Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases
Abstract
1. Introduction
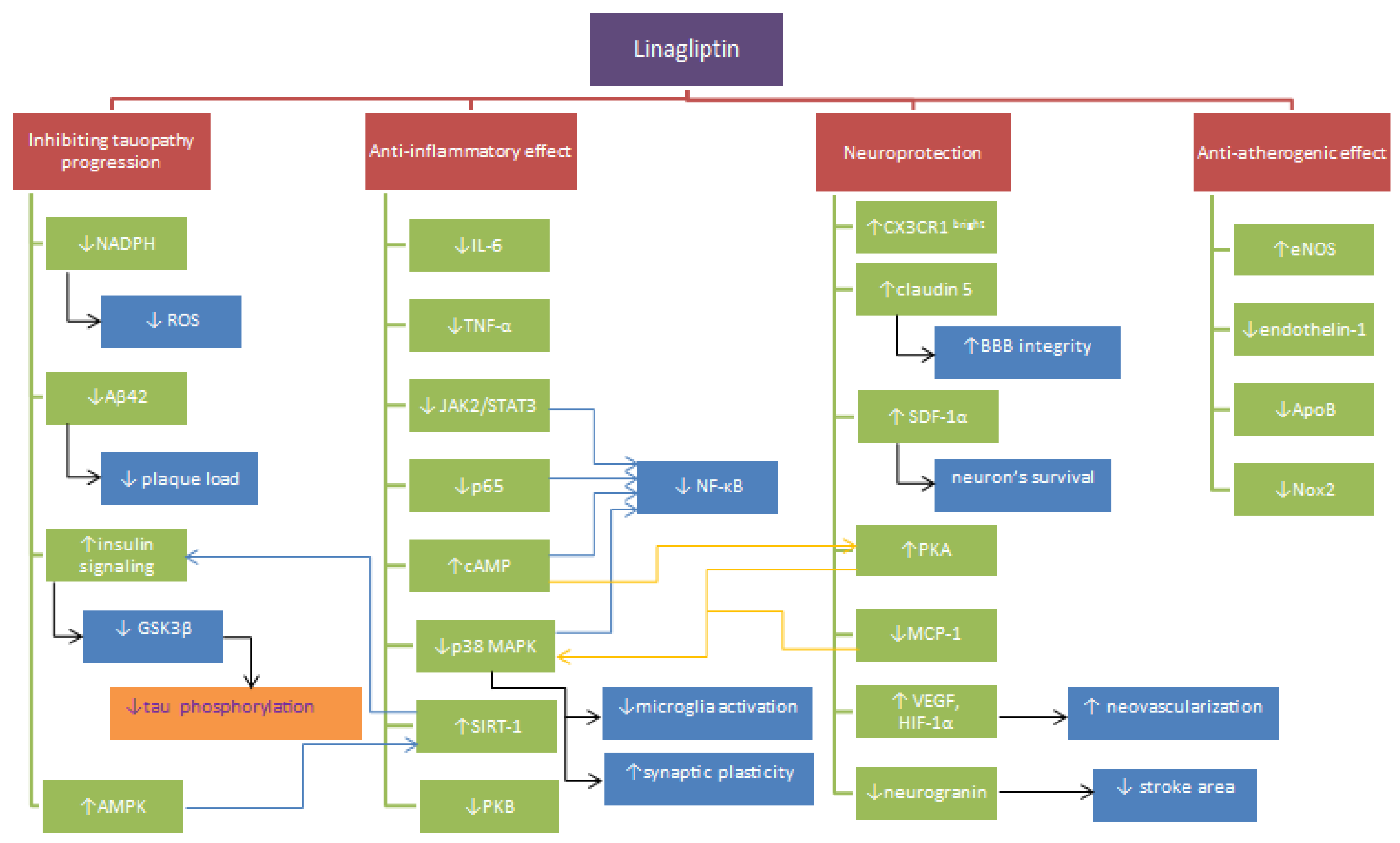
2. Inflammation and Oxidative Stress
3. Cerebral Blood Flow
4. Neurodegeneration
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015; World Health Organization: Geneva, Switzerland, 2016.
