Decreased Level of Neurotrophic Factor Neuritin 1 in Women with Ovarian Endometriosis after Receiving Gonadotropin-Releasing Hormone Agonist Treatment
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. NRN1 mRNA Expression in Endometriotic Tissues of Patients Treated with GnRHa
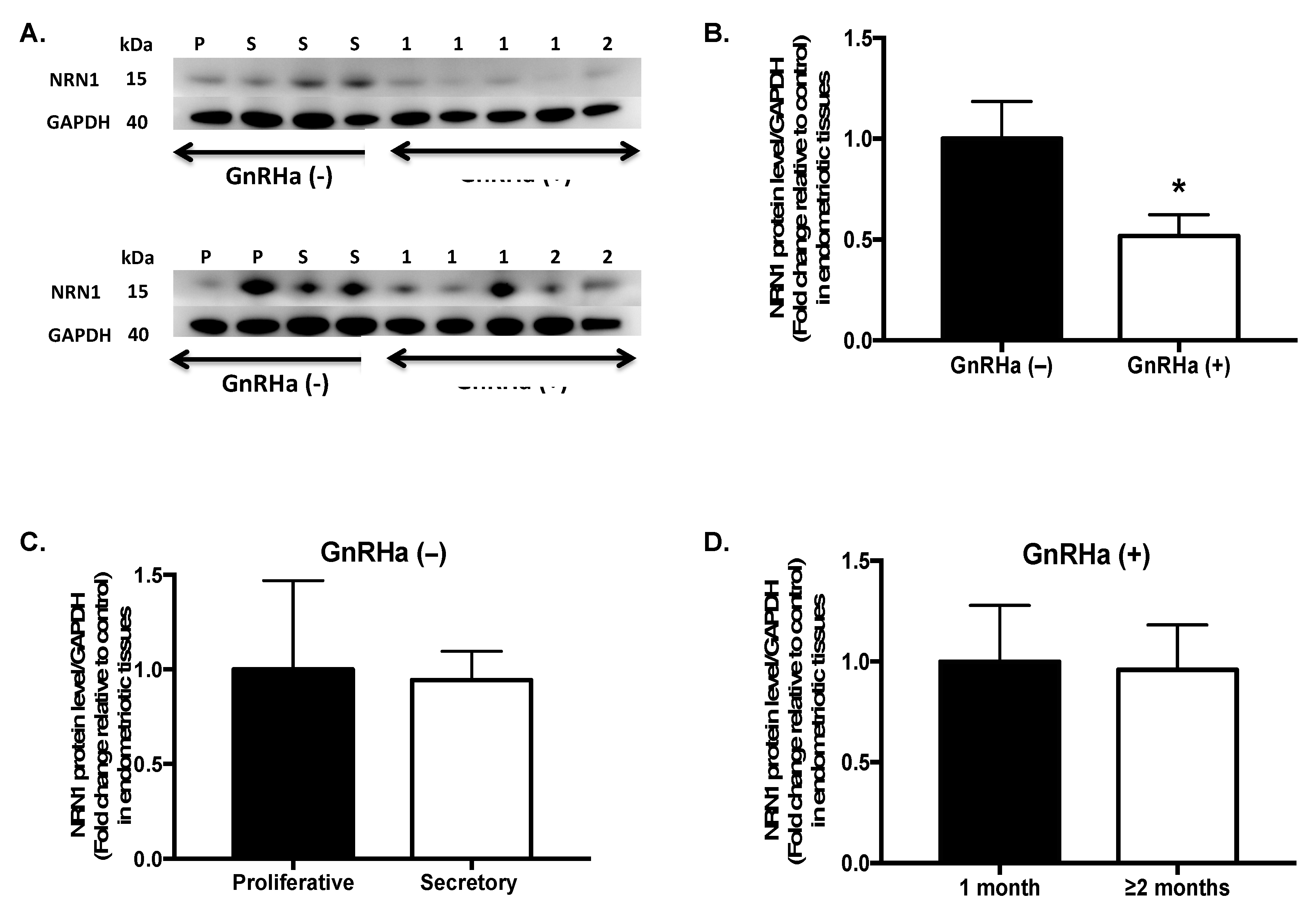
2.3. NRN1 Protein Expression in Patients Treated with GnRHa
2.4. Localization of NRN1 in Endometrial Tissues of Women with Endometriosis
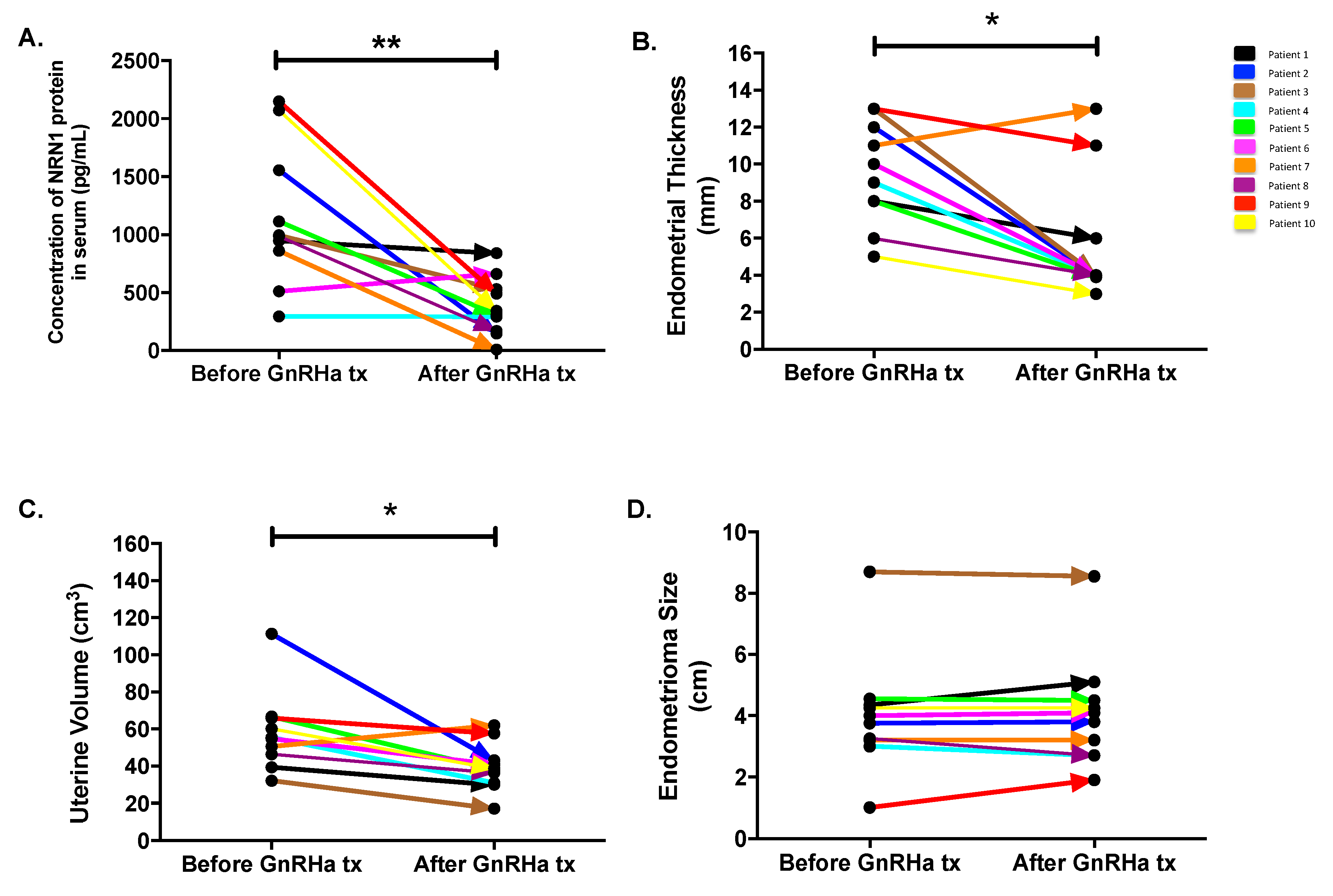
2.5. Follow-Up Study
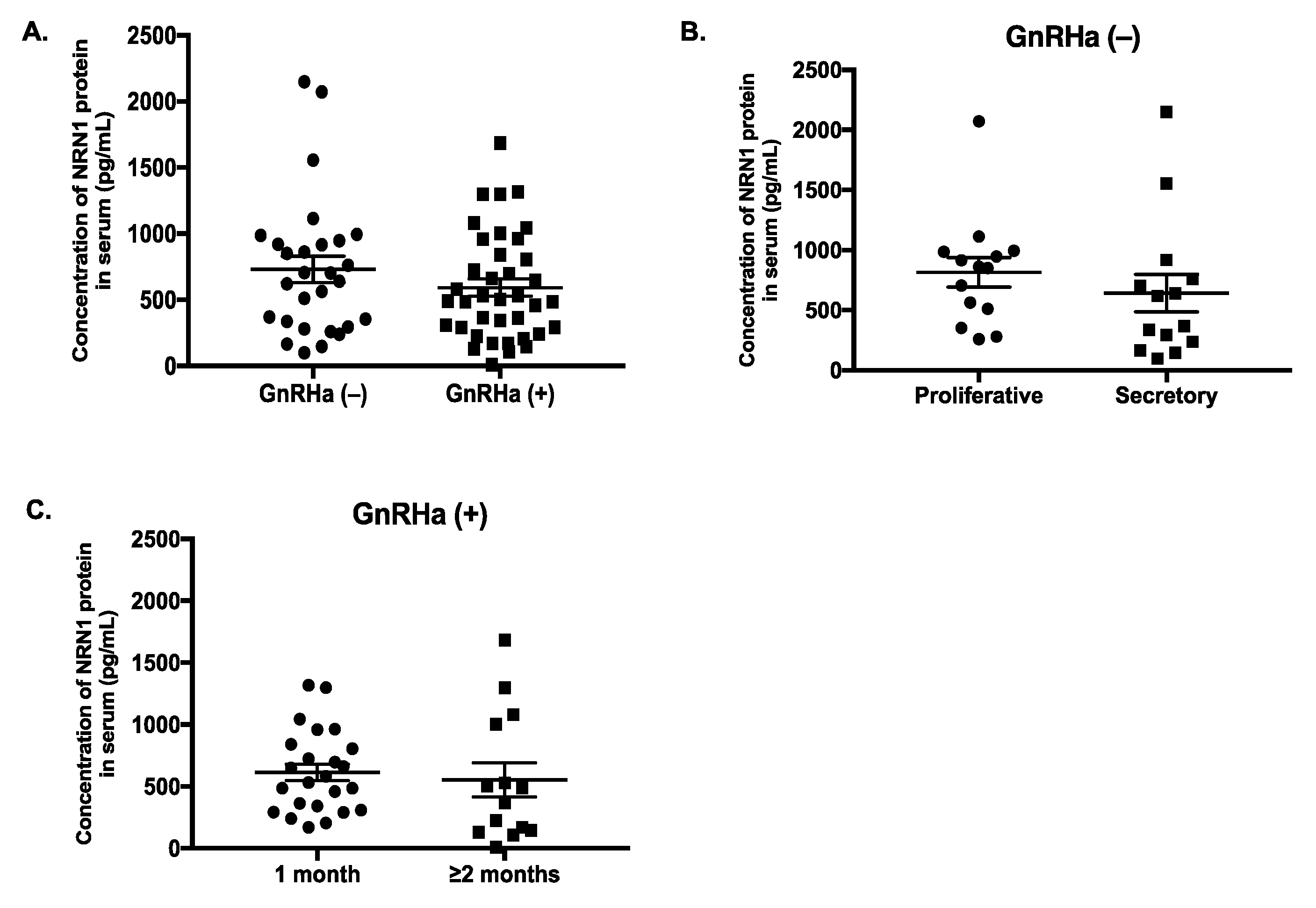
2.6. Serum Level of NRN1 in Women with Endometriosis
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment and Specimen Collection
4.2. RNA Isolation and qPCR Analysis
4.3. Western Blot Analysis
4.4. Immunohistochemistry
4.5. Enzyme-Linked Immunosorbent Assay
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NRN1 | Neuritin 1 |
| GnRHa | Gonadotropin-releasing hormone agonist |
| cDNA | Complementary deoxyribonucleic acid |
| JIRB | Joint International Review Board |
| qPCR | Quantitative polymerase chain reaction |
| mRNA | Messenger ribonucleic acid |
| WB | Western blotting |
| IHC | Immunohistochemistry |
| ELISA | Enzyme-linked immunosorbent assay |
| TBP | TATA-box-binding protein |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| DAB | Diaminobenzidine |
| IgG | Immunoglobulin |
References
- Hickey, M.; Ballard, K.; Farquhar, C. Endometriosis. BMJ 2014, 348, g1752. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Fuldeore, M.J.; Soliman, A.M. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol. Obstet. Investig. 2017, 82, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Farquhar, C. Endometriosis: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2014, 3, CD009590. [Google Scholar] [CrossRef] [PubMed]
- Berkley, K.J.; Rapkin, A.J.; Papka, R.E. The pains of endometriosis. Science 2005, 308, 1587–1589. [Google Scholar] [CrossRef]
