Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals
Abstract
1. Introduction
2. The Need for Newer Anti-Inflammatories
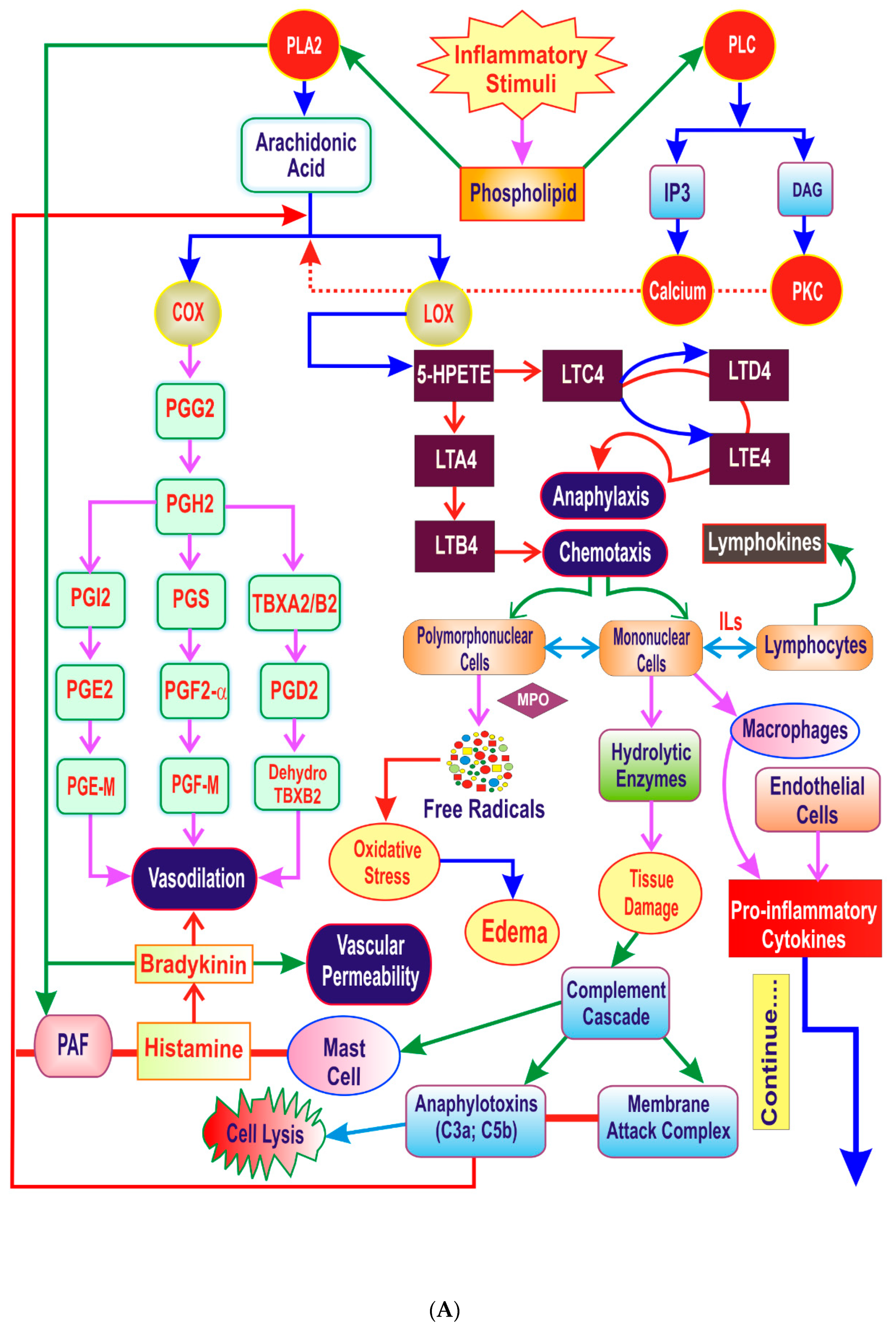
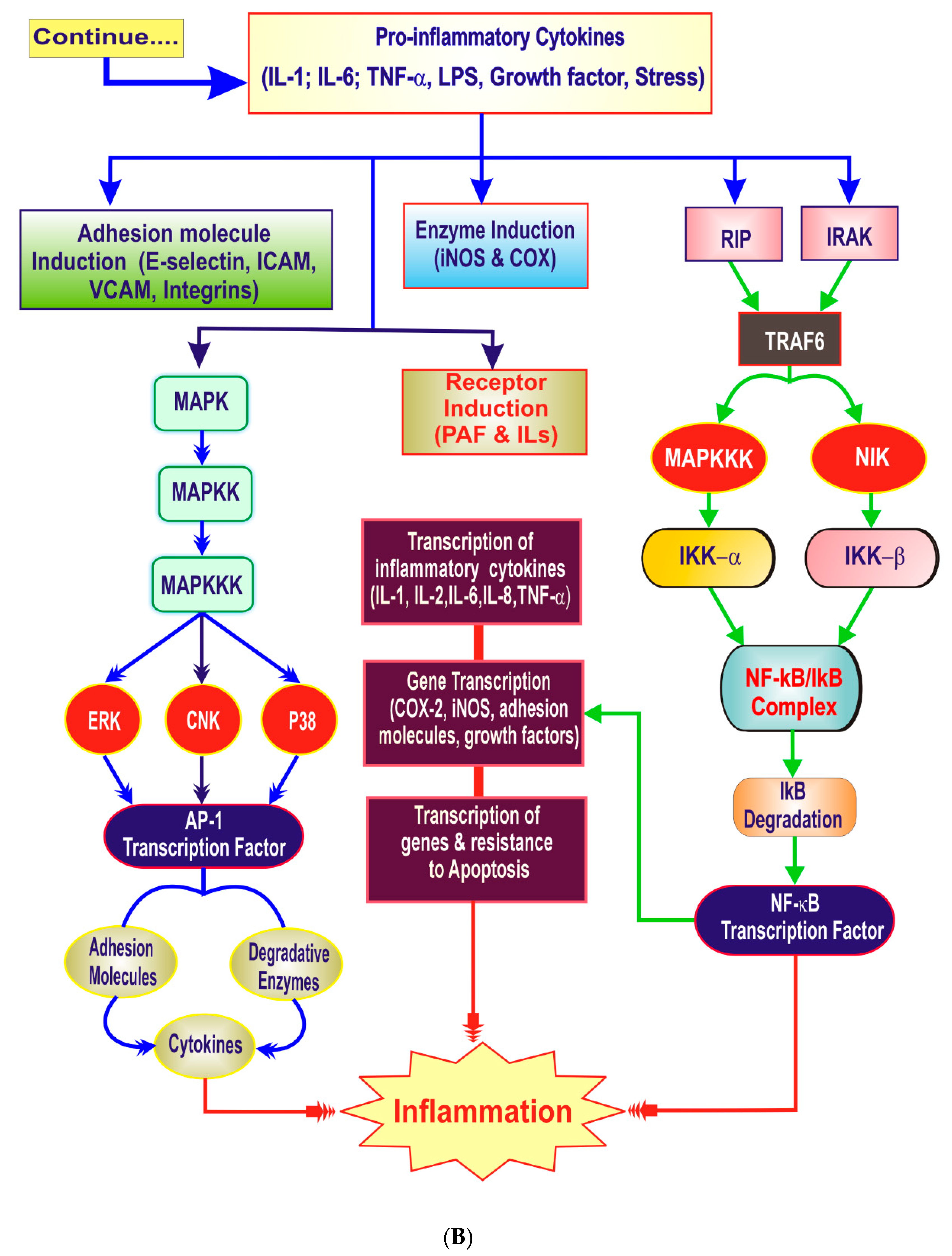
3. The Inflammatory Cascade
4. Key Players of Inflammation
4.1. Lipid Derived Mediators
4.2. Proinflammatory Cytokines
4.3. Vasoactive Mediators
4.4. Hydrolytic Enzymes
4.5. Reactive Oxygen Species (ROS)
4.6. Transcription Factors
4.7. Complement System
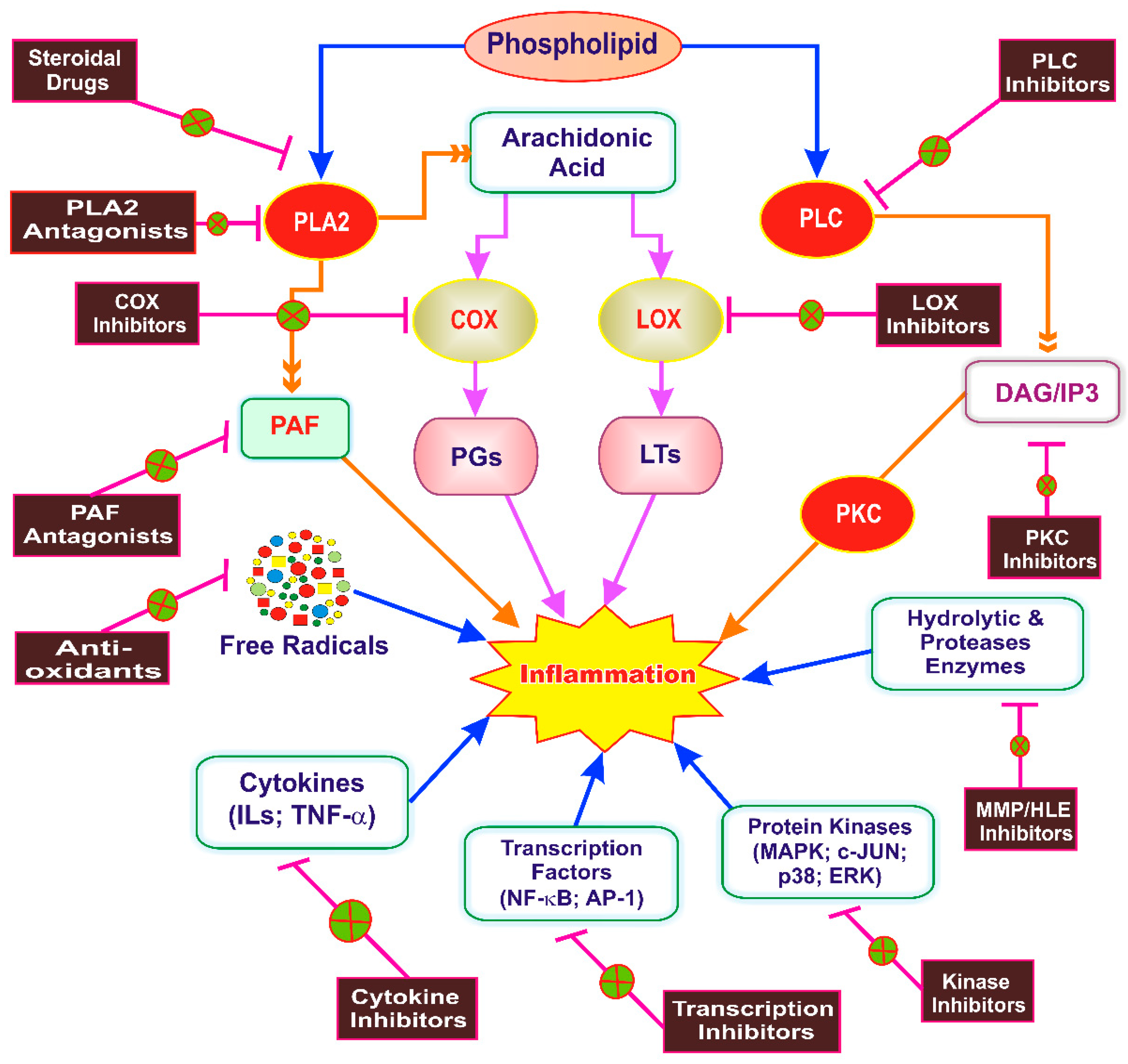
5. Inflammation as a Therapeutic Target of Phytoconstituents
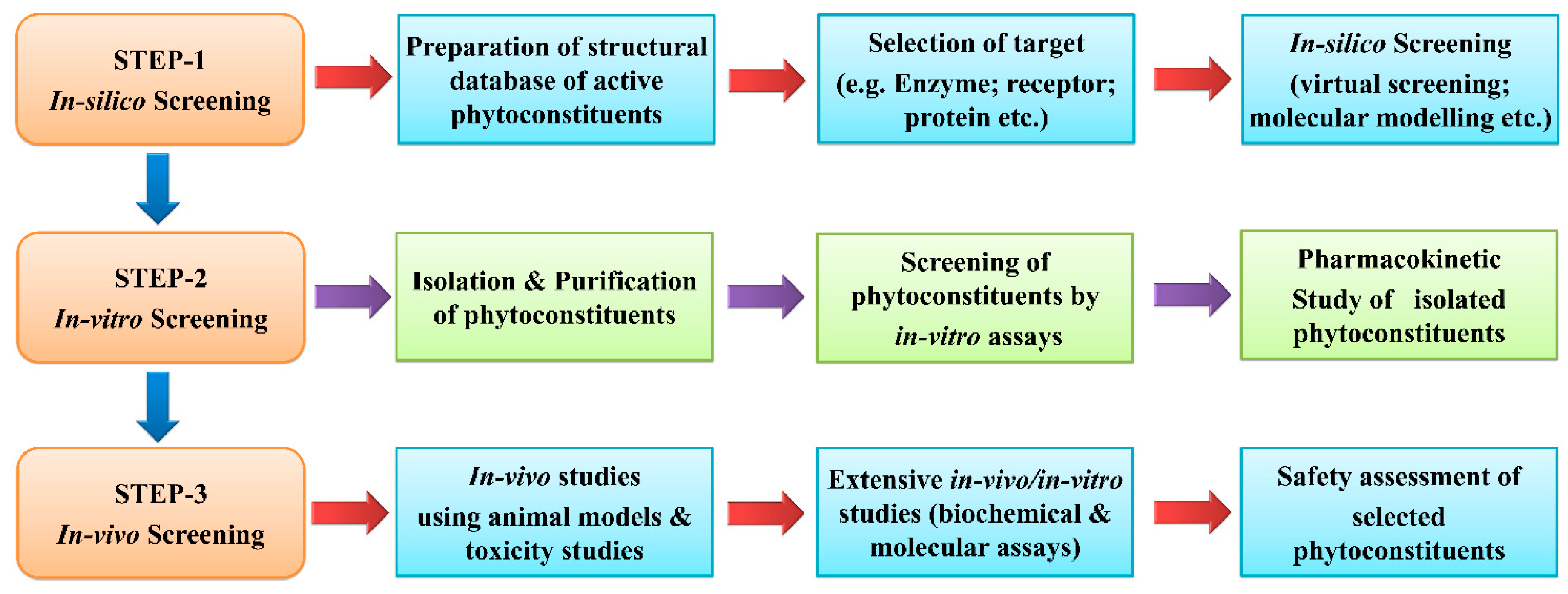
6. Anti-Inflammatory Drug Discovery from Phytoconstituents: Current Status and Systemic Approach
7. Anti-Inflammatory Drug Development from Natural Products: Current Status and Systematic Approach in Use of Different Animal Models for Evaluations
7.1. Animal Models and Mechanisms for Screening of Anti-Inflammatory Activity
7.1.1. Acute Inflammation
7.1.2. Sub-Acute Inflammation
7.1.3. Chronic Inflammation
7.2. Advantages and Limitations of Animal Models of Inflammation
7.2.1. Carrageenan-Induced Paw Edema
- Advantages: Widely used and well-established working model of the inflammation [109]. Inflammation-induced by carrageenan is acute, non-immune, and reproducible [109]. Involvement of multiple mechanisms allow this model as a preliminary test for the screening of anti-inflammatory drugs [160]. Biphasic response after subplantar carrageenan injection enable this model to predict the probable biological targets of test drug in the inflammation. This model is sensitive to cyclooxygenase inhibitors and suitable for the assessment of NSAIDs that act by the cyclooxygenase inhibition which is involved in prostaglandin synthesis [109,161].
- Limitations: To eradicate the effects of stress, animals should be acclimatized at least one week before the commencement of an experiment [162]. The investigator should be trained to record the stable and reproducible paw volumes using sophisticated equipment like plethysmometer. Rise in paw edema is based on the concentration of injected carrageenan. Typically the maximum edema response produced by carrageenan is too difficult to inhibit. Therefore, the carrageenan type and preparation of its solution needs careful attention [160,162].
7.2.2. Histamine/5-HT-induced Paw Edema
- Advantages: Histamine/5-HT-induced paw edema methods are convenient to appraise the acute anti-inflammatory effect of substances. These models can be used as secondary models to authorize the results of carrageenan-induced paw edema model, especially for those drugs that showed effect at the first phase of carrageenan-induced inflammation. These models are suitable for the assessment of those drugs that act through the histamine and/or 5-HT inhibition [163,164].
- Limitations: Inflammation or paw edema induced by injection of histamine or 5-HT is minimal and transient. These models are rather inappropriate for the assessment of drugs like prostaglandin inhibitors, which act by the mechanisms excluding histamine and/or 5-HT [163].
7.2.3. Bradykinin-induced Paw Edema
- Advantages: It is an animal model of the acute inflammation. Results of this model can be correlated with the results of carrageenan-induced paw edema model. Drugs inhibiting prostaglandins are effective in this model.
7.2.4. Dextran-induced Edema
- Advantages: This model includes both histamine and the serotonin for the induction of edema. Therefore, it is suitable to assess the anti-inflammatory effect of anti-histaminic or anti-serotonin drugs. This model is employed to reinforce the results of carrageenan-induced paw inflammation model.
7.2.5. Lipopolysaccharide-Induced Paw Edema
- Advantages: This model is very suitable for the recognition of anti-inflammatory agents that acts through cytokine modulation [129,165]. Besides, paw inflammation, lipopolysaccharide also induces inflammatory hyperalgesia. Therefore, this model is advantageous for the simultaneous assessment of analgesic and anti-inflammatory drugs [128].
7.2.6. Arachidonic Acid-Induced Ear Edema
- Advantages: Valid model of topical acute inflammation. This is beneficial for the recognition of anti-inflammatory compounds that acts through eicosanoids inhibition.