- Ahmad, M.; Dar, N.J.; Bhat, Z.S.; Hussain, A.; Shah, A.; Liu, H.; Graham, S.H. Inflammation in ischemic stroke: Mechanisms, consequences and possible drug targets. CNS Neurol. Disord. Drug Targets 2014, 13, 1378–1396. [Google Scholar] [CrossRef] [PubMed]
- Doupis, J. Linagliptin: From bench to bedside. Drug Des. Devel. Ther. 2014, 8, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Vella, A. Mechanism of action of DPP-4 inhibitors--new insights. J. Clin. Endocrinol. Metab. 2012, 97, 2626–2628. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Wódkiewicz, E.; Słupski, M.; Walczak, M.; Socha, M.; Malinowski, B.; Pawlak-Osińska, K. Neuroprotective Activity of Sitagliptin via Reduction of Neuroinflammation beyond the Incretin Effect: Focus on Alzheimer’s Disease. Biomed. Res. Int. 2018, 2018, 6091014. [Google Scholar] [CrossRef] [PubMed]
- Vella, A.; Bock, G.; Giesler, P.D.; Burton, D.B.; Serra, D.B.; Saylan, M.L.; Deacon, C.F.; Foley, J.E.; Rizza, R.A.; Camilleri, M. The effect of dipeptidyl peptidase-4 inhibition on gastric volume, satiation and enteroendocrine secretion in type 2 diabetes: A double-blind, placebo-controlled crossover study. Clin. Endocrinol. 2008, 69, 737–744. [Google Scholar] [CrossRef]
- Omar, B.; Ahrén, B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes 2014, 63, 2196–2202. [Google Scholar] [CrossRef]
- Retlich, S.; Duval, V.; Graefe-Mody, U.; Friedrich, C.; Patel, S.; Jaehde, U.; Staab, A. Population Pharmacokinetics and Pharmacodynamics of Linagliptin in Patients with Type 2 Diabetes Mellitus. Clin. Pharmacokinet. 2015, 54, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Graefe-Mody, U.; Retlich, S.; Friedrich, C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin. Pharmacokinet. 2012, 51, 411–427. [Google Scholar] [CrossRef]
- Metzmann, K.; Schnell, D.; Jungnik, A.; Ring, A.; Theodor, R.; Hohl, K.; Meinicke, T.; Friedrich, C. Effect of food and tablet-dissolution characteristics on the bioavailability of linagliptin fixed-dose combination with metformin: Evidence from two randomized trials. Int. J. Clin. Pharmacol. Ther. 2014, 52, 549–563. [Google Scholar] [CrossRef]
- Kornelius, E.; Lin, C.L.; Chang, H.H.; Li, H.H.; Huang, W.N.; Yang, Y.S.; Lu, Y.L.; Peng, C.H.; Huang, C.N. DPP-4 Inhibitor Linagliptin Attenuates Aβ-induced Cytotoxicity through Activation of AMPK in Neuronal Cells. CNS Neurosci. Ther. 2015, 21, 549–557. [Google Scholar] [CrossRef]
- Ma, M.; Hasegawa, Y.; Koibuchi, N.; Toyama, K.; Uekawa, K.; Nakagawa, T.; Lin, B.; Kim-Mitsuyama, S. DPP-4 inhibition with linagliptin ameliorates cognitive impairment and brain atrophy induced by transient cerebral ischemia in type 2 diabetic mice. Cardiovasc. Diabetol. 2015, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Mi, D.H.; Fang, H.J.; Zheng, G.H.; Liang, X.H.; Ding, Y.R.; Liu, X.; Liu, L.P. DPP-4 inhibitors promote proliferation and migration of rat brain microvascular endothelial cells under hypoxic/high-glucose conditions, potentially through the SIRT1/HIF-1/VEGF pathway. CNS Neurosci. Ther. 2018, 25, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Inagaki, M.; Tsuji, M.; Gocho, T.; Handa, K.; Hasegawa, H.; Yura, A.; Kawakami, T.; Ohsawa, I.; Goto, Y.; et al. Linagliptin has Wide-Ranging Anti-Inflammatory Points of Action in Human Umbilical Vein Endothelial Cells. Jpn. Clin. Med. 2016, 7, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Waltereit, R.; Weller, M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol. Neurobiol. 2003, 27, 99–106. [Google Scholar] [CrossRef]
- Tarkowski, E.; Rosengren, L.; Blomstrand, C.; Wikkelsö, C.; Jensen, C.; Ekholm, S.; Tarkowski, A. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin. Exp. Immunol. 1997, 110, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Salheen, S.M.; Panchapakesan, U.; Pollock, C.A.; Woodman, O.L. The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Preserves Endothelial Function in Mesenteric Arteries from Type 1 Diabetic Rats without Decreasing Plasma Glucose. PLoS ONE 2015, 10, e0143941. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Ortsäter, H.; Olverling, A.; Darlöf, E.; Wolbert, P.; Nyström, T.; Klein, T.; Sjöholm, Å.; Patrone, C. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: A comparison with glimepiride. Diabetes 2013, 62, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Olverling, A.; Larsson, M.; Mansouri, S.; Nathanson, D.; Nyström, T.; Klein, T.; Sjöholm, Å.; Patrone, C. Linagliptin enhances neural stem cell proliferation after stroke in type 2 diabetic mice. Regul. Pept. 2014, 190–191, 25–31. [Google Scholar] [CrossRef]