- Yan, D.; Liu, X.; Guo, S.W. Nerve fibers and endometriotic lesions: Partners in crime in inflicting pains in women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 209, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Fraser, I.S. The importance of pelvic nerve fibers in endometriosis. Women’s Health 2015, 11, 611–618. [Google Scholar] [CrossRef]
- Bokor, A.; Kyama, C.M.; Vercruysse, L.; Fassbender, A.; Gevaert, O.; Vodolazkaia, A.; De Moor, B.; Fülöp, V.; D’Hooghe, T. Density of small diameter sensory nerve fibres in endometrium: A semi-invasive diagnostic test for minimal to mild endometriosis. Hum. Reprod. 2009, 24, 3025–3032. [Google Scholar] [CrossRef]
- Aghaey, M.F.; Mehdizadeh, K.A.; Zare, M.A.; Ghajarie, B.A.M.; Shariati, B.A.; Zolali, B.; Najafi, L. Diagnosis of endometrial nerve fibers in women with endometriosis. Arch. Gynecol. Obstet. 2011, 28, 1157–1162. [Google Scholar] [CrossRef]
- Triolo, O.; Lagana, A.S.; Sturlese, E. Chronic pelvic pain in endometriosis: An overview. J. Clin. Med. Res. 2013, 5, 153–163. [Google Scholar] [CrossRef]
- Al-Jefout, M.; Dezarnaulds, G.; Cooper, M.; Tokushige, N.; Luscombe, G.M.; Markham, R.; Fraser, I.S. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: A double blind study. Hum. Reprod. 2009, 24, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Barcena de Arellano, M.L.; Arnold, J.; Sacher, F.; Blochle, M.; Staube, M.; Bartley, J.; Vercellino, G.F.; Chiantera, V.; Schneider, A.; Mechsner, S. Eutopic endometrium from women with endometriosis does not exhibit neurotrophic properties. J. Neuroimmunol. 2012, 249, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.A.; Pergola, G.M.; Tekmal, R.R.; Montoya, I.A.; Dey, T.D.; Schenken, R.S. Cytokine regulation of cellular proliferation in endometriosis. Ann. N. Y. Acad. Sci. 1994, 734, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Anaf, V.; Simon, P.; El Nakadi, I.; Fayt, I.; Simonart, T.; Buxant, F.; Noel, J.C. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum. Reprod. 