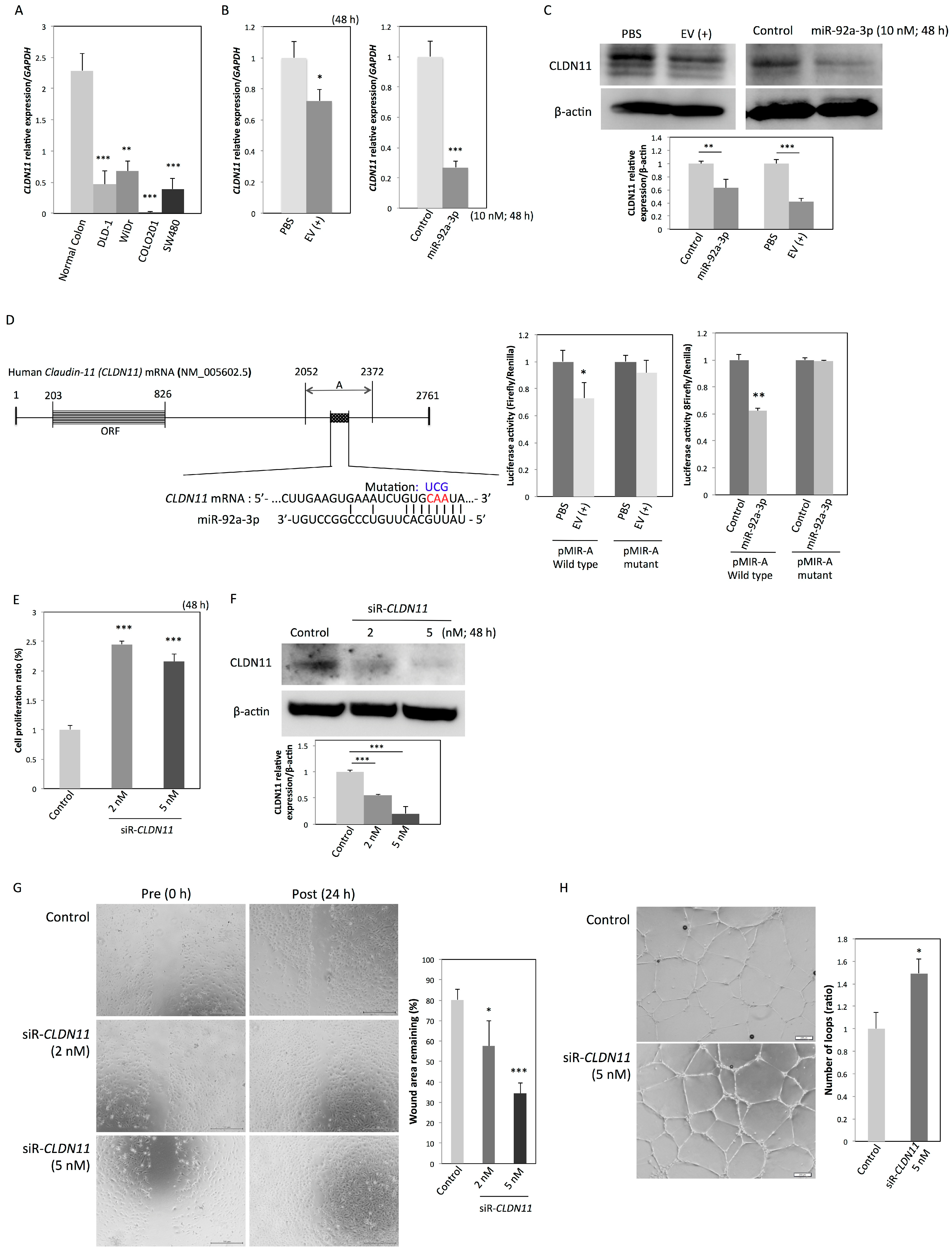
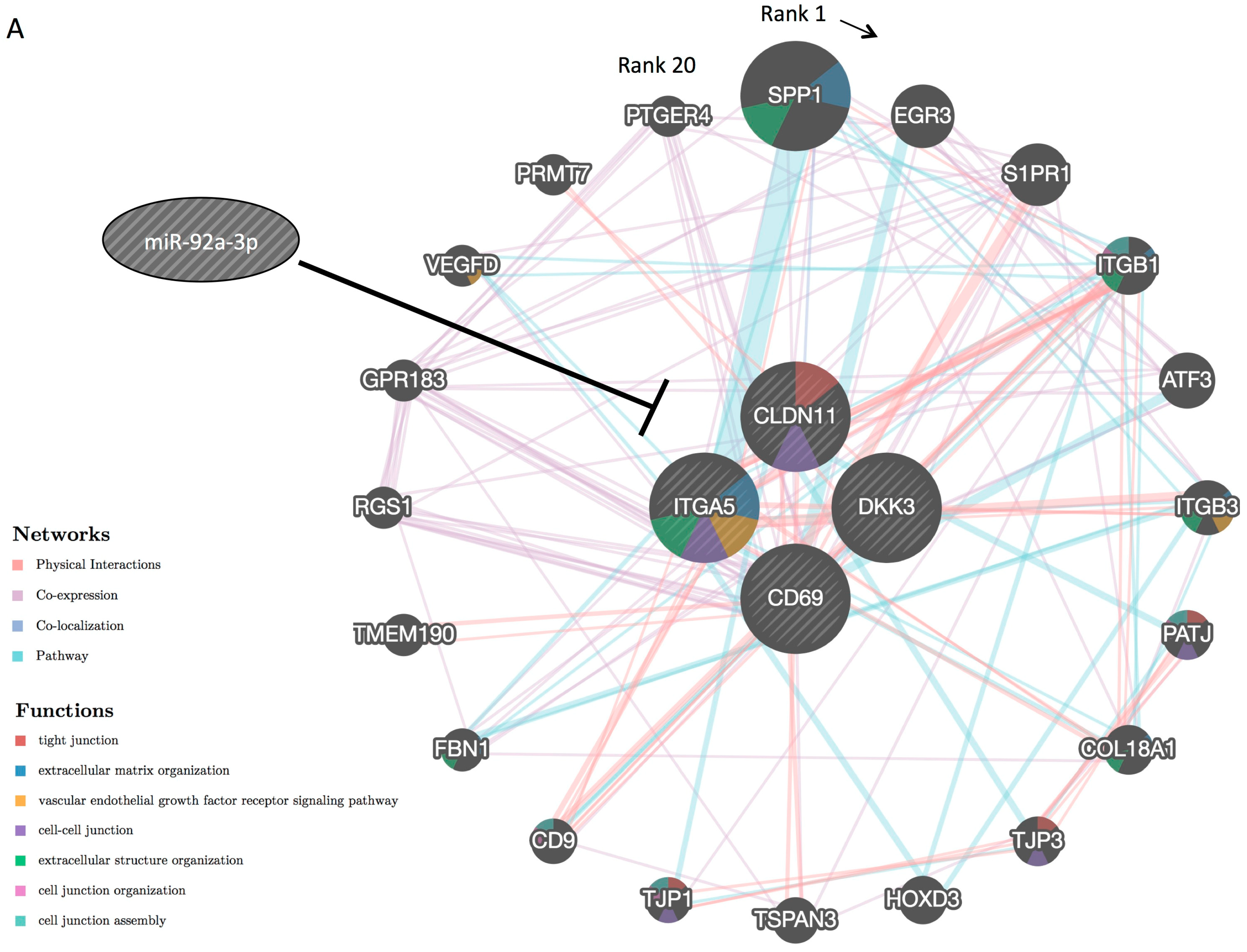
| No. | Gene Name | Symbol | RefSeq ID | Fold Change (miR-92a-3p/Control) |
|---|---|---|---|---|
| 1 | regulator of G-protein signaling 7 | RGS7 | NM_001282773.1 | 6.940 |
| 2 | sterile alpha motif domain containing 15 | SAMD15 | NM_001010860.1 | 6.147 |
| 3 | brain expressed X-linked 1 | BEX1 | NM_018476.3 | 6.144 |
| 4 | Yip1 interacting factor homolog B, membrane trafficking protein | YIF1B | NM_001145461.1 | 5.812 |
| 5 | IQ motif containing D | IQCD | XM_011537864.1 | 5.713 |
| 6 | leukemia NUP98 fusion partner 1 | LNP1 | NM_001085451.1 | 5.566 |
| 7 | phosphorylase kinase, alpha 2 (liver) | PHKA2 | NM_000292.2 | 5.448 |
| 8 | olfactory receptor family 2 subfamily T member 1 | OR2T1 | NM_030904.1 | 5.130 |
| 9 | chromosome 15 open reading frame 48 | C15orf48 | NM_197955.2 | 5.092 |
| 10 | kinesin family member 15 | KIF15 | NM_020242.2 | 4.765 |
| 11 | dual adaptor of phosphotyrosine and 3-phosphoinositides | DAPP1 | NM_001306151.1 | 4.626 |
| 12 | DNA replication and sister chromatid cohesion 1 | DSCC1 | XM_005251065.2 | 4.596 |
| 13 | proline/serine-rich coiled-coil 1 | PSRC1 | XM_011542306.1 | 4.579 |
| 14 | glycerol-3-phosphate acyltransferase 2, mitochondrial | GPAT2 | NM_207328.2 | 4.511 |
| 15 | olfactory receptor, family 10, subfamily G, member 6 | OR10G6 | - | 4.497 |
| 16 | TYMS opposite strand | TYMSOS | NM_001012716.2 | 4.448 |
| 17 | apolipoprotein O | APOO | NR_026545.2 | 4.444 |
| 18 | membrane protein, palmitoylated 3 | MPP3 | XM_006721917.2 | 4.340 |
| 19 | TNF alpha induced protein 8 like 2 | TNFAIP8L2 | NM_024575.4 | 4.338 |