- Elbaz, E.M.; Senousy, M.A.; El-Tanbouly, D.M.; Sayed, R.H. Neuroprotective effect of linagliptin against cuprizone-induced demyelination and behavioural dysfunction in mice: A pivotal role of AMPK/SIRT1 and JAK2/STAT3/NF-κB signalling pathway modulation. Toxicol. Appl. Pharmacol. 2018, 352, 153–161. [Google Scholar] [CrossRef]
- Kosaraju, J.; Holsinger, R.M.D.; Guo, L.; Tam, K.Y. Linagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Mitigates Cognitive Deficits and Pathology in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6074–6084. [Google Scholar] [CrossRef]
- Salim, H.M.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Dipeptidyl peptidase-4 inhibitor, linagliptin, ameliorates endothelial dysfunction and atherogenesis in normoglycemic apolipoprotein-E deficient mice. Vascul. Pharmacol. 2016, 79, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hardigan, T.; Abdul, Y.; Ergul, A. Linagliptin reduces effects of ET-1 and TLR2-mediated cerebrovascular hyperreactivity in diabetes. Life Sci. 2016, 159, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chiazza, F.; Tammen, H.; Pintana, H.; Lietzau, G.; Collino, M.; Nyström, T.; Klein, T.; Darsalia, V.; Patrone, C. The effect of DPP-4 inhibition to improve functional outcome after stroke is mediated by the SDF-1α/CXCR4 pathway. Cardiovasc. Diabetol. 2018, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Hasegawa, H.; Tsuji, M.; Oguchi, T.; Mihara, M.; Suzuki, H.; Nishida, K.; Inoue, M.; Shimizu, T.; Ohsawa, I.; et al. Linagliptin inhibits lipopolysaccharide-stimulated interleukin-6 production, intranuclear p65 expression, and p38 mitogen-activated protein kinase phosphorylation in human umbilical vein endothelial cells. Ren. Replace. Ther. 2016, 2, 17. [Google Scholar] [CrossRef][Green Version]
- Shigiyama, F.; Kumashiro, N.; Miyagi, M.; Iga, R.; Kobayashi, Y.; Kanda, E.; Uchino, H.; Hirose, T. Linagliptin improves endothelial function in patients with type 2 diabetes: A randomized study of linagliptin effectiveness on endothelial function. J. Diabetes Investig. 2017, 8, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.L.; Kielian, T. Toll-like receptors in health and disease in the brain: Mechanisms and therapeutic potential. Clin. Sci. 2011, 121, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Cho, S. Microglia and Monocyte-Derived Macrophages in Stroke. Neurotherapeutics 2016, 13, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Saqib, U.; Sarkar, S.; Suk, K.; Mohammad, O.; Baig, M.S.; Savai, R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 2018, 9, 17937–17950. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Yamadera, S.; Nakamura, Y.; Inagaki, M.; Kenmotsu, S.; Nohara, T.; Sato, N.; Oguchi, T.; Tsuji, M.; Ohsawa, I.; Gotoh, H.; et al. Linagliptin inhibits lipopolysaccharide-induced inflammation in human U937 monocytes. Inflamm. Regen. 2018, 38, 13. [Google Scholar] [CrossRef] [PubMed]
- Vila, N.; Castillo, J.; Dávalos, A.; Chamorro, A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 2000, 31, 2325–2329. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, J.; Losy, J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol. Scand. 2001, 104, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. 2007, 7, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Dai, D.; Wang, X.; Ding, Z.; Mehta, J.L. DPP-4 inhibitors repress NLRP3 inflammasome and interleukin-1beta via GLP-1 receptor in macrophages through protein kinase C pathway. Cardiovasc. Drugs Ther. 2014, 28, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Bonora, B.M.; Cappellari, R.; Menegazzo, L.; Vedovato, M.; Iori, E.; Marescotti, M.C.; Albiero, M.; Avogaro, A. Acute Effects of Linagliptin on Progenitor Cells, Monocyte Phenotypes, and Soluble Mediators in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 748–756. [Google Scholar] [CrossRef]