2002, 17, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Anaf, V.; Chapron, C.; El Nakadi, I.; De Moor, V.; Simonart, T.; Noel, J.C. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil. Steril. 2006, 86, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, H.; Huang, X.; Lu, B.; Xu, H.; Zhou, C. Nerve fibres in ovarian endometriotic lesions in women with ovarian endometriosis. Hum. Reprod. 2010, 25, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, J. Neuritin, a neurotrophic factor in nervous system physiology. Curr. Med. Chem. 2014, 21, 1212–1219. [Google Scholar] [CrossRef]
- Dong, H.; Luo, X.; Niu, Y.; Yu, N.; Gao, R.; Wang, H.; Yang, L.; Huang, J. Neuritin 1 expression in human normal tissues and its association with various human cancers. Int. J. Clin. Exp. Pathol. 2018, 11, 1956–1964. [Google Scholar]
- Zhao, Q.R.; Lu, J.M.; Li, Z.Y.; Mei, Y.A. Neuritin promotes neurite and spine growth in rat cerebellar granule cells via L-type calcium channel-mediated calcium influx. J. Neurochem. 2018, 147, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Javaherian, A.; Cline, H.T. Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron 2005, 45, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xie, M.; Lu, H.; Zhang, H.; Shi, M.; Zhang, Y.; Xi, C.; Li, J.; Yang, T. Anti-Apoptotic Effect of IGF1 on Schwann Exposed to Hyperglycemia is Mediated by Neuritin, a Novel Neurotrophic Factor. Mol. Neurobiol. 2018, 55, 495–505. [Google Scholar] [CrossRef]
- Francis, S.M.; Larsen, J.E.; Pavey, S.J.; Bowman, R.V.; Hayward, N.K.; Fong, K.M.; Yang, I.A. Expression profiling identifies genes involved in emphysema severity. Respir. Res. 2009, 10, 81. [Google Scholar] [CrossRef]
- Kojima, N.; Shiojiri, N.; Sakai, Y.; Miyajima, A. Expression of neuritin during liver maturation and regeneration. FEBS Lett. 2005, 579, 4562–4566. [Google Scholar] [CrossRef][Green Version]
- Rahmawati, E.; Maurya, P.K.; Kao, A.P.; Chen, H.W.; Tzeng, C.R. The biosignatures of human endometriosis with and without GnRH agonist treatment. Fertil. Steril. 2013, 100, S362–S363. [Google Scholar] [CrossRef]