7.2.7. Croton Oil/TPA-Induced Ear Edema
- Advantages: These models are appropriate for the screening of steroidal and non-steroidal anti-inflammatory drugs. In the TPA induced ear edema model more potent and less potent results are linked with COX and LOX inhibitors, respectively.
- Limitations: As multiple mechanisms are involved in the croton oil/TPA induced ear edema model, it is rational to use these models to only predict rather than to approve the mode of action of anti-inflammatory compounds. Usually, animals are sacrificed at the completion of the experimental protocol to harvest the tissue samples and the auricular lymph nodes for detailed investigations [130,132].
7.2.8. Oxazolone-Induced Ear Edema
- Advantages: Well recognized model of delayed-type hypersensitivity (immune inflammation).
7.2.9. Acetic Acid/ Compound 48/80-Induced Vascular Permeability
- Advantages: Appropriate experimental model for the assessment of acute anti-inflammatory effect. The ant-inflammatory activity of drugs against compound 48/80-induced vascular permeability is linked with mast cell stabilization or anti-histaminic activity of drugs.
7.2.10. Pleurisy Tests
- Advantages: These tests are suitable to screen the acute anti-inflammatory activity. This test enables to evaluate the inflammatory phenomenon like fluid extravasation, the leukocyte migration and biochemical parameters in the exudate.
7.2.11. Granuloma Pouch Model
- Advantages: Model of sub-acute inflammation. It is beneficial to administer the test substances into the air pouch because it causes direct contact of test compounds with the target cells.
- Limitations: Subcutaneous injection of a large volume of air induces pain to experimental animals. The procedure requires anesthesia. Usually, animals are sacrificed at the end of the experiment [144].
7.2.12. Cotton Pellet-Induced Granuloma
- Advantages: Widely used model of the chronic inflammation. Wet/moist weight of granuloma correlates with the amount of transudate. The dry weight of granuloma relates with the granulomatous tissue formation. Generally, steroidal drugs inhibit dry weight of granuloma. The biochemical analysis of granuloma provides information about the proliferative changes occurring due to chronic irritation and inflammation. Hence, this model provides a window to additional markers of the inflammatory process.
- Limitations: Implantation and removal of cotton pellet and granuloma needs anaesthetics and surgical skills. The implantations may cause localized sepsis and this may confound the observations. Repeated handling of the animals, removal of surgical stitches, and need of sacrifice of the animals to collect the granuloma are other shortcomings of this model [150,151].
7.2.13. Formalin-Induced Paw Edema
- Advantages: This is the experimental model of chronic inflammation that closely resemble human arthritis. Depending on the capacity of the drug to act on the neurogenic phase, the inflammatory phase or on both the phases it is possible to predict the involvement of central or peripheral components in the anti-inflammatory effect of drugs.
7.2.14. CFA-Induced Arthritis
- Advantages: Well-characterized model of the chronic inflammation and arthritic alterations. Though seronegative in nature, CFA-induced inflammation involves immune-inflammatory components. Inflammation in the CFA-injected (primary lesions) and non-injected (secondary lesions) paws represent the clinical symptoms of human inflammation and arthritis, respectively. This model can be used to assess the effect of test drugs against acute, chronic and immune-inflammatory and arthritic conditions.
- Limitations: Preparation and induction of CFA may affect the severity of arthritic response and therefore need careful attention. The evaluations need sophisticated instruments like plethysmometer and Von-Frey apparatus to measure alterations in the paw volume and pain threshold. As the experimental duration is more and it involves painful and inflammatory pathology, it is stressful to the animals. Generally, animals are sacrificed at the completion of the protocol [154,157].
