Ring Formation and Hydration Effects in Electron Attachment to Misonidazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment
2.2. Theory
3. Results and Discussion
3.1. Molecular Fragmentation
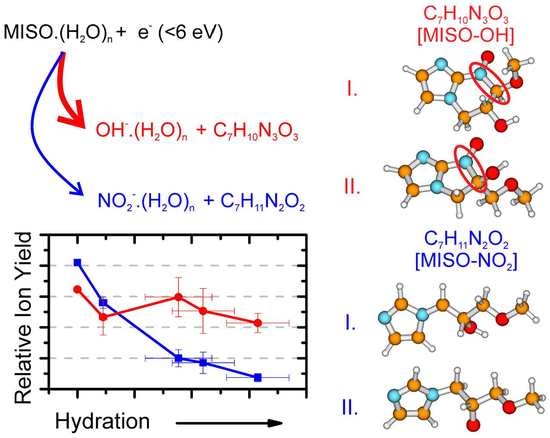
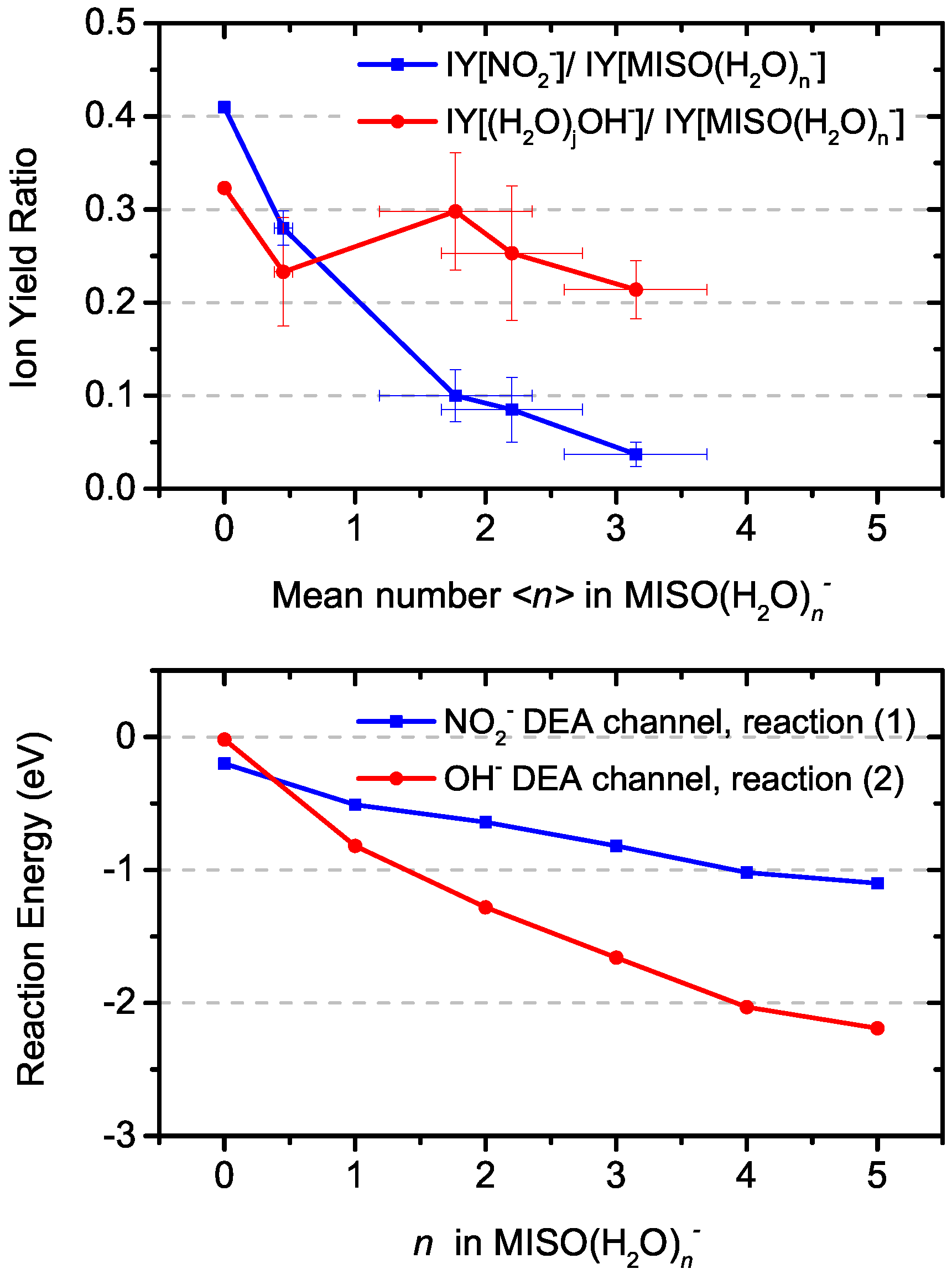
3.2. Water Solvent Effects
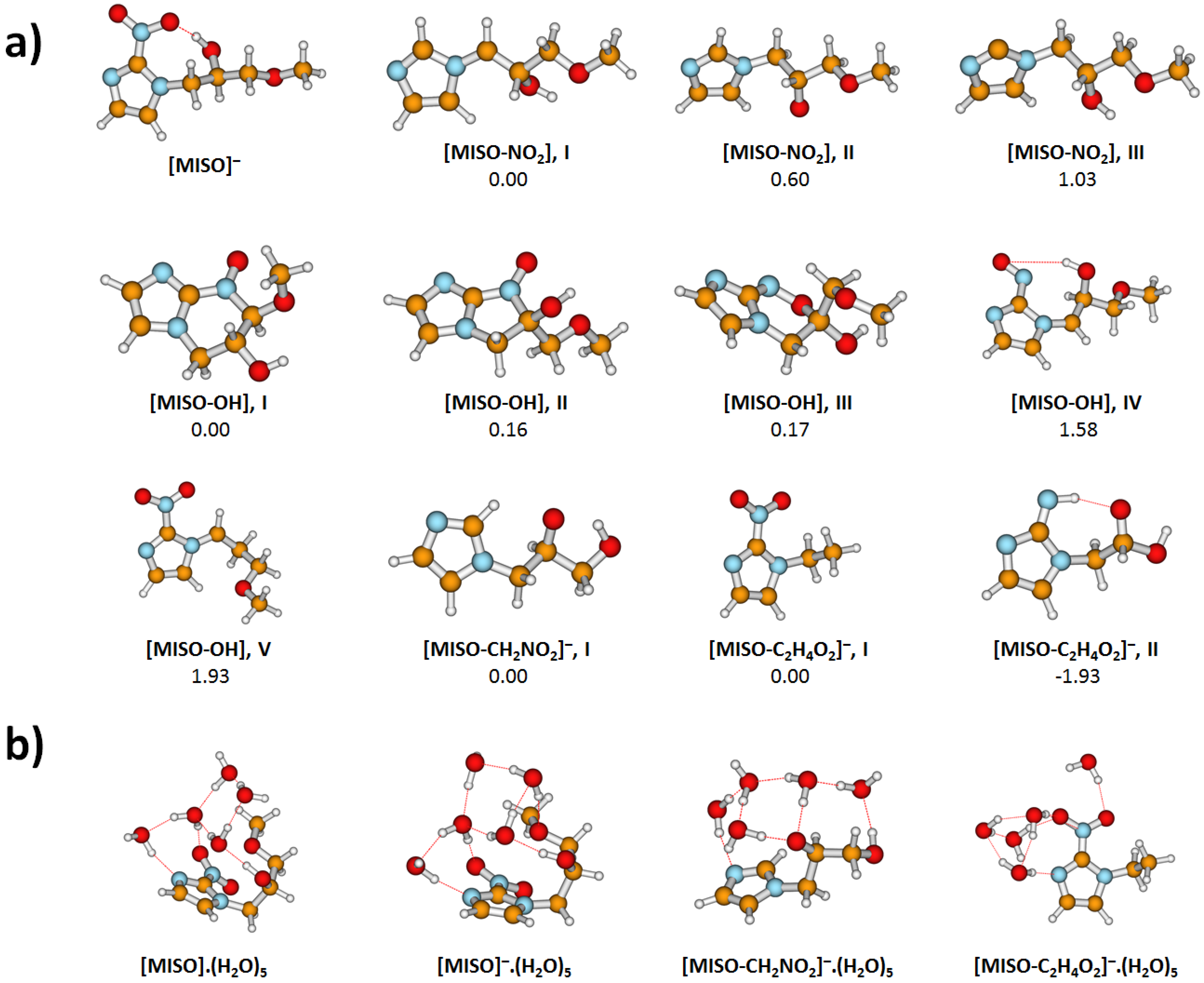
3.3. Theoretical Model for DEA from MISO
- (1)
- MISO.(HO) NO.(HO) + [MISO-NO]
- (2)
- MISO.(HO) OH.(HO) + [MISO-OH]
- (3a)
- MISO.(HO) [MISO-CHNO].(HO) + CHNO
- (3b)
- MISO.(HO) [MISO-CHO].(HO) + CHOH + CO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DEA | Dissociative Electron Attachment |
| RTOF | Reflectron Time of Flight Mass Spectrometer |
| MISO | Misonidazole |
| DMSO | dimethyl sulfoxide |
References
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular Damage Induced by Ionizing Radiation: The Direct and Indirect Effects of Low-Energy Electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Tejedor, G.G.; Fuss, M.C. Radiation Damage in Biomolecular Systems; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Alizadeh, E.; Sanche, L. Precursors of Solvated Electrons in Radiobiological Physics and Chemistry. Chem. Rev. 2012, 112, 5578–5602. [Google Scholar] [CrossRef] [PubMed]
- Mucke, M.; Braune, M.; Barth, S.; Förstel, M.; Lischke, T.; Ulrich, V.; Arion, T.; Becker, U.; Bradshaw, A.; Hergenhahn, U. A hitherto unrecognized source of low-energy electrons in water. Nat. Phys. 2010, 6, 143. [Google Scholar] [CrossRef]
- Kumar, A.; Walker, J.A.; Bartels, D.M.; Sevilla, M.D. A Simple ab Initio Model for the Hydrated Electron That Matches Experiment. J. Phys. Chem. 2015, 119, 9148–9159. [Google Scholar] [CrossRef]
