A Concise Review on the Molecular Structure and Function Relationship of β-Glucan
Abstract
:1. Introduction
2. The Molecular Structure of β-Glucan
2.1. Molecular Weight
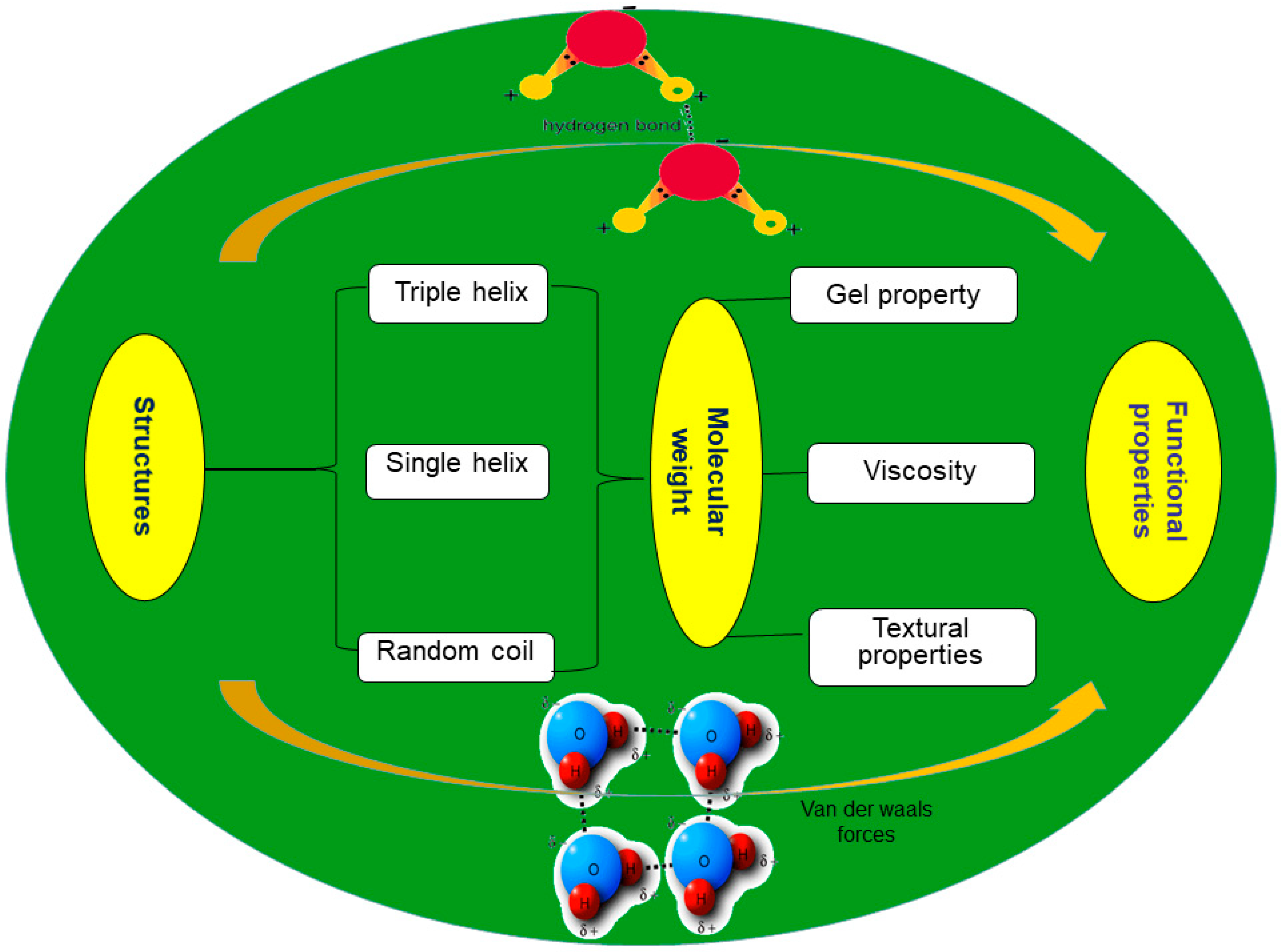
2.2. Conformation
2.3. Branching Degree
2.4. Monosaccharide Composition
3. Functionalities of β-Glucan
3.1. Physicochemical Properties of β-Glucan
3.1.1. Bile Acid-Binding Capacity
3.1.2. Solubility of β-Glucan
3.2. Rheological Properties of β-Glucan
3.2.1. Gel Property
3.2.2. Viscosity
3.2.3. Textural Properties
4. Industrial Application of β-Glucan
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AsFIFFF | Asymmetrical flow field-flow fractionation |
| DLS | Dynamic light scattering |
| DS | Degree of substitution |
| HPGPC | High-performance gel permeation chromatography |
| LS | Light scattering |
| MALS | Multi-angle light scattering |
| Mw | Molecular weight |
| NMR | Nuclear magnetic resonance |
| PBS | Phosphate buffer saline |
| RI | Refractive index |
| SEC | Size-exclusion chromatography |
| SLS | Static light scattering |
| HPLC | High performance liquid chromatography |
| MALLS | Multiangle laser light scattering method |
| DRI | Differential refractive index |
| HPSEC | High-performance size exclusion chromatography |
| SE-HPLC | Size-exclusion high-performance liquid chromatography |
| LLS | Laser light scattering |
| Pd | Polydispersity index |
| VS | Viscosity detector |
| RALLS | Right angle laser light scattering detector |
| DV | Differential viscometer |
| DP | Differential pressure |
References
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. β-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Du, B.; Bian, Z.; Xu, B. Skin health promotion effects of natural β-glucan derived from cereals and microorganisms: A review. Phyther. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.; Izydorczyk, M.S. Cereal β-glucans: Structure, physical properties and physiological functions. In Functional Food Carbohydrates, 1st ed.; Biliaderis, C.G., Izydorczyk, M.S., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Naumann, E.; van Rees, A.B.; Önning, G.; Öste, R.; Wydra, M.; Mensink, R.P. β-Glucan incorporated into a fruit drink effectively lowers serum LDL-cholesterol concentrations. Am. J. Clin. Nutr. 2006, 83, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.R.; Fincher, G.B.; Stone, B.A. Water soluble (1→3) (1→4)-β-d-glucans from barley (Hordeum vulgare) endosperm. II. Fine structure. Carbohydr Polym. 1983, 3, 207–225. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, X.; Cai, F.; Zhang, L. Renaturation of triple helical polysaccharide lentinan in water-diluted dimethylsulfoxide solution. Carbohydr. Res. 2010, 345, 419–424. [Google Scholar] [CrossRef]
- Ohno, N.; Miura, N.N.; Chiba, N.; Adachi, Y.; Yadomae, T. Comparison of the immunopharmacological activities of triple and single-helical Schizophyllan in mice. Biol. Pharm. Bull. 1995, 18, 1242–1247. [Google Scholar] [CrossRef]
- Banchathanakij, R.; Suphantharika, M. Effect of different β-glucans on the gelatinisation and retrogradation of rice starch. Food Chem. 2009, 114, 5–14. [Google Scholar] [CrossRef]
