Trefoil Factor 3 (TFF3) Is Involved in Cell Migration for Skeletal Repair
Abstract
1. Introduction
2. Results
2.1. Expression of TFF3 in Human Joint Tissue
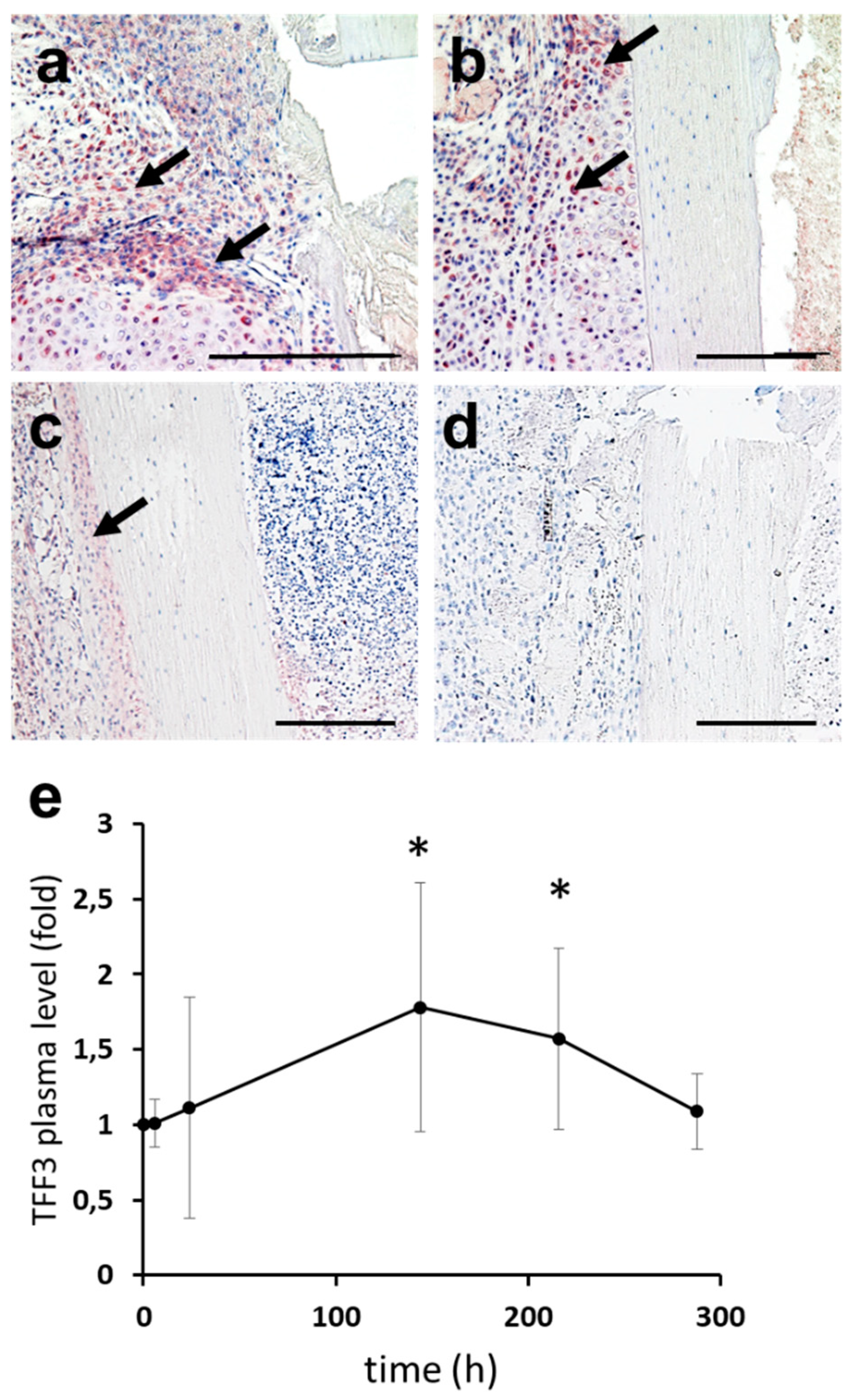
2.2. Expression of TFF3 in Murine Fracture Callus
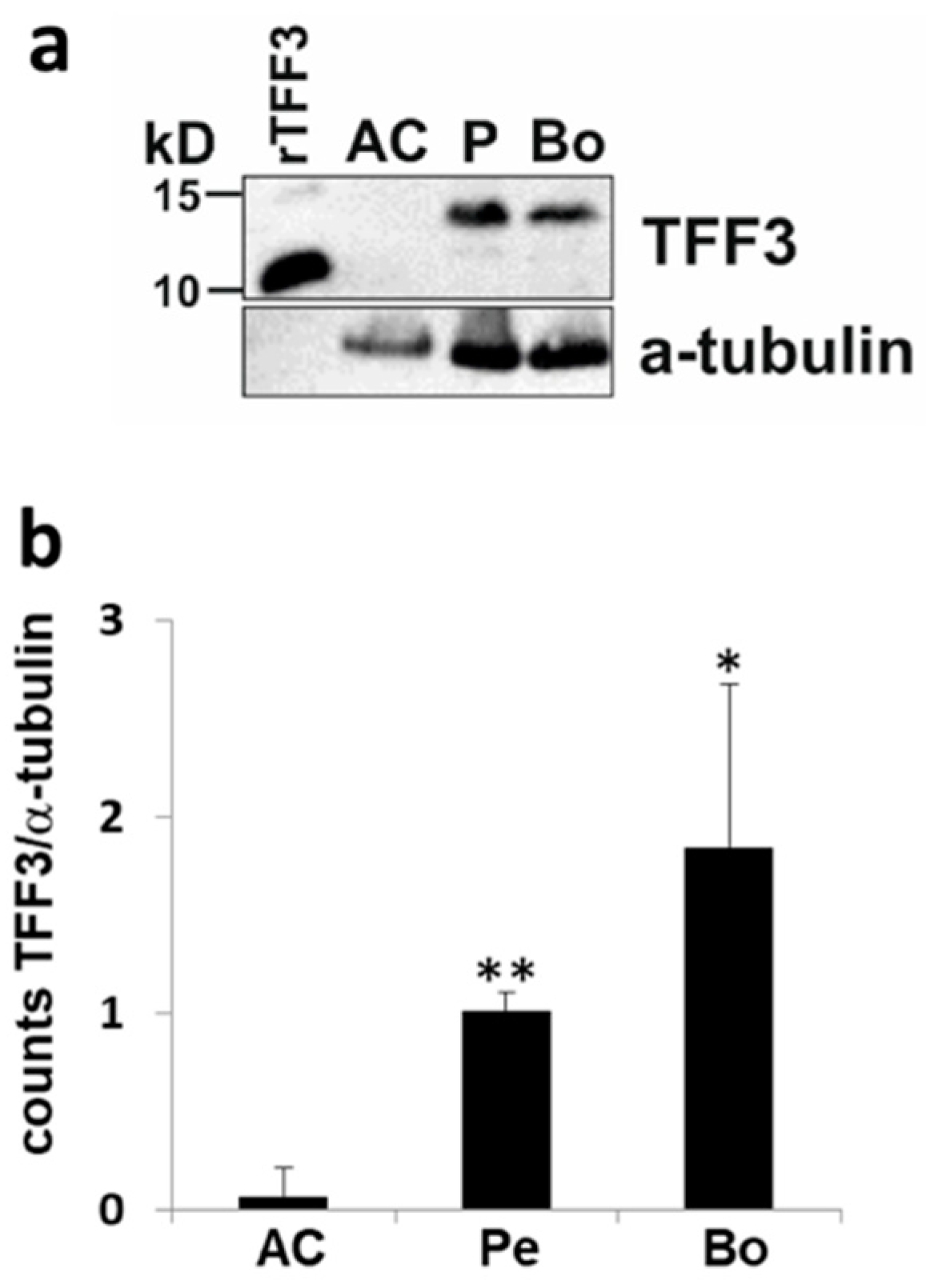
2.3. Upstream Regulators of TFF3: Induction of TFF3 by Trauma
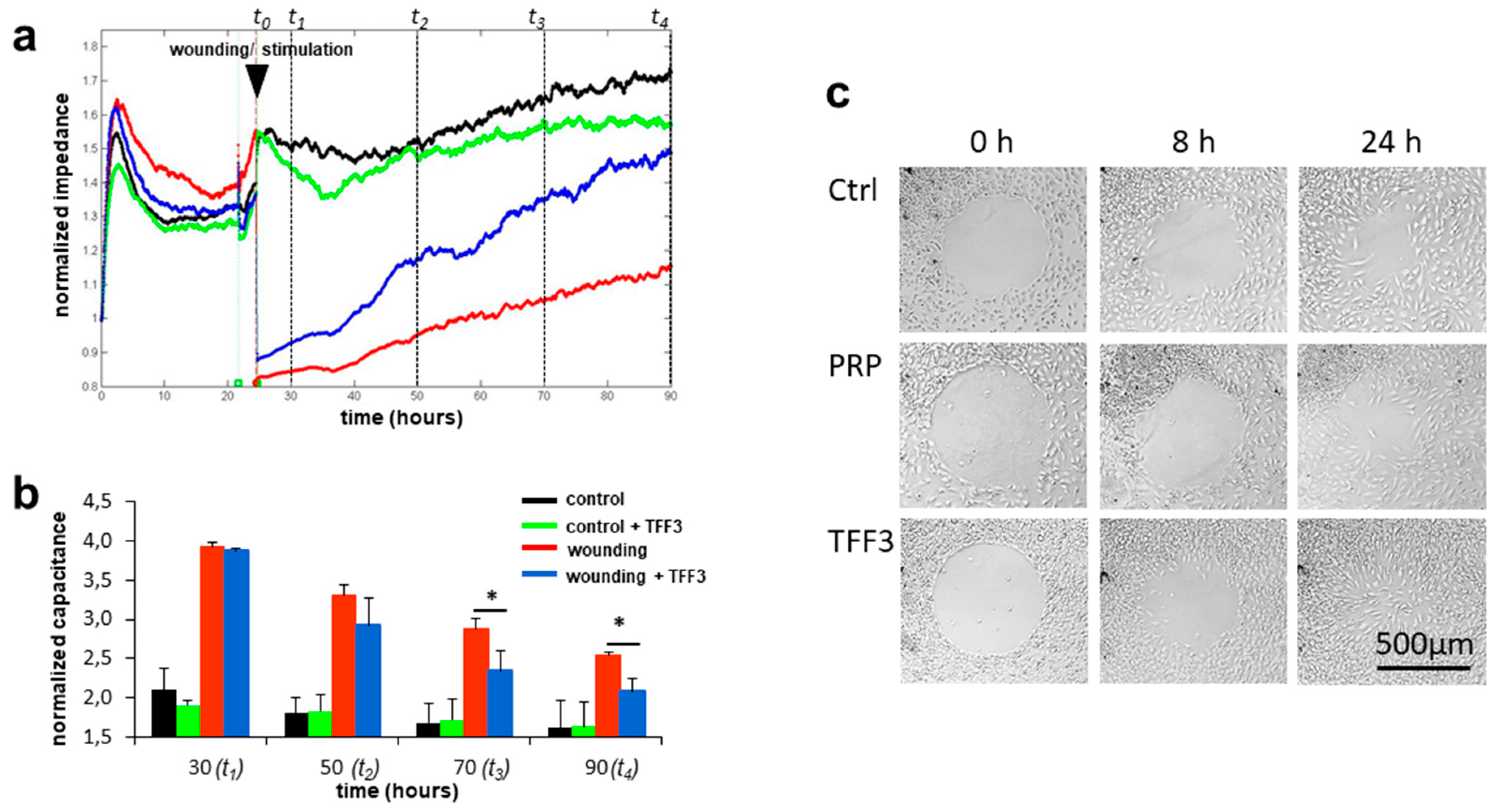
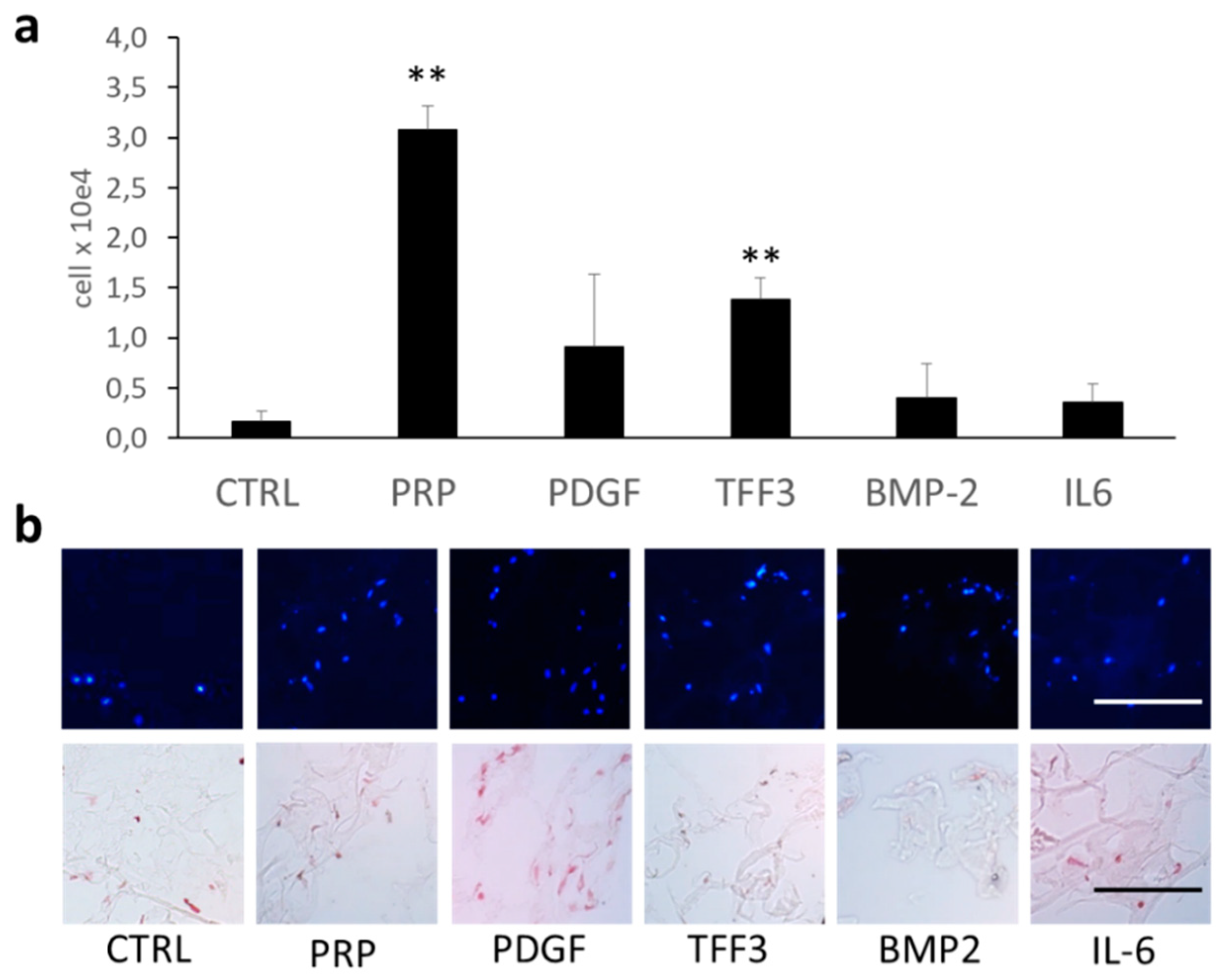
2.4. Effects of TFF3 on Cell Migration and Proliferation of MPCs
2.5. Radius Cell Migration Assay
2.6. Cell Proliferation and Cell Viability
2.7. Chemotaxis Assay
2.8. Immunofluorescence
2.9. RT2 Profiler PCR Array Analysis
3. Discussion
4. Methods
4.1. Human Tissue Samples
4.2. Immunoblot Analysis
4.3. Murine Fracture Callus
4.4. Immunohistochemical Analysis
4.5. Human Serum Samples
4.6. Cell Culture Experiments
4.7. RT2 Profiler PCR Array of Human Cell Motility
4.8. Electric Cell-Substrate Impedance Sensing (ECIS)
4.9. Radius Cell Migration Assay
4.10. Proliferation Assay
4.11. Chemotaxis Assay
4.12. Immunofluorescence
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Thim, L.; May, F.E. Structure of mammalian trefoil factors and functional insights. Cell Mol. Life Sci. 2005, 62, 2956–2973. [Google Scholar] [CrossRef] [PubMed]
- Albert, T.K.; Laubinger, W.; Muller, S.; Hanisch, F.G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human intestinal tff3 forms disulfide-linked heteromers with the mucus-associated fcgbp protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, N.; Belovari, T.; Baus Loncar, M. Trefoil factor family protein 3 (tff3) is present in cartilage during endochondral ossification in the developing mouse fetus. Acta Histochem. 