Astrochemistry and Astrobiology: Materials Science in Wonderland?
Abstract
1. Introduction
2. Complex Organic Molecules
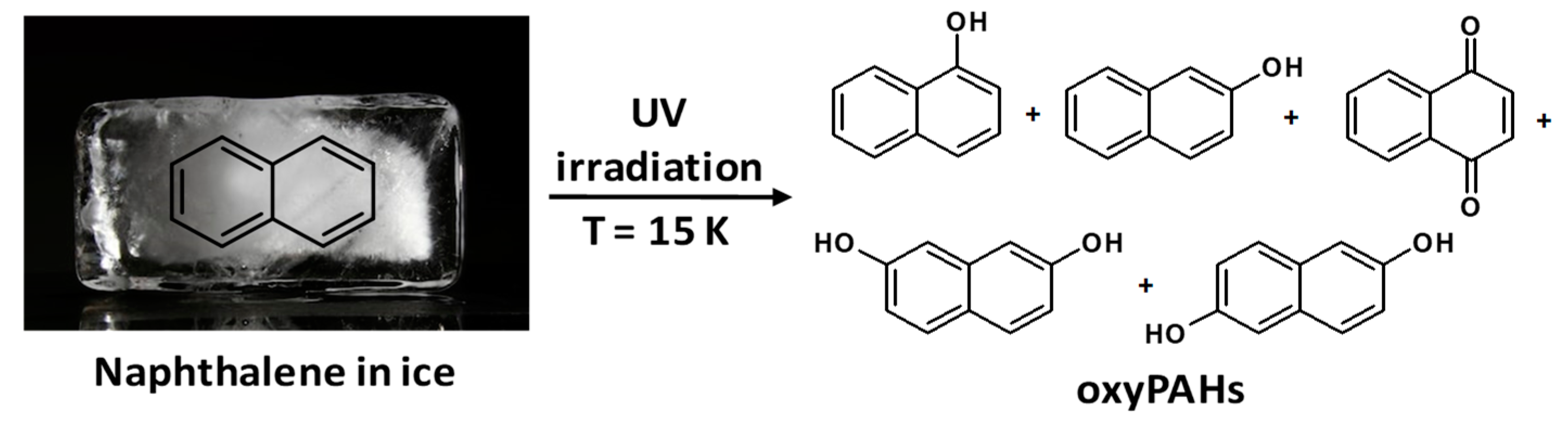
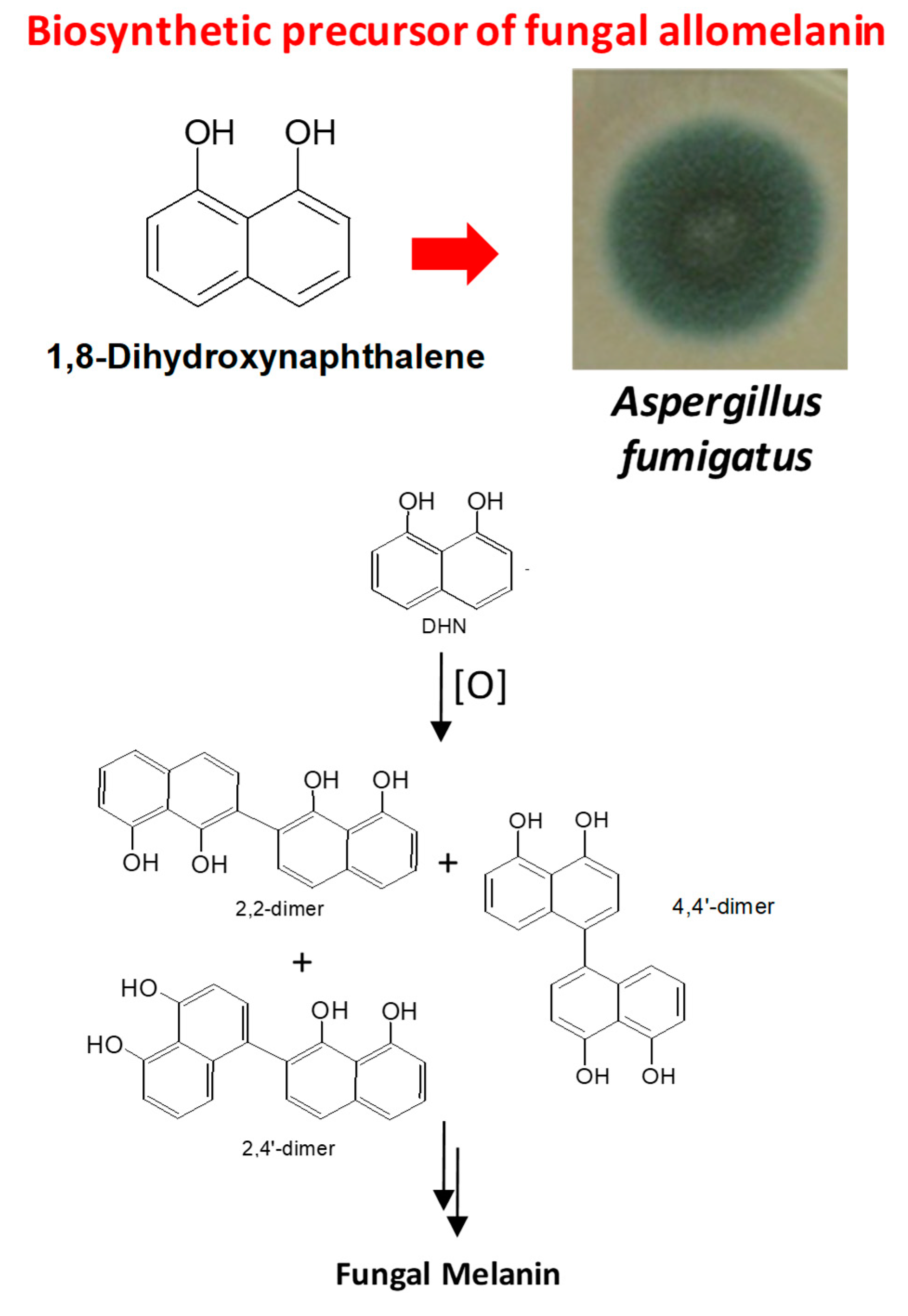
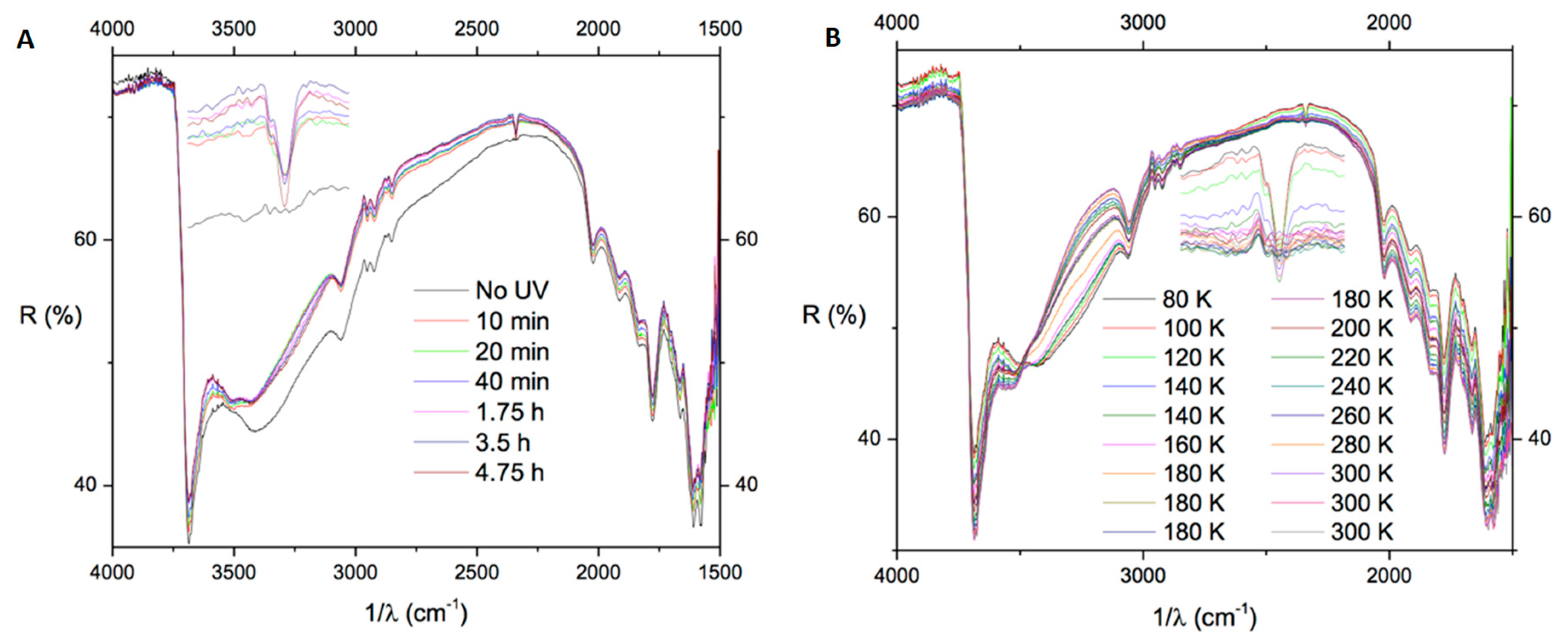
2.1. Polycyclic Aromatic Hydrocarbons (PAHs)