7.3. In-Vitro Detection of Inflammatory Biomarkers
7.4. Biomarkers for Prediction of Side Effects of Anti-Inflammatory Drugs
7.4.1. Biomarkers for Prediction of Gastrointestinal Side Effects
7.4.2. Biomarkers for Prediction of Cardiovascular Side Effects
7.4.3. Metabolomics for Prediction of Side Effects
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 12-HETE | 12-Hydroxyeicosatetraenoic acid |
| 12-HPETE | 5-hydroperoxyeicosatetraenoic acid |
| 5-HT | 5-hydroxytryptamine |
| AA | Arachidonic acid |
| Akt | Serine/threonine-specific protein kinase |
| AP-1 | Activator protein 1 |
| COX | Cyclooxygenase |
| DAG | Diacylglycerol |
| EETs | Epoxyeicosatrienic acids |
| EIA | Enzyme immunoassay |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinase; |
| HLE | Human leukocyte elastase |
| IFN-γ | Interferon-γ |
| IKK | IκB kinase |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IP3 | Inositol triphosphate |
| IRAK | Interleukin-1 receptor-associated kinase |
| JNKs | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| LPX | Lipoxygenase |
| LTB4 | Leukotriene B4 |
| LTs | Leukotrienes |
| LXs | Lipoxins |
| MAPKs | Mitogen-activated protein kinases |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MMPs | Matrix metalloproteinases |
| MPO | Myeloperoxidase |
| NF-κB | Nuclear factor kappa-beta |
| NIK | NF-κB inducing kinase |
| NO | Nitric oxide |
| NOS-2 | Nitric oxide synthase-2 |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| p38 kinase | p38 mitogen-activated protein kinases |
| PAF | Platelet activating factor |
| PGE2 | Prostaglandin E2 |
| PGs | Prostaglandins |
| PI3K | Phosphatidylinositol-3-kinase |
| PKC | Protein kinase C |
| PLA2 | Phospholipase A2 |
| PLC | Phospholipase C |
| PMNL | Polymorphonuclear leucocyte |
| RIP | Receptor-interacting protein |
| ROS | Reactive oxygen species |
| TBX | Thromboxane |
| TNF-α | Tumor necrosis factor alpha |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
References
- Dewanjee, S.; Dua, T.K.; Sahu, R. Potential anti-inflammatory effect of Leea macrophylla Roxb. leaves: A wild edible plant. Food Chem. Toxicol. 2013, 59, 514–520. [Google Scholar] [CrossRef]
- Kulkarni, R.G.; Achaiah, G.; Sastry, G.N. Novel targets for antiinflammatory and antiarthritic agents. Curr. Pharm. Des. 2006, 12, 2437–2454. [Google Scholar] [CrossRef]
- Calixto, J.B.; Otuki, M.F.; Santos, A.R.S. Anti-Inflammatory Compounds of Plant Origin. Part I. Action on Arachidonic Acid Pathway, Nitric Oxide and Nuclear Factor κ B (NF-κB). Planta Med. 2003, 69, 973–983. [Google Scholar]
- Chung, H.-J.; Lee, H.-S.; Shin, J.-S.; Lee, S.-H.; Park, B.-M.; Youn, Y.-S.; Lee, S.K. Modulation of acute and chronic inflammatory processes by a traditional medicine preparation GCSB-5 both in vitro and in vivo animal models. J. Ethnopharmacol. 2010, 130, 450–459. [Google Scholar] [CrossRef]
- Simmons, D.L. What makes a good anti-inflammatory drug target? Drug Discov. Today 2006, 11, 210–219. [Google Scholar] [CrossRef]
- Debnath, S.; Ghosh, S.; Hazra, B. Inhibitory effect of Nymphaea pubescens Willd. flower extract on carrageenan-induced inflammation and CCl4-induced hepatotoxicity in rats. Food Chem. Toxicol. 2013, 59, 485–491. [Google Scholar] [CrossRef]
- Fangkrathok, N.; Junlatat, J.; Sripanidkulchai, B. In vivo and in vitro anti-inflammatory activity of Lentinus polychrous extract. J. Ethnopharmacol. 2013, 147, 631–637. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef]
- Divya, T.; Latha, P.; Usha, K.; Anuja, G.; Suja, S.; Shyamal, S.; Shine, V.; Sini, S.; Shikha, P.; Rajasekharan, S. Anti-inflammatory, analgesic and anti-lipid peroxidative properties of Wattakaka volubilis (Linn. f.) Stapf. Indian J. Nat. Prod. Resour. 2009, 8, 137–141. [Google Scholar]
- Jo, W.S.; Yang, K.M.; Choi, Y.J.; Jeong, C.H.; Ahn, K.J.; Nam, B.H.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. In vitro and in vivo anti-inflammatory effects of pegmatite. Mol. Cell. Toxicol. 2010, 6, 195–202. [Google Scholar] [CrossRef]
- Sofidiya, M.O.; Imeh, E.; Ezeani, C.; Aigbe, F.R.; Akindele, A.J. Antinociceptive and anti-inflammatory activities of ethanolic extract of Alafia barteri. Rev. Bras. De Farm. 2014, 24, 348–354. [Google Scholar] [CrossRef]
- Kumari, K.; Weerakoon, T.; Handunnetti, S.; Samarasinghe, K.; Suresh, T. Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 2014, 151, 1202–1208. [Google Scholar] [CrossRef]
- Qandil, A.M. Prodrugs of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), More Than Meets the Eye: A Critical Review. Int. J. Mol. Sci. 2012, 13, 17244–17274. [Google Scholar] [CrossRef]
- De Oliveira, R.G.; Mahon, C.P.A.N.; Ascêncio, P.G.M.; Ascêncio, S.D.; Balogun, S.O.; Martins, D.T.D.O. Evaluation of anti-inflammatory activity of hydroethanolic extract of Dilodendron bipinnatum Radlk. J. Ethnopharmacol. 2014, 155, 387–395. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Gorzalczany, S.; López, P.; Acevedo, C.; Ferraro, G. Anti-inflammatory effect of Lithrea molleoides extracts and isolated active compounds. J. Ethnopharmacol. 2011, 133, 994–998. [Google Scholar] [CrossRef]
- Uddin, G.; Rauf, A.; Siddiqui, B.S.; Muhammad, N.; Khan, A.; Shah, S.U.A.; Rauf, D.A. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine 2014, 21, 954–959. [Google Scholar] [CrossRef]
- Nagori, K.; Singh, M.K.; Dewangan, D.; Verma, V.; Tripathi, D. Anti-inflammatory activity and chemo profile of plants used in traditional medicine: A review. J. Chem. Pharm. Res. 2010, 2, 122–130. [Google Scholar]
- Bellik, Y.; Boukraa, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular Mechanism Underlying Anti-Inflammatory and Anti-Allergic Activities of Phytochemicals: An Update. Molecules 2012, 18, 322–353. [Google Scholar] [CrossRef]
- Lipsky, P.E. The clinical potential of cyclooxygenase-2–specific inhibitors. Am. J. Med. 1999, 106, 51S–57S. [Google Scholar] [CrossRef]
- Gupta, M.; Mazumder, U.K.; Gomathi, P.; Selvan, V.T. Antiinflammatory evaluation of leaves of Plumeria acuminata. BMC Complement. Altern. Med. 2006, 6, 36. [Google Scholar] [CrossRef]
- Eddouks, M.; Chattopadhyay, D.; Zeggwagh, N.A. Animal Models as Tools to Investigate Antidiabetic and Anti-Inflammatory Plants. Evid.-Based Complement. Altern. Med. 2012, 2012, 142087. [Google Scholar] [CrossRef]
- Roome, T.; Dar, A.; Naqvi, S.; Ali, S.; Choudhary, M.I. Aegiceras corniculatum extract suppresses initial and late phases of inflammation in rat paw and attenuates the production of eicosanoids in rat neutrophils and human platelets. J. Ethnopharmacol. 2008, 120, 248–254. [Google Scholar] [CrossRef]
- Dubois, C.; Abeele, F.V.; Lehen’Kyi, V.; Gkika, D.; Guarmit, B.; Lepage, G.; Slomianny, C.; Borowiec, A.S.; Bidaux, G.; Benahmed, M.; et al. Remodeling of Channel-Forming ORAI Proteins Determines an Oncogenic Switch in Prostate Cancer. Cancer Cell 2014, 26, 19–32. [Google Scholar] [CrossRef]
- Stables, M.J.; Gilroy, D.W. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 2011, 50, 35–51. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Benito, P.B.; Lanza, A.M.D.; Sen, A.M.S.; Matellano, L.F.; Gómez, A.S.; Martínez, M.J.A.; Galindez, J.D.S. Effects of Some Iridoids from Plant Origin on Arachidonic Acid Metabolism in Cellular Systems. Planta Med. 2000, 66, 324–328. [Google Scholar] [CrossRef]
- Safayhi, H.; Sailer, E.-R. Anti-Inflammatory Actions of Pentacyclic Triterpenes. Planta Med. 1997, 63, 487–493. [Google Scholar] [CrossRef]
- Busse, W.W. Leukotrienes and inflammation. Am. J. Respir. Crit. Care Med. 1998, 157, S210–S213. [Google Scholar] [CrossRef]
- Schinella, G.; Máñez, S.; Ríos, J.L.; Prieto, J.M.; Giner, R.M.; Recio, M.C. Diphyllin Acetylapioside, A 5-Lipoxygenase Inhibitor from Haplophyllum hispanicum. Planta Med. 2002, 68, 359–360. [Google Scholar]
- Cho, J.Y.; Yoo, E.S.; Baik, K.U.; Park, M.H.; Han, B.H. In Vitro Inhibitory Effect of Protopanaxadiol Ginsenosides on Tumor Necrosis Factor (TNF)-α Production and its Modulation by Known TNF-α Antagonists. Planta Med. 2001, 67, 213–218. [Google Scholar] [CrossRef]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Larsen, G.L.; Henson, P.M. Mediators of inflammation. Annu. Rev. Immunol. 1983, 1, 335–359. [Google Scholar] [CrossRef]
- Hersh, E.M.; Bodey, G.P. Leukocytic Mechanisms in Inflammation. Annu. Rev. Med. 1970, 21, 105–132. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease; Elsevier Health Sciences: London, UK, 2014. [Google Scholar]
- Anderson, M.T.; Staal, F.; Gitler, C.; Herzenberg, L.A. Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 11527–11531. [Google Scholar] [CrossRef]
- Flohé, L.; Brigelius-Flohé, R.; Saliou, C.; Traber, M.G.; Packer, L. Redox Regulation of NF-kappa B Activation. Free. Radic. Boil. Med. 1997, 22, 1115–1126. [Google Scholar] [CrossRef]
- Heras, B.D.L.; Hortelano, S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy-Drug Targets 2009, 8, 28–39. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, J.K.; Noh, M.S.; Hwang, B.Y.; Hong, Y.S.; Lee, J.J. Anti-inflammatory effect of kamebakaurin in in vivo animal models. Planta Med. 2004, 70, 526–530. [Google Scholar] [CrossRef]
- Guo, R.-F.; Ward, P.A. role of C5A in inflammatory responses. Annu. Rev. Immunol. 2005, 23, 821–852. [Google Scholar] [CrossRef]
- MacGlashan, D. Histamine: A mediator of inflammation. J. Allergy Clin. Immunol. 2003, 112, 53. [Google Scholar] [CrossRef]
- Maleki, N.; Nayebi, A.M.; Garjani, A. Effects of central and peripheral depletion of serotonergic system on carrageenan-induced paw oedema. Int. Immunopharmacol. 2005, 5, 1723–1730. [Google Scholar] [CrossRef]
- Laupattarakasem, P.; Houghton, P.; Hoult, J.; Itharat, A. An evaluation of the activity related to inflammation of four plants used in Thailand to treat arthritis. J. Ethnopharmacol. 2003, 85, 207–215. [Google Scholar] [CrossRef]