| 20 | WD repeat domain 63 | WDR63 | NM_001288563.1 | 4.328 |
| 21 | pleckstrin | PLEK | NM_002664.2 | 4.324 |
| 22 | solute carrier family 7 member 14 | SLC7A14 | NM_020949.2 | 4.306 |
| 23 | MCM3AP antisense RNA 1 | MCM3AP-AS1 | NR_110565.1 | 4.220 |
| 24 | zinc finger protein 114 | ZNF114 | NM_001301062.1 | 4.210 |
| 25 | zinc finger RNA binding protein 2 | ZFR2 | NM_015174.1 | 4.188 |
| 26 | neuralized E3 ubiquitin protein ligase 1 | NEURL1 | XM_011540334.1 | 4.143 |
| 27 | mitogen-activated protein kinase 6 pseudogene 4 | MAPK6PS4 | - | 4.132 |
| 28 | chromosome 12 open reading frame 76 | C12orf76 | XM_005253882.2 | 4.039 |
| 29 | zinc finger protein 485 | ZNF485 | XM_011539498.1 | 4.034 |
| 30 | tripartite motif containing 45 | TRIM45 | XM_011542199.1 | 4.034 |
| No. | Gene Name | Symbol | RefSeq ID | Fold Change (miR-92a-3p/Control) |
|---|---|---|---|---|
| 1 | interleukin 1 receptor like 1 | IL1RL1 | NR_104167.1 | 0.076 |
| 2 | interferon induced protein with tetratricopeptide repeats 2 | IFIT2 | NM_001547.4 | 0.076 |
| 3 | thymidine phosphorylase | TYMP | NM_001257989.1 | 0.096 |
| 4 | MX dynamin like GTPase 2 | MX2 | NM_002463.1 | 0.101 |
| 5 | keratin 19, type I | KRT19 | NM_002276.4 | 0.104 |
| 6 | C-X-C motif chemokine ligand 10 | CXCL10 | NM_001565.3 | 0.117 |
| 7 | C-X-C motif chemokine ligand 11 | CXCL11 | NM_001302123.1 | 0.118 |
| 8 | radical S-adenosyl methionine domain containing 2 | RSAD2 | NM_080657.4 | 0.122 |
| 9 | mannosidase alpha class 2A member 1 | MAN2A1 | NM_002372.3 | 0.133 |
| 10 | interferon induced protein with tetratricopeptide repeats 1 | IFIT1 | NM_001548.4 | 0.133 |