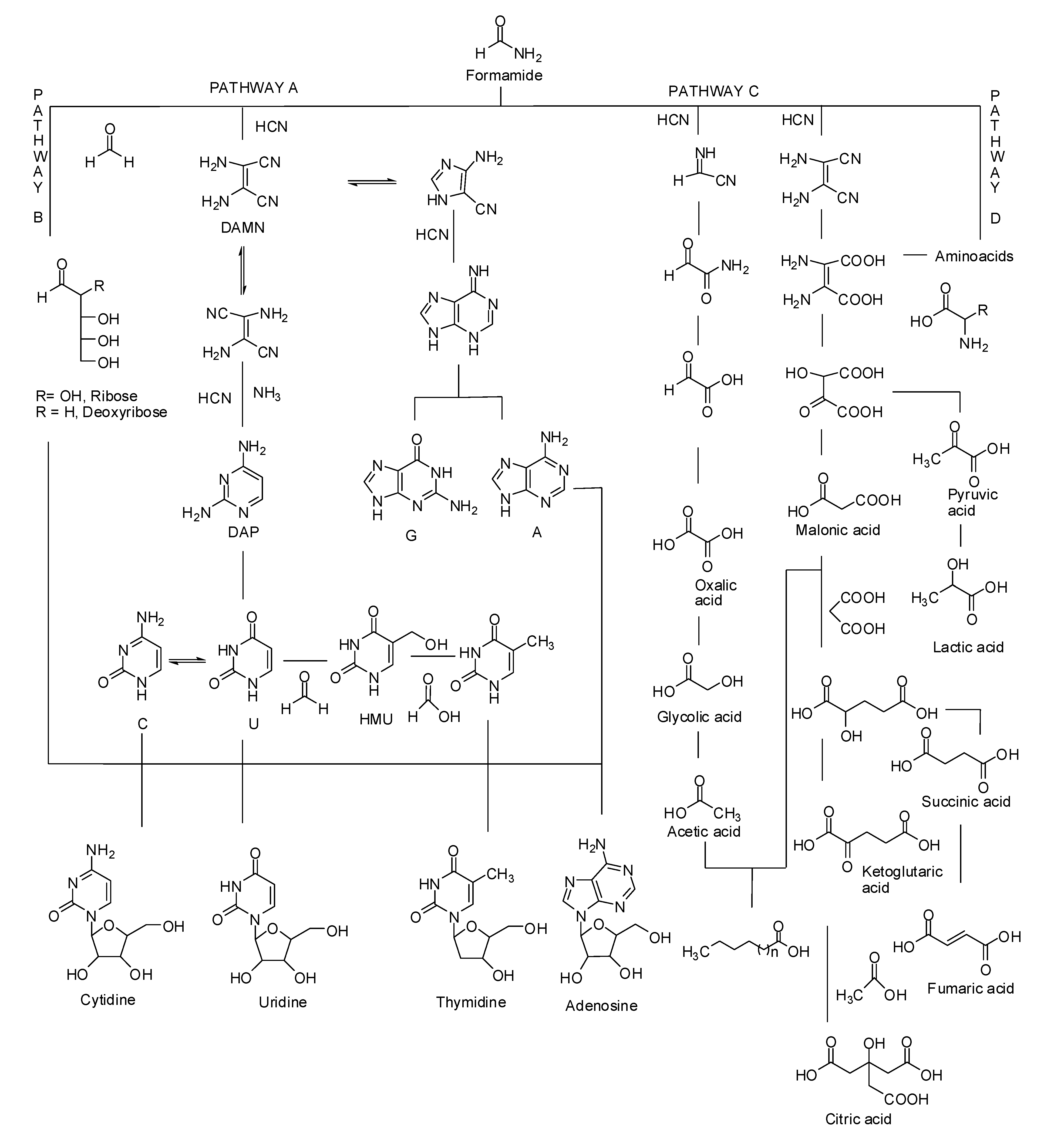
2.2. Hydrogen Cyanide (HCN) Oligomers and Polymers: Aminomalononitrile
- (i)
- they constitute a versatile coating technology, able to coat almost all known materials, even teflon, with a conformal nano-coating with a controllable thickness;
- (ii)