- Barcena de Arellano, M.L.; Arnold, J.; Lang, H.; Vercellino, G.F.; Chiantera, V.; Schneider, A.; Mechsner, S. Evidence of neurotrophic events due to peritoneal endometriotic lesions. Cytokine 2013, 62, 253–261. [Google Scholar] [CrossRef]
- Browne, A.S.; Yu, J.; Huang, R.P.; Francisco, A.M.; Sidell, N.; Taylor, R.N. Proteomic identification of neurotrophins in the eutopic endometrium of women with endometriosis. Fertil. Steril. 2012, 98, 713–719. [Google Scholar] [CrossRef][Green Version]
- Wessels, J.M.; Kay, V.R.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil. Steril. 2016, 105, 119–128. [Google Scholar] [CrossRef]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 12, CD008475. [Google Scholar] [CrossRef]
- Aranyi, Z.; Polyak, I.; Toth, N.; Vermes, G.; Gocsei, Z. Ultrasonography of sciatic nerve endometriosis. Muscle Nerve 2016, 54, 500–505. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J. Hum. Reprod. Sci. 2014, 7, 170–174. [Google Scholar] [CrossRef]
- Rahmawati, E.; Yang, W.V.; Lei, Y.P.; Maurya, P.K.; Chen, H.W.; Tzeng, C.R. Gonadotropin-releasing hormone agonist induces downregulation of tensin 1 in women with endometriosis. Acta. Obstet. Gynecol. Scand. 2019, 98, 222–231. [Google Scholar] [CrossRef]
- Sallam, H.N.; Garcia-Velasco, J.A.; Dias, S.; Arici, A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst. Rev. 2006, 1, CD004635. [Google Scholar] [CrossRef]
- Van der Houwen, L.E.; Schreurs, A.M.; Schats, R.; Heymans, M.W.; Lambalk, C.B.; Hompes, P.G.; Mijatovic, V. Efficacy and safety of intrauterine insemination in patients with moderate-to-severe endometriosis. Reprod. Biomed. Online 2014, 28, 590–598. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.A.; Kim, M.R.; Lee, J.H.; Kim, J.Y.; Hwang, K.J.; Kim, H.S.; Lee, E.S. Gonadotropin-releasing hormone agonist reduces aromatase cytochrome P450 and cyclooxygenase-2 in ovarian endometrioma and eutopic endometrium of patients with endometriosis. Gynecol. Obstet. Investig. 2009, 68, 73–81. [Google Scholar] [CrossRef]
