1. Introduction
Alcoholic liver disease (ALD) is a complex condition that includes a wide range of conditions from simple fatty liver (steatosis) to more severe liver injuries, such as alcoholic hepatitis, cirrhosis, and hepatocellular carcinoma. Excess alcohol consumption is the primary cause of global liver-related mortality [
1]. Lesions associated with this condition may occur separately, simultaneously, or sequentially in patients. Alcoholic fatty liver is the first hepatic response to binge drinking or chronic alcohol consumption. The alcohol-induced fatty liver might recover after alcohol withdrawal; however, more severe liver injuries, such as liver inflammation and hepatitis or steatohepatitis, may persist even after alcohol withdrawal. In-progress hepatic diseases may result in the development of liver fibrosis, which is characterized by excessive scarring, vascular alterations, and functional failure of the liver [
2]. In general, recovery from alcohol-induced liver injury after cessation of alcohol consumption is associated with disease severity [
3].
Astaxanthin (Asta) is a xanthophyll carotenoid sourced from various marine creatures, such as shrimp, salmon, and the algae
Haematococcus pluvialis. Asta has been demonstrated to exhibit numerous biological activities, including anti-inflammatory, antitumor, and free radical-clearing actions [
4,
5]. Over the past decade, several studies have investigated the preventive or therapeutic effects of Asta against various disorders or diseases, such as cancer, age-related macular degeneration, inflammation, and cardiovascular oxidative stress, and have examined its effects for the promotion of immune responses [
6]. Asta has also been found to be capable of preventing diet-induced obesity and hepatic steatosis in vivo, as evidenced by its stronger preventive and nonalcoholic steatohepatitis (NASH) therapeutic effects than those exerted by vitamin E on a mouse model [
7,
8]. Asta is unstable; it drastically decomposes on exposure to heat, light, and oxygen; and is poorly water soluble and dispersible in aqueous solutions. These characteristics cause pure Asta compounds to exhibit low bioavailability, which limits their pharmaceutical or biomedical applications. Therefore, biomaterial manipulations, such as encapsulating Asta into a hydrophilic polymer matrix or incorporating Asta into liposomes by using emulsion or suspensions, have been investigated and developed to efficiently improve the solubility and stability of Asta.
Liposomes, which comprise a colloidal and vesicular structure, are composed of one or more lipid bilayers [
9]. They have been widely used as drug carriers for peptides/proteins, hormones, antibiotics, and anticancer agents in the drug delivery system. Liposomes are biocompatible, biodegradable, and nontoxic, and their structure is similar to that of cell membranes. Thus, liposomes have become a suitable alternative for drug vehicles to increase the stability of encapsulated agents and improve the pharmacodynamics or therapeutic index of drugs [
10,
11]. Among liposome classes, phosphatidylcholine liposomes are the most widely used because their phospholipid components are similar to the relevant components of the cell membrane. The phospholipid 1,2-distearoyl-
sn-glycero-3-phosphocholine (DSPC) has been used for liposome preparation for years; we have successfully fabricated DSPC liposome-encapsulated propranolol, which stimulated human osteoblastic cell proliferation and differentiation as effectively as did pure propranolol [
12]. Furthermore, the prepared liposomal propranolol significantly increased bone volume density of the tibia and spine in ovariectomized rats (OVX rats) at a low dose of one-tenth to 1% of the therapeutic range of propranolol, thus demonstrating that the established liposome encapsulation can increase the bioavailability and potency of the target compound in vivo [
13]. Thus, DSPC-based liposomes were employed as carriers for low bioavailable and unstable Asta in the current study. The encapsulation efficiency, drug release, and cytotoxic properties of Asta-encapsulated liposomes (Asta-lipo) were assessed. The improvement in steatohepatitis and liver fibrosis induced by alcohol intake was further evaluated through two administration routes—intraperitoneal injection (i.p.) and oral administration (Per os, p.o)—on a mouse model. Alanine aminotransferase (ALT) is known as serum glutamate pyruvate transaminase. ALT is primarily produced in the liver, and its activity is much lower in other tissues; thus, ALT is specifically measured to assess liver injury or hepatic diseases. Elevated ALT levels are highly associated with the presence of damaged liver cells in liver injury and hepatitis. Aspartate aminotransferase (AST), or originally called serum glutamic oxaloacetic transaminase, is present in the mitochondria and cytoplasm of liver cells. When liver cells are damaged, AST is released. AST is also present in other tissues such as the heart, skeletal muscle, and kidney. The combined measurements of these two liver enzyme activities are essential for the diagnosis and assessment of liver disease or injury. Therefore, the preventive and reparative effects of Asta-lipo were examined and verified using histopathology and serum ALT and AST analyses in this study.
3. Discussion
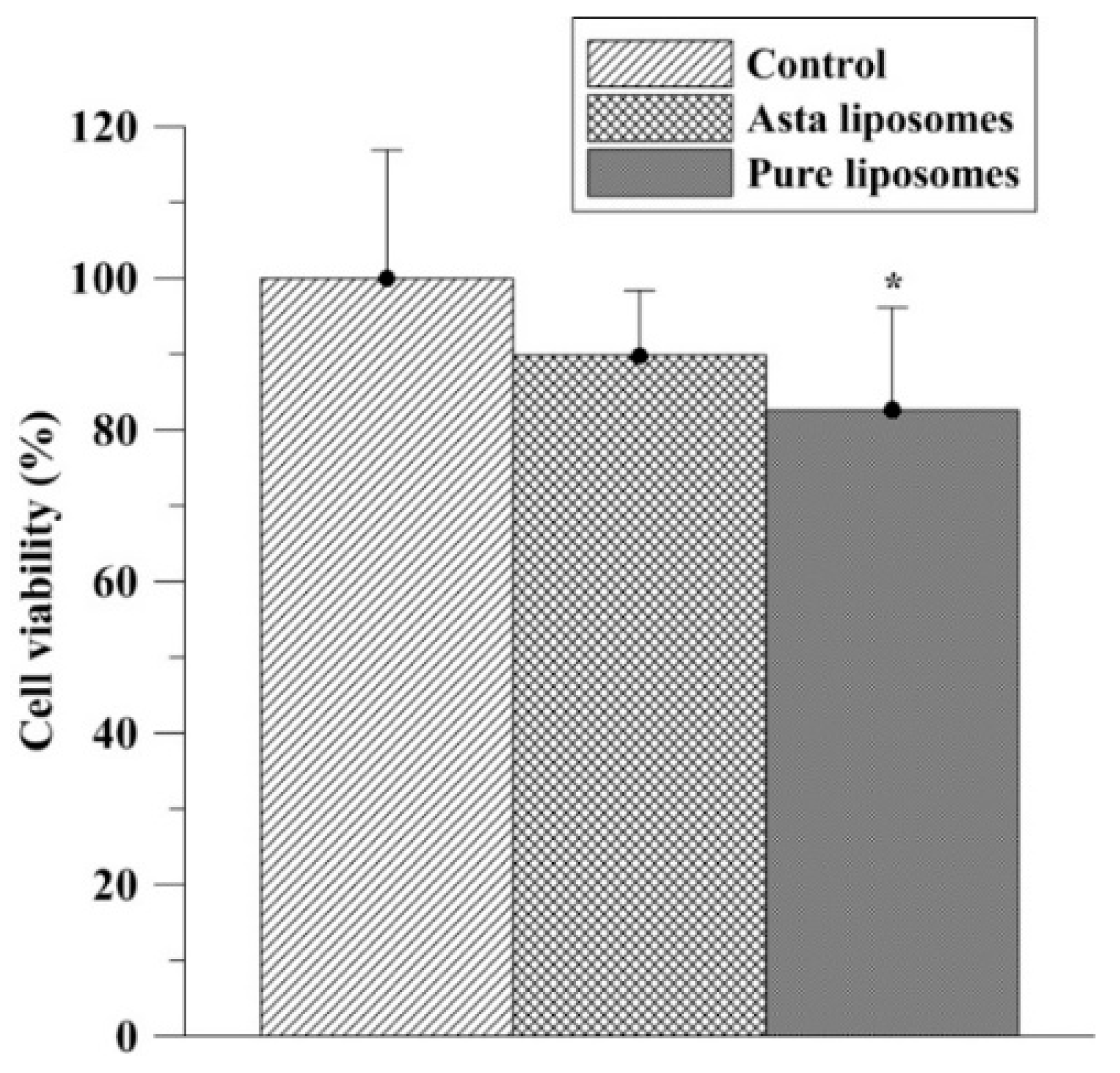
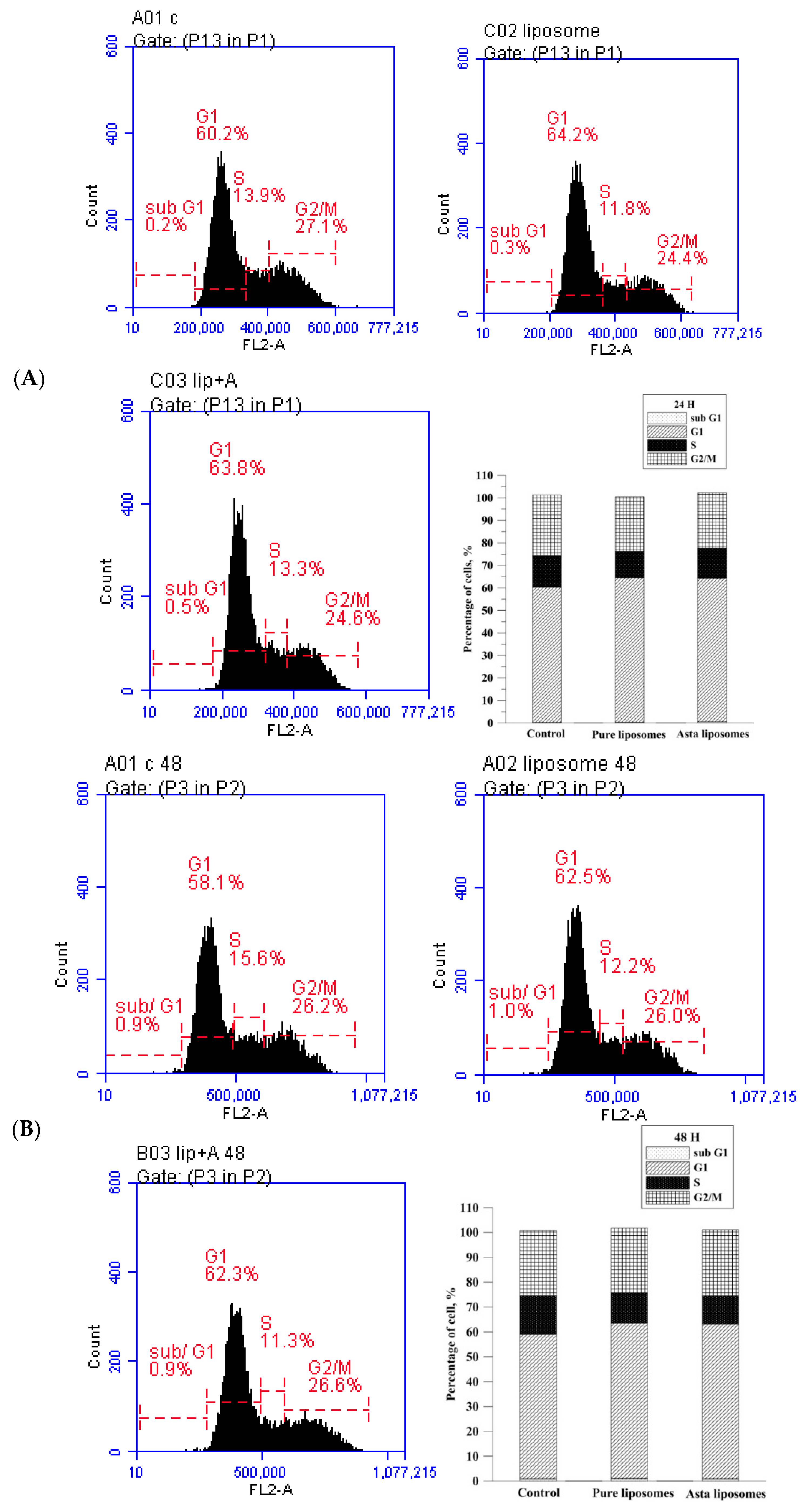
In the present study, the preventive and reparative effects of Asta-lipo and possible influences of liposomes on hepatic repair were examined and verified in vitro and in vivo using MTT assay, cell cycle analysis, histopathological and serum biochemical analyses. No significant influences were found in the cell cycle phases prior to treatment with pure liposomes or Asta-lipo. The in vitro results on the effects of Asta-lipo on the cell cycle of normal cells were in accordance with the data of the MTT assay despite slight decrease in L929 viability resulted from treatment with 0.01 mg/mL pure liposomes. These results are in line with our previous in vitro findings [
12]. The investigation also showed that significant IC
50 values below 0.5 mM were detected in vitro for phosphatidylcholines (PCs) like PC(12:0/12:0) and PC(14:1/14:1)trans [
15]. Furthermore, the slight but significant inhibitory effects of pure liposomes on normal fibroblast viability could be because their inhibitory effects are non-cell cycle–specific instead of cell cycle–specific. These are in accordance with the minimal deteriorated influences of Asta-lipo on normal cells in the current study.