- they have the ability to reduce metal cations like Ag+ [26];
- (iii)
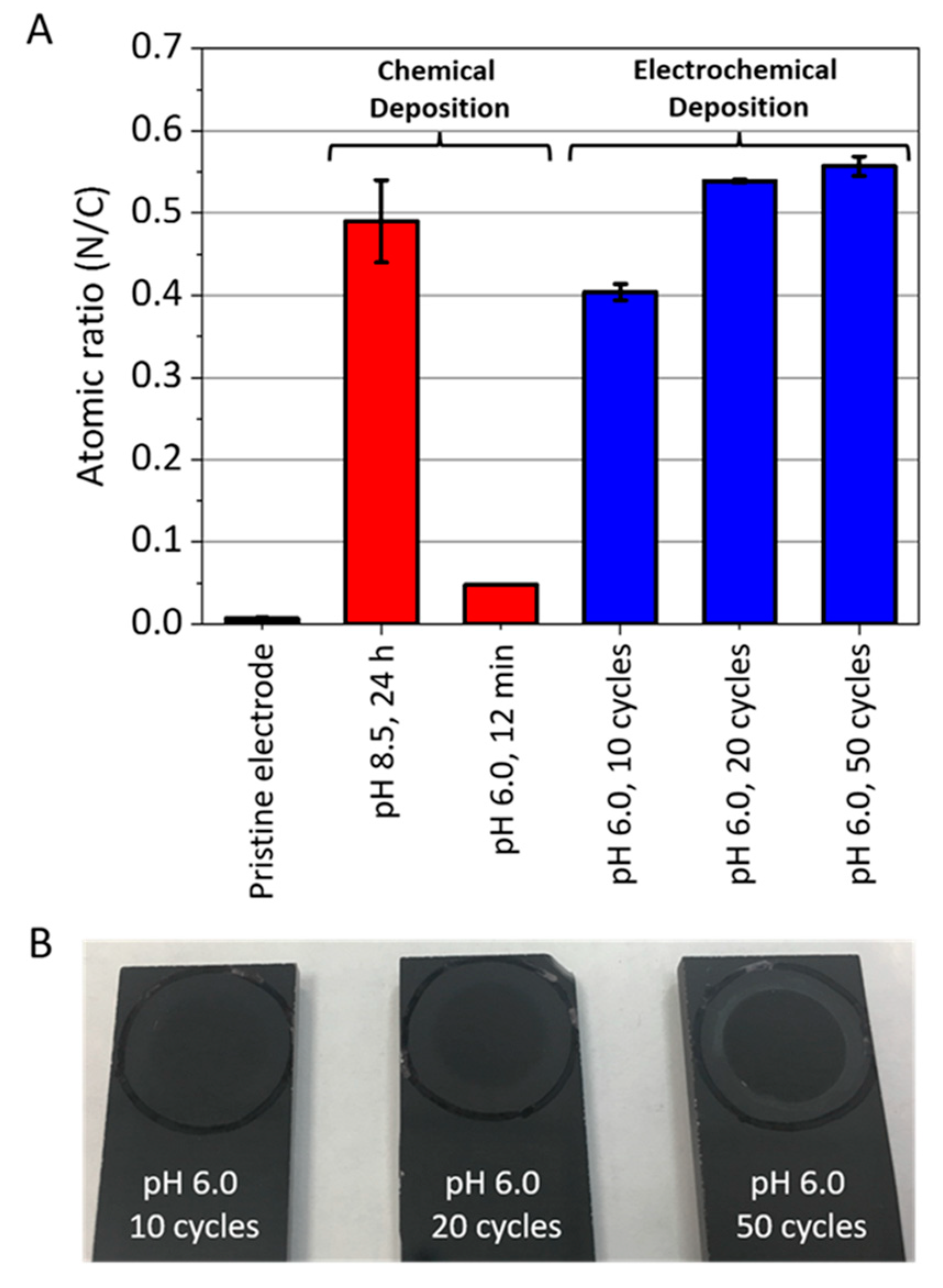
- they can be deposited on conductive substrates by means of cyclic voltammetry (CV)or chronoamperometry from solutions in which no chemical transformation of the monomers occur, namely at pH = 6 [31].