- Huhtinen, K.; Desai, R.; Stahle, M.; Salminen, A.; Handelsman, D.J.; Perheentupa, A.; Poutanen, M. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. Int. J. Clin. Endocrinol. Metab. 2012, 97, 4228–4235. [Google Scholar] [CrossRef]
- Tetzlaff, J.E.; Huppenbauer, C.B.; Tanzer, L.; Alexander, T.D.; Jones, K.J. Motoneuron injury and repair: New perspectives on gonadal steroids as neurotherapeutics. J. Mol. Neurosci. 2006, 28, 53–64. [Google Scholar] [CrossRef]
- Sheth, S.S.; Hajari, A.R.; Lulla, C.P.; Kshirsagar, D. Sonographic evaluation of uterine volume and its clinical importance. J. Obstet. Gynaecol. Res. 2017, 43, 185–189. [Google Scholar] [CrossRef]
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]


| Women with Endometriosis GnRHa (−) | Women with Endometriosis GnRHa (+) | p-Value | |
|---|---|---|---|
| Total (n) | 37 | 45 | |
| Age (years) | 32.4 ± 5.9 (21–44) | 34.4 ± 4.2 (25–48) | 0.08 |
| BMI (kg/m2) | 21.6 ± 3.8 (16–35) | 20.9 ± 2.7 (16–29) | 0.35 |
| Dysmenorrhea (%) | 30 (81.1%) | 39 (86.7%) | 0.49 |
| Analgesics (%) | 8 (21.6%) | 14 (31.1%) | 0.33 |
| Smoking (%) | 9 (24.3%) | 17 (37.8%) | 0.19 |
| Alcohol (%) | 5 (13.5%) | 7 (15.6%) | 0.79 |
| Proliferative phase | 16 (43.2%) | ||
| Secretory phase | 21 (56.8%) |
| Patients | Age (y) | BMI (kg/m2) | Duration of GnRHa Tx | Diagnosis at Laparoscopy | Endometrial Thickness (mm) | Uterine Volume (mm3) | Chocolate Cyst Size (cm) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Before GnRHa Tx | After GnRHa Tx | Before GnRHa Tx | After GnRHa Tx | Before GnRHa Tx | After GnRHa Tx | |||||
| 1 | 32 | 19.88 | 1 | Stage IV endometriosis | 8 | 6 | 39.421 | 30.055 | 4.35 | 5.1 |
| 2 | 33 | 22.04 | 2 | Stage III endometriosis | 12 | 4 | 111.368 | 43.156 | 3.75 | 3.8 |
| 3 | 32 | 20.57 | 2 | Stage III endometriosis | 13 | 4 | 32.236 | 17.199 | 8.7 | 8.55 |