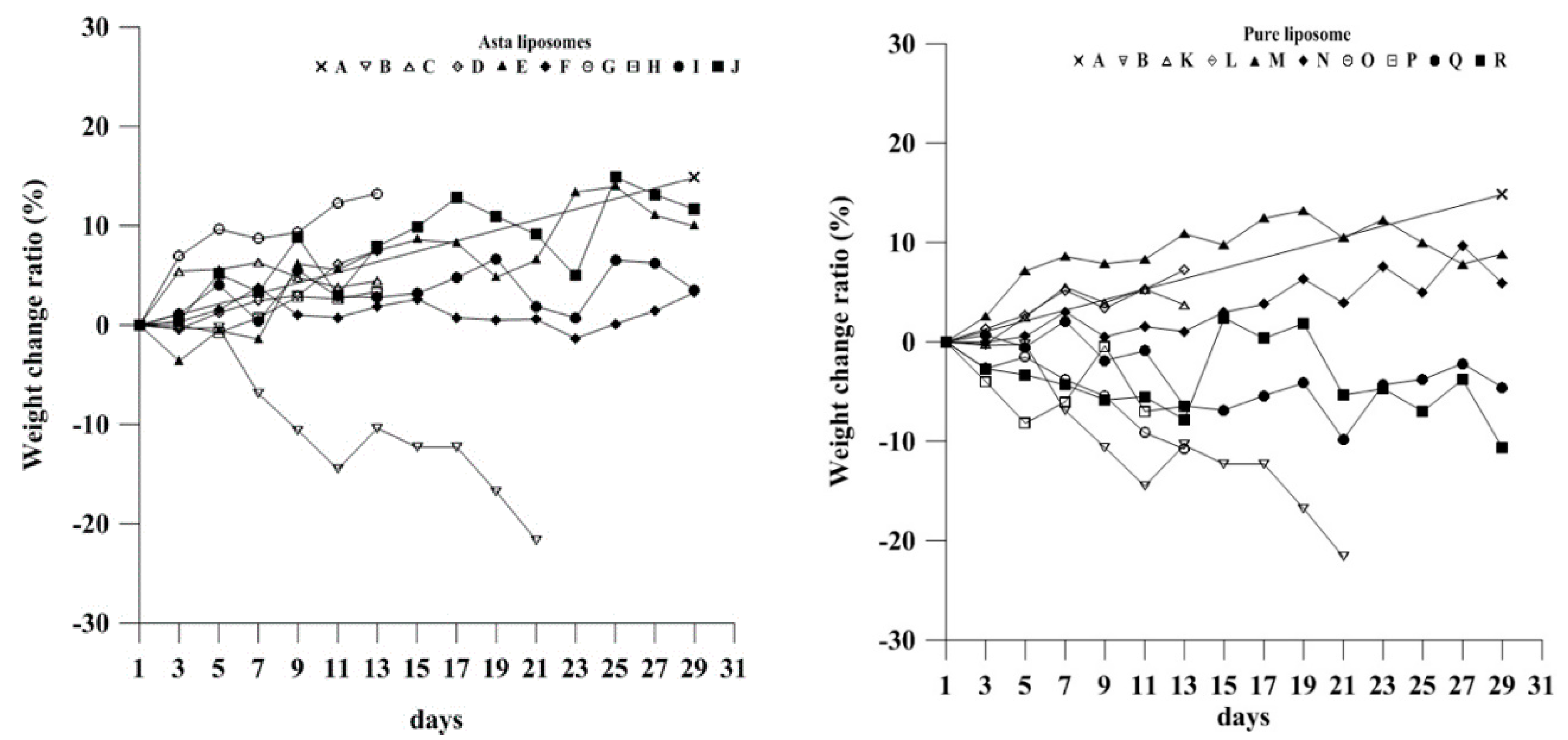
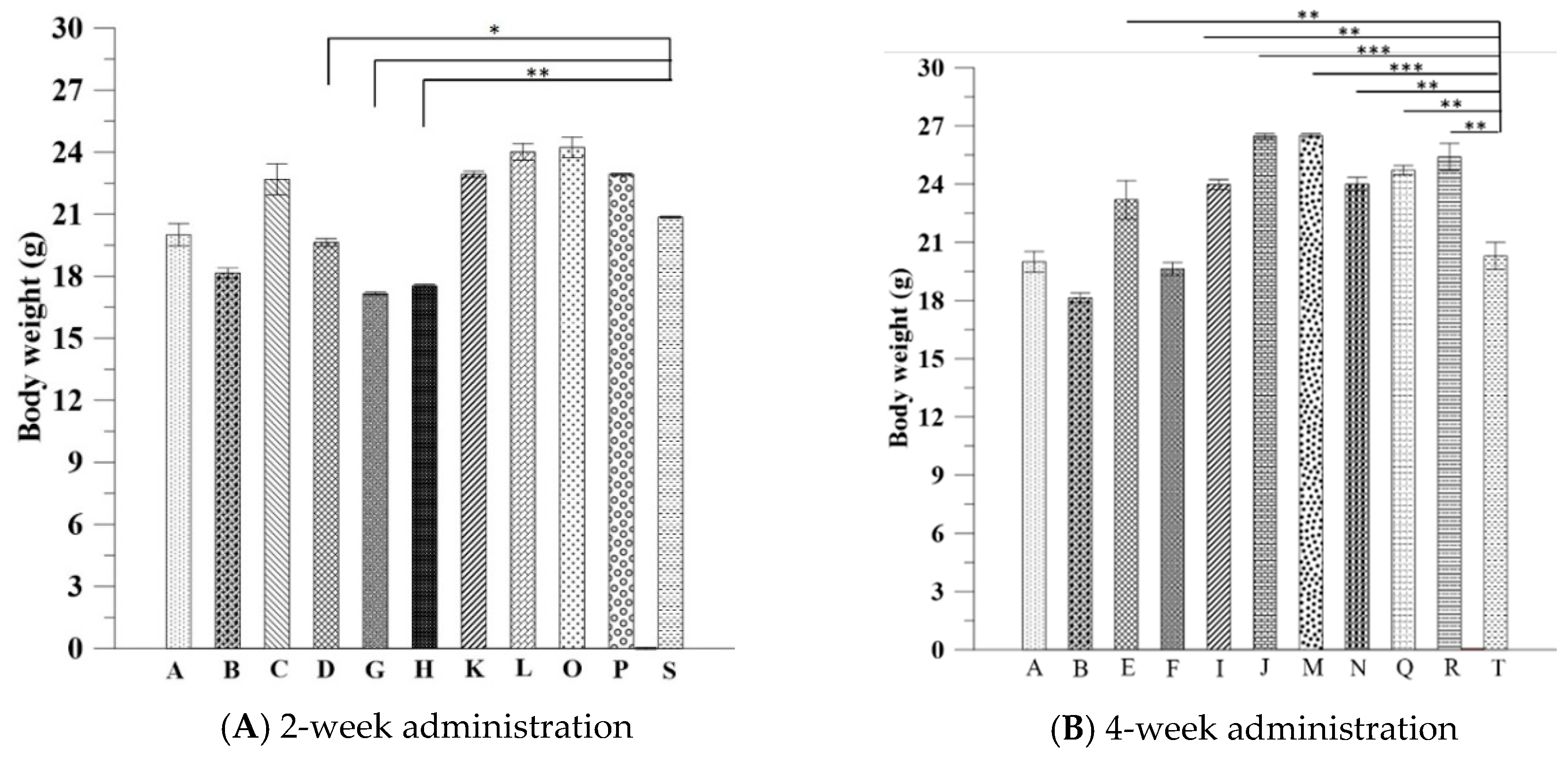
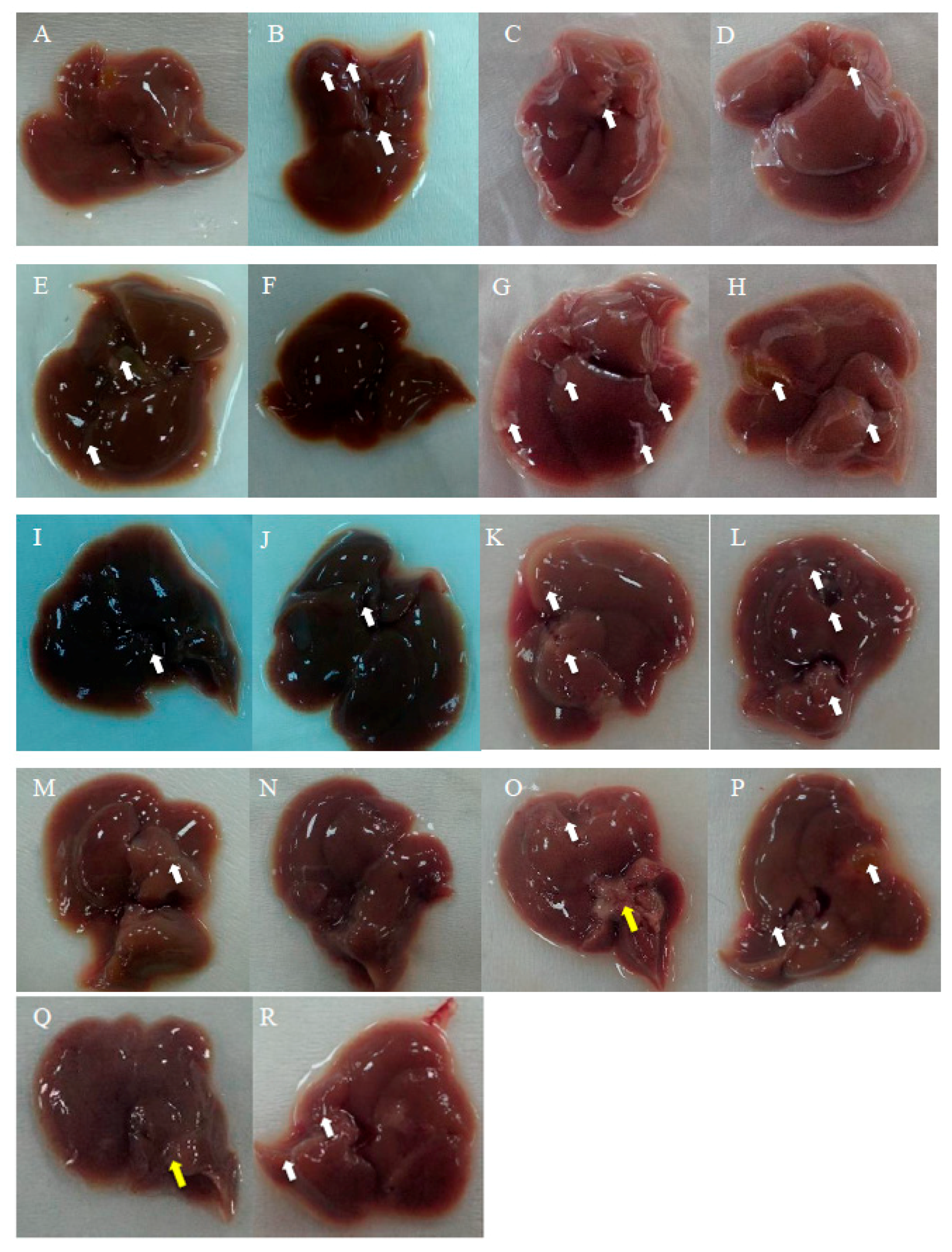
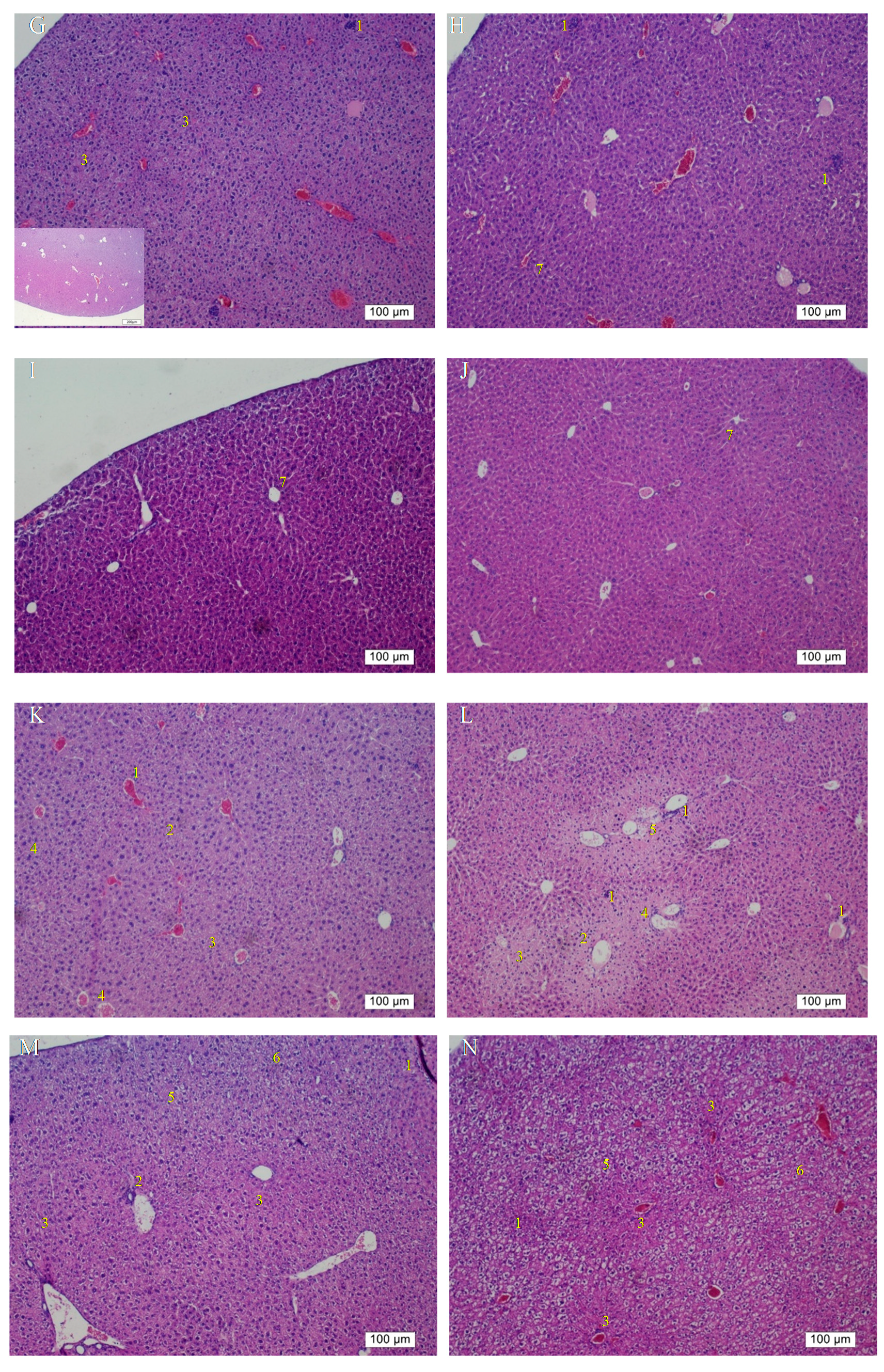
The body weight data of pure liposome-treated mice suggested that pure liposomes alone are not capable of exerting protective or therapeutic effects against repeated alcohol consumption, although liposomes themselves are not harmful to mice. The effects of Asta-lipo and pure liposomes in the mice prior to 3-week 30% alcohol with or without feeding with second dose of 30% alcohol were grossly and