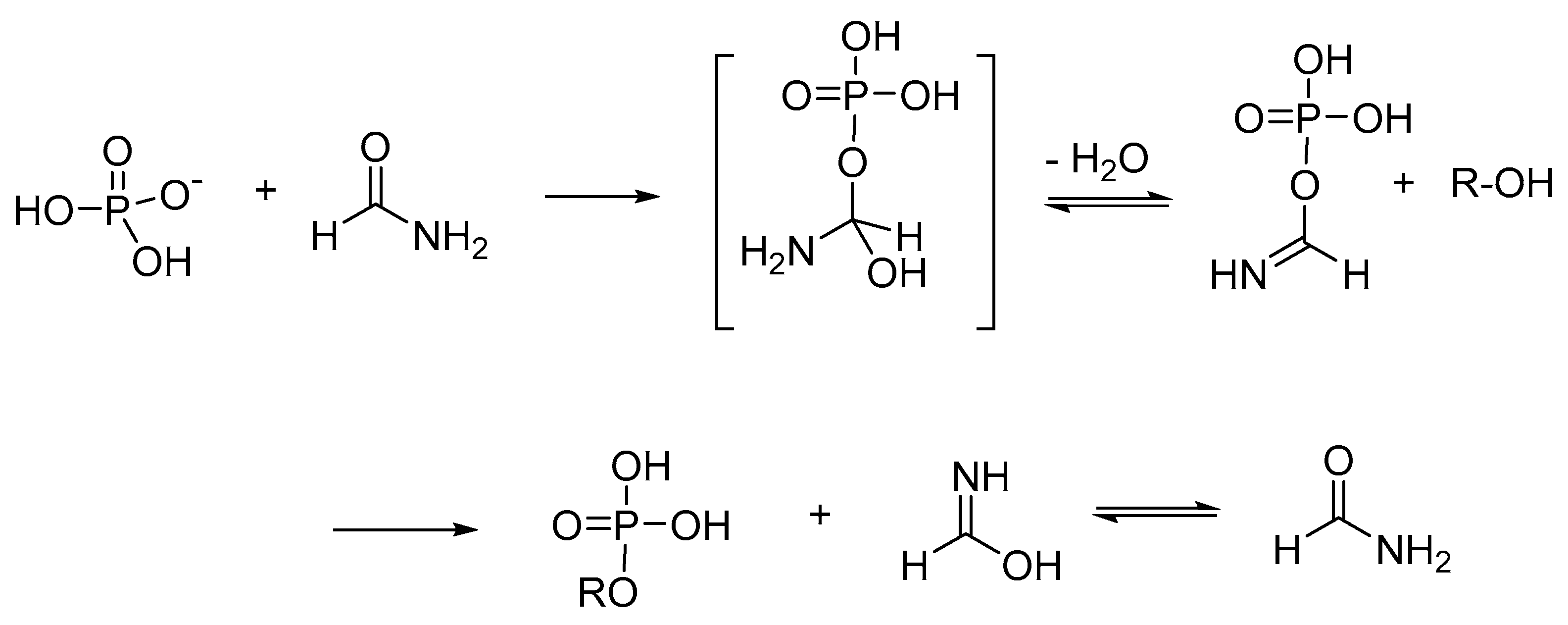
2.3. Formamide-Based Prebiotic Chemistry in Phosphorylation Processes
2.4. Biochemistry of Extremophilic Microorganisms
3. Astrochemically Relevant Processes
3.1. Solid-State Reactions on Minerals and Meteorite Surface
3.2. Proton Beam Bombardment on Minerals
4. Miscellanea
- (i)
- the methane/nitrogen ratio;
- (ii)
- the energy source;
- (iii)
- the pressure and temperature of the gas mixture;
- (iv)
- oxygen, CO2, and water contamination.