2013, 115, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Rosler, S.; Haase, T.; Claassen, H.; Schulze, U.; Schicht, M.; Riemann, D.; Brandt, J.; Wohlrab, D.; Muller-Hilke, B.; Goldring, M.B.; et al. Trefoil factor 3 is induced during degenerative and inflammatory joint disease, activates matrix metalloproteinases, and enhances apoptosis of articular cartilage chondrocytes. Arthritis Rheum. 2010, 62, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, F.P.; Berry, M.S. Mucins and tff peptides of the tear film and lacrimal apparatus. Prog. Histochem. Cytochem. 2006, 41, 1–53. [Google Scholar] [CrossRef]
- Dubeykovskaya, Z.; Si, Y.; Chen, X.; Worthley, D.L.; Renz, B.W.; Urbanska, A.M.; Hayakawa, Y.; Xu, T.; Westphalen, C.B.; Dubeykovskiy, A.; et al. Neural innervation stimulates splenic tff2 to arrest myeloid cell expansion and cancer. Nat. Commun. 2016, 7, 10517. [Google Scholar] [CrossRef]
- Dieckow, J.; Brandt, W.; Hattermann, K.; Schob, S.; Schulze, U.; Mentlein, R.; Ackermann, P.; Sel, S.; Paulsen, F.P. Cxcr4 and cxcr7 mediate tff3-induced cell migration independently from the erk1/2 signaling pathway. Investig. Ophthalmol. Vis. Sci. 2016, 57, 56–65. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil factor family (tff) peptides and chemokine receptors: A promising relationship. J. Med. Chem. 2009, 52, 6505–6510. [Google Scholar] [CrossRef]