- Higgs, G.; Moncada, S.; Salmon, J.A.; Seager, K. The source of thromboxane and prostaglandins in experimental inflammation. Br. J. Pharmacol. 1983, 79, 863–868. [Google Scholar] [CrossRef]
- Chung, K.; Barnes, P. Platelet-activating factor: A potent mediator of inflammation. Postgrad. Med. J. 1989, 65, 420–421. [Google Scholar] [CrossRef]
- Cabellos, C.; E Macintyre, D.; Forrest, M.; Burroughs, M.; Prasad, S.; Tuomanen, E. Differing roles for platelet-activating factor during inflammation of the lung and subarachnoid space. The special case of Streptococcus pneumoniae. J. Clin. Investig. 1992, 90, 612–618. [Google Scholar] [CrossRef]
- Keeble, J.; Blades, M.; Pitzalis, C.; Da Rocha, F.A.C.; Brain, S.D. The role of substance P in microvascular responses in murine joint inflammation. Br. J. Pharmacol. 2005, 144, 1059–1066. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkubo, T.; Takahashi, H.; Inoki, R. Interaction of bradykinin with substance P on vascular permeability and pain response. Jpn. J. Pharmacol. 1986, 41, 427–429. [Google Scholar] [CrossRef]
- Pan, Z.K.; Zuraw, B.L.; Lung, C.C.; Prossnitz, E.R.; Browning, D.D.; Ye, R.D. Bradykinin stimulates NF-kappaB activation and interleukin 1beta gene expression in cultured human fibroblasts. J. Clin. Investig. 1996, 98, 2042–2049. [Google Scholar] [CrossRef]
- Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [CrossRef]
- Saukkonen, K.; Sande, S.; Cioffe, C.; Wolpe, S.; Sherry, B.; Cerami, A.; Tuomanen, E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J. Exp. Med. 1990, 171, 439–448. [Google Scholar] [CrossRef]
- Levi, M. Current understanding of disseminated intravascular coagulation. Br. J. Haematol. 2004, 124, 567–576. [Google Scholar] [CrossRef]
- Strande, J.L.; Phillips, S.A. Thrombin increases inflammatory cytokine and angiogenic growth factor secretion in human adipose cells in vitro. J. Inflamm. 2009, 6, 4. [Google Scholar] [CrossRef]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef]
- Sautebin, L. Prostaglandins and nitric oxide as molecular targets for anti-inflammatory therapy. Fitoterapia 2000, 71, S48–S57. [Google Scholar] [CrossRef]
- Conner, E.M.; Grisham, M.B. Inflammation, free radicals, and antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef]
- Okoli, C.O.; Akah, P.A. Mechanisms of the anti-inflammatory activity of the leaf extracts of Culcasia scandens P. Beauv (Araceae). Pharmacol. Biochem. Behav. 2004, 79, 473–481. [Google Scholar] [CrossRef]
- Dahanukar, S.; Kulkarni, R.; Rege, N. Pharmacology of medicinal plants and natural products. Indian J. Pharmacol. 2000, 32, S81–S118. [Google Scholar]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Selendang Ayu Oil Spill Lessons Learn. 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Mitra, S.; Kar, S.; Surajlata, K.; Banerjee, E.R. Screening of Novel Natural Product Derived Compounds for Drug Discovery in Inflammation. J. Plant Biochem. Physiol. 2016, 3, 159. [Google Scholar] [CrossRef]
- Paul, A.T.; Gohil, V.M.; Bhutani, K.K. Modulating TNF-α signaling with natural products. Drug Discov. Today 2006, 11, 725–732. [Google Scholar] [CrossRef]
- Oh, S.-W.; Cha, J.-Y.; Jung, J.-E.; Chang, B.-C.; Kwon, H.-J.; Lee, B.-R.; Kim, D.-Y. Curcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-κB inhibition. J. Ethnopharmacol. 2011, 136, 414–421. [Google Scholar] [CrossRef]
- Chan, M.M. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem. Pharmacol. 1995, 49, 1551–1556. [Google Scholar] [CrossRef]
- Cao, H.; Yu, R.; Choi, Y.; Ma, Z.-Z.; Zhang, H.; Xiang, W.; Lee, D.Y.-W.; Berman, B.M.; Moudgil, K.D.; Fong, H.H.; et al. Discovery of cyclooxygenase inhibitors from medicinal plants used to treat inflammation. Pharmacol. Res. 2010, 61, 519–524. [Google Scholar] [CrossRef]
- Kumari, R.; Meyyappan, A.; Selvamani, P.; Mukherjee, J.; Jaisankar, P. Lipoxygenase inhibitory activity of crude bark extracts and isolated compounds from Commiphora berryi. J. Ethnopharmacol. 2011, 138, 256–259. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, P.S.; Park, J.; Yoo, E.S.; Baik, K.U.; Kim, Y.-K.; Park, M.H. Inhibitor of tumor necrosis factor-α production in lipopolysaccharide-stimulated RAW264.7 cells from Amorpha fruticosa. J. Ethnopharmacol. 2000, 70, 127–133. [Google Scholar] [CrossRef]
- Scuro, L.S.; Simioni, P.U.; Grabriel, D.L.; Saviani, E.E.; Modolo, L.V.; Tamashiro, W.M.S.C.; Salgado, I. Suppression of nitric oxide production in mouse macrophages by soybean flavonoids accumulated in response to nitroprusside and fungal elicitation. BMC Biochem. 2004, 5, 5. [Google Scholar] [CrossRef][Green Version]
- Ko, H.-H.; Weng, J.-R.; Tsao, L.-T.; Yen, M.-H.; Wang, J.-P.; Lin, C.-N. Anti-inflammatory flavonoids and pterocarpanoid from Crotalaria pallida and C. assamica. Bioorganic Med. Chem. Lett. 2004, 14, 1011–1014. [Google Scholar] [CrossRef]
- Bernardes, N.R.; Heggdorne-Araújo, M.; Borges, I.F.; Almeida, F.M.; Amaral, E.P.; Lasunskaia, E.B.; Muzitano, M.F.; Oliveira, D.B. Nitric oxide production, inhibitory, antioxidant and antimycobacterial activities of the fruits extract and flavonoid content of Schinus terebinthifolius. Rev. Bras. De Farm. 2014, 24, 644–650. [Google Scholar] [CrossRef]
- Sharififar, F.; Dehghn-Nudeh, G.; Mirtajaldini, M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009, 112, 885–888. [Google Scholar] [CrossRef]
- Ruangnoo, S.; Jaiaree, N.; Makchuchit, S.; Panthong, S.; Thongdeeying, P.; Itharat, A. An in vitro inhibitory effect on RAW 264.7 cells by anti-inflammatory compounds from Smilax corbularia Kunth. Asian Pac. J. Allergy Immunol. 2012, 30, 268. [Google Scholar]
- Abad, M.J.; Bermejo, P.; Villar, A. The activity of flavonoids extracted from Tanacetum microphyllum DC. (Compositae) on soybean lipoxygenase and prostaglandin synthetase. Gen. Pharmacol. Vasc. Syst. 1995, 26, 815–819. [Google Scholar] [CrossRef]
- Guabiraba, R.; Campanha-Rodrigues, A.L.; Souza, A.L.; Santiago, H.C.; Lugnier, C.; Alvarez-Leite, J.; Lemos, V.S.; Teixeira, M.M. The flavonoid dioclein reduces the production of pro-inflammatory mediators in vitro by inhibiting PDE4 activity and scavenging reactive oxygen species. Eur. J. Pharmacol. 2010, 633, 85–92. [Google Scholar] [CrossRef]
- Xagorari, A.; Papapetropoulos, A.; Mauromatis, A.; Economou, M.; Fotsis, T.; Roussos, C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 2001, 296, 181–187. [Google Scholar]
- Moscatelli, V.; Hnatyszyn, O.; Acevedo, C.; Megías, J.; Alcaraz, M.; Ferraro, G. Flavonoids from Artemisia copa with Anti-Inflammatory Activity. Planta Med. 2006, 72, 72–74. [Google Scholar] [CrossRef]
- Wang, G.-J.; Chen, Y.-M.; Wang, T.-M.; Lee, C.-K.; Chen, K.-J.; Lee, T.-H. Flavonoids with iNOS inhibitory activity from Pogonatherum crinitum. J. Ethnopharmacol. 2008, 118, 71–78. [Google Scholar] [CrossRef]
- Li, Y.-C.; Yeh, C.-H.; Yang, M.-L.; Kuan, Y.-H. Luteolin Suppresses Inflammatory Mediator Expression by Blocking the Akt/NFκB Pathway in Acute Lung Injury Induced by Lipopolysaccharide in Mice. Evidence-Based Complement. Altern. Med. 2011, 2012, 1–8. [Google Scholar] [CrossRef]
- Grace-Lynn, C.; Darah, I.; Chen, Y.; Latha, L.Y.; Jothy, S.L.; Sasidharan, S. In Vitro Antioxidant Activity Potential of Lantadene A, a Pentacyclic Triterpenoid of Lantana Plants. Molecules 2012, 17, 11185–11198. [Google Scholar] [CrossRef]
- Loke, W.M.; Proudfoot, J.M.; Stewart, S.; McKinley, A.J.; Needs, P.W.; Kroon, P.A.; Hodgson, J.M.; Croft, K.D. Metabolic transformation has a profound effect on anti-inflammatory activity of flavonoids such as quercetin: Lack of association between antioxidant and lipoxygenase inhibitory activity. Biochem. Pharmacol. 2008, 75, 1045–1053. [Google Scholar] [CrossRef]
- Saragusti, A.C.; Ortega, M.G.; Cabrera, J.L.; Estrin, D.A.; Marti, M.A.; Chiabrando, G.A. Inhibitory effect of quercetin on matrix metalloproteinase 9 activity Molecular mechanism and structure–activity relationship of the flavonoid–enzyme interaction. Eur. J. Pharmacol. 2010, 644, 138–145. [Google Scholar] [CrossRef]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Koo, H.-N.; Na, H.-J.; Kim, M.-S.; Hong, S.-H.; Eom, J.-W.; Kim, K.-S.; Shin, T.-Y.; Kim, H.-M. inhibition of tnf-α and il-6 production by aucubin through blockade of nf-κB activation in rbl-2H3 mast cells. Cytokine 2002, 18, 252–259. [Google Scholar] [CrossRef]
- Burnett, B.; Jia, Q.; Zhao, Y.; Levy, R. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J. Med. Food 2007, 10, 442–451. [Google Scholar] [CrossRef]
- Facino, R.M.; Carini, M.; Stefani, R.; Aldini, G.; Saibene, L. Anti-Elastase and Anti-Hyaluronidase Activities of Saponins and Sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: Factors Contributing to their Efficacy in the Treatment of Venous Insufficiency. Arch. Der Pharm. 1995, 328, 720–724. [Google Scholar] [CrossRef]
- Diaz, A.M.; Abad, M.J.; Fernández, L.; Recuero, C.; Villaescusa, L.; Silván, A.M.; Bermejo, P. In Vitro Anti-Inflammatory Activity of Iridoids and Triterpenoid Compounds Isolated from Phillyrea latifolia L. Boil. Pharm. Bull. 2000, 23, 1307–1313. [Google Scholar] [CrossRef]
- Reddy, A.M.; Lee, J.-Y.; Seo, J.H.; Kim, B.H.; Chung, E.Y.; Ryu, S.Y.; Kim, Y.S.; Lee, C.-K.; Min, K.R.; Kim, Y. Artemisolide from Artemisia asiatica: Nuclear Factor-κB (NF-κB) inhibitor suppressing prostaglandin E2 and nitric oxide production in macrophages. Arch. Pharmacal Res. 2006, 29, 591–597. [Google Scholar] [CrossRef]