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Leger, A.; Puget, J.L. Identification of the ‘unidentified’ IR emission features of interstellar dust? Astron. Astrophys. 1984, 137, L5–L8. [Google Scholar]
- Allamandola, L.J.; Tielens, A.G.G.M.; Barker, J.R. Polycyclic Aromatic Hydrocarbons and the unidentified infrared emission bands: Auto exhaust along the milky way! Astrophys. J. 1985, 290, L25–L28. [Google Scholar] [CrossRef]
- Blasberger, A.; Behar, E.; Perets, H.B.; Brosch, N.; Tielens, A.G.G.M. Observational Evidence Linking Interstellar UV Absorption to PAH Molecules. Astrophys. J. 2017, 173, 836. [Google Scholar] [CrossRef]
- López-Puertas, M.; Dinelli, B.M.; Adriani, A.; Funke, B.; García-Comas, M.; Moriconi, M.L.; D’Aversa, E.; Boersma, C.; Allamandola, L.J. Large abundances of polycyclic aromatic hydrocarbons in Titan’s upper atmosphere. Astrophys. J. 2013, 770, 2. [Google Scholar] [CrossRef]
- Peeters, E.; Hony, S.; Van Kerckhoven, C.; Tielens, A.G.G.M.; Allamandola, L.J.; Hudgins, D.M.; Bauschlicher, C.W. The rich 6 to 9 µm spectrum of interstellar PAHs. Astron. Astrophys. 2002, 390, 1089–1113. [Google Scholar] [CrossRef]
- Bauschlicher, C.W., Jr.; Peeters, E.; Allamandola, L.J. The Infrared Spectra of Very Large Irregular Polycyclic Aromatic Hydrocarbons (PAHs): Observational Probes of Astronomical PAH Geometry, Size, and Charge. Astrophys. J. 2009, 697, 311–327. [Google Scholar] [CrossRef]
- Rapacioli, M.; Joblin, C.; Boissel, P.; Calvo, F.; Spiegelman, F. Spectroscopy of Polycyclic Aromatic Hydrocarbons and Very Small Grains in Photodissociation Regions. Astron. Astrophys. 2005, 429, 193–204. [Google Scholar] [CrossRef]
- Simon, A.; Joblin, C. Photodissociation of [Fex(C24H12)y]+ Complexes in the PIRENEA Setup: Iron-Polycyclic Aromatic Hydrocarbon Clusters as Candidates for Very Small Interstellar Grains. J. Phys. Chem. 2009, 113, 4878–4888. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.J.F.; Kazandjian, M.V.; van der Werf, P.P.; Israel, F.P.; Meijerink, R.; Weiß, A.; Requena-Torres, M.A.; Güsten, R. Radiative and mechanical feedback into the molecular gas of NGC 253. Astron. Astrophys. 2014, 564. [Google Scholar] [CrossRef]
- Galué, H.A.; DíazLeines, G. Origin of Spectral Band Patterns in the Cosmic Unidentified Infrared Emission. Phys. Rev. Lett. 2017, 119, 171102. [Google Scholar] [CrossRef]
- Parker, D.S.N.; Zhang, F.; Kim, Y.S.; Kaiser, R.I.; Landera, A.; Kislov, V.V.; Mebel, A.M.; Tielens, A.G.G.M. Low temperature formation of naphthalene and its role in the synthesis of PAHs (Polycyclic Aromatic Hydrocarbons) in the interstellar medium. Proc. Natl. Acad. Sci. USA 2012, 109, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Merino, P.; Švec, M.; Martinez, J.I.; Jelinek, P.; Lacovig, P.; Dalmiglio, M.; Lizzit, S.; Soukiassian, P.; Cernicharo, J.; Martin-Gago, J.A. Graphene etching on SiC grains as a path to interstellar polycyclic aromatic hydrocarbons formation. Nature Commun. 2014, 5, 3054. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, J.; Mattioda, A.L.; Linnartz, H.; Allamandola, L.J. Photochemistry of Polycyclic Aromatic Hydrocarbons in Cosmic Water Ice I. Mid-IR Spectroscopy and Photoproducts. Astron. Astrophys. 2011, 525. [Google Scholar] [CrossRef]
- Bernstein, M.P.; Sandford, S.A.; Allamandola, L.J.; Gillette, J.S.; Clemett, S.J.; Zare, R.N. UV Irradiation of Polycyclic Aromatic Hydrocarbons in Ices: Poduction of Alcohols, Quinones, and Ethers. Science 1999, 283, 1135–1138. [Google Scholar] [CrossRef]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Allamandola, L.J. Ultraviolet Irradiation of Naphthalene in H2O Ice: Implications for Meteorites and Biogenesis. Meteorit. Planet. Sci. 2001, 36, 351–358. [Google Scholar] [CrossRef]
- Cecchini, M.M.; Reale, S.; Manini, P.; d’Ischia, M.; De Angelis, F. Modeling Fungal Melanin Buildup: Biomimetic Polymerization of 1,8-Dihydroxynaphthalene Mapped by Mass Spectrometry. Chem. Eur. J. 2017, 23, 8092–8098. [Google Scholar] [CrossRef] [PubMed]
- Manini, P.; Bietti, M.; Galeotti, M.; Salamone, M.; Lanzalunga, O.; Cecchini, M.M.; Reale, S.; Crescenzi, O.; Napolitano, A.; De Angelis, F.; et al. Characterization and Fate of Hydrogen-Bonded Free-Radical Intermediates and Their Coupling Products from the Hydrogen Atom Transfer Agent 1,8-Naphthalenediol. ACS Omega 2018, 3, 3918–3927. [Google Scholar] [CrossRef]
- Manini, P.; Lino, V.; Franchi, P.; Gentile, G.; Sibillano, T.; Giannini, C.; Picardi, E.; Napolitano, A.; Valgimigli, L.; Chiappe, C.; et al. A Robust Fungal Allomelanin Mimic: An Antioxidant and Potent π-Electron Donor with Free-Radical Properties that can be Tuned by Ionic Liquids. ChemPlusChem 2019, 84. [Google Scholar] [CrossRef]