histopathologically analyzed and evaluated. These gross pathological observations primarily showed that Asta-lipo exerted ameliorative effects on alcoholic liver fibrosis in vivo. In the mice given treatment prior to 3-weeks of 30% alcohol consumption, our findings indicated a more complete restoration of hepatic tissues exerted by the 4-week treatment with intraperitoneal Asta-lipo (
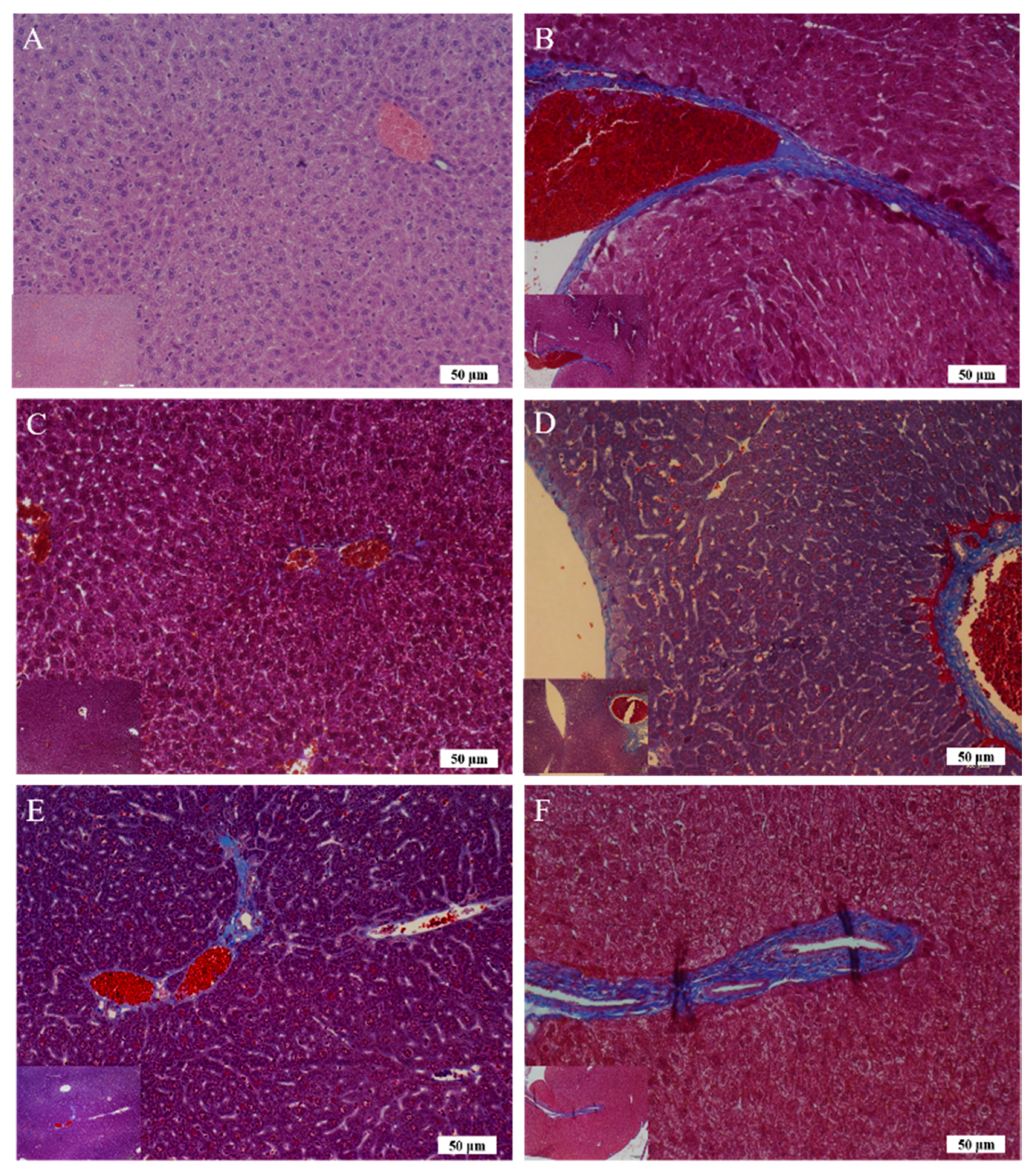
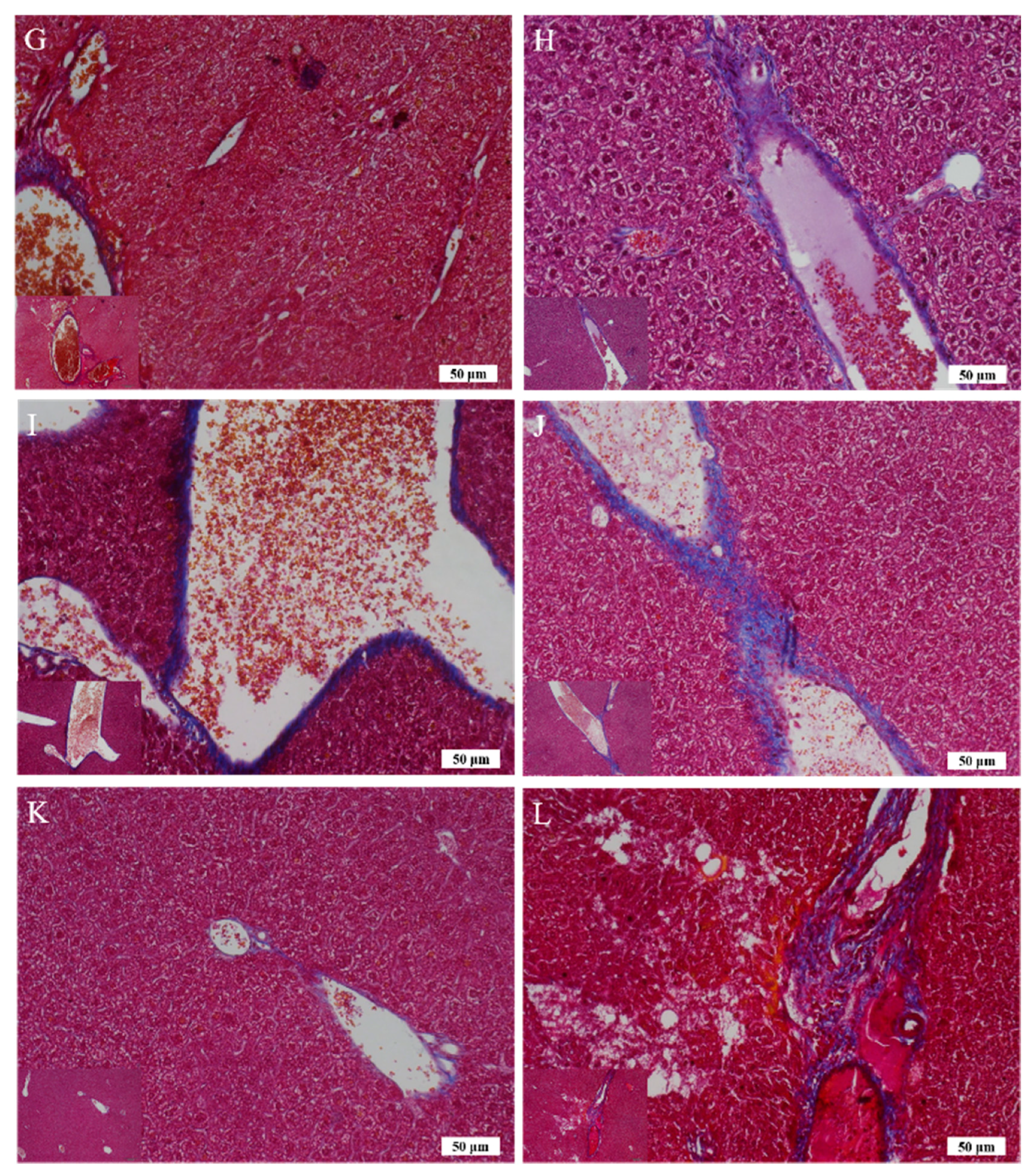
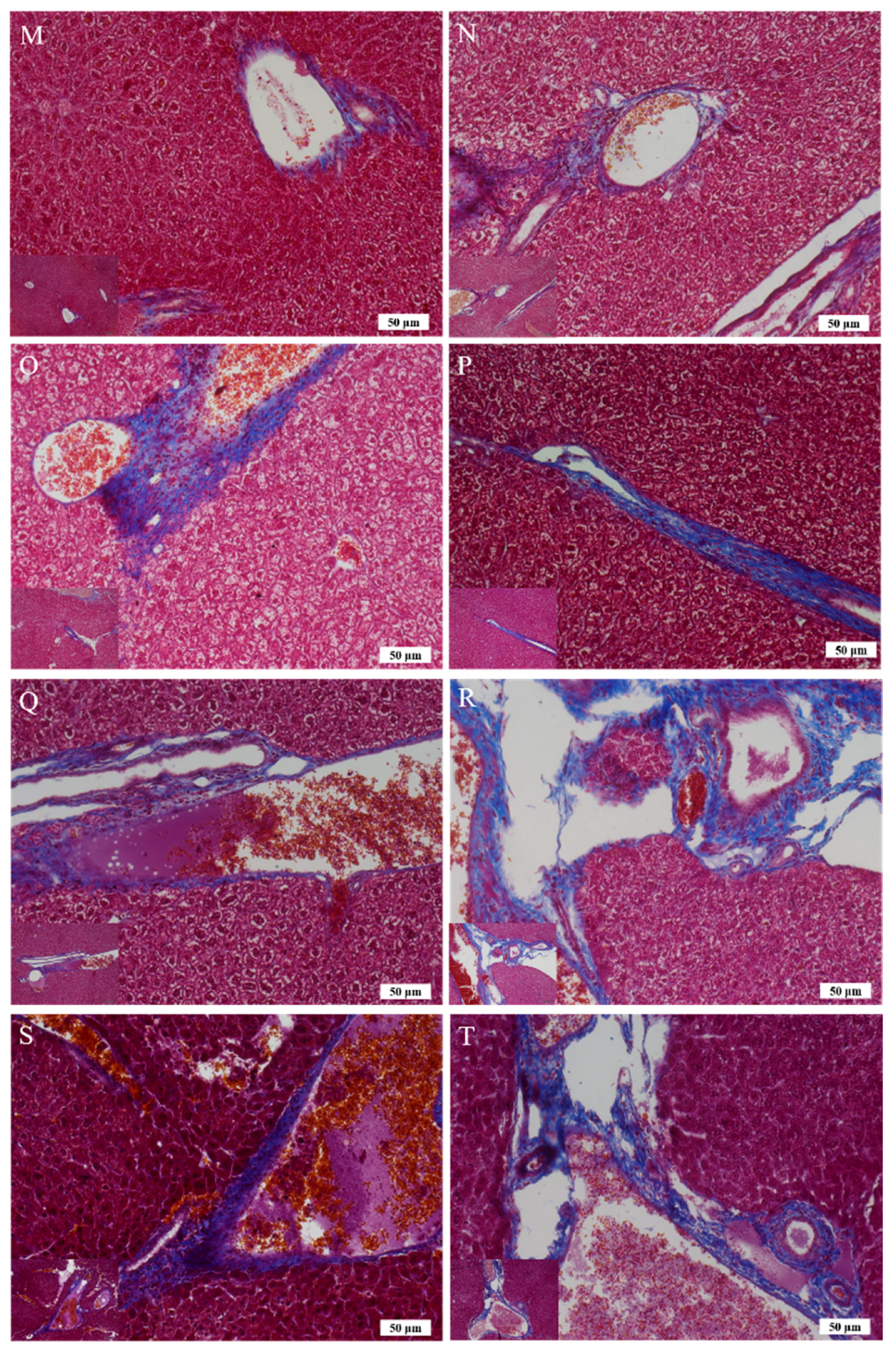
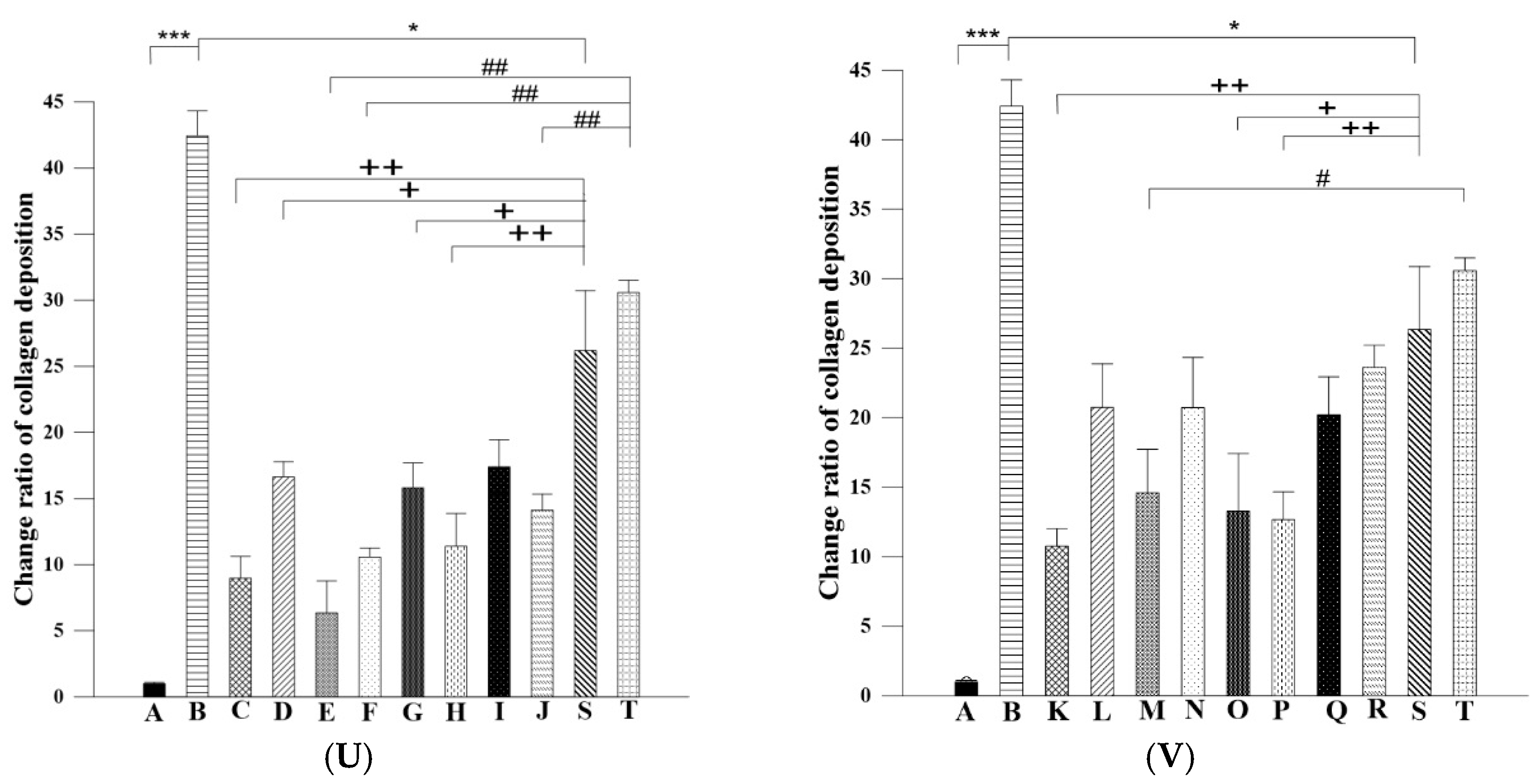
Figure 6F), but overall, this effect was weaker than that observed in mice treated with Asta-lipo orally for the same duration. Moreover, Masson’s Trichrome staining and semi-quantitation of collagen content also showed the lowest collagen level in the group orally administered Asta-lipo for 4 weeks (Group E,
Figure 7U). Therefore, our histopathological findings in the mice prior to 3-weeks of 30% alcohol consumption suggested that oral administration of Asta-lipo for 2 and 4 weeks had reparative effects against alcoholic liver disease prior to alcohol consumption for 3 weeks.