- Chomicz, L.; Zdrowowicz, M.; Kasprzykowski, F.; Rak, J.; Buonaugurio, A.; Wang, Y.; Bowen, K.H. How to Find Out Whether a 5-Substituted Uracil Could Be a Potential DNA Radiosensitizer. J. Phys. Chem. Lett. 2013, 4, 2853–2857. [Google Scholar] [CrossRef]
- Meißner, R.; Kočišek, J.; Feketeová, L.; Fedor, J.; Fárník, M.; Limao-Vieira, P.; Illenberger, E.; Denifl, S. Low-energy electrons transform the nimorazole molecule into a radiosensitiser. Nat. Commun. 2019, 10, 2388. [Google Scholar] [CrossRef]
- Poštulka, J.; Slavíček, P.; Fedor, J.; Fárník, M.; Kočišek, J. Energy Transfer in Microhydrated Uracil, 5-Fluorouracil, and 5-Bromouracil. J. Phys. Chem. 2017, 121, 8965–8974. [Google Scholar] [CrossRef]
- Workman, P.; Strafford, I.J. The experimental development of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev. 1993, 12, 73–82. [Google Scholar] [CrossRef]
- Liu, J.n.; Bu, W.; Shi, J. Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. Chem. Rev. 2017, 117, 6160–6224. [Google Scholar] [CrossRef]
- Lopci, E.; Grassi, I.; Chiti, A.; Nanni, C.; Cicoria, G.; Toschi, L.; Fonti, C.; Lodi, F.; Mattioli, S.; Fanti, S. PET radiopharmaceuticals for imaging of tumor hypoxia: A review of the evidence. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 365–384. [Google Scholar]
- Paterson, I.; Dawes, P.; Henk, J.; Moore, J. Pilot study of radiotherapy with misonidazole in head and neck cancer. Clin. Radiol. 1981, 32, 225–229. [Google Scholar] [CrossRef]
- Fu, K.K.; Cooper, J.S.; Marcial, V.A.; Laramore, G.E.; Pajak, T.F.; Jacobs, J.; Al-Sarraf, M.; Forastiere, A.A.; Cox, J.D. Evolution of the radiation therapy oncology group clinical trials for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 425–438. [Google Scholar] [CrossRef]