| 11 | cytidine/uridine monophosphate kinase 2 | CMPK2 | NR_046236.1 | 0.147 |
| 12 | complement factor B | CFB | NM_001710.5 | 0.148 |
| 13 | interferon stimulated exonuclease gene 20kDa | ISG20 | XM_011521521.1 | 0.150 |
| 14 | indoleamine 2,3-dioxygenase 1 | IDO1 | NM_002164.5 | 0.155 |
| 15 | nidogen 2 | NID2 | XM_005267407.3 | 0.160 |
| 16 | interferon, gamma-inducible protein 30 | IFI30 | NM_006332.4 | 0.166 |
| 17 | C-C motif chemokine ligand 5 | CCL5 | NM_001278736.1 | 0.168 |
| 18 | 2′-5′-oligoadenylate synthetase-like | OASL | NM_001261825.1 | 0.169 |
| 19 | dickkopf WNT signaling pathway inhibitor 3 | DKK3 | XM_006718178.2 | 0.170 |
| 20 | general transcription factor IIE subunit 2 | GTF2E2 | XM_011544510.1 | 0.172 |
| 21 | beta-2-microglobulin | B2M | NM_004048.2 | 0.174 |
| 22 | matrix metallopeptidase 10 | MMP10 | NM_002425.2 | 0.174 |
| 23 | prolyl 3-hydroxylase 3 | P3H3 | NM_014262.4 | 0.179 |
| 24 | keratin 15, type I | KRT15 | XM_005257345.2 | 0.181 |
| 25 | interferon induced protein 35 | IFI35 | XM_005257302.3 | 0.191 |
| 26 | basic leucine zipper ATF-like transcription factor 2 | BATF2 | XM_011544750.1 | 0.194 |
| 27 | nuclear protein 1, transcriptional regulator | NUPR1 | NM_012385.2 | 0.196 |
| 28 | interferon induced protein with tetratricopeptide repeats 3 | IFIT3 | NM_001549.5 | 0.197 |
| 29 | interferon induced transmembrane protein 1 | IFITM1 | NM_003641.3 | 0.198 |
| 30 | solute carrier family 15 member 3 | SLC15A3 | XM_011545095.1 | 0.199 |
| 31 | oleoyl-ACP hydrolase | OLAH | XM_006717456.2 | 0.200 |
| 32 | mitogen-activated protein kinase-activated protein kinase 2 | MAPKAPK2 | NM_032960.3 | 0.201 |