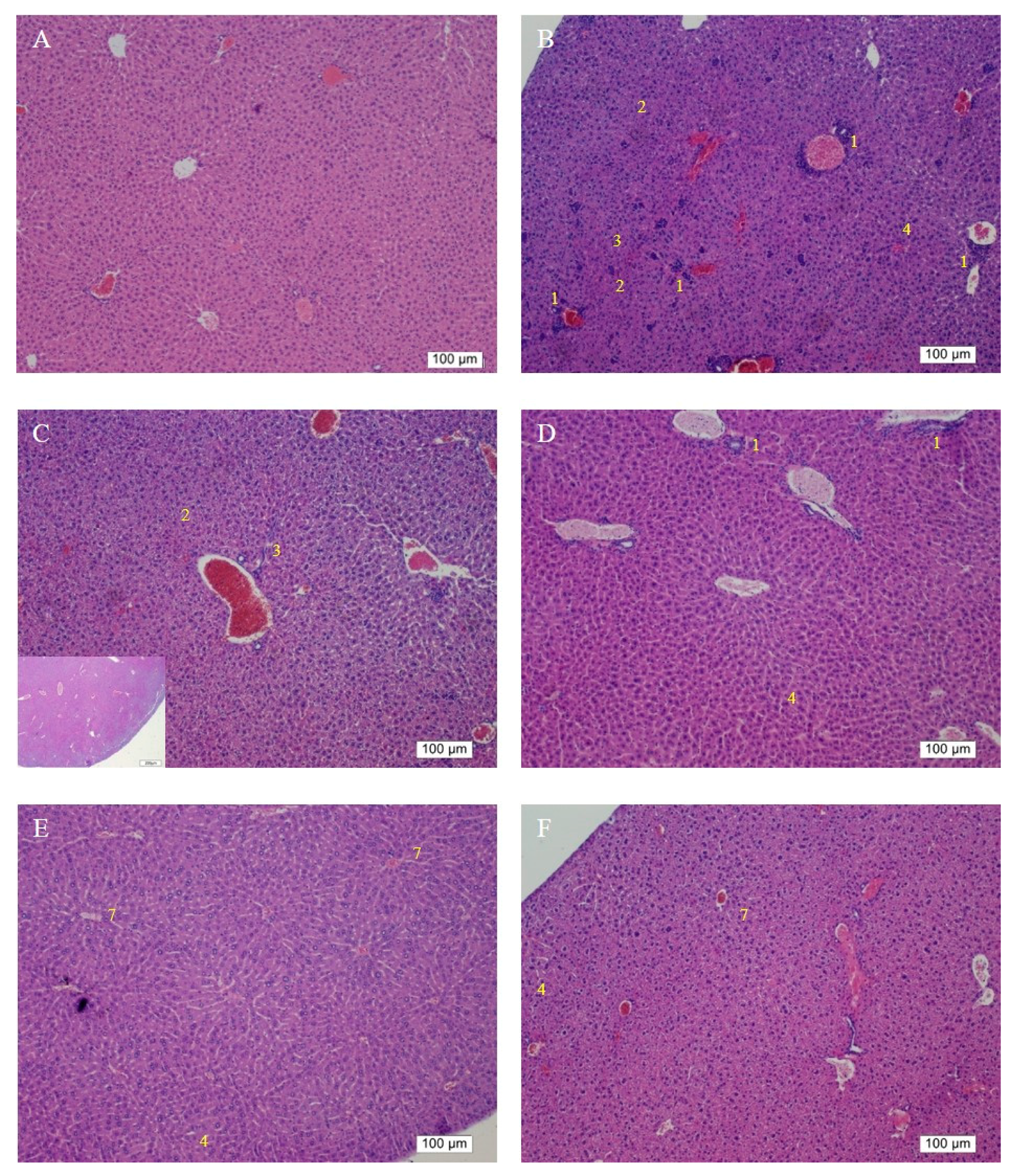
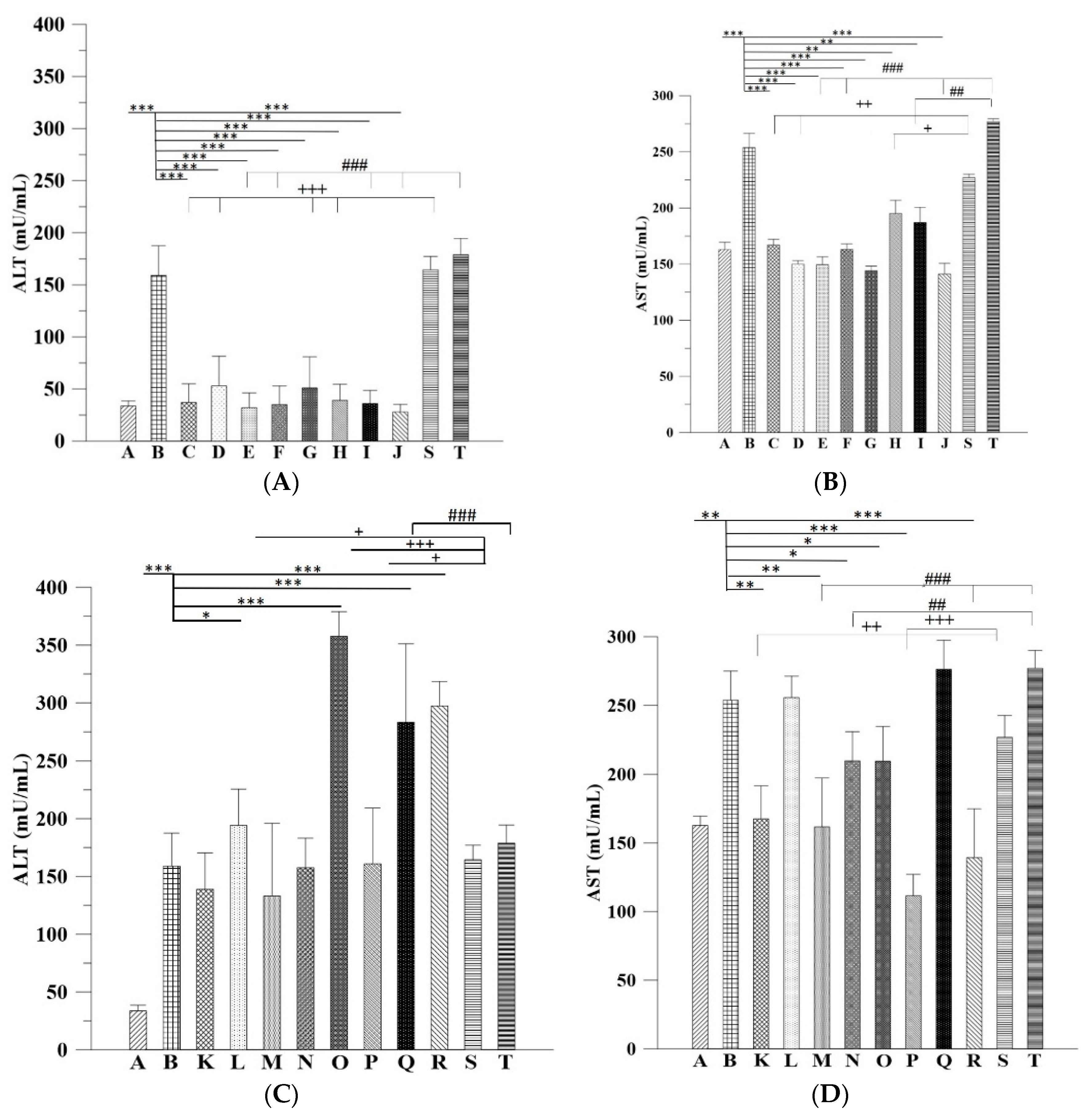
On the other hand, the H&E findings in Groups C and H were in accordance with their ALT levels and indicated that their ALT levels returned within the normal range. However, the ALT levels in Group G significantly decreased but were still higher than the normal range compared with that in the group without any treatment; this result is coherent with the histopathological findings. The histopathological observations in the groups that received the 3-week alcohol treatment plus second-dose alcohol intake with intraperitoneal injection of Asta-lipo for 2 and 4 weeks (Groups H and J) revealed an obvious reduction in fibrotic areas, improvement in hepatocyte swelling, the rearrangement of hepatocytes, and the appearance of normal hepatic structures, such as the central vein and sinusoids, demonstrating that liver repair and recovery from the existing alcoholic liver disease plus repeated alcohol consumption were promoted and accelerated by intraperitoneal treatment with Asta-lipo (Groups H and J). These features were also found in the group that received second-dose alcohol and oral treatment with Asta-lipo for 4 weeks (Group I). Consistently, the ALT levels of Groups H, I, and J, drastically dropped to levels lower than their normal range, as collagen content of these groups were also clearly decreased (
Figure 7U), which strongly evidenced that intraperitoneal treatment with Asta-lipo is capable of restoring liver injury and fibrosis in alcoholic liver disease with subsequent alcohol consumption, and this therapeutic effect can be active even in a relatively short period of 2 weeks.