| 4 | 38 | 18.61 | 1 | Stage III endometriosis | 9 | 4 | 55.561 | 31.063 | 3 | 2.7 |
| 5 | 40 | 20.83 | 1 | Stage III endometriosis | 8 | 3.9 | 66.695 | 38.322 | 4.55 | 4.5 |
| 6 | 33 | 20.78 | 1 | Stage IV endometriosis | 10 | 4 | 54.843 | 41.047 | 4 | 4.1 |
| 7 | 33 | 22.59 | 2 | Stage III endometriosis | 11 | 13 | 50.544 | 62.057 | 3.2 | 3.2 |
| 8 | 33 | 18.56 | 2 | Stage III endometriosis | 6 | 4 | 46.476 | 36.504 | 3.25 | 2.7 |
| 9 | 35 | 17.27 | 2 | Stage IV endometriosis | 13 | 11 | 65.869 | 57.678 | 1 | 1.9 |
| 10 | 32 | 23.05 | 1 | Stage IV endometriosis | 5 | 3 | 60.191 | 38.788 | 4.25 | 4.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmawati, E.; Yang, W.-C.V.; Lei, Y.-P.; Maurya, P.K.; Chen, H.-W.; Tzeng, C.-R. Decreased Level of Neurotrophic Factor Neuritin 1 in Women with Ovarian Endometriosis after Receiving Gonadotropin-Releasing Hormone Agonist Treatment. Int. J. Mol. Sci. 2019, 20, 4352. https://doi.org/10.3390/ijms20184352
Rahmawati E, Yang W-CV, Lei Y-P, Maurya PK, Chen H-W, Tzeng C-R. Decreased Level of Neurotrophic Factor Neuritin 1 in Women with Ovarian Endometriosis after Receiving Gonadotropin-Releasing Hormone Agonist Treatment. International Journal of Molecular Sciences. 2019; 20(18):4352. https://doi.org/10.3390/ijms20184352
Chicago/Turabian StyleRahmawati, Endah, Wei-Chung Vivian Yang, Yen-Ping Lei, Pawan Kumar Maurya, Huei-Wen Chen, and Chii-Ruey Tzeng. 2019. "Decreased Level of Neurotrophic Factor Neuritin 1 in Women with Ovarian Endometriosis after Receiving Gonadotropin-Releasing Hormone Agonist Treatment" International Journal of Molecular Sciences 20, no. 18: 4352. https://doi.org/10.3390/ijms20184352
APA StyleRahmawati, E., Yang, W.-C. V., Lei, Y.-P., Maurya, P. K., Chen, H.-W., & Tzeng, C.-R. (2019). Decreased Level of Neurotrophic Factor Neuritin 1 in Women with Ovarian Endometriosis after Receiving Gonadotropin-Releasing Hormone Agonist Treatment. International Journal of Molecular Sciences, 20(18), 4352. https://doi.org/10.3390/ijms20184352