- Paulsen, F.P.; Woon, C.W.; Varoga, D.; Jansen, A.; Garreis, F.; Jager, K.; Amm, M.; Podolsky, D.K.; Steven, P.; Barker, N.P.; et al. Intestinal trefoil factor/tff3 promotes re-epithelialization of corneal wounds. J. Biol. Chem. 2008, 283, 13418–13427. [Google Scholar] [CrossRef]
- Schulze, U.; Hampel, U.; Sel, S.; Contreras-Ruiz, L.; Schicht, M.; Dieckow, J.; Diebold, Y.; Paulsen, F. Trefoil factor family peptide 3 (tff3) is upregulated under experimental conditions similar to dry eye disease and supports corneal wound healing effects in vitro. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory tff peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–181. [Google Scholar] [PubMed]
- Baker, C.E.; Moore-Lotridge, S.N.; Hysong, A.A.; Posey, S.L.; Robinette, J.P.; Blum, D.M.; Benvenuti, M.A.; Cole, H.A.; Egawa, S.; Okawa, A.; et al. Bone fracture acute phase response-a unifying theory of fracture repair: Clinical and scientific implications. Clin. Rev. Bone Miner. Metab. 2018, 16, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Jagla, W.; Wiede, A. Molecular medicine of tff-peptides: From gut to brain. Histol. Histopathol. 2001, 16, 319–334. [Google Scholar] [PubMed]
- Durer, U.; Hartig, R.; Bang, S.; Thim, L.; Hoffmann, W. Tff3 and egf induce different migration patterns of intestinal epithelial cells in vitro and trigger increased internalization of e-cadherin. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2007, 20, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, Y.P.; Xiao, C.X.; Ren, J.L.; Guleng, B. Serum tff3 may be a pharamcodynamic marker of responses to chemotherapy in gastrointestinal cancers. BMC Clin. Pathol. 