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical patrolling monocyte function in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Bellavance, M.A.; Gosselin, D.; Yong, V.W.; Stys, P.K.; Rivest, S. Patrolling monocytes play a critical role in CX3CR1-mediated neuroprotection during excitotoxicity. Brain Struct. Funct. 2015, 220, 1759–1776. [Google Scholar] [CrossRef]
- Audoy-Rémus, J.; Richard, J.F.; Soulet, D.; Zhou, H.; Kubes, P.; Vallières, L. Rod-Shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J. Neurosci. 2008, 28, 10187–10199. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.A. Role of the vascular endothelium and plaque in acute ischemic stroke. Neurology 2012, 79 (Suppl. 1), S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and Beneficial Role of ROS. Oxid. Med. Cell. Longev. 2016, 2016, 7909186. [Google Scholar] [CrossRef] [PubMed]
- Lastra, G.; Syed, S.; Kurukulasuriya, L.R.; Manrique, C.; Sowers, J.R. Type 2 diabetes mellitus and hypertension: An update. Endocrinol. Metab. Clin. N. Am. 2014, 43, 103–122. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Asgary, S.; Adelnia, A.; Setorki, M.; Khazaei, M.; Kazemi, S.; Shamsi, F. The effects of cornelian cherry on atherosclerosis and atherogenic factors in hypercholesterolemic rabbits. J. Med. Plants Res. 2011, 5, 2670–2676. [Google Scholar]
- Kibbe, M.; Billiar, T.; Tzeng, E. Inducible nitric oxide synthase and vascular injury. Cardiovasc. Res. 1999, 43, 650–657. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Terpolilli, N.A.; Moskowitz, M.A.; Plesnila, N. Nitric oxide: Considerations for the treatment of ischemic stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1332–1346. [Google Scholar] [CrossRef]
- Vellecco, V.; Mitidieri, E.; Gargiulo, A.; Brancaleone, V.; Matassa, D.; Klein, T.; Esposito, F.; Cirino, G.; Bucci, M. Vascular effects of linagliptin in non-obese diabetic mice are glucose-independent and involve positive modulation of the endothelial nitric oxide synthase (eNOS)/caveolin-1 (CAV-1) pathway. Diabetes Obes. Metab. 2016, 18, 1236–1243. [Google Scholar] [CrossRef]
- Jyoti, U.; Kansal, S.K.; Kumar, P.; Goyal, S. Possible vasculoprotective role of linagliptin against sodium arsenite-induced vascular endothelial dysfunction. Naunyn Schmiedeberg’s Arch Pharmacol. 2016, 389, 167–175. [Google Scholar] [CrossRef]
- Theodorou, K.; Boon, R.A. Endothelial Cell Metabolism in Atherosclerosis. Front. Cell Dev. Biol. 2018, 6, 82. [Google Scholar] [CrossRef]
- Soler, E.P.; Ruiz, V.C. Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: Similarities and differences. Curr. Cardiol. Rev. 2010, 6, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Hajra, L.; Evans, A.I.; Chen, M.; Hyduk, S.J.; Collins, T.; Cybulsky, M.I. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl. Acad. Sci. USA 2000, 97, 9052–9057. [Google Scholar] [CrossRef]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.M.; Luo, Q.Q.; Huang, S.; Yang, Q.W.; Wang, F.X.; Ke, Y.; Qian, Z.M. CX3CL1/CX3CR1-mediated microglia activation plays a detrimental role in ischemic mice brain via p38MAPK/PKC pathway. J. Cereb. Blood Flow Metab. 2015, 35, 1623–1631. [Google Scholar] [CrossRef]
- Yuan, B.; Shi, H.; Zheng, K.; Su, Z.; Su, H.; Zhong, M.; He, X.; Zhou, C.; Chen, H.; Xiong, Q.; et al. MCP-1-mediated activation of microglia promotes white matter lesions and cognitive deficits by chronic cerebral hypoperfusion in mice. Mol. Cell Neurosci. 2017, 78, 52–58. [Google Scholar] [CrossRef]
- Gao, H.H.; Gao, L.B.; Wen, J.M. Correlations of MCP-1 −2518A>G polymorphism and serum levels with cerebral infarction risk: A meta-analysis. DNA Cell Biol. 2014, 33, 522–530. [Google Scholar] [CrossRef]
- Grozdanov, V.; Bliederhaeuser, C.; Ruf, W.P.; Roth, V.; Fundel-Clemens, K.; Zondler, L.; Brenner, D.; Martin-Villalba, A.; Hengerer, B.; Kassubek, J.; et al. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 2014, 128, 651–663. [Google Scholar] [CrossRef]