- Mason, R.P.; Holtzman, J.L. Mechanism of microsomal and mitochondrial nitroreductase. Electron spin resonance evidence for nitroaromatic free radical intermediates. Biochemistry 1975, 14, 1626–1632. [Google Scholar] [CrossRef]
- Knox, R.J.; Knight, R.C.; Edwards, D.I. Studies on the action of nitroimidazole drugs: The products of nitroimidazole reduction. Biochem. Pharmacol. 1983, 32, 2149–2156. [Google Scholar] [CrossRef]
- Wardman, P. The mechanism of radiosensitization by electron-affinic compounds. Int. J. Radiat. Appl. Instrum. Part Radiat. Phys. Chem. 1987, 30, 423–432. [Google Scholar] [CrossRef]
- Varghese, A.J.; Whitmore, G.F. Modification of Guanine Derivatives by Reduced 2-Nitroimidazoles. Cancer Res. 1983, 43, 78–82. [Google Scholar] [PubMed]
- Edwards, D.I. Reduction of nitroimidazoles in vitro and DNA damage. In Bioreduction in the Activation of Drugs; Alexander, P., Gielen, J., Sartorelli, A.C., Eds.; Pergamon Press Ltd.: Oxford, UK, 1986; p. 53. [Google Scholar]
- Tanzer, K.; Feketeová, L.; Puschnigg, B.; Scheier, P.; Illenberger, E.; Denifl, S. Reactions in Nitroimidazole Triggered by Low-Energy (0–2 eV) Electrons: Methylation at N1-H Completely Blocks Reactivity. Angew. Chem. Int. Ed. 2014, 53, 12240–12243. [Google Scholar] [CrossRef]
- Ribar, A.; Fink, K.; Probst, M.; Huber, S.E.; Feketeová, L.; Denifl, S. Isomer Selectivity in Low-Energy Electron Attachment to Nitroimidazoles. Chem. Eur. J. 2017, 23, 12892–12899. [Google Scholar] [CrossRef]
- Meißner, R.; Feketeová, L.; Illenberger, E.; Denifl, S. Reactions in the Radiosensitiser Misonidazole Induced by Low-Energy (0–10 eV) Electrons. Int. J. Mol. Sci. 2019, 20, 3496. [Google Scholar] [CrossRef]
- Böhler, E.; Warneke, J.; Swiderek, P. Control of chemical reactions and synthesis by low-energy electrons. Chem. Soc. Rev. 2013, 42, 9219–9231. [Google Scholar] [CrossRef]