- Han, J.W.; Lee, B.G.; Kim, Y.K.; Yoon, J.W.; Jin, H.K.; Hong, S.; Lee, H.Y.; Lee, K.R.; Lee, H.W. Ergolide, sesquiterpene lactone from Inula britannica, inhibits inducible nitric oxide synthase and cyclo-oxygenase-2 expression in RAW 264.7 macrophages through the inactivation of NF-κB. Br. J. Pharmacol. 2001, 133, 503–512. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Lignan constituents of Tilia amurensis and their biological evaluation on antitumor and anti-inflammatory activities. Food Chem. Toxicol. 2012, 50, 3680–3686. [Google Scholar] [CrossRef]
- Liao, C.-R.; Ho, Y.-L.; Huang, G.-J.; Yang, C.S.; Chao, C.-Y.; Chang, Y.-S.; Kuo, Y.-H. One Lignanoid Compound and Four Triterpenoid Compounds with Anti-Inflammatory Activity from the Leaves of Elaeagnus oldhamii Maxim. Molecules 2013, 18, 13218–13227. [Google Scholar] [CrossRef]
- Hernandez, V.; Mánez, S.; Recio, M.; Giner, R.; Rios, J. Anti-inflammatory profile of dehydrocostic acid, a novel sesquiterpene acid with a pharmacophoric conjugated diene. Eur. J. Pharm. Sci. 2005, 26, 162–169. [Google Scholar] [CrossRef]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, J.C.; Hyun, C.-G.; Lee, N.H. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J. Med. Plants Res. 2009, 3, 914–920. [Google Scholar]
- Huang, J.; Wang, Y.; Li, C.; Wang, X.; He, X. Anti-Inflammatory Oleanolic Triterpenes from Chinese Acorns. Molecules 2016, 21, 669. [Google Scholar] [CrossRef]
- Raju, R.; Gunawardena, D.; Ahktar, M.A.; Low, M.; Reddell, P.; Münch, G. Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules 2016, 21, 1521. [Google Scholar] [CrossRef]
- Mooi, L.Y.; Yew, W.T.; Hsum, Y.W.; Soo, K.K.; Hoon, L.S.; Chieng, Y.C. Suppressive effect of maslinic acid on PMA-induced protein kinase C in human B-lymphoblastoid cells. Asian Pac. J. Cancer Prev. 2012, 13, 1177–1182. [Google Scholar] [CrossRef]
- Campana, P.; Coleman, C.; Sousa, L.; Teixeira, M.; Ferreira, D.; Braga, F. Faculdade de Farmácia Universidade Federal de Minas Gerais Belo Horizonte MG–Brazil; Department of Biomolecular Sciences University of Mississippi University United States Mansoin F: TNF-α Inhibiting Heterotrimeric Flavonoid from Mansoa Hirsuta DC. Planta Med. 2016, 82, 5. [Google Scholar]
- Sagrawat, H.; Mann, A.; Kharya, M. Pharmacological potential of Eugenia jambolana: A review. Pharmacogn. Mag. 2006, 2, 96. [Google Scholar]
- Jachak, S.M.; Saklani, A. Challenges and opportunities in drug discovery from plants. Curr. Sci. 2007, 92, 1251. [Google Scholar]
- Pan, S.Y.; Pan, S.; Yu, Z.-L.; Ma, D.-L.; Chen, S.-B.; Fong, W.-F.; Han, Y.-F.; Ko, K.-M. New perspectives on innovative drug discovery: An overview. J. Pharm. Pharm. Sci. 2010, 13, 450–471. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Butterweck, V.; Nahrstedt, A. What Is the Best Strategy for Preclinical Testing of Botanicals? A Critical Perspective. Planta Med. 2012, 78, 747–754. [Google Scholar] [CrossRef]
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Boominathan, R.; Parimaladevi, B.; Mandal, S.; Ghoshal, S. Anti-inflammatory evaluation of Ionidium suffruticosam Ging. in rats. J. Ethnopharmacol. 2004, 91, 367–370. [Google Scholar] [CrossRef]
- Panthong, A.; Norkaew, P.; Kanjanapothi, D.; Taesotikul, T.; Anantachoke, N.; Reutrakul, V. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook f. J. Ethnopharmacol. 2007, 111, 335–340. [Google Scholar] [CrossRef]
- Mahajan, A.; Hardyal, S.; Tung, B. Effect of concurrent use of cimetidine and anti-inflammatory agents in experimental models of peptic ulcer and inflammation. Indian J. Pharmacol. 1984, 16, 132. [Google Scholar]
- Sarkhel, S. Evaluation of the anti-inflammatory activities of Quillaja saponaria Mol. saponin extract in mice. Toxicol. Rep. 2016, 3, 1–3. [Google Scholar] [CrossRef]
- Osadebe, P.; Okoye, F. Anti-inflammatory effects of crude methanolic extract and fractions of Alchornea cordifolia leaves. J. Ethnopharmacol. 2003, 89, 19–24. [Google Scholar] [CrossRef]
- Fernandez, A.; Alvarez, A.; García, M.; Sáenz, M. Anti-inflammatory effect of Pimenta racemosa var. ozua and isolation of the triterpene lupeol. Il Farm. 2001, 56, 335–338. [Google Scholar] [CrossRef]
- Duwiejua, M.; Woode, E.; Obiri, D. Pseudo-akuammigine, an alkaloid from Picralima nitida seeds, has anti-inflammatory and analgesic actions in rats. J. Ethnopharmacol. 2002, 81, 73–79. [Google Scholar] [CrossRef]
- Gepdiremen, A.; Mshvildadze, V.; Suleyman, H.; Elias, R. Acute and chronic antiinflammatory effects of Hedera colchica in rats. J. Ethnopharmacol. 2004, 94, 191–195. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiinflammatory Drugs. Exp. Boil. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Perianayagam, J.B.; Sharma, S.; Pillai, K. Anti-inflammatory activity of Trichodesma indicum root extract in experimental animals. J. Ethnopharmacol. 2006, 104, 410–414. [Google Scholar] [CrossRef]
- Patil, K.R.; Patil, C.R. Anti-inflammatory activity of bartogenic acid containing fraction of fruits of Barringtonia racemosa Roxb. in acute and chronic animal models of inflammation. J. Tradit. Complementary Med. 2017, 7, 86–93. [Google Scholar] [CrossRef]
- Vasudevan, M.; Gunnam, K.K.; Parle, M. Antinociceptive and anti-inflammatory effects of Thespesia populnea bark extract. J. Ethnopharmacol. 2007, 109, 264–270. [Google Scholar] [CrossRef]
- Singh, B.; Bani, S.; Gupta, D.; Chandan, B.; Kaul, A. Anti-inflammatory activity of ‘TAF’ an active fraction from the plant Barleria prionitis Linn. J. Ethnopharmacol. 2003, 85, 187–193. [Google Scholar] [CrossRef]
- Ben, I.O.; Etim, O.E.; Udo, N.M. Anti-inflammatory effects of Napoleona imperialis P. Beauv. (Lecythidaceae) on rat model of inflammation. Indian J. Health Sci. 2016, 9, 89. [Google Scholar] [CrossRef]
- Al-Haboubi, H.A.; Zeitlin, I.J. Re-appraisal of the role of histamine in carrageenan-induced paw oedema. Eur. J. Pharmacol. 1983, 88, 169–176. [Google Scholar] [CrossRef]
- Raud, J.; Konrad, D.; Dahlén, S.-E. Delayed anti-inflammatory action of nedocromil sodium in the rat paw is dependent on de novo protein synthesis. Eur. J. Pharmacol. 1995, 282, 207–211. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.; Matos, F.J. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Katz, L.B.; Theobald, H.M.; Bookstaff, R.C.; Peterson, R.E. Characterization of the enhanced paw edema response to carrageenan and dextran in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats. J. Pharmacol. Exp. Ther. 1984, 230, 670–677. [Google Scholar]
- Vilar, M.; de Souza, G.; Vilar, D.; Leite, J.; Raffin, F.; Barbosa-Filho, J.; Nogueira, F.; Rodrigues-Mascarenhas, S.; Moura, T. Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark. Molecules 2016, 21, 1087. [Google Scholar] [CrossRef]
- Coura, C.O.; Souza, R.B.; Rodrigues, J.A.G.; Vanderlei, E.D.S.O.; De Araújo, I.W.F.; Ribeiro, N.A.; Frota, A.F.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.A.; et al. Mechanisms Involved in the Anti-Inflammatory Action of a Polysulfated Fraction from Gracilaria cornea in Rats. PLoS ONE 2015, 10, e0119319. [Google Scholar] [CrossRef]
- Lo, T.N.; Almeida, A.P.; Beaven, M.A. Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J. Pharmacol. Exp. Ther. 1982, 221, 261–267. [Google Scholar]
- Babu, N.P.; Pandikumar, P.; Ignacimuthu, S. Anti-inflammatory activity of Albizia lebbeck Benth., an ethnomedicinal plant, in acute and chronic animal models of inflammation. J. Ethnopharmacol. 2009, 125, 356–360. [Google Scholar] [CrossRef]
- Calil, I.L.; Zarpelon, A.C.; Guerrero, A.T.; Alves-Filho, J.C.; Ferreira, S.H.; Cunha, F.Q.; Cunha, T.M.; Verri, W.A., Jr. Lipopolysaccharide induces inflammatory hyperalgesia triggering a TLR4/MyD88-dependent cytokine cascade in the mice paw. PLoS ONE 2014, 9, e90013. [Google Scholar] [CrossRef]
- Vajja, B.N.; Juluri, S.; Kumari, M.; Kole, L.; Chakrabarti, R.; Joshi, V.D. Lipopolysaccharide-induced paw edema model for detection of cytokine modulating anti-inflammatory agents. Int. Immunopharmacol. 2004, 4, 901–909. [Google Scholar] [CrossRef]
- Boller, S.; Soldi, C.; Marques, M.C.; Santos, E.P.; Cabrini, D.A.; Pizzolatti, M.G.; Zampronio, A.R.; Otuki, M.F. Anti-inflammatory effect of crude extract and isolated compounds from Baccharis illinita DC in acute skin inflammation. J. Ethnopharmacol. 2010, 130, 262–266. [Google Scholar] [CrossRef]
- Tamura, E.K.; Jimenez, R.S.; Waismam, K.; Gobbo-Neto, L.; Lopes, N.P.; Malpezzi-Marinho, E.A.; Marinho, E.A.; Farsky, S.H. Inhibitory effects of Solidago chilensis Meyen hydroalcoholic extract on acute inflammation. J. Ethnopharmacol. 2009, 122, 478–485. [Google Scholar] [CrossRef]
- Inoue, H.; Mori, T.; Shibata, S.; Koshihara, Y. Modulation by glycyrrhetinic acid derivatives of TPA-induced mouse ear oedema. Br. J. Pharmacol. 1989, 96, 204–210. [Google Scholar] [CrossRef]
- Moreno, J. Effect of aristolochic acid on arachidonic acid cascade and in vivo models of inflammation. Immunopharmacology 1993, 26, 1–9. [Google Scholar] [CrossRef]
- Nonato, F.R.; Nogueira, T.M.O.; Barros, T.A.D.A.; Lucchese, A.M.; Oliveira, C.E.C.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Antinociceptive and antiinflammatory activities of Adiantum latifolium Lam.: Evidence for a role of IL-1β inhibition. J. Ethnopharmacol. 2011, 136, 518–524. [Google Scholar] [CrossRef]
- Sadeghi, H.; Parishani, M.; Touri, M.A.; Ghavamzadeh, M.; Barmak, M.J.; Zarezade, V.; Delaviz, H.; Sadeghi, H. Pramipexole reduces inflammation in the experimental animal models of inflammation. Immunopharmacol. Immunotoxicol. 2017, 39, 1–7. [Google Scholar] [CrossRef]
- Bralley, E.E.; Greenspan, P.; Hargrove, J.L.; Wicker, L.; Hartle, D.K. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J. Inflamm. 2008, 5, 1. [Google Scholar] [CrossRef]
- Bas, E.; Recio, M.C.; Máñez, S.; Giner, R.M.; Escandell, J.M.; López-Ginés, C.; Ríos, J.-L. New insight into the inhibition of the inflammatory response to experimental delayed-type hypersensitivity reactions in mice by scropolioside A. Eur. J. Pharmacol. 2007, 555, 199–210. [Google Scholar] [CrossRef]