In addition to inflammation and progressive fibrosis, hepatic angiogenesis has been found in chronic liver fibrosis regardless of its etiology. Evidence has shown that long-term ethanol intake is associated with angiogenesis through corresponding actions of several mediators, such as vascular endothelial growth factor (VEGF) and Vascular endothelial growth factor receptor 2 (VEGFR-2), in the liver [
16,
17]. In the group that received oral administration of pure liposomes for 2 weeks, obvious angiogenesis; inflammatory cell infiltration; and hepatocyte swelling, degeneration, and necrosis were observed in the mouse liver (Group K) (
Figure 6K). Moreover, the liver of mice treated with intraperitoneal injection of pure liposomes for 2 weeks exhibited severe hepatocyte degeneration and necrosis as well as obvious hepatic angiogenesis, inflammatory cell infiltration, and fatty degeneration-like areas (Group L). Histopathological findings in Groups K and L indicated that treatment with pure liposomes, either through oral administration or intraperitoneal injection, could not restore liver injury and fibrosis in alcoholic liver disease. Instead, microscopic observations revealed that additional phospholipids from liposomal administration appeared to deteriorate liver injury and promote angiogenesis in existing alcoholic liver disease. With the continuation of liposomal treatment to week 4, the appearance of fibrotic tissues, inflammatory cell infiltration, hepatocyte degeneration and necrosis, and angiogenesis to certain extent remained in the liver (Groups M and N) (
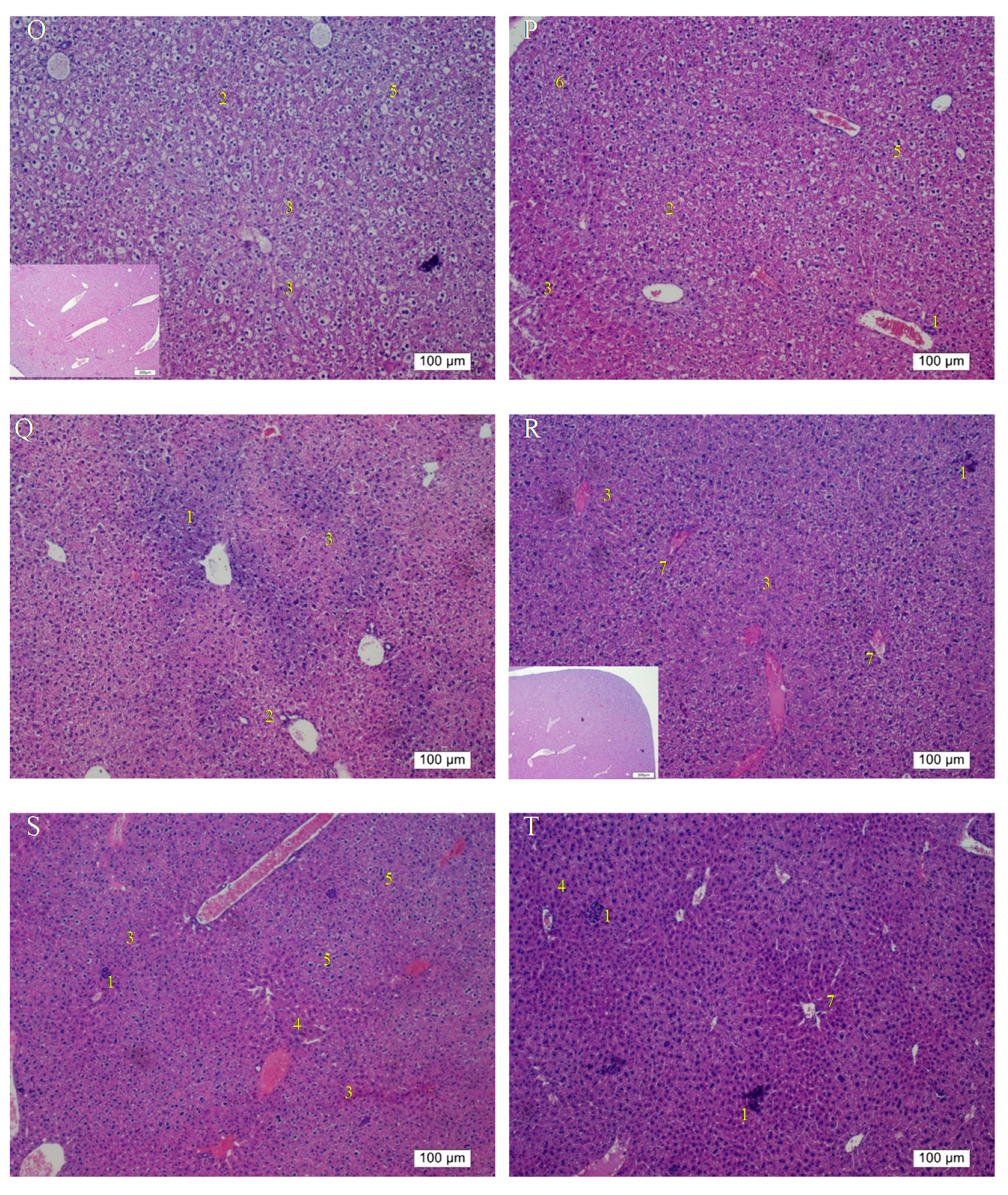
Figure 6M,N), showing that the administration of pure liposomes for a longer period could not exert any reparative effects on alcoholic liver disease. The ALT levels in these groups decreased but were still higher than the normal range, indicating the persistence of injury and loss of function in the liver. In addition, the livers of mice that received a repeated intake of 30% ethanol with oral administration of pure liposomes (Group O) for 2 weeks exhibited large areas of fatty liver change coupled with clear and possibly more acute hepatocyte swelling. In the counterpart as well as in the second-dose 30% ethanol intake group treated intraperitoneally with pure liposomes for 2 weeks (Group P), the livers presented with complex lesions of hepatocyte swelling, degeneration, and necrosis together with rather fibrotic tissues and inflammatory cell infiltration, implicating somewhat hepatic damage caused by intraperitoneally injected liposomes for a short period of 2 weeks. Group O showed an ALT level nearly equal to that of the nontherapy group, implicating acute toxicity mainly due to the oral intake of second-dose ethanol and to some degree through oral intake of pure liposomes. Oral administration with both treatments may more severely affect liver function or lead to larger burden for lipid metabolism and alcohol detoxification via portal circulation in a shorter period of 2 weeks in Group O than in Group P (i.p. treatment with pure liposomes). As pure liposomes were administered for 4 weeks (Group Q and R) through both oral and intraperitoneal routes in the experimental groups treated with 3-week alcohol intake plus a second dose of alcohol intake, severe hepatocyte necrosis and chronic hepatic fibrosis was noted, further suggesting that not only the loading of external lipids is ineffective in recovery from alcoholic liver disease caused by repeated alcohol consumption but also the extra lipids may worsen the conditions or hamper the natural reparative progress in the liver. This is fully supported by the finding that the ALT levels in Groups Q and R reached as high as that of the group without any treatment. The results obtained from Groups O to R revealed that treatment with pure liposomes is not only incapable of repairing existing alcohol-induced liver injury with or without ongoing ethanol intake but also that lipid-based liposomes cause more severe fatty degeneration and hepatocyte degeneration and necrosis, probably resulting from more oxidative stress and liver dysfunction [
18,
19]. (
Figure 6O–R). By contrast, treatment with Asta-lipo through either administration routes for 4 weeks can obviously restore and attenuate hepatic necrosis, fibrosis, and fatty changes caused by 3-week alcohol intake, whereas oral administration of Asta-lipo exerted more rapid and efficient effects on hepatic repair, probably because oral administration enables Asta-lipo to directly undergo first-pass metabolism in the liver.