- Whiteley, W.; Jackson, C.; Lewis, S.; Lowe, G.; Rumley, A.; Sandercock, P.; Wardlaw, J.; Dennis, M.; Sudlow, C. Inflammatory markers and poor outcome after stroke: A prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009, 6, e1000145. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Edvinsson, L.; Xu, C.B. Apolipoprotein B of low-density lipoprotein impairs nitric oxide-mediated endothelium-dependent relaxation in rat mesenteric arteries. Eur. J. Pharmacol. 2014, 725, 10–17. [Google Scholar] [CrossRef]
- Dong, H.; Chen, W.; Wang, X.; Pi, F.; Wu, Y.; Pang, S.; Xie, Y.; Xia, F.; Zhang, Q. Apolipoprotein A1, B levels, and their ratio and the risk of a first stroke: A meta-analysis and case-control study. Metab. Brain Dis. 2015, 30, 1319–1330. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; de Winther, M.P.; Weber, C.; Daemen, M.J.; Lutgens, E.; Soehnlein, O. Atherosclerotic plaque destabilization: Mechanisms, models, and therapeutic strategies. Circ. Res. 2014, 114, 214–226. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, P.; Zhu, Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediat. Inflamm. 2013, 2013, 124614. [Google Scholar] [CrossRef]
- Ziegler, G.; Harhausen, D.; Schepers, C.; Hoffmann, O.; Röhr, C.; Prinz, V.; König, J.; Lehrach, H.; Nietfeld, W.; Trendelenburg, G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem. Biophys. Res. Commun. 2007, 359, 574–579. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Y.; Zhang, J.; Sun, L.; Liu, Z.; Zhang, S.; Wang, B.; Su, D.; Su, Z. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience 2011, 176, 162–172. [Google Scholar] [CrossRef]
- Heiss, W.D. The ischemic penumbra: How does tissue injury evolve? Ann. N. Y. Acad Sci. 2012, 1268, 26–34. [Google Scholar] [CrossRef]
- Pushie, M.J.; Crawford, A.M.; Sylvain, N.J.; Hou, H.; Hackett, M.J.; George, G.N.; Kelly, M.E. Revealing the Penumbra through Imaging Elemental Markers of Cellular Metabolism in an Ischemic Stroke Model. ACS Chem. Neurosci. 2018, 9, 886–893. [Google Scholar] [CrossRef]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Schneider, J.A.; Wilson, R.S.; Beck, T.L.; Evans, D.A.; Carli, C.D. Characteristics of MR infarcts associated with dementia and cognitive function in the elderly. Neuroepidemiology 2012, 38, 41–47. [Google Scholar] [CrossRef]
- Zhang, L.; Chopp, M.; Zhang, Y.; Xiong, Y.; Li, C.; Sadry, N.; Rhaleb, I.; Lu, M.; Zhang, Z.G. Diabetes Mellitus Impairs Cognitive Function in Middle-Aged Rats and Neurological Recovery in Middle-Aged Rats After Stroke. Stroke 2016, 47, 2112–2118. [Google Scholar] [CrossRef]
- Darsalia, V.; Larsson, M.; Lietzau, G.; Nathanson, D.; Nyström, T.; Klein, T.; Patrone, C. Gliptin-mediated neuroprotection against stroke requires chronic pretreatment and is independent of glucagon-like peptide-1 receptor. Diabetes Obes. Metab. 2016, 18, 537–541. [Google Scholar] [CrossRef]
- Thangarajah, H.; Yao, D.; Chang, E.I.; Shi, Y.; Jazayeri, L.; Vial, I.N.; Galiano, R.D.; Du, X.L.; Grogan, R.; Galvez, M.G.; et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 13505–13510. [Google Scholar] [CrossRef]
- Craige, S.M.; Chen, K.; Pei, Y.; Li, C.; Huang, X.; Chen, C.; Shibata, R.; Sato, K.; Walsh, K.; Keaney, J.F., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 2011, 124, 731–740. [Google Scholar] [CrossRef]
- Joo, H.Y.; Yun, M.; Jeong, J.; Park, E.R.; Shin, H.J.; Woo, S.R.; Jung, J.K.; Kim, Y.M.; Park, J.J.; Kim, J.; et al. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1α (HIF-1α) via direct interactions during hypoxia. Biochem. Biophys. Res. Commun. 2015, 462, 294–300. [Google Scholar] [CrossRef]
- Laemmle, A.; Lechleiter, A.; Roh, V.; Schwarz, C.; Portmann, S.; Furer, C.; Keogh, A.; Tschan, M.P.; Candinas, D.; Vorburger, S.A.; et al. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1α protein under hypoxic conditions. PLoS ONE 2012, 7, e33433. [Google Scholar] [CrossRef]
- Wiciński, M.; Socha, M.; Walczak, M.; Wódkiewicz, E.; Malinowski, B.; Rewerski, S.; Górski, K.; Pawlak-Osińska, K. Beneficial Effects of Resveratrol Administration-Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Rostène, W.; Kitabgi, P.; Parsadaniantz, S.M. Chemokines: A new class of neuromodulator? Nat. Rev. Neurosci. 2007, 8, 895–903. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, H.; Zhang, X.; Zhao, S.; Zhou, Z.; Mu, X.; Zhao, C.; Teng, W. The Role of SDF-1/CXCR4/CXCR7 in Neuronal Regeneration after Cerebral Ischemia. Front. Neurosci. 2017, 11, 590. [Google Scholar] [CrossRef]