- Bas, E.; Recio, M.C.; Abdallah, M.; Máñez, S.; Giner, R.M.; Cerda-Nicolas, M.; Ríos, J.-L. Inhibition of the pro-inflammatory mediators’ production and anti-inflammatory effect of the iridoid scrovalentinoside. J. Ethnopharmacol. 2007, 110, 419–427. [Google Scholar] [CrossRef]
- Fujii, Y.; Takeuchi, H.; Tanaka, K.; Sakuma, S.; Ohkubo, Y.; Mutoh, S. Effects of FK506 (tacrolimus hydrate) on chronic oxazolone-induced dermatitis in rats. Eur. J. Pharmacol. 2002, 456, 115–121. [Google Scholar] [CrossRef]
- Barker, J.N.; Allen, M.H.; Macdonald, D.M. The Effect of In Vivo Interferon-Gamma on the Distribution of LFA-1 and ICAM-1 in Normal Human Skin. J. Investig. Dermatol. 1989, 93, 439–442. [Google Scholar] [CrossRef]
- Hossen, M.A.; Inoue, T.; Shinmei, Y.; Minami, K.; Fujii, Y.; Kamei, C. Caffeic acid inhibits compound 48/80-induced allergic symptoms in mice. Boil. Pharm. Bull. 2006, 29, 64–66. [Google Scholar] [CrossRef]
- Segawa, S.; Takata, Y.; Kaneda, H.; Watari, J. Effects of a Hop Water Extract on the Compound 48/80-Stimulated Vascular Permeability in ICR Mice and Histamine Release from OVA-Sensitized BALB/c Mice. Biosci. Biotechnol. Biochem. 2007, 71, 1577–1581. [Google Scholar] [CrossRef][Green Version]
- Li, C.-W.; Wu, X.-L.; Zhao, X.-N.; Su, Z.-Q.; Chen, H.-M.; Wang, X.-F.; Zhang, X.-J.; Zeng, H.-F.; Chen, J.-N.; Li, Y.-C.; et al. Anti-Inflammatory Property of the Ethanol Extract of the Root and Rhizome of Pogostemon cablin (Blanco) Benth. Sci. World J. 2013, 2013, 1–12. [Google Scholar]
- Patel, M.; Murugananthan, G.; Gowda, K. In vivo animal models in preclinical evaluation of anti-inflammatory activity—A review. Int. J. Pharm. Res. Allied Sci. 2012, 1, 1–5. [Google Scholar]
- Rachmawati, H.; Safitri, D.; Pradana, A.T.; Adnyana, I.K. TPGS-Stabilized Curcumin Nanoparticles Exhibit Superior Effect on Carrageenan-Induced Inflammation in Wistar Rat. Pharmaceutics 2016, 8, 24. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, C.; Song, L.; Li, X.; Shi, S.; Mo, J.; Chen, H.; Bai, H.; Wu, X.; Zhao, J.; et al. Effect of total phenolics from Laggera alata on acute and chronic inflammation models. J. Ethnopharmacol. 2006, 108, 243–250. [Google Scholar] [CrossRef]
- Meshram, G.G.; Kumar, A.; Rizvi, W.; Tripathi, C.; Khan, R. Evaluation of the anti-inflammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeck in rats. J. Tradit. Complementary Med. 2016, 6, 172–175. [Google Scholar] [CrossRef]
- Neto, A.G.; Costa, J.M.; Belati, C.C.; Vinhólis, A.H.; Possebom, L.S.; Filho, A.D.S.; Cunha, W.R.; Carvalho, J.C.; Bastos, J.K.; E Silva, M.; et al. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen. J. Ethnopharmacol. 2005, 96, 87–91. [Google Scholar] [CrossRef]
- Amresh, G.; Reddy, G.; Rao, C.; Singh, P. Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. J. Ethnopharmacol. 2007, 110, 526–531. [Google Scholar] [CrossRef]
- Gupta, M.; Mazumder, U.; Kumar, R.S.; Gomathi, P.; Rajeshwar, Y.; Kakoti, B.B.; Selven, V.T. Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. J. Ethnopharmacol. 2005, 98, 267–273. [Google Scholar] [CrossRef]
- Panthong, A.; Kanjanapothi, D.; Taesotikul, T.; Wongcome, T.; Reutrakul, V. Anti-inflammatory and antipyretic properties of Clerodendrum petasites S. Moore. J. Ethnopharmacol. 2003, 85, 151–156. [Google Scholar] [CrossRef]
- Juma, K.M.; Ahmed, Z.A.; Numan, I.T.; Hussain, S.A.R. Dose-dependent anti-inflammatory effect of silymarin in experimental animal model of chronic inflammation. Afr. J. Pharm. Pharmacol. 2009, 3, 242–247. [Google Scholar]
- Lalrinzuali, K.; Vabeiryureilai, M.; Jagetia, G.C. Investigation of the Anti-Inflammatory and Analgesic Activities of Ethanol Extract of Stem Bark of Sonapatha Oroxylum indicum In Vivo. Int. J. Inflamm. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Mbiantcha, M.; Almas, J.; Shabana, S.U.; Nida, D.; Aisha, F. Anti-arthritic property of crude extracts of Piptadeniastrum africanum (Mimosaceae) in complete Freund’s adjuvant-induced arthritis in rats. BMC Complement. Altern. Med. 2017, 17, 269. [Google Scholar] [CrossRef]
- Bauerova, K.; Bezek, S. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen. Physiol. Biophys. 2000, 18, 15–20. [Google Scholar]
- Cascão, R.; Vidal, B.; Raquel, H.; Neves-Costa, A.; Figueiredo, N.; Gupta, V.; Fonseca, J.E.; Moita, L.F. Potent Anti-Inflammatory and Antiproliferative Effects of Gambogic Acid in a Rat Model of Antigen-Induced Arthritis. Mediat. Inflamm. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Kshirsagar, A.D.; Panchal, P.V.; Harle, U.N.; Nanda, R.K.; Shaikh, H.M. Anti-Inflammatory and Antiarthritic Activity of Anthraquinone Derivatives in Rodents. Int. J. Inflamm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar]
- Carroll, A.R.; Kweifio-Okai, G.; Kweifio-Okai, G. Antiarthritic effect of lupeol acetate. Phytother. Res. 1993, 7, 213–215. [Google Scholar]
- Winyard, P.G.; Willoughby, D.A.; Morris, C.J. Carrageenan-Induced Paw Edema in the Rat and Mouse. In Inflammation Protocols; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2003; Volume 225, pp. 115–122. [Google Scholar]
- Seibert, K.; Masferrer, J.L. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor 1994, 4, 17–23. [Google Scholar]
- Whiteley, P.E.; Dalrymple, S.A. Models of Inflammation: Carrageenan-Induced Paw Edema in the Rat. Curr. Protoc. Pharmacol. 2001, 5–6. [Google Scholar] [CrossRef]
- Cole, H.W.; Brown, C.E.; Magee, D.E.; Magee, C.; Roudebush, R.E.; Bryant, H.U. Serotonin-induced paw edema in the rat: Pharmacological profile. Gen. Pharmacol. Vasc. Syst. 1995, 26, 431–436. [Google Scholar] [CrossRef]
- Nakamura, H.; Shimizu, M. Early and delayed phases of hind paw edema in rats. Jpn. J. Pharmacol. 1974, 24, 393–405. [Google Scholar] [CrossRef][Green Version]
- Kostadinov, I.; Delev, D.; Petrova, A.; Stanimirova, I.; Draganova, K.; Kostadinova, I.; Murdjeva, M. Study on anti-inflammatory and immunomodulatory effects of clomipramine in carrageenan-and lipopolysaccharide-induced rat models of inflammation. Biotechnol. Biotechnol. Equip. 2014, 28, 552–558. [Google Scholar] [CrossRef]
- Agarwal, A.; D’Souza, P.; Johnson, T.S.; Dethe, S.M.; Chandrasekaran, C. Use of in vitro bioassays for assessing botanicals. Curr. Opin. Biotechnol. 2014, 25, 39–44. [Google Scholar] [CrossRef]
- Liao, C.-H.; Guo, S.-J.; Lin, J.-Y. Characterisation of the chemical composition and in vitro anti-inflammation assessment of a novel lotus (Nelumbo nucifera Gaertn) plumule polysaccharide. Food Chem. 2011, 125, 930–935. [Google Scholar] [CrossRef]
- Sahota, T.; Sanderson, I.; Danhof, M.; Della Pasqua, O. Model-based prediction of the acute and long-term safety profile of naproxen in rats. Br. J. Pharmacol. 2015, 172, 3861–3874. [Google Scholar] [CrossRef]
- Gosselin, K.B.; Feldman, H.A.; Sonis, A.L.; Bechard, L.J.; Kellogg, M.D.; Gura, K.; Venick, R.; Gordon, C.M.; Guinan, E.C.; Duggan, C. Serum citrulline as a biomarker of gastrointestinal function during hematopoietic cell transplantation (HCT) in children. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 709–714. [Google Scholar] [CrossRef]
- Crenn, P.; Vahedi, K.; Lavergne-Slove, A.; Cynober, L.; Matuchansky, C.; Messing, B. Plasma citrulline: A marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 2003, 124, 1210–1219. [Google Scholar] [CrossRef]
- Crenn, P.; Messing, B.; Cynober, L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin. Nutr. 2008, 27, 328–339. [Google Scholar] [CrossRef]
- Carr, D.F.; Ayehunie, S.; Davies, A.; Duckworth, C.A.; French, S.; Hall, N.; Hussain, S.; Mellor, H.R.; Norris, A.; Park, B.K.; et al. Towards better models and mechanistic biomarkers for drug-induced gastrointestinal injury. Pharmacol. Ther. 2017, 172, 181–194. [Google Scholar] [CrossRef]
- Banerjee, A. Gastrointestinal toxicity biomarkers. Biomark. Toxicol. 2014, 269–277. [Google Scholar] [CrossRef]
- Kindt, E.; Wu, A.; Vitsky, A.; Scott, W.; Gross, C.; Yang, A.H.; Schaiff, W.T.; Ramaiah, S.K.; John-Baptiste, A.; Huang, W. Evaluation of Potential Gastrointestinal Biomarkers in a PAK4 Inhibitor-treated Preclinical Toxicity Model to Address Unmonitorable Gastrointestinal Toxicity. Toxicol. Pathol. 2012, 40, 482–490. [Google Scholar]
- Luk, G.D.; Bayless, T.M.; Baylin, S.B. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J. Clin. Investig. 1980, 66, 66–70. [Google Scholar] [CrossRef]
- Miyoshi, J.; Miyamoto, H.; Goji, T.; Taniguchi, T.; Tomonari, T.; Sogabe, M.; Kimura, T.; Kitamura, S.; Okamoto, K.; Fujino, Y.; et al. Serum diamine oxidase activity as a predictor of gastrointestinal toxicity and malnutrition due to anticancer drugs. J. Gastroenterol. Hepatol. 2015, 30, 1582–1590. [Google Scholar] [CrossRef]
- Moriyama, K.; Kouchi, Y.; Morinaga, H.; Irimura, K.; Hayashi, T.; Ohuchida, A.; Goto, T.; Yoshizawa, Y. Diamine oxidase, a plasma biomarker in rats to GI tract toxicity of oral fluorouracil anti-cancer drugs. Toxicology 2006, 217, 233–239. [Google Scholar] [CrossRef]
- Wofoekamp, M.; De Bruin, R.; Wolvekamp, M. Diamine Oxidase: An Overview of Historical, Biochemical and Functional Aspects. Dig. Dis. 1994, 12, 2–14. [Google Scholar] [CrossRef]
- Biegański, T. Biochemical, physiological and pathophysiological aspects of intestinal diamine oxidase. Acta Physiol. Pol. 1983, 34, 139–154. [Google Scholar]
- Fattaha, N.A.A.; Abdel-Rahman, M.S. Effects of omeprazole on ethanol lesions. Toxicol. Lett. 2000, 118, 21–30. [Google Scholar] [CrossRef]
- Yáñez, J.A.; Teng, X.W.; Roupe, K.A.; Fariss, M.W.; Davies, N.M. Chemotherapy induced gastrointestinal toxicity in rats: Involvement of mitochondrial DNA, gastrointestinal permeability and cyclooxygenase-2. J. Pharm. Pharm. Sci. 2003, 6, 308–314. [Google Scholar]