| 33 | ISG15 ubiquitin-like modifier | ISG15 | NM_005101.3 | 0.203 |
| 34 | AXL receptor tyrosine kinase | AXL | NM_001278599.1 | 0.203 |
| 35 | atypical chemokine receptor 3 | ACKR3 | NM_020311.2 | 0.207 |
| 36 | complement component 3a receptor 1 | C3AR1 | NM_004054.2 | 0.207 |
| 37 | MX dynamin like GTPase 1 | MX1 | NM_001144925.2 | 0.208 |
| 38 | tumor necrosis factor superfamily member 13b | TNFSF13B | XM_005254029.3 | 0.208 |
| 39 | isocitrate dehydrogenase 1 (NADP+) | IDH1 | NM_005896.3 | 0.208 |
| 40 | PDZ domain containing 2 | PDZD2 | NM_178140.3 | 0.210 |
| 41 | integrin subunit alpha 5 | ITGA5 | NM_002205.3 | 0.214 |
| 42 | tumor-associated calcium signal transducer 2 | TACSTD2 | NM_002353.2 | 0.219 |
| 43 | HLA complex P5 (non-protein coding) | HCP5 | NR_040662.1 | 0.221 |
| 44 | olfactory receptor family 9 subfamily I member 1 | OR9I1 | NM_001005211.1 | 0.222 |
| 45 | fibrillin 1 | FBN1 | NM_000138.4 | 0.222 |
| 46 | phospholipase A1 member A | PLA1A | NM_001293225.1 | 0.223 |
| 47 | CD69 molecule | CD69 | NM_001781.2 | 0.224 |
| 48 | integral membrane protein 2B | ITM2B | NM_021999.4 | 0.226 |
| 49 | DnaJ heat shock protein family (Hsp40) member B9 | DNAJB9 | NM_012328.2 | 0.228 |
| 50 | scavenger receptor class B member 2 | SCARB2 | NM_001204255.1 | 0.230 |
| 51 | sterile alpha motif domain containing 9 | SAMD9 | NM_017654.3 | 0.230 |
| 52 | insulin like growth factor binding protein 6 | IGFBP6 | NM_002178.2 | 0.231 |
| 53 | claudin 11 | CLDN11 | NM_001185056.1 | 0.232 |
| 54 | unc-93 homolog B1 (C. elegans) | UNC93B1 | XM_011545291.1 | 0.238 |
| 55 | FRY microtubule binding protein | FRY | XM_006719749.2 | 0.239 |