- Doitsidou, M.; Reichman-Fried, M.; Stebler, J.; Köprunner, M.; Dörries, J.; Meyer, D.; Esguerra, C.V.; Leung, T.; Raz, E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell 2002, 111, 647–659. [Google Scholar] [CrossRef]
- Laske, C.; Stellos, K.; Stransky, E.; Seizer, P.; Akcay, O.; Eschweiler, G.W.; Leyhe, T.; Gawaz, M. Decreased plasma and cerebrospinal fluid levels of stem cell factor in patients with early Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 15, 451–460. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; He, X.; Tang, G.; Tang, Y.H.; Liu, Y.; Lin, X.; Lu, Y.; Yang, G.Y.; Wang, Y. Postacute stromal cell-derived factor-1α expression promotes neurovascular recovery in ischemic mice. Stroke 2014, 45, 1822–1829. [Google Scholar] [CrossRef]
- Selvaraj, U.M.; Ortega, S.B.; Hu, R.; Gilchrist, R.; Kong, X.; Partin, A.; Plautz, E.J.; Klein, R.S.; Gidday, J.M.; Stowe, A.M. Preconditioning-induced CXCL12 upregulation minimizes leukocyte infiltration after stroke in ischemia-tolerant mice. J. Cereb. Blood Flow Metab. 2017, 37, 801–813. [Google Scholar] [CrossRef]
- Kwon, H.S.; Kim, Y.S.; Park, H.H.; Choi, H.; Lee, K.Y.; Lee, Y.J.; Heo, S.H.; Chang, D.I.; Koh, S.H. Increased VEGF and decreased SDF-1α in patients with silent brain infarction are associated with better prognosis after first-ever acute lacunar stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 704–710. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Tang, Y.; Tang, G.; Yang, G.Y.; Wang, Y. CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke 2013, 44, 190–197. [Google Scholar] [CrossRef]
- De Vos, A.; Bjerke, M.; Brouns, R.; De Roeck, N.; Jacobs, D.; Van den Abbeele, L.; Guldolf, K.; Zetterberg, H.; Blennow, K.; Engelborghs, S.; et al. Neurogranin and tau in cerebrospinal fluid and plasma of patients with acute ischemic stroke. BMC Neurol. 2017, 17, 170. [Google Scholar] [CrossRef]
- Gallwitz, B.; Rosenstock, J.; Rauch, T.; Bhattacharya, S.; Patel, S.; von Eynatten, M.; et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: A randomised, double-blind, non-inferiority trial. Lancet 2012, 380, 475–483. [Google Scholar] [CrossRef]
- Li, Y.R.; Tsai, S.S.; Chen, D.Y.; Chen, S.T.; Sun, J.H.; Chang, H.Y.; Chen, T.H. Linagliptin and cardiovascular outcomes in type 2 diabetes after acute coronary syndrome or acute ischemic stroke. Cardiovasc. Diabetol. 2018, 17, 2. [Google Scholar] [CrossRef]
- Marx, N.; Rosenstock, J.; Kahn, S.E.; Zinman, B.; Kastelein, J.J.; Lachin, J.M.; Patel, S. Design and baseline characteristics of the CARdiovascular outcome trial of LINAgliptin versus glimepiride in type 2 diabetes (CAROLINA®). Diabetes Vasc. Dis. Res. 2015, 12, 164–174. [Google Scholar] [CrossRef]
- Rosenstock, J.; Perkovic, V.; Alexander, J.H.; Cooper, M.E.; Marx, N.; Pencina, M.J.; Pfarr, E. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): A randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc. Diabetol. 2018, 17, 39. [Google Scholar]
- Cunningham, E.L.; McGuinness, B.; Herron, B.; Passmore, A.P. Dementia. Ulster Med. J. 2015, 84, 79–87. [Google Scholar]
- Iqbal, K.; Liu, F.; Gong, C.X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef]
- Yilmaz, U. [Alzheimer’s disease]. Radiologe 2015, 55, 386–388. [Google Scholar] [CrossRef]
- Wiciński, M.; Socha, M.; Malinowski, B.; Wódkiewicz, E.; Walczak, M.; Górski, K.; Słupski, M.; Pawlak-Osińska, K. Liraglutide and its Neuroprotective Properties-Focus on Possible Biochemical Mechanisms in Alzheimer’s Disease and Cerebral Ischemic Events. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Hong, J.T. NF-kB as a mediator of brain inflammation in AD. CNS Neurol. Disord. Drug Targets 2017. [Google Scholar] [CrossRef]
- van Dam, P.S.; Aleman, A. Insulin-like growth factor-I, cognition and brain aging. Eur. J. Pharmacol. 2004, 490, 87–95. [Google Scholar] [CrossRef]
- Stockhorst, U.; de Fries, D.; Steingrueber, H.J.; Scherbaum, W.A. Insulin and the CNS: Effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol. Behav. 2004, 83, 47–54. [Google Scholar] [CrossRef]
- Craft, S.; Watson, G.S. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurol. 2004, 3, 169–178. [Google Scholar] [CrossRef]