- Wardill, H.R.; Bowen, J.M.; Gibson, R.J. Biomarkers of small intestinal mucosal damage induced by chemotherapy: An emerging role for the 13C sucrose breath test. J. Supportive Oncol. 2013, 11, 61–67. [Google Scholar] [CrossRef]
- Meling, T.R.; Aabakken, L.; Røseth, A.; Osnes, M. Faecal Calprotectin Shedding after Short-Term Treatment with Non-Steroidal Anti-Inflammatory Drugs. Scand. J. Gastroenterol. 1996, 31, 339–344. [Google Scholar] [CrossRef]
- Røseth, A.G.; Schmidt, P.N.; Fagerhol, M.K. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 1999, 34, 50–54. [Google Scholar]
- Guerrant, R.L.; Araujo, V.; Soares, E.; Kotloff, K.; Lima, A.A.; Cooper, W.H.; Lee, A.G. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J. Clin. Microbiol. 1992, 30, 1238–1242. [Google Scholar]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar]
- Boyd, S.D. Everything you wanted to know about small RNA but were afraid to ask. Lab. Investig. 2008, 88, 569–578. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Stoner, L.; Lucero, A.A.; Palmer, B.R.; Jones, L.M.; Young, J.M.; Faulkner, J. Inflammatory biomarkers for predicting cardiovascular disease. Clin. Biochem. 2013, 46, 1353–1371. [Google Scholar] [CrossRef]
- Wang, J.; Tan, G.-J.; Han, L.-N.; Bai, Y.-Y.; He, M.; Liu, H.-B. Novel biomarkers for cardiovascular risk prediction. J. Geriatr. Cardiol. JGC 2017, 14, 135. [Google Scholar] [CrossRef]
- Bassuk, S.S.; Rifai, N.; Ridker, P.M. High-sensitivity C-reactive protein: Clinical importance. Curr. Probl. Cardiol. 2004, 29, 439–493. [Google Scholar]
- Devaraj, S.; Xu, D.Y.; Jialal, I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: Implications for the metabolic syndrome and atherothrombosis. Circulation 2003, 107, 398–404. [Google Scholar] [CrossRef]
- Brennan, M.-L.; Penn, M.S.; Aviles, R.J.; Goormastic, M.; Pepoy, M.L.; McErlean, E.S.; Nissen, S.E.; Van Lente, F.; Nambi, V.; Shishehbor, M.H.; et al. Prognostic Value of Myeloperoxidase in Patients with Chest Pain. N. Engl. J. Med. 2003, 349, 1595–1604. [Google Scholar] [CrossRef]
- Zakynthinos, E.; Pappa, N. Inflammatory biomarkers in coronary artery disease. J. Cardiol. 2009, 53, 317–333. [Google Scholar] [CrossRef]
- Strobel, N.A.; Fassett, R.G.; Marsh, S.A.; Coombes, J.S. Oxidative stress biomarkers as predictors of cardiovascular disease. Int. J. Cardiol. 2011, 147, 191–201. [Google Scholar] [CrossRef]
- Sies, H.; Cadenas, E. Oxidative stress: Damage to intact cells and organs. Philos. Transactions R. Soc. Lond. B Biol. Sci. 1985, 311, 617–631. [Google Scholar] [CrossRef]
- Zhang, Z.-J. Systematic review on the association between F2-isoprostanes and cardiovascular disease. Ann. Clin. Biochem. 2013, 50, 108–114. [Google Scholar] [CrossRef]
- Epstein, F. Beyond choresterol. Modifications of low-density-lipoprotein that increase its atherosclerosis. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef]
- Zalewski, A.; Macphee, C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: Biology, epidemiology, and possible therapeutic target. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 923–931. [Google Scholar] [CrossRef]
- Mohiuddin, I.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Nitrotyrosine and Chlorotyrosine: Clinical Significance and Biological Functions in the Vascular System. J. Surg. Res. 2006, 133, 143–149. [Google Scholar] [CrossRef]
- Dröge, W.; Breitkreutz, R. Glutathione and immune function. Proc. Nutr. Soc. 2000, 59, 595–600. [Google Scholar] [CrossRef]
- De Chiara, B.; Mafrici, A.; Campolo, J.; Famoso, G.; Sedda, V.; Parolini, M.; Cighetti, G.M.; Lualdi, A.; Fiorentini, C.; Parodi, O. Low plasma glutathione levels after reperfused acute myocardial infarction are associated with late cardiac events. Coron. Artery Dis. 2007, 18, 77–82. [Google Scholar] [CrossRef]
- Campolo, J.; De Maria, R.; Caruso, R.; Accinni, R.; Turazza, F.; Parolini, M.; Roubina, E.; De Chiara, B.; Cighetti, G.M.; Frigerio, M.; et al. Blood glutathione as independent marker of lipid peroxidation in heart failure. Int. J. Cardiol. 2007, 117, 45–50. [Google Scholar] [CrossRef]
- Rhee, E.P.; Gerszten, R.E. Metabolomics and cardiovascular biomarker discovery. Clin. Chem. 2012, 58, 139–147. [Google Scholar] [CrossRef]
- Shah, S.H.; Kraus, W.E.; Newgard, C.B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: Form and function. Circulation 2012, 126, 1110–1120. [Google Scholar] [CrossRef]
- Kordalewska, M.; Markuszewski, M.J. Metabolomics in cardiovascular diseases. J. Pharm. Biomed. Anal. 2015, 113, 121–136. [Google Scholar] [CrossRef]
- Um, S.Y.; Chung, M.W.; Kim, K.-B.; Kim, S.H.; Oh, J.S.; Oh, H.Y.; Lee, H.J.; Choi, K.H. Pattern Recognition Analysis for the Prediction of Adverse Effects by Nonsteroidal Anti-Inflammatory Drugs Using1H nmr-Based Metabolomics in Rats. Anal. Chem. 2009, 81, 4734–4741. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.-Y.; Qiu, H.; Harris, T.R.; Sirish, P.; Hammock, B.D.; Chiamvimonvat, N. Use of Metabolomic Profiling in the Study of Arachidonic Acid Metabolism in Cardiovascular Disease. Congest. Hear. Fail. 2011, 17, 42–46. [Google Scholar] [CrossRef]

| Category | Mediator | Source Organ/Cells | Mechanism | Reference |
|---|---|---|---|---|
| Vasoactive amines | Histamine | Basophils, gastric cells, enterochromaffin cells, histaminergic nerve cells | Vasodilatation and increased vascular permeability | [43] |
| Serotonin | Intestine, blood, spleen, nervous system | Vasodilatation and increased vascular permeability (less potent than histamine) | [44] | |
| Arachidonic acid metabolites | Prostaglandins (PGs) | Formed by the metabolism of arachidonic acid by cyclooxygenases (COX) | Enhanced vascular permeability, fever, sensory nerve stimulation, and pain amplification. PGE2 causes production of edema and erythema | [45] |
| Leukotrienes (LTs) | Formed by the metabolism of arachidonic acid by lipoxygenase (LOX) | LTB4 stimulates neutrophil chemotaxis, enhanced neutrophil-endothelial interactions, neutrophil activation, degranulation and release of various inflammatory mediators, enzymes and free radicals | [29] | |
| Thromboxane (TX) | Granuloma tissues, macrophages, human synovial tissues, Thromboxane A2 (TXA2) is present in platelets and circulating leukocytes | Platelet aggregation, smooth muscle contraction | [46] | |
| Platelet activating factor (PAF) | PAF | Liberated by macrophages, endothelial cells, platelets and neutrophils | Initiates cardinal features of inflammation, expression of adhesion molecules, platelet aggregation, formation of leukotrienes, chemotaxis, sensitization of sensory nerves to pain | [47,48] |
| Kinins | Substance p (Sub-p) | Released from sensory nerves | Increased microvascular permeability, neutrophil accumulation, potentiates responses to bradykinin, serotonin, prostaglandin, and ATP | [49,50] |
| Bradykinin | Plasma precursor protein kininogen produces bradykinin through kallikrein | Increased vascular permeability, sensory nerve ending stimulation, inflammatory mediator release, activation of NF-κB, induction of cytokine gene expression | [50,51] | |
| Cytokines | Interleukins (ILs) | Produced by activated lymphocytes and macrophages | Up-regulation of adhesion molecule expression, stimulation of pro-inflammatory mediator release | [52,53] |
| Tumor Necrosis Factor-α and β (TNF-α, β) | Activated macrophages/monocytes, fibroblasts, mast cells, natural killer (NK) cells, T and B lymphocytes | Stimulation of PGE2, collagenase, IL-1 production, fever, induction of acute-phase reactant protein production, adhesion molecule up-regulation, cytokine induction, chemokine synthesis | [52,53] | |
| Transforming Growth Factor-β (TGF-β) | T cells, platelets, monocytes | Attraction of monocytes and other leukocytes to the site of injury, increased cell adhesion | [52] | |
| Interferons (IFN-α and β) | IFN-α is a produced by leukocytes and IFN-β is a produced by fibroblasts | Activation of macrophages and mononuclear phagocytes | [52] | |
| Clotting system | Thrombin | Blood | Mobilization of p-selectin, the release of chemokines, adhesion molecule expression, induction of COX-2, production of PGs, PAF and nitric oxide | [54,55] |
| Complement system | Anaphylotoxins C3a and C5a | Complement proteins reside as inactive forms in plasma | Potentiate inflammation by binding to receptors on mast cells, basophils, phagocytic cells, and endothelial cells | [42] |
| Miscellaneous | Nitric oxide (NO) | Leukocytes, endothelial cells, sensory nerve cells | Vasodilation and cytotoxicity | [56,57] |
| Reactive Oxygen Species (ROS) | Phagocytic leukocytes like, neutrophils, monocytes, macrophages, eosinophils | Vascular leakage, chemotaxis, endothelial damage, oxidative stress, activation of transcription factors like nuclear transcription factor-κB (NF-κB) | [58] |
| Compound | Target | Stimulus/system | IC50 | Reference |
|---|---|---|---|---|
| Alkaloid | ||||
| Lycoricidinol | TNF-α | LPS-stimulated murine macrophages | 0.002 mg/mL | [64] |
| Lycorine | TNF-α | LPS-stimulated murine macrophages | 0.2 mg/mL | [64] |
| Curcuminoids | ||||
| Curcumin | NF-κB | NF-κB dependent transcription inhibition in reporter assays in A549 cells | 21.5 μM | [65] |
| Curcumin | TNF-α and IL-1 | LPS-induced production of TNF and IL-lβ by a human monocytic macrophage cell line Mono Mac 6 | 5 μM | [66] |
| Diterpene | ||||
| Cryptotanshinone | COX-2 | Pulsed ultrafiltration LC–MS screening | 22 μM | [67] |
| Nimbiol | LOX | In-vitro soybean lipoxygenase assay | 106 μM | [68] |
| Sugiol | LOX | In-vitro soybean lipoxygenase assay | 60.7 μM | [68] |
| Flavonoid | ||||
| Amoradicin | TNF-α | LPS-stimulated TNF-α release in RAW 264.7 cells | 28.51 μM | [69] |
| Apigenin | NO | LPS-stimulated macrophages | 2.8 μM | [70] |