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishibashi, Y.; Ohtsu, H. Serum tff1 and tff3 but not tff2 are higher in women with breast cancer than in women without breast cancer. Sci. Rep. 2017, 7, 4846. [Google Scholar] [CrossRef]
- Braga Emidio, N.; Hoffmann, W.; Brierley, S.M.; Muttenthaler, M. Trefoil factor family: Unresolved questions and clinical perspectives. Trends Biochem. Sci. 2019, 44, 387–390. [Google Scholar] [CrossRef]
- Vemula, S.; Shi, J.; Hanneman, P.; Wei, L.; Kapur, R. Rock1 functions as a suppressor of inflammatory cell migration by regulating pten phosphorylation and stability. Blood 2010, 115, 1785–1796. [Google Scholar] [CrossRef]
- Guo, J.; Xu, L.; Teng, X.; Sun, M. Microrna-7-5p regulates the proliferation and migration of intestinal epithelial cells by targeting trefoil factor 3 via inhibiting the phosphoinositide 3-kinase/akt signalling pathway. Int. J. Mol. Med. 2017, 40, 1435–1443. [Google Scholar] [CrossRef]
- Storesund, T.; Schenck, K.; Osmundsen, H.; Roed, A.; Helgeland, K.; Kolltveit, K.M. Signal transduction and gene transcription induced by tff3 in oral keratinocytes. Eur. J. Oral Sci. 2009, 117, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Taupin, D.R.; Itoh, H.; Podolsky, D.K. Distinct pathways of cell migration and antiapoptotic response to epithelial injury: Structure-function analysis of human intestinal trefoil factor. Mol. Cell Biol. 2000, 20, 4680–4690. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, H.; Dai, J.; Zhang, W.; Zhang, J.; Xue, G.; Wu, J. Tff3 contributes to epithelial-mesenchymal transition (emt) in papillary thyroid carcinoma cells via the mapk/erk signaling pathway. J. Cancer 2018, 9, 4430–4439. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Zhang, D.Y.; Zhao, Y.; Qiu, W.; Wang, P.; Sun, Y. Itf promotes migration of intestinal epithelial cells through crosstalk between the erk and jak/stat3 pathways. Sci. Rep. 2016, 6, 33014. [Google Scholar] [CrossRef] [PubMed]
- Graness, A.; Chwieralski, C.E.; Reinhold, D.; Thim, L.; Hoffmann, W. Protein kinase c and erk activation are required for tff-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-alpha-induced interleukin-6 (il-6) and il-8 secretion. J. Biol. Chem. 2002, 277, 18440–18446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Gao, J.; Li, H.; Guo, W.; Mao, Q.; Gao, E.; Zhu, Y.Q. Trefoil factor 3 peptide regulates migration via a twist-dependent pathway in gastric cell. Biochem. Biophys. Res. Commun. 2013, 438, 6–12. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Zhou, Y.; Mao, X.; Deng, X. Cloning and characterization of the human trefoil factor 3 gene promoter. PLoS ONE 2014, 9, e95562. [Google Scholar] [CrossRef]
- Maycock, N.J.; Marshall, J. Genomics of corneal wound healing: A review of the literature. Acta Ophthalmol. 2014, 92, e170–e184. [Google Scholar] [CrossRef]