- Lafosse, A.; Bertin, M.; Azria, R. Electron driven processes in ices: Surface functionalization and synthesis reactions. Prog. Surf. Sci. 2009, 84, 177–198. [Google Scholar] [CrossRef]
- Sajeev, Y. Cycloaddition of molecular dinitrogens: formation of tetrazete anion (N4-; D2h) through associative electron attachment. Mol. Phys. 2019, 117, 2162–2166. [Google Scholar] [CrossRef]
- Davis, D.; Sajeev, Y. Low energy electron catalyst: the electronic origin of catalytic strategies. Phys. Chem. Chem. Phys. 2016, 18, 27715–27720. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, N.; Ha, D.G.; Kim, S.K. “Associative” Electron Attachment to Azabenzene-(CO2)n van der Waals Complexes: Stepwise Formation of Covalent Bonds with Additive Electron Affinities. J. Am. Chem. Soc. 2008, 130, 16241–16244. [Google Scholar] [CrossRef] [PubMed]
- Kočišek, J.; Pysanenko, A.; Fárník, M.; Fedor, J. Microhydration Prevents Fragmentation of Uracil and Thymine by Low-Energy Electrons. J. Phys. Chem. Lett. 2016, 7, 3401–3405. [Google Scholar] [CrossRef] [PubMed]
- Kočišek, J.; Lengyel, J.; Fárník, M. Ionization of large homogeneous and heterogeneous clusters generated in acetylene–Ar expansions: Cluster ion polymerization. J. Chem. Phys. 2013, 138, 124306. [Google Scholar] [CrossRef]
- Fárník, M.; Lengyel, J. Mass spectrometry of aerosol particle analogues in molecular beam experiments. Mass Spectrom. Rev. 2018, 37, 630–651. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision A.03, 2016; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hollas, D.; Svoboda, O.; Ončák, M.; Slavíček, P. ABIN, Source Code. Available online: https://github.com/PHOTOX/ABIN (accessed on 1 September 2019).