| Apigenin | NO | LPS and TNF-γ-stimulated macrophages | 10.4 μM | [70] |
| Apigenin | NO | LPS-stimulated RAW 264.7 macrophages | 10.7 μM | [71] |
| Apigenin | NO | LPS-stimulated macrophages | 19.2 μg/mL | [72] |
| Apigenin | Free radical | DPPH radical scavenging assay | 30.3 μg/mL | [73] |
| Astilbin | PGE2 | LPS-stimulated RAW 264.7 cells | 19.6 μg/mL (43.5 μM) | [74] |
| Centaureidin | LOX | Soybean lipoxygenase assay | 20 μM | [75] |
| Centaureidin | LOX | In-vitro cyclooxygenase assay | 318 μM | [75] |
| Cirsiliol | 5-LOX | 5-LOX inhibition in rat basophilic leukemia cells | 0.1 μM | [3] |
| Dioclein | PDE4 | LPS-stimulated macrophages | 16.8 μM | [76] |
| Daidzein | NO | LPS-stimulated macrophages | 40.0 μM | [70] |
| Daidzein | NO | LPS and TNF-γ-stimulated macrophages | 81.4 μM | [70] |
| Engeletin | PGE2 | LPS-stimulated RAW 264.7 cells | 14.4 μg/mL (33.2 μM) | [74] |
| Genistein | NO | LPS-stimulated macrophages | 16.6 μM | [70] |
| Genistein | NO | LPS and TNF-γ-stimulated macrophages | 34.5 μM | [70] |
| Genistein | NO | LPS-stimulated TNF-α release in RAW 264.7 cells | 5 μM | [77] |
| Jaceosidin | COX-2 | LPS-stimulated RAW 264.7 cells | 2.8 μM | [78] |
| Kaempferol | NO | LPS activated macrophages | 10.6 μM | [79] |
| Kaempferol | NO | LPS induced Akt phosphorylation | 30.5 μM/kg | [80] |
| Lantadene A | Free radical | DPPH radical scavenging activity | 6.5 mg/mL | [81] |
| Lantadene A | Hydroxy radical | Hydroxyl radical scavenging activity | 42.4 mg/mL | [81] |
| Lantadene A | Superoxide anion | Superoxide anion radical scavenging activity | 2.5 mg/mL | [81] |
| Lantadene A | NO | NO scavenging assay | 98.0 µg/mL | [81] |
| Luteolin | NO | LPS-stimulated macrophages | 10.4 μM | [70] |
| Luteolin | NO | LPS and TNF-γ-stimulated macrophages | 38.6 μM | [70] |
| Luteolin | NO | LPS-induced NF-κB activation | 35.1 μM/kg | [80] |
| Luteolin | NO | LPS-stimulated TNF-α release in RAW 264.7 cells | 1 μM | [77] |
| Luteolin | NO | LPS-stimulated macrophages | 10.4 μM | [79] |
| Quercetin | NO | LPS-stimulated RAW 264.7 cells | 11.2 μg/mL (37.1 μM) | [74] |
| Quercetin | PGE2 | LPS-stimulated RAW 264.7 cells | 19.9 μg/mL (65.8 μM) | [74] |
| Quercetin | TNF-α | LPS-stimulated RAW 264.7 cells | 1.25 μg/mL (4.14 μM) | [74] |
| Quercetin | TNF-α | LPS-stimulated TNF-α release in RAW 264.7 cells | 1 μM | [77] |
| Quercetin | LTB4 | Peripheral blood mononuclear cells | 2 μM | [82] |
| Quercetin | MMP-9 | Fluorescent gelatin dequenching assay and gelatin zymography | 22 μM | [83] |
| Rutin | Free radicals | DPPH radical scavenging assay | 23.7 μg/mL | [83] |
| Velutin | NF-κB | Secreted embryonic alkaline phosphatase reporter assay | 2 μM | [84] |
| Glycoside | ||||
| Aucubin | TNF-α | Ag-stimulated TNF-α release in rat basophilic leukemia (RBL)-2H3 mast cells | 0.101 μg/ml | [85] |
| Aucubin | IL-6 | Ag stimulated IL-6 production in rat basophilic leukemia (RBL) -2H3 mast cells | 0.19 μg/mL | [85] |
| Baicalin | COX-1 | Human osteosarcoma cell line | 9.8 μg/mL | [86] |
| Baicalin | COX-2 | Human osteosarcoma cell line | 7.3 μg/mL | [86] |
| Hederagenin | Hyaluronidase | Hyaluronidase activity assay | 280.4 μM | [87] |
| Hederagenin | Elastase | Porcine pancreatic elastase | 40.6 μM | [87] |
| Ligustroside | PGE2 | Calcium ionophores stimulated mouse peritoneal macrophages | 48.5 μM | [88] |
| Ligustroside | TXB2 | TXB2 release induced by calcium ionophore in human platelets | 122.6 μM | [88] |
| Oleuropeoside | PGE2 | Calcium ionophores stimulated mouse peritoneal macrophages | 47 μM | [88] |
| Lactone | ||||
| Artemisolide | NF-κB | LPS-stimulated RAW 264.7 cells | 5.8 μM | [89] |
| Artemisolide | PGE2 | LPS-stimulated RAW 264.7 cells | 8.7 μM | [89] |
| Artemisolide | NO | LPS-stimulated RAW 264.7 cells | 6.4 μM | [89] |
| Desmethoxyyangonin | TNF-α | Okadaic acid-stimulated TNF-α release from BALB/3T3 cells | 17 μM | [64] |
| Ergolide | NO | LPS/IFN-γ-stimulated RAW 264.7 macrophages | 1.95 μM | [90] |
| Ergolide | PGE2 | LPS/IFN-γ-stimulated RAW 264.7 macrophages | 3 μM | [90] |
| Yangonin | TNF-α | Okadaic acid-stimulated TNF-α release from BALB/3T3 cells | 40 μM | [64] |
| Lignan | ||||
| (−)-pinoresinol 4-O-b-D-glucopyranoside | NO | LPS-stimulated murine microglia BV-2 | 34.35 μM | [91] |
| (−)-syringaresinol | NO | LPS-stimulated murine microglia BV-2 | 15.05 μM | [91] |
| Isoamericanol B | NO | LPS-stimulated RAW 264.7 cells | 10.3 μg/mL | [92] |
| Sesquiterpene acid | ||||
| Dehydrocostic acid | LTB4 | Leukotriene B4 generation by rat leukocytes | 22 μM | [93] |
| Dehydrocostic acid | Elastase | Elastase activity assay | 43 μM | [93] |
| Dehydrocostic acid | PLA2 | Bee venom PLA2 activity | 17 μM | [93] |
| Stilbenoid | ||||
| Piceatannol | Elastase | Procine pancreatic elastase assay | 15.6 µg/mL | [91] |
| Resveratrol | NO | Cytokine stimulated inducible NO synthase expression and nitrite production in human primary airway epithelial cells | 3.6 μM | [94] |
| Resveratrol | GMCS | GMCS factor release in airway epithelial cells | 0.44 μM | [94] |
| Resveratrol | IL-8 | IL-8 release in airway epithelial cells. | 4.7 μM | [94] |
| Tannin | ||||
| (+)-catechin | COX-1 | Human osteosarcoma cell line | 2.8 μg/mL | [86] |
| (+)-catechin | COX-2 | Human osteosarcoma cell line | 10.5 μg/mL | [86] |
| Catechin | Elastase | Procine pancreatic elastase assay | 20.2 µg/mL | [95] |
| Triterpenoid | ||||
| 2,3,19-trihydroxy-24-oxo-olean-12-en-28-oic acid | NO | LPS-induced NO production in RAW 264.7 macrophages | 5.4 μM | [96] |
| Alphitolic acid | NO | LPS+ IFN-γ activated RAW264.7 macrophages | 17.6 µM | [97] |
| Alphitolic acid | TNF-α | LPS+ IFN-γ activated RAW264.7 macrophages | 22.7 µM | [97] |
| Arjunic acid/Arjuntriterpenic acid | NO | LPS-induced NO production in RAW 264.7 macrophages | 20.1 μM | [96] |
| Arjunolic acid | NO | LPS induced NO production in RAW 264.7 macrophages | 13.0 μM | [96] |
| Betulinic acid | Elastase | Procine pancreatic elastase assay | 21.6 µg/mL | [95] |
| Betulinic acid | NO | LPS+IFN-γ activated RAW264.7 macrophages | 8.3 µM | [97] |
| Betulinic acid | TNF-α | LPS+IFN-γ activated RAW264.7 macrophages | 23.5 µM | [97] |
| Cis-coumaroyl alphitolic acid | NO | LPS+IFN-γ activated RAW264.7 macrophages | 3.5 µM | [97] |
| Cis-coumaroyl alphitolic acid | TNF-α | LPS + IFN-γ activated RAW264.7 macrophages | 5.6 µM | [97] |
| Emmolic acid/Ceanothic acid | NO | LPS+IFN-γ activated RAW264.7 macrophages | >36 µM | [97] |
| Emmolic acid/Ceanothic acid | TNF-α | LPS+IFN-γ activated RAW264.7 macrophages | >36 µM | [97] |
| Emmolic acid acetate | NO | LPS+IFN-γ activated RAW264.7 macrophages | 14.7 µM | [97] |
| Emmolic acid acetate | TNF-α | LPS+IFN-γ activated RAW264.7 macrophages | >36 µM | [97] |
| Friedelin | LOX | In-vitro soybean lipoxygenase assay | 35.8 μM | [68] |
| Maslinic acid | PKC | Non-radioactive detection of PKC using Raji cells | 11.5 µM | [98] |
| Oleanolic acid | PGE2 | Calcium ionophores stimulated mouse peritoneal macrophages | 23.5 μM | [88] |
| Oleanolic acid | Hyaluronidase | Hyaluronidase activity assay | 280.4 μM | [87] |
| Oleanolic acid | Elastase | Porcine pancreatic elastase assay | 5.1 μM | [87] |
| Oleanolic acid | Elastase | Procine pancreatic elastase assay | 3 µg/mL | [95] |
| Oleanolic acid | PKC | Non-radioactive detection of PKC using Raji cells | 39.29 µM | [98] |
| Oleanolic acid | NO | LPS-induced NO production in RAW 264.7 macrophages | 7.8 μM | [96] |
| Paradrymoniside | NO | LPS-induced NO production in RAW 264.7 macrophages | 10.1 μM | [96] |
| Pyracrenic acid | Elastase | Procine pancreatic elastase assay | 1.5 µg/mL | [95] |
| Sericic acid | NO | LPS-induced NO production in RAW 264.7 macrophages | 17.2 μM | [96] |
| Trans-coumaroyl alphitolic acid | NO | LPS+IFN-γ activated RAW264.7 macrophages | 1.7 µM | [97] |
| Trans-coumaroyl alphitolic acid | TNF-α | LPS+IFN-γ activated RAW264.7 macrophages | 10.9 µM | [97] |
| Ursolic acid | PGE2 | Calcium ionophore stimulated mouse peritoneal macrophages | 60.9 μM | [88] |
| Ursolic acid | TXB2 | TXB2 release induced by calcium ionophore in human platelets | 50.2 μM | [88] |
| Ursolic acid | LOX | In vitro soybean lipoxygenase assay | 92.8 μM | [68] |
| Ursolic acid | PKC | Non-radioactive detection of PKC using Raji cells | 27.93 µM | [98] |
| Daucosterol | LOX | In vitro soybean lipoxygenase assay | 108.7 μM | [68] |
| Escin | Hyaluronidase | Hyaluronidase activity assay | 149.9 μM | [87] |
| Miscellaneous | ||||
| Crotafuran E | NO | LPS+IFN-γ-stimulated N9 microglial cells | 13.9 μM | [71] |
| Escinol | Hyaluronidase | Hyaluronidase activity assay | 1.65 mM | [87] |
| Mansoins F | TNF-α | LPS-stimulated THP-1 cells | 19.3 μM | [99] |
| Senkyunolide O | COX-2 | Pulsed ultrafiltration LC–MS screening | 5 μM | [67] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. https://doi.org/10.3390/ijms20184367
Patil KR, Mahajan UB, Unger BS, Goyal SN, Belemkar S, Surana SJ, Ojha S, Patil CR. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. International Journal of Molecular Sciences. 2019; 20(18):4367. https://doi.org/10.3390/ijms20184367
Chicago/Turabian StylePatil, Kalpesh R., Umesh B. Mahajan, Banappa S. Unger, Sameer N. Goyal, Sateesh Belemkar, Sanjay J. Surana, Shreesh Ojha, and Chandragouda R. Patil. 2019. "Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals" International Journal of Molecular Sciences 20, no. 18: 4367. https://doi.org/10.3390/ijms20184367
APA StylePatil, K. R., Mahajan, U. B., Unger, B. S., Goyal, S. N., Belemkar, S., Surana, S. J., Ojha, S., & Patil, C. R. (2019). Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. International Journal of Molecular Sciences, 20(18), 4367. https://doi.org/10.3390/ijms20184367