- Xie, J.; Han, Z.Y.; Matsuda, T. Mechanical compressive loading stimulates the activity of proximal region of human col2a1 gene promoter in transfected chondrocytes. Biochem. Biophys. Res. Commun. 2006, 344, 1192–1199. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the american rheumatism association. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Paulsen, F.; Varoga, D.; Paulsen, A.; Tsokos, M. Trefoil factor family (tff) peptides of normal human vater’s ampulla. Cell Tissue Res. 2005, 321, 67–74. [Google Scholar] [CrossRef]
- Rontgen, V.; Blakytny, R.; Matthys, R.; Landauer, M.; Wehner, T.; Gockelmann, M.; Jermendy, P.; Amling, M.; Schinke, T.; Claes, L.; et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J. Orthop. Res. 2010, 28, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Muller-Graf, F.; Matthys, R.; Abaei, A.; Jonas, R.; Gebhard, F.; Rasche, V.; Ignatius, A. In vivo evaluation of fracture callus development during bone healing in mice using an mri-compatible osteosynthesis device for the mouse femur. J. Vis. Exp. JoVE 2017. [Google Scholar] [CrossRef] [PubMed]
- Probst, J.C.; Skutella, T.; Muller-Schmid, A.; Jirikowski, G.F.; Hoffmann, W. Molecular and cellular analysis of rp1.B in the rat hypothalamus: In situ hybridization and immunohistochemistry of a new p-domain neuropeptide. Brain Res. Mol. Brain Res. 1995, 33, 269–276. [Google Scholar] [CrossRef]
- Zimmermann, R.; Reske, S.; Metzler, P.; Schlegel, A.; Ringwald, J.; Eckstein, R. Preparation of highly concentrated and white cell-poor platelet-rich plasma by plateletpheresis. Vox Sang. 2008, 95, 20–25. [Google Scholar] [CrossRef] [PubMed]


| Gene | Fold Change | p-Value |
|---|---|---|
| CAPN1 | 0.630 | 0.151 |
| ARHGDIA | 0.793 | 0.109 |