| 56 | myosin VIIA and Rab interacting protein | MYRIP | NR_104316.1 | 0.240 |
| 57 | selectin E | SELE | NM_000450.2 | 0.240 |
| 58 | interferon regulatory factor 7 | IRF7 | XM_005252909.2 | 0.244 |
| 59 | HECT and RLD domain containing E3 ubiquitin protein ligase 5 | HERC5 | NM_016323.3 | 0.244 |
| 60 | secreted and transmembrane 1 | SECTM1 | XM_011523588.1 | 0.244 |
| 61 | laminin subunit gamma 2 | LAMC2 | NM_005562.2 | 0.248 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, N.O.; Heishima, K.; Akao, Y.; Senda, T. Extracellular Vesicles Containing MicroRNA-92a-3p Facilitate Partial Endothelial-Mesenchymal Transition and Angiogenesis in Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4406. https://doi.org/10.3390/ijms20184406
Yamada NO, Heishima K, Akao Y, Senda T. Extracellular Vesicles Containing MicroRNA-92a-3p Facilitate Partial Endothelial-Mesenchymal Transition and Angiogenesis in Endothelial Cells. International Journal of Molecular Sciences. 2019; 20(18):4406. https://doi.org/10.3390/ijms20184406
Chicago/Turabian StyleYamada, Nami O., Kazuki Heishima, Yukihiro Akao, and Takao Senda. 2019. "Extracellular Vesicles Containing MicroRNA-92a-3p Facilitate Partial Endothelial-Mesenchymal Transition and Angiogenesis in Endothelial Cells" International Journal of Molecular Sciences 20, no. 18: 4406. https://doi.org/10.3390/ijms20184406
APA StyleYamada, N. O., Heishima, K., Akao, Y., & Senda, T. (2019). Extracellular Vesicles Containing MicroRNA-92a-3p Facilitate Partial Endothelial-Mesenchymal Transition and Angiogenesis in Endothelial Cells. International Journal of Molecular Sciences, 20(18), 4406. https://doi.org/10.3390/ijms20184406