- Kočišek, J.; Grygoryeva, K.; Lengyel, J.; Fárník, M.; Fedor, J. Effect of Cluster Environment on the Electron Attachment to 2-Nitrophenol. Eur. Phys. J. 2016, 70, 98. [Google Scholar] [CrossRef]
- Smiglak, M.; Hines, C.C.; Reichert, W.M.; Vincek, A.S.; Katritzky, A.R.; Thrasher, J.S.; Sun, L.; McCrary, P.D.; Beasley, P.A.; Kelley, S.P.; et al. Synthesis, limitations, and thermal properties of energetically-substituted, protonated imidazolium picrate and nitrate salts and further comparison with their methylated analogs. New J. Chem. 2012, 36, 702–722. [Google Scholar] [CrossRef]
- Solov’yov, A.V.; Surdutovich, E.; Scifoni, E.; Mishustin, I.; Greiner, W. Physics of ion beam cancer therapy: A multiscale approach. Phys. Rev. E 2009, 79, 011909. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Feketeová, L.; Albright, A.L.; Sørensen, B.S.; Horsman, M.R.; White, J.; O’Hair, R.A.; Bassler, N. Formation of radical anions of radiosensitizers and related model compounds via electrospray ionization. Int. J. Mass Spectrom. 2014, 365–366, 56–63. [Google Scholar] [CrossRef]
- Overgaard, J.; Overgaard, M.; Nielsen, O.S.; Pedersen, A.K.; Timothy, A.R. A comparative investigation of nimorazole and misonidazole as hypoxic radiosensitizers in a C3H mammary carcinoma in vivo. Br. J. Cancer 1982, 46, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Pshenichnyuk, S.A.; Modelli, A.; Komolov, A.S. Interconnections between dissociative electron attachment and electron-driven biological processes. Int. Rev. Phys. Chem. 2018, 37, 125–170. [Google Scholar] [CrossRef]
- Fedor, J.; Cicman, P.; Coupier, B.; Feil, S.; Winkler, M.; Głuch, K.; Husarik, J.; Jaksch, D.; Farizon, B.; Mason, N.J.; et al. Fragmentation of Transient Water Anions Following Low-Energy Electron Capture by H2O/D2O. J. Phys. At. Mol. Opt. Phys. 2006, 39, 3935. [Google Scholar] [CrossRef]
- Kočišek, J.; Sedmidubská, B.; Indrajith, S.; Fárník, M.; Fedor, J. Electron Attachment to Microhydrated Deoxycytidine Monophosphate. J. Phys. Chem. 2018, 122, 5212–5217. [Google Scholar] [CrossRef] [PubMed]



| Ion | Relative Ion Yield | |||
|---|---|---|---|---|
| (i) isolated [21] | (ii) dry | (iii) hydrated | ||
| 201 | MISO | 50 | 75 | 100 |
| 141 | [MISO-CHNO] or [MISO-CHO] | 25 | 100 | 50 |
| 46 | NO | 100 | 30 | 3 |
| 17 | OH | - | 22 | 20 |
| n | VEA | AEA | R. (1) | R. (2) | R. (3a) | R. (3b) |
|---|---|---|---|---|---|---|
| 0 | 0.84 (0.81) | 1.45 (1.42) | −0.20 (−0.06) | −0.02 (0.13) | 0.41 (0.49) | −0.83 (−0.76) |
| 1 | 1.01 (0.96) | 1.63 (1.66) | −0.51 (−0.43) | −0.82 (−0.73) | 0.05 (0.23) | −1.09 (−1.05) |
| 2 | 1.09 (0.97) | 1.84 (1.77) | −0.64 (−0.49) | −1.28 (−1.13) | −0.12 (0.07) | −1.33 (−1.23) |
| 3 | 1.22 (1.19) | 2.07 (1.99) | −0.82 (−0.70) | −1.66 (−1.55) | −0.27 (−0.09) | −1.45 (−1.33) |
| 4 | 1.48 (1.45) | 2.17 (2.08) | −1.02 (−0.92) | −2.03 (−1.94) | −0.62 (−0.41) | −1.71 (−1.61) |
| 5 | 1.35 (1.33) | 2.14 (2.06) | −1.10 (−1.04) | −2.19 (−2.14) | −0.58 (−0.32) | −1.81 (−1.71) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ončák, M.; Meißner, R.; Arthur-Baidoo, E.; Denifl, S.; Luxford, T.F.M.; Pysanenko, A.; Fárník, M.; Pinkas, J.; Kočišek, J. Ring Formation and Hydration Effects in Electron Attachment to Misonidazole. Int. J. Mol. Sci. 2019, 20, 4383. https://doi.org/10.3390/ijms20184383
Ončák M, Meißner R, Arthur-Baidoo E, Denifl S, Luxford TFM, Pysanenko A, Fárník M, Pinkas J, Kočišek J. Ring Formation and Hydration Effects in Electron Attachment to Misonidazole. International Journal of Molecular Sciences. 2019; 20(18):4383. https://doi.org/10.3390/ijms20184383
Chicago/Turabian StyleOnčák, Milan, Rebecca Meißner, Eugene Arthur-Baidoo, Stephan Denifl, Thomas F. M. Luxford, Andriy Pysanenko, Michal Fárník, Jiří Pinkas, and Jaroslav Kočišek. 2019. "Ring Formation and Hydration Effects in Electron Attachment to Misonidazole" International Journal of Molecular Sciences 20, no. 18: 4383. https://doi.org/10.3390/ijms20184383
APA StyleOnčák, M., Meißner, R., Arthur-Baidoo, E., Denifl, S., Luxford, T. F. M., Pysanenko, A., Fárník, M., Pinkas, J., & Kočišek, J. (2019). Ring Formation and Hydration Effects in Electron Attachment to Misonidazole. International Journal of Molecular Sciences, 20(18), 4383. https://doi.org/10.3390/ijms20184383