The ALT and AST data combined with in vivo histopathological findings also implicated that shorter-term 2-week administration of Asta-lipo via either administration routes was not sufficiently effective to steadily restore normal status from repeated alcohol consumption-induced hepatic damage. Based on the histopathological observations and serum ALT results, 4-week intraperitoneal or oral administration of Asta-lipo could effectively improve hepatic function and repair injured liver tissues caused by incurred and repeated consumption of alcohol. This could be because Asta-lipo administered intraperitoneally can distribute faster in the mice abdominal cavity, and the rescue of more severe, repeated alcohol-induced liver injuries can be more effectively attained. By contrast, oral administration of Asta-lipo may be capable of providing more steady and gradual liver function-recovering effects, which is more suitable for the treatment of mice that received 3-week 30% alcohol without repeated 30% alcohol intake. Moreover, recent investigations using animal models have shown different serum biochemistry data to the findings in humans [
20]. Feeding male C57BL/6 mice with a Lieber-DeCarli diet containing 5% ethanol for 10 days, followed by a single dose of ethanol (5 g/kg body weight) by gavage, induces significant fatty liver and liver injury with peak serum levels of approximately 250 IU/L alanine aminotransferase and 420 IU/L aspartate aminotransferase 9 hours after gavage. Meanwhile, a second dose of 20% ethanol clearly led to increased ALT and AST [
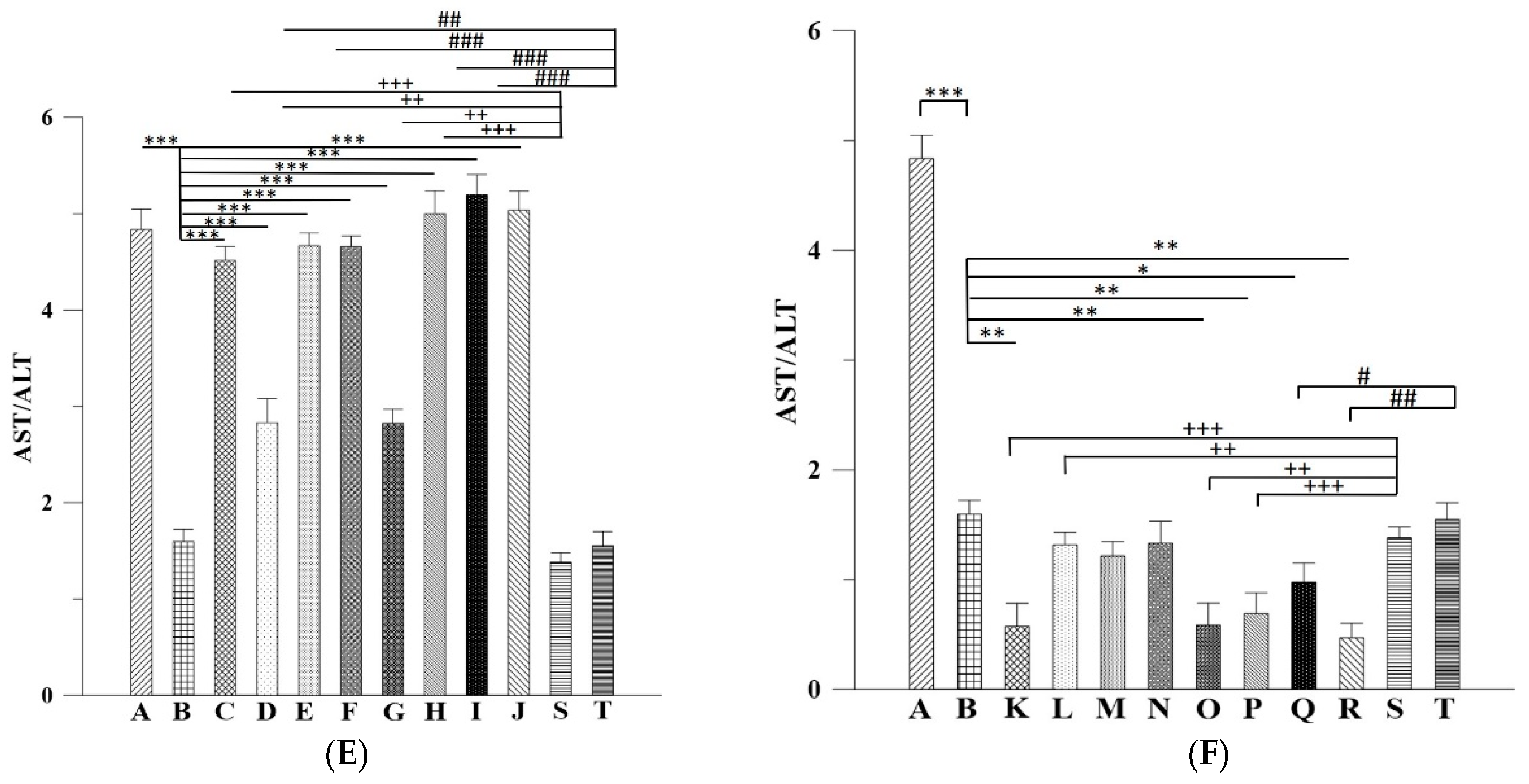
21]. Therefore, it is not surprising that in our study the mice that received 30% ethanol orally for 21 days showed ALT around 150 IU/L coupled with AST about 250 IU/L. Also, previous studies in humans have shown that the ratio of aspartate aminotransferase to alanine aminotransferase <1 suggest nonalcoholic steatohepatitis (NASH), a ratio of ≥2 is strongly suggestive of alcoholic liver disease [
22]. In the mice p.o. or i.p. administered with Asta-lipo prior to 3-week 30% alcohol with or without feeding with second dose of 30% alcohol presented with AST: ALT values principally higher than 2, which is coherent with the previous conclusions by Sorbi et al. (1999) [
22]. As to those mice administered with pure liposomes, Groups K, O, P and R showed the AST:ALT ratio less than 1, suggesting that subsequent administration with liposomes could be metabolic burdens under the liver conditions of 3-week 30% alcohol with or without feeding with second dose of 30% alcohol, leading to the AST:ALT ratio closer to NASH. However, longer-term (4 week) administration with liposomes prior to 3-week 30% alcohol with or without feeding with second dose of 30% alcohol, like the values shown in Groups M, N and Q, resulted in increased ratio of AST: ALT. This could be associate with the longer halflife of mitochondrial AST released in response to alcohol and the coexistence of deficiency of pyridoxal-6-phosphate in alcoholics, which is a cofactor for the enzymatic activity of ALT [
23]. In addition, involvement of the non-invasive fibrosis markers for evaluation of ALD such as hyaluronan (HA) and Terminal peptide of procollagen III (PCIIINP) has been reported [
24]. Although several non-invasive fibrosis markers have been suggested as alternatives to liver biopsy in patients with ALD, none has been sufficiently ascertained [
25]. Despite the limitation of AST/ALT ratio in ALD patients together with severe fibrosis, animal studies showed different serum biochemistry data to these previous findings in humans [
20] and our serum AST and ALT results showed the coherence to some extent with those found previously in mice [
21]. The current study has extensively investigated and evaluated the therapeutic activities of Asta-lipo on the gross, histopathologic, and serum biochemical changes. The histopathologic observations and serum biochemical changes after Asta-lipo administration were evident and convincing in ALD on the mice model.
Asta has been shown to possess anti-inflammatory, free radical-clearing, and angiogenesis-inducing effects [
26,
27,
28]. The liver is an organ with abundant blood supply and is in charge of clearing harmful substances in the circulatory system. Therefore, it is reasonable that previous studies have found that Asta has protective and therapeutic capabilities against liver fibrosis, steatohepatitis, and nonalcoholic fatty liver disease on murine models [
8,
29,
30]. These actions have been found to be related with some growth factors such as TGF-β1, autophagy, and inhibition of the Smad3 pathway activation in hepatic stellate cells [
31,
32]. Furthermore, Asta has also been shown to protect liver injury induced by CCl
4 in rats by suppressing oxidative stress parameters like malondialdehyde (MDA) and nitric oxide (NO), and enhancing the activities of physiological antioxidant system such as superoxide dismutase (SOD) and catalase (CAT) [
33]. These protective effects of Asta against CCl
4-induced liver damage are similar with those exerted by angiotensin-converting enzyme inhibitors [
34]. To date, few studies have investigated the preventive and therapeutic effects of Asta on alcoholic liver diseases. The current data successfully highlighted that Asta-lipo administered intraperitoneally and orally possesses the capability to restore alcohol-induced liver injuries and has preventive actions on the repeated alcohol consumption status in vivo. We previously found that 2-week intraperitoneal administration of HA nanoparticles aggregated Asta was capable of restoring the retrorsine-CCl
4-induced liver fibrosis and necrosis in a murine model, demonstrating that nanoparticle-encapsulated Asta can repair fibrotic and necrotic liver disease caused by toxicants in vivo [
35]. Notably, in our previous study and in the current study, shorter-term (2 weeks) treatment with nanoparticle-encapsulated Asta through the intraperitoneal route exerted reparative effects on alcohol- or toxicant-induced liver injuries, indicating that the low bioavailability of Asta can be improved after nanoparticle encapsulation, and intraperitoneal is a feasible administration route for nanoparticle-encapsulated Asta to exhibit ameliorating actions against liver fibrotic and necrotic diseases.
4. Materials and Methods
4.1. Materials and Animals
Asta was produced and provided by Bioptik Technology Inc (Miaoli, Taiwan). Asta was extracted according to the method described in the US patent US8030523B2, filed by Bioptik Technology Inc. In brief,
Pomacae canaliculata eggs were mixed proportionally in deionized water and homogenized (Polytron PT-2100, Bestway, Taiwan). The egg shells were then removed to obtain a solution of glycoprotein carotenoid. Proteins, sugars, and lipids in the glycoprotein carotenoid solution were sequentially removed to prepare the carotenoid solution. Finally, pure Asta was extracted using 95% ethanol, and the purity of Asta was quantified using the method described by Skrede et al. [
36]. Cholesterol, lecithin, DSPC (molecular weight: 790.15 Da), chloroform, and methanol were obtained from Sigma (St. Louis, MO, USA). All chemicals used in this study were of reagent grade.
The animal research was approved by the Institutional Animal Care and Use Committee of I-Shou University, Taiwan (AUP-105-50-01), date (20-06-2016). A total of 90 wild-type C57BL/6J male mice (age, 12 weeks) were used in the mouse liver fibrosis model (average weight, 24 ± 2 g).
4.2. Production and Characterization of Astaxanthin-Encapsulated Liposomes
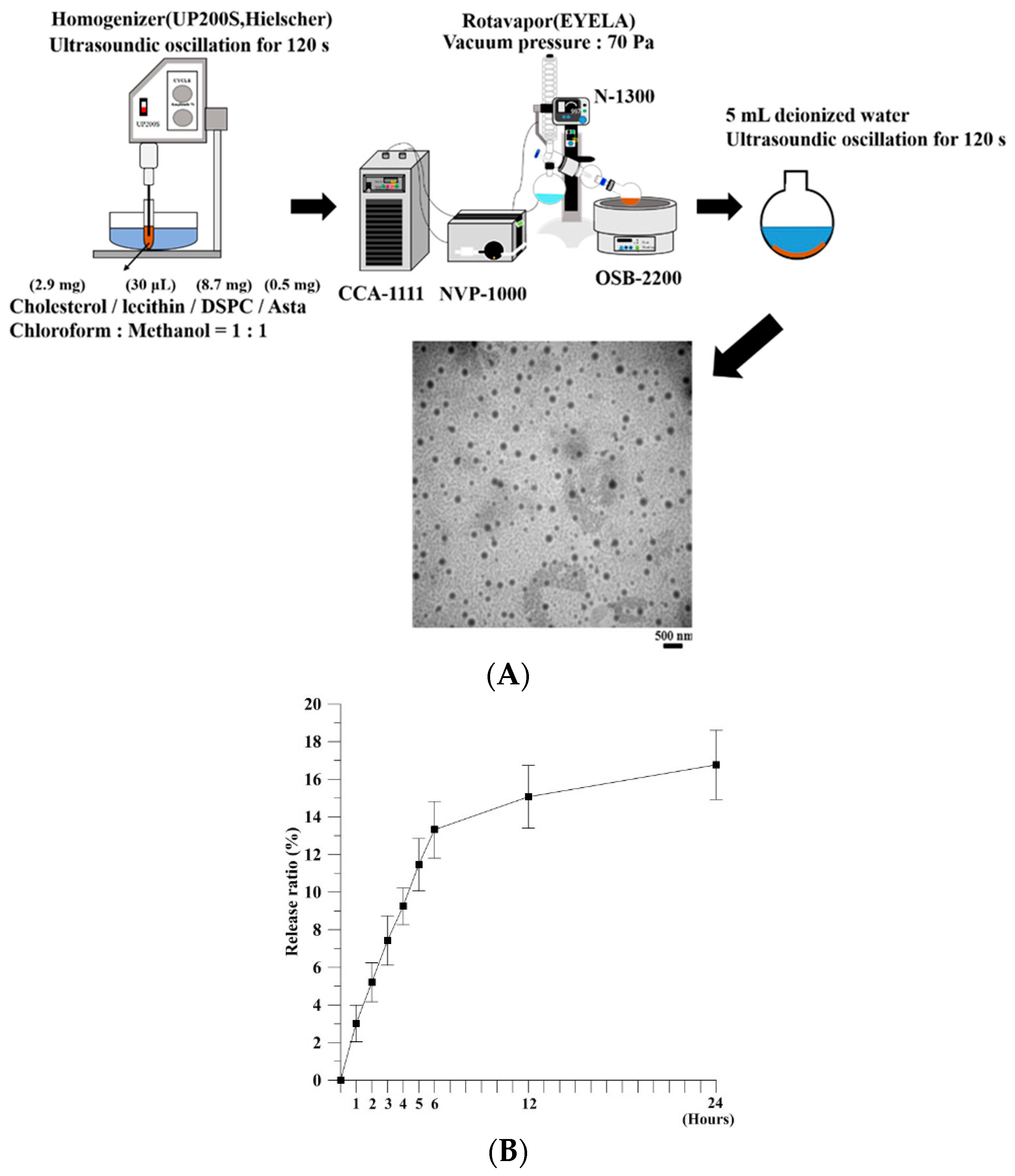
Asta-lipo was prepared using the evaporation sonication method with some modification [
9]. Briefly, phospholipids used for liposomes were a mixture of DSPC (8.7 mg), cholesterol (2.9 mg), lecithin (30 μL), and Asta (0.5 mg). DSPC, cholesterol, lecithin, and Asta were dissolved in methanol:chloroform (1:1,
v/v) and then loaded into a flask. The mixture in a flask was homogenized for 120 s (UP 200S, Germany) and dried using a rotary evaporator (N-1300, Japan) to form a thin layer of film. The produced film was rehydrated with deionized water (5 mL) and subjected to sonication for 20 min (
Figure 1A). The size of Asta-lipo was calculated on a random sampling basis for approximately 100–150 individual liposomes through transmission electron microscopy (TEM). The entrapment efficiency (EE) of Asta in liposomes was determined as follows: 1 mL of the solution containing the prepared Asta liposomes was centrifuged at 14,000 rpm for 60 min, and the amount of nonencapsulated Asta in the supernatant was measured through high-performance liquid chromatography (HPLC, Agilent 1100 series). A standard concentration curve of Asta was established for determining the amount of Asta encapsulated in liposomes. The EE was calculated using the following formula:
The in vitro release of Asta from liposomes was assessed. A total of 1 mL of Asta liposomes was added to a 1.5-mL microcentrifuge tube. The tube was then placed on a shaker with the temperature and shaking rate set at 37 °C and 40 rpm, respectively. At designated time points, the sample was centrifuged at 14,000 rpm for 60 min. The amount of nonencapsulated Asta in the supernatant was determined through HPLC. The independent experiment was repeated three times (
n = 3). The in vitro release rate was calculated as follows:
4.3. In Vitro Cell Viability Study
L929 fibroblasts were suspended at a density of 1 × 104 cells/mL and seeded onto 96-well plates. Asta-lipo, pure liposomes or control was added to each well in triplicate. The cell viability of L929 normal fibroblasts was examined using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After a 24-h treatment, 20 μL of MTT solution was added to cells, and cells were then incubated for 3 h. The formazan precipitate was dissolved in 200 μL dimethyl sulfoxide (DMSO), and absorbance was measured at 570 nm on a multiplate reader (Thermo Scientific, Waltham, MA, USA).