- Bedse, G.; Di Domenico, F.; Serviddio, G.; Cassano, T. Aberrant insulin signaling in Alzheimer’s disease: Current knowledge. Front. Neurosci. 2015, 9, 204. [Google Scholar] [CrossRef]
- Pérez, A.; Morelli, L.; Cresto, J.C.; Castaño, E.M. Degradation of soluble amyloid beta-peptides 1-40, 1-42, and the Dutch variant 1-40Q by insulin degrading enzyme from Alzheimer disease and control brains. Neurochem. Res. 2000, 25, 247–255. [Google Scholar] [CrossRef]
- De Felice, F.G.; Vieira, M.N.; Bomfim, T.R.; Decker, H.; Velasco, P.T.; Lambert, M.P.; Viola, K.L.; Zhao, W.Q.; Ferreira, S.T.; Klein, W.L. Protection of synapses against Alzheimer’s-linked toxins: Insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc. Natl. Acad. Sci. USA 2009, 106, 1971–1976. [Google Scholar] [CrossRef]
- Tokutake, T.; Kasuga, K.; Yajima, R.; Sekine, Y.; Tezuka, T.; Nishizawa, M.; Ikeuchi, T. Hyperphosphorylation of Tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3β signaling pathway. J. Biol. Chem. 2012, 287, 35222–35233. [Google Scholar] [CrossRef]
- Metaxas, A.; Kempf, S.J. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen. Res. 2016, 11, 1579–1581. [Google Scholar] [CrossRef]
- Dziedzic, T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev. Neurother. 2015, 15, 523–531. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef]
- Holmes, C. Review: Systemic inflammation and Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2013, 39, 51–68. [Google Scholar] [CrossRef]
- Elwood, E.; Lim, Z.; Naveed, H.; Galea, I. The effect of systemic inflammation on human brain barrier function. Brain Behav. Immun. 2017, 62, 35–40. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Yan, Z.; Gibson, S.A.; Buckley, J.A.; Qin, H.; Benveniste, E.N. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2018, 189, 4–13. [Google Scholar] [CrossRef]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The role of JAK-STAT signaling within the CNS. JAK-STAT 2013, 2, e22925. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Balakin, K.V.; Lavrovsky, Y. Small molecule inhibitors of NF-kB and JAK/STAT signal transduction pathways as promising anti-inflammatory therapeutics. Mini Rev. Med. Chem. 2011, 11, 55–78. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef]
- Greco, S.J.; Hamzelou, A.; Johnston, J.M.; Smith, M.A.; Ashford, J.W.; Tezapsidis, N. Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and β-amyloid in neurons. Biochem. Biophys. Res. Commun. 2011, 414, 170–174. [Google Scholar] [CrossRef]
- Vázquez-Manrique, R.P.; Farina, F.; Cambon, K.; Dolores Sequedo, M.; Parker, A.J.; Millán, J.M.; Weiss, A.; Déglon, N.; Neri, C. AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease. Hum. Mol. Genet. 2016, 25, 1043–1058. [Google Scholar] [CrossRef]
- Wiciński, M.; Malinowski, B.; Węclewicz, M.M.; Grześk, E.; Grześk, G. Anti-atherogenic properties of resveratrol: 4-week resveratrol administration associated with serum concentrations of SIRT1, adiponectin, S100A8/A9 and VSMCs contractility in a rat model. Exp. Ther. Med. 2017, 13, 2071–2078. [Google Scholar] [CrossRef]
- Stefano, G.B.; Challenger, S.; Kream, R.M. Hyperglycemia-associated alterations in cellular signaling and dysregulated mitochondrial bioenergetics in human metabolic disorders. Eur. J. Nutr. 2016, 55, 2339–2345. [Google Scholar] [CrossRef]
- Khan, M.A.; Schultz, S.; Othman, A.; Fleming, T.; Lebrón-Galán, R.; Rades, D.; Clemente, D.; Nawroth, P.P.; Schwaninger, M. Hyperglycemia in Stroke Impairs Polarization of Monocytes/Macrophages to a Protective Noninflammatory Cell Type. J. Neurosci. 2016, 36, 9313–9325. [Google Scholar] [CrossRef]
- Shao, B.; Bayraktutan, U. Hyperglycaemia promotes human brain microvascular endothelial cell apoptosis via induction of protein kinase C-ßI and prooxidant enzyme NADPH oxidase. Redox Biol. 2014, 2, 694–701. [Google Scholar] [CrossRef]
- Wijesekara, N.; Ahrens, R.; Sabale, M.; Wu, L.; Ha, K.; Verdile, G.; Fraser, P.E. Amyloid-β and islet amyloid pathologies link Alzheimer’s disease and type 2 diabetes in a transgenic model. FASEB J. 2017, 31, 5409–5418. [Google Scholar] [CrossRef]