- Longo, D.L.; Stefania, R.; Aime, S.; Oraevsky, A. Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications. Int. J. Mol. Sci. 2017, 18, 1719. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Barra, M.; Musto, A.; Navarra, A.; Alfè, M.; Manini, P.; Parisi, S.; Cassinese, A.; Criscuolo, V.; d’Ischia, M. Stem cell-compatible eumelaninbiointerface fabricated by chemically controlled solid state polymerization. Mater. Horiz. 2015, 2, 212–220. [Google Scholar] [CrossRef]
- Ferris, J.P.; Hagan, W.J., Jr. HCN and chemical evolution: The possible role of cyano compounds in prebiotic synthesis. Tetrahedron 1984, 40, 1093–1120. [Google Scholar] [CrossRef]
- Matthews, C.N.; Minard, R.D. Hydrogen cyanide polymers, comets and the origin of life. Faraday Discuss. 2006, 133, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in prebiotic chemistry. II Synthesis of purine precursors and amino acids from aqueous hydrogen cyanide. J. Mol. Biol. 1967, 30, 223–253. [Google Scholar] [PubMed]
- Raulin, F.; Fonsalas, F.; Wolny, M. Aminomalononitrile: Some new data of prebiotic interest. Orig. Life 1984, 14, 151–156. [Google Scholar] [CrossRef]
- Thissen, H.; Koegler, A.; Salwiczek, M.; Easton, C.D.; Qu, Y.; Lithgow, T.; Evans, R.A. Prebiotic-chemistry inspired polymer coatings for biomedical and materials science applications. NPG Asia Mater. 2015, 7, e225. [Google Scholar] [CrossRef]
- Menzies, D.J.; Ang, A.; Thissen, H.; Evans, R.A. Adhesive prebiotic chemistry inspired coatings for bone contacting applications. ACS Biomater. Sci. Eng. 2017, 3, 793–806. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Kang, S.M.; Rho, J.; Choi, I.S.; Messersmith, P.B.; Lee, H. Norepinephrine: Material independant, multifunctional surface modification reagent. J. Am. Chem. Soc. 2009, 131, 13224–13225. [Google Scholar] [CrossRef]
- d’Ischia, M.; Palumbo, A.; Prota, G. Adrenalinoxidationrevisited. New products beyond the adrenochrome stage. Tetrahedron 1988, 4, 6441–6446. [Google Scholar] [CrossRef]
- Ball, V.; Toh, R.J.; Voelcker, N.; Thissen, H.; Evans, R. Electrochemical deposition of aminomalonotrile based films. Colloids Surf. A 2008, 552, 124–129. [Google Scholar] [CrossRef]
- Saitta, A.M.; Saija, F. Miller experiments in atomistic computer simulations. Proc. Natl. Acad. Sci. USA 2014, 111, 13768–13773. [Google Scholar] [CrossRef] [PubMed]
- Adande, G.R.; Woolf, N.J.; Ziurys, L.M. Observations of interstellar formamide: Availability of a prebiotic precursor in the galactic habitable zone. Astrobiology 2013, 13, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; Di Mauro, E. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Pino, S.; Costanzo, G.; Di Mauro, E. Genetics first or metabolismfirst? The formamide clue. Chem. Soc. Rev. 2012, 41, 5526–5565. [Google Scholar] [CrossRef]
- Šponer, J.E.; Šponer, J.; Nováková, O.; Brabec, V.; Šedo, O.; Zdráhal, Z.; Costanzo, G.; Pino, S.; Saladino, R.; Di Mauro, E. Emergence of the First Catalytic Oligonucleotides in a Formamide-Based Origin Scenario. A review. Chem. Eur. J. 2016, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Bizzarri, B.M.; Di Mauro, E.; Garcia Ruiz, J.M. A global scale scenario for prebiotic chemistry: Silica-based self-asssembled mineral structures and formamide. Biochemistry 2016, 55, 2806–2811. [Google Scholar] [CrossRef]
- Gull, M. Prebiotic Phosphorylation Reactions on the Early Earth. Challenges 2014, 5, 193–212. [Google Scholar] [CrossRef]
- Yamagata, Y.; Matsukawa, T.; Mohri, T.; Inomata, K. Phosphorylation of adenosine in aqueous solution by electric discharges. Nature 1979, 282, 284–286. [Google Scholar] [CrossRef]
- Schwartz, A.; Ponnamperuma, C. Phosphorylation on the Primitive Earth: Phosphorylation of Adenosine with Linear Polyphosphate Salts in Aqueous Solution. Nature 1968, 218, 443. [Google Scholar] [CrossRef]
- Graaf, R.; Visscher, J.; Schwartz, A.W. A plausibly prebiotic synthesis of phosphonic acids. Nature 1995, 378, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A.; Kim, H.J.; Carrigan, M.A. Asphalt, Water, and the Prebiotic Synthesis of Ribose, Ribonucleosides, and RNA. Acc. Chem. Res. 2012, 45, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Schoffstall, A.M.; Mahone, S.M. Formate ester formation in amide solutions. Orig. Life Evol. Biosph. 1988, 18, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Schoffstall, A.M.; Laing, E.M. Phosphorylation mechanisms in chemical evolution. Orig. Life Evol. Biosph. 1985, 15, 141–150. [Google Scholar] [CrossRef]
- Yamagata, Y.; Watanabe, H.; Saitoh, M.; Namba, T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 1991, 352, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; Di Mauro, E. Nucleoside Phosphorylation by PhosphateMinerals. J. Biol. Chem. 2007, 282, 16729–16735. [Google Scholar] [CrossRef] [PubMed]
- Mamajanov, I.; Engelhart, A.E.; Bean, H.D.; Hud, N.V. DNA and RNA in anhydrous media: Duplex, triplex, and G-quadruplex secondary structures in a deep eutectic solvent. Angew. Chem. Int. Ed. 2010, 49, 6310–6314. [Google Scholar] [CrossRef]