4.4. Cell Cycle Analysis
The cell cycle of L929 fibroblasts was analyzed through flow cytometry after 24- and 48-h Asta-lipo treatment. L929 fibroblasts (1.5 × 105 cells/mL) were seeded onto the flask for 24 h; then, 500 μL of Asta-lipo or pure liposomes was added. The cells were incubated (37 °C, 5% CO2) for 24 or 48 h before harvest. Thereafter, the cells were centrifuged at 1200 rpm for 5 min and washed with phosphate-buffered saline, followed by staining with PI/RNase staining buffer (BD Bioscience, San Jose, CA, USA) for 30 min at room temperature. The cell cycle was analyzed through flow cytometry (Accuri C6, BD Bioscience). The cell cycle distribution was measured from 10,000 cells by using the ModFit LT software (Topsham, ME, USA).
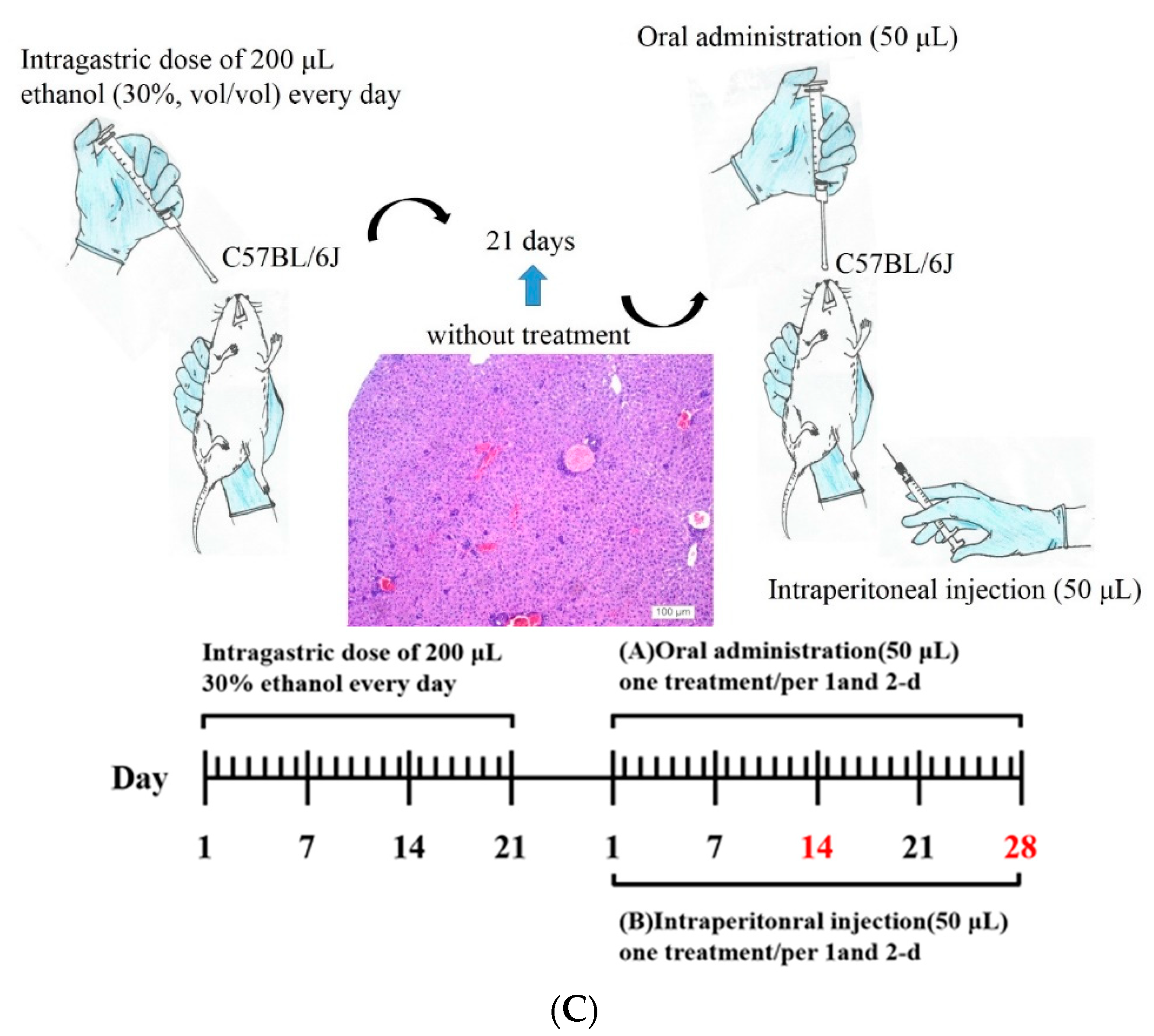
4.5. Animal Experimental Design
Mice received an intragastric dose of 30% ethanol (
v/
v) at a dose of 3 g/kg body weight every day for 21 days through a 24-gauge stainless feeding tube. Mice were weighed on alternate days and housed under standard conditions, with food and water provided ad libitum. Mice with alcohol-induced liver fibrosis were categorized into different experimental groups according to their treatment period, treatment type (Asta-lipo or pure liposomes), and administration route, as shown in
Table 2. Intraperitoneal injection or oral administration of Asta-lipo/pure liposomes were executed as shown in
Figure 1B. Before intraperitoneal injection of treatments, mice were anaesthetized with Zoletil
® (tiletamine with zolazepam, 40 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). At the end of experiments, the weight of mice in each group was measured; then, mice were euthanized through an overdose of CO
2. Blood and liver samples were harvested for serum biochemical and histopathological analyses, respectively.
Figure 1C showed schematic representation and time scale of the establishment of alcoholic disease and administration routes on the mouse model.
4.6. Histopathological Analysis
After a 2-week and 4-week treatment with Asta-lipo or pure liposomes, the livers of mice in the respective groups were collected. The liver lobes were removed, and liver tissues were fixed in 10% neutral buffered formalin. The liver cuts were dehydrated in ethanol and then embedded in wax. Hematoxylin and Eosin (H&E) staining was performed in dishes, starting with a dewaxing procedure in xylene. Sequential incubations in 100% and 95% ethanol and tap water were conducted to rehydrate the liver tissues. The liver tissues were stained with Mayer’s hematoxylin (Merck, Darmstadt, Germany) for 15 min, followed by a 3-min wash in tap water. The tissues were then stained with eosin–phloxine solution (Merck) for 30 s and washed in tap water for 30 min. The liver tissue samples were then dehydrated with sequential incubations in 50%, 70%, 80%, 90%, 95%, and 100% ethanol and xylene.
Histological sections were further stained with Masson’s Trichrome to assess collagen content in mice liver after induction of alcoholic liver disease with or without treatment with Asta-lipo or pure liposomes. The liver tissue sections were deparaffinized in xylene and then hydrated in graded ethanol, xylene, and distilled water. Weigert’s iron hematoxylin solution was used to stain the liver tissues. Tissue slides were then sequentially incubated in Biebrich scarlet-acid fuchsin (Sigma) for 5 min, followed by treatment with phosphomolybdic–phosphotungstic acid solution and aniline blue (Sigma). ImageJ software was applied to measure the collagen content in each group [
37]. In brief, liver samples from experimental groups were stained and compared to Group B, S or T. The color settings in the ImageJ software was maintained at all times between the calculations of the blue-stained areas in samples. Magnification 100× was employed to evaluate the samples and the calculation was repeated in four microscopic fields.
4.7. Measurement of Alanine Aminotransferase and Aspartate Aminotransferase
We executed the ALT and AST assays and calculate the ratio of AST:ALT to further clinically evaluate and validate the status or recovery from alcohol-induced liver fibrosis and damage after treatment with Asta-lipo or pure liposomes in the experimental groups. ALT or AST samples were prepared according to the instruction protocol (AAT Bioquest, CA, USA). Each 100 μL mixture of the sample and ALT/AST enzyme on the plate were incubated at 37 °C for 20–30 min in the dark. The absorbance was measured at 575 nm on a microplate reader.
4.8. Statistical Analysis
Data are presented as the mean ± standard error of the mean (SEM) unless stated otherwise. Results were analyzed using one-way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparisons test on SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) to determine whether significant differences existed between the control and experimental groups (
P < 0.05) [
38].