| STAT3 | 0.851 | 0.219 |
| BAIAP2 | 0.866 | 0.443 |
| ROCK1 | 0.868 | 0.043 * |
| SH3PXD2A | 0.869 | 0.456 |
| PLD1 | 0.871 | 0.562 |
| IGF1 | 0.872 | 0.690 |
| BCAR1 | 0.874 | 0.499 |
| PRKCA | 0.879 | 0.478 |
| PTK2 | 0.881 | 0.073 |
| PTEN | 0.882 | 0.499 |
| ACTN3 | 1.091 | 0.554 |
| ACTB | 1.101 | 0.567 |
| MMP9 | 1.145 | 0.916 |
| EGF | 1.155 | 0.991 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, K.; Schmid, S.; Paulsen, F.; Ignatius, A.; Klinger, P.; Hotfiel, T.; Swoboda, B.; Gelse, K. Trefoil Factor 3 (TFF3) Is Involved in Cell Migration for Skeletal Repair. Int. J. Mol. Sci. 2019, 20, 4277. https://doi.org/10.3390/ijms20174277
Krüger K, Schmid S, Paulsen F, Ignatius A, Klinger P, Hotfiel T, Swoboda B, Gelse K. Trefoil Factor 3 (TFF3) Is Involved in Cell Migration for Skeletal Repair. International Journal of Molecular Sciences. 2019; 20(17):4277. https://doi.org/10.3390/ijms20174277
Chicago/Turabian StyleKrüger, Katharina, Sebastian Schmid, Friedrich Paulsen, Anita Ignatius, Patricia Klinger, Thilo Hotfiel, Bernd Swoboda, and Kolja Gelse. 2019. "Trefoil Factor 3 (TFF3) Is Involved in Cell Migration for Skeletal Repair" International Journal of Molecular Sciences 20, no. 17: 4277. https://doi.org/10.3390/ijms20174277
APA StyleKrüger, K., Schmid, S., Paulsen, F., Ignatius, A., Klinger, P., Hotfiel, T., Swoboda, B., & Gelse, K. (2019). Trefoil Factor 3 (TFF3) Is Involved in Cell Migration for Skeletal Repair. International Journal of Molecular Sciences, 20(17), 4277. https://doi.org/10.3390/ijms20174277