- Ciaramella, M.; Cannio, R.; Moracci, M.; Pisani, F.M.; Rossi, M. Molecular biology of extremophiles. World J. Microbiol. Biotechnol. 1995, 11, 71–84. [Google Scholar] [CrossRef]
- Rimmer, P.B.; Shorttle, O. Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents. Life 2019, 9, 12. [Google Scholar] [CrossRef]
- van de Vossenberg, J.L.; Driessen, A.J.; Konings, W.N. The essence of being extremophilic: The role of the unique archaeal membrane lipids. Extremophiles 1998, 2, 163–170. [Google Scholar] [CrossRef]
- de Rosa, M.; Gambacorta, A.; Millonig, G.; Bu’Lock, J.D. Convergent characters of extremely thermophilic acidophilic bacteria. Experientia 1974, 30, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Guagliardi, A.; Cerchia, L.; Moracci, M.; Rossi, M. The chromosomal protein sso7d of the CrenarchaeonSulfolobus solfataricus rescues aggregated proteins in an ATP hydrolysis-dependent manner. J. Biol. Chem. 2000, 275, 31813–31818. [Google Scholar] [CrossRef]
- Pouwels, J.; Moracci, M.; Cobucci-Ponzano, B.; Perugino, G.; Van der Oost, J.; Kaper, T.; Lebbink, J.; de Vos, W.; Ciaramella, M.; Rossi, M. Activity and stability of hyperthermophilic enzymes: A comparative study on two archaeal ß-glycosidases. Extremophiles 2000, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Sanderson, I.; Moracci, M.; Ciaramella, M.; Nucci, R.; Rossi, M.; Pearl, L.H. Crystal structure of the ß-glycosidase from the hyperthermophilic Archaeon Sulfolobussolfataricus: Resilience as a key factor in thermostability. J. Mol. Biol. 1997, 271, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Atalah, J.; Cáceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Ariel, S.; Askari, S.; Evans, S.V.; Hwang, C.; Jay, J.; Scheffer, J.R.; Trotter, J.; Walsh, L.; Wong, Y.F. Reaction selectivity in solid state photochemistry. Tetrahedron 1987, 43, 1253–1272. [Google Scholar] [CrossRef]
- Lange, R.Z.; Hofer, G.; Weber, T.; Schlüter, A.D. A Two-Dimensional Polymer Synthesized through Topochemical [2 + 2]-Cycloaddition on the MultigramScale. J. Am. Chem. Soc. 2017, 139, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y. Solid-State [2 + 2] Photodimerization and Photopolymerization of α,ω-Diarylpolyene Monomers: Effective Utilization of Noncovalent Intermolecular Interactions in Crystals. Molecules 2011, 16, 119–148. [Google Scholar] [CrossRef] [PubMed]
- Barentsen, H.M.; van Dijk, M.; Zuilhof, H.; Sudhölter, E.J.R. Thermal and Photoinduced Polymerization of Thin Diacetylene Films. 1. Phthalimido-Substituted Diacetylenes. Macromolecules 2000, 33, 766–774. [Google Scholar] [CrossRef]
- Badarau, C.; Wang, Z.Y. Synthesis and Optical Properties of Thermally and Photochemically Cross-Linkable Diacetylene-Containing Polymers. Macromolecules 2004, 371, 147–153. [Google Scholar] [CrossRef]
- Matsumoto, A.; Odani, T. Topochemical Polymerization of 1,3-Diene Monomers and Features of Polymer Crystals as Organic Intercalation Materials. Macromol. Rapid Commun. 2001, 22, 1195–1215. [Google Scholar] [CrossRef]
- Matsumoto, A. Stereospecific Polymerization of 1,3-Diene Monomers in the Crystalline State. Progr. React. Kinet. Mech. 2001, 26, 59–109. [Google Scholar] [CrossRef]
- Potenti, S.; Manini, P.; Fornaro, T.; Poggiali, G.; Crescenzi, O.; Napolitano, A.; Brucato, J.R.; Barone, V.; d’Ischia, M. Solid State Photochemistry of HydroxylatedNaphthalenes on Forsterite: Probing Polycyclic Aromatic Hydrocarbon-Mineral Interactions under Astrochemically-Relevant UV Irradiation Conditions. ACS Earth Space Chem. 2018, 2, 977–1000. [Google Scholar] [CrossRef]
- Carota, E.; Botta, G.; Rotelli, L.; Di Mauro, E.; Saladino, R. Current Advances in Prebiotic Chemistry Under Space Conditions. Curr. Org. Chem. 2015, 19, 1963–1979. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Neri, V.; Brucato, J.R.; Colangeli, L.; Ciciriello, F.; Di Mauro, E.; Costanzo, G. Synthesis and Degradation of Nucleic Acid components by formamide and cosmic dust analogues. ChemBioChem 2005, 6, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Cossetti, C.; Di Mauro, E.; Deamer, D. Catalytic effects of Murchison Material: Prebiotic Synthesis and Degradation of RNA Precursors. Orig. Life Evol. Biosph. 2011, 41, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Delfino, M.; Di Mauro, E. Meteorites as catalysts for prebiotic chemistry. Chem. Eur. J. 2013, 19, 16916–16922. [Google Scholar] [CrossRef]
- Rotelli, L.; Trigo-Rodríguez, J.M.; Moyano-Cambero, C.M.; Carota, E.; Botta, L.; Di Mauro, E.; Saladino, R. The key role of meteorites in the formation of relevant prebiotic molecules in a formamide/water environment. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Saladino, R.; Carota, E.; Botta, G.; Kapralov, M.; Timoshenko, G.N.; Rozanov, A.Y.; Krasavin, E.; Di Mauro, E. Meteorite-catalyzed syntheses of nucleosides and of other prebiotic compounds from formamide under proton irradiation. Proc. Natl. Acad. Sci. USA 2015, 112, E2746–E2755. [Google Scholar] [CrossRef]