- Bruno, A.; Williams, L.S.; Kent, T.A. How important is hyperglycemia during acute brain infarction? Neurologist 2004, 10, 195–200. [Google Scholar] [CrossRef]
- Mi, D.; Wang, P.; Yang, B.; Pu, Y.; Yang, Z.; Liu, L. Correlation of hyperglycemia with mortality after acute ischemic stroke. Ther. Adv. Neurol. Disord. 2017, 11, 1756285617731686. [Google Scholar] [CrossRef]
- Carvalho, C.; Katz, P.S.; Dutta, S.; Katakam, P.V.; Moreira, P.I.; Busija, D.W. Increased susceptibility to amyloid-β toxicity in rat brain microvascular endothelial cells under hyperglycemic conditions. J. Alzheimer’s Dis. 2014, 38, 75–83. [Google Scholar] [CrossRef]
- Huang, J.; Liu, B.; Yang, C.; Chen, H.; Eunice, D.; Yuan, Z. Acute hyperglycemia worsens ischemic stroke-induced brain damage via high mobility group box-1 in rats. Brain Res. 2013, 1535, 148–155. [Google Scholar] [CrossRef]
- Sato, T.; Iwaki, M.; Shimogaito, N.; Wu, X.; Yamagishi, S.; Takeuchi, M. TAGE (toxic AGEs) theory in diabetic complications. Curr. Mol. Med. 2006, 6, 351–358. [Google Scholar] [CrossRef]
| Authors | Subject of Study | Dose of Linagliptin | Results |
|---|---|---|---|
| Kornelius et al. (2015) [11] | SK-N-MC human neuronal cells | 10–100 μM of linagliptin for 24 h | ↓ Aβ-induced cytotoxicity, ↓ GSK3β, ↓ ROS, ↓ hyper p-tau |
| Ma et al. (2015) [12] | rBMVECs | 0.083 g/kg diet for 8 weeks ater BCCAO | ↓ cognitive impairment, ↓ stroke volume, ↓ COS |
| Mi et al. (2018) [13] | rBMVECs | 40 nM | ↑ VEGF, ↑ eNOS, ↑ HIF-1α, ↑ SIRT1, |
| Nakamura et al. (2016) [14] | HUVECs | 1, 5, 10, 50, and 100 nM 1 h prior to incubation with LPS | ↓ IL-6, ↓ p-p38 MAPK ↓ p65 |
| Nakamura et al. (2016) [15] | HUVECs | 1 h 50 nM after 1 h 1 μg/mL LPS together with 50 nM linagliptin, | ↑ PKA, ↑ PKC, ↑ cAMP, ↓ PKB ↓ ROS |
| Yamadera et al. (2018) [16] | U937 cells | 1, 5, 10, 50, or 100 nM | ↓ IL-6, ↓ TNF-α |
| Salheen et al. (2015) [17] | STZ-induced diabetic rats | 2 mg/kg/ day for 4 weeks | ↑ NO, ↑ EDR, ↓ NADPH, ↑ Nox2 |
| Darsalia et al. (2013) [18] | C57BL/6 mice | 10 mg/kg/day for 4 weeks before and 3 weeks after MCAO | ↑ survival of neurons |
| Darsalia et al. (2014) [19] | C57BL/6 mice | 10 mg/kg/day for 4 weeks before and 3 weeks after MCAO | ↑ NSCs proliferation |
| Elbaz et al. (2018) [20] | C57BL/6 mice | 10 mg/kg/day for 3 weeks after 2 (from 3) weeks cuprizone administration | ↓ p-JAK2, ↑ p-AMPK, ↓ p-STAT3, ↓ NF-κB p65, ↑ SIRT1. |
| Kosaraju et al. (2017) [21] | 3xTg-AD mouse | 5, 10, and 20 mg/kg/day for 8 weeks. | ↑ Cognitive Performance, ↓ Aβ42, ↓ hyper p-tau |
| Salim et al. (2016) [22] | ApoE(−/−) mice | 10 mg/kg/day for 20 weeks | ↓ VCAM-1 ↓ MCP-1 ↓ NADPH |
| Hardigan et al. (2016) [23] | Male type-2 diabetic GK rats | 83 mg/kg for one week, next 166mg/kg for three weeks | ↓ ET-1, ↓ TLR2 |
| Chiazza al. (2018) [24] | C57BL/6 mice | varied at every stage of the experiment | ↑ post stroke rehabilitation ↑ SDF-1α ↓ stroke volume |
| Fadini et al. (2016) [25] | Diabetes type 2 patients | 5 mg per day for 4 days | ↑ SDF-1α, ↑ CX3CR1bright, ↓ MCP-1, ↓ CCL22, ↓ IL-12 |
| Shigiyama et al. (2015) [26] | Diabetes type 2 patients | 750 mg/day metformin + 5 mg/day linagliptin for 16 weeks | ↓ Apo B |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Górski, K.; Walczak, M.; Wódkiewicz, E.; Słupski, M.; Pawlak-Osińska, K.; Malinowski, B. Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 4052. https://doi.org/10.3390/ijms20164052
Wiciński M, Górski K, Walczak M, Wódkiewicz E, Słupski M, Pawlak-Osińska K, Malinowski B. Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases. International Journal of Molecular Sciences. 2019; 20(16):4052. https://doi.org/10.3390/ijms20164052
Chicago/Turabian StyleWiciński, Michał, Karol Górski, Maciej Walczak, Eryk Wódkiewicz, Maciej Słupski, Katarzyna Pawlak-Osińska, and Bartosz Malinowski. 2019. "Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases" International Journal of Molecular Sciences 20, no. 16: 4052. https://doi.org/10.3390/ijms20164052
APA StyleWiciński, M., Górski, K., Walczak, M., Wódkiewicz, E., Słupski, M., Pawlak-Osińska, K., & Malinowski, B. (2019). Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases. International Journal of Molecular Sciences, 20(16), 4052. https://doi.org/10.3390/ijms20164052