- Dalgarno, A. The galactic cosmic ray ionization rate. Proc. Natl. Acad. Sci. USA 2012, 109, 5967–5971. [Google Scholar] [CrossRef]
- Hudson, J.S.; Eberle, J.F.; Vachani, R.H.; Rogers, L.C.; Wade, J.H.; Krishnamurthy, R.; Springsteen, G.A. A unified mechanism for abiotic adenine and purine synthesis in formamide. Angew. Chem. Int. Ed. 2012, 51, 5134–5137. [Google Scholar] [CrossRef]
- Jeilani, Y.A.; Nguyen, H.T.; Newallo, D.; Dimandja, J.M.D.; Nguyen, M.T. Free radical routes for prebiotic formation of DNA nucleobases from formamide. Phys. Chem. Chem. Phys. 2013, 15, 21084–21093. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Michalcikovà, R.; Shestivskà, V.; Sponer, J.; Sponer, J.E. High Energy chemistry of formamide: A simpler way for nucleobase formation. J. Phys. Chem. A 2014, 118, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Civis, S.; Mladek, A.; Sponer, J.; Juha, L.; Sponer, J.E. On the road from formamide ices to nucleobases: IR spectroscopic observation of a direct reaction between cyano radicals and formamide in a high-energy impact event. J. Am. Chem. Soc. 2012, 134, 20788–20796. [Google Scholar] [CrossRef]
- Saladino, R.; Bizzarri, B.M.; Botta, L.; Šponer, J.; Šponer, J.E.; Georgelin, T.; Jaber, M.; Rigaud, B.; Kapralov, M.; Timoshenko, G.N.; et al. Proton irradiation: A key to the challenge of N-glycosidic bond formation in a prebiotic context. Sci. Rep. 2017, 7, 14709–14716. [Google Scholar] [CrossRef]
- Botta, L.; Bizzarri, B.M.; Piccinino, D.; Fornaro, T.; Brucato, J.R.; Saladino, R. Prebiotic synthesis of carboxylic acids, amino acids and nucleic acid bases from formamide under photochemical condition. Eur. Phys. J. Plus 2017, 132, 317. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, L.; Di Mauro, E. The Prevailing Catalytic Role of Meteorites in Formamide Prebiotic Processes. Life 2018, 8, 6. [Google Scholar] [CrossRef]
- Saladino, R.; Carota, E.; Botta, G.; Kapralov, M.; Timoshenko, G.N.; Rozanov, A.; Krasavin, E.; Di Mauro, E. First evidence on the role of heavy ions irradiation of meteorites and formamide in the origin of biomolecule. Orig. Life Evol. Biosph. 2016, 46, 515–521. [Google Scholar] [CrossRef]
- Niemann, H.B.; Atreya, S.K.; Bauer, S.J.; Carignan, G.R.; Demick, J.E.; Frost, R.L.; Gautier, D.; Haberman, J.A.; Harpold, D.N.; Hunten, D.M.; et al. The abundances of constituents of Titan’s atmosphere from the GCMS instrument on the Huygens probe. Nature 2005, 438, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Waite Jr, J.H.; Young, D.T.; Cravens, T.E.; Coates, A.J.; Crary, F.J.; Magee, B.; Westlake, J. The Process of Tholin Formation in Titan’s Upper Atmosphere. Science 2007, 316, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Saladino, R.; Bizzarri, B.M.; Cobucci-Ponzano, B.; Iacono, R.; Avino, R.; Caliro, S.; Carandente, A.; Lorenzini, F.; Tortora, A.; et al. Formamide-basedprebioticchemistry in the PhlegreanFields. Adv. Space Res. 2018, 62, 2372–2379. [Google Scholar] [CrossRef]
- Puzzarini, C.; Barone, V. Diving for Accurate Structures in the Ocean of Molecular Systems with the Help of Spectroscopy and Quantum Chemistry. Acc. Chem. Res. 2018, 51, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Puzzarini, C.; Barone, V. A never-ending story in the sky: The secrets of chemical evolution. Phys. Life Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vallance, C. Astrochemistry: From the Big Bang to the Present Day; World Scientific Publishing Europe Ltd.: London, UK, 2017. [Google Scholar]
- Islam, S.; Powner, M.W. Prebiotic system chemistry: Complexity overcoming clutter. Chem 2017, 2, 470–501. [Google Scholar] [CrossRef]
- Ashkenasy, G.; Hermans, T.H.; Ottoc, S.; Taylor, A.F. Systems Chemistry. Chem. Soc. Rev. 2017, 46, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Ischia, M.; Manini, P.; Moracci, M.; Saladino, R.; Ball, V.; Thissen, H.; Evans, R.A.; Puzzarini, C.; Barone, V. Astrochemistry and Astrobiology: Materials Science in Wonderland? Int. J. Mol. Sci. 2019, 20, 4079. https://doi.org/10.3390/ijms20174079
d’Ischia M, Manini P, Moracci M, Saladino R, Ball V, Thissen H, Evans RA, Puzzarini C, Barone V. Astrochemistry and Astrobiology: Materials Science in Wonderland? International Journal of Molecular Sciences. 2019; 20(17):4079. https://doi.org/10.3390/ijms20174079
Chicago/Turabian Styled’Ischia, Marco, Paola Manini, Marco Moracci, Raffaele Saladino, Vincent Ball, Helmut Thissen, Richard A. Evans, Cristina Puzzarini, and Vincenzo Barone. 2019. "Astrochemistry and Astrobiology: Materials Science in Wonderland?" International Journal of Molecular Sciences 20, no. 17: 4079. https://doi.org/10.3390/ijms20174079
APA Styled’Ischia, M., Manini, P., Moracci, M., Saladino, R., Ball, V., Thissen, H., Evans, R. A., Puzzarini, C., & Barone, V. (2019). Astrochemistry and Astrobiology: Materials Science in Wonderland? International Journal of Molecular Sciences, 20(17), 4079. https://doi.org/10.3390/ijms20174079