- Ulmius, M.; Önning, G.; Nilsson, L. Solution behavior of barley β-glucan as studied with asymmetrical flow field-flow fractionation. Food Hydrocoll. 2012, 26, 175–180. [Google Scholar] [CrossRef]
- Surenjav, U.; Zhang, L.; Xu, X.; Zhang, X.; Zeng, F. Effects of molecular structure on antitumor activities of (1→3)-β-d-glucans from different Lentinus edodes. Carbohydr. Polym. 2006, 63, 97–104. [Google Scholar] [CrossRef]
- Lei, N.; Wang, M.; Zhang, L.; Xiao, S.; Fei, C.; Wang, X.; Zhang, K.; Zheng, W.; Wang, C.; Yang, R.; et al. Effects of low molecular weight yeast β-glucan on antioxidant and immunological activities in mice. Int. J. Mol. Sci. 2015, 16, 21575–21590. [Google Scholar] [CrossRef]
- Suárez, E.R.; Syvitski, R.; Kralovec, J.A.; Noseda, M.D.; Barrow, C.J.; Ewart, H.S.; Lumsden, M.D.; Grindley, T.B. Immunostimulatory polysaccharides from Chlorella p yrenoidosa. A new galactofuranan. Measurement of molecular weight and molecular weight dispersion by DOSY NMR. Biomacromolecules 2006, 7, 2368–2376. [Google Scholar] [CrossRef]
- Volikakis, P.; Biliaderis, C.G.; Vamvakas, C.; Zerfiridis, G.K. Effects of a commercial oat-β-glucan concentrate on the chemical, physico-chemical and sensory attributes of a low-fat white-brined cheese product. Food Res. Int. 2004, 37, 83–94. [Google Scholar] [CrossRef]
- Bamforth, C.W. Barley β-glucans: Their role in malting and brewing. Brewers Digest. 1982, 35, 22–27. [Google Scholar]
- Zhong, K.; Zhang, Q.; Tong, L.; Liu, L.; Zhou, X.; Zhou, S. Molecular weight degradation and rheological properties of schizophyllan under ultrasonic treatment. Ultrason. Sonochem. 2015, 23, 75–80. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Physicochemical properties and antitumor activities for sulfated derivatives of lentinan. Carbohydr. Res. 2009, 344, 2209–2216. [Google Scholar] [CrossRef]
- Byun, E.-H.; Kim, J.-H.; Sung, N.-Y.; Choi, J.; Lim, S.-T.; Kim, K.-H.; Yook, H.-S.; Byun, M.-W.; Lee, J.-W. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat. Phys. Chem. 2008, 77, 781–786. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Masoodi, F.A.; Amin, F.; Wani, I.A.; Khanday, F.A.; Gani, A. Structural, thermal, functional, antioxidant & antimicrobial properties of β-d-glucan extracted from baker’s yeast (Saccharomyces cereviseae)—Effect of γ-irradiation. Carbohydr. Polym. 2016, 140, 442–450. [Google Scholar]
- Chang, Y.J.; Lee, S.; Yoo, M.A.; Lee, H.G. Structural and biological characterization of sulfated-derivatized oat β-glucan. J. Agric. Food Chem. 2006, 54, 3815–3818. [Google Scholar] [CrossRef]
- de Souza, N.L.; Bartz, J.; Eda R, Z.; de Oliveira, P.D.; da Silva, W.S.V.; Alves, G.H.; Dias, A.R.G. Functional, thermal and rheological properties of oat β-glucan modified by acetylation. Food Chem. 2015, 178, 243–250. [Google Scholar] [CrossRef]
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An insight into anti-inflammatory effects of fungal β-glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Du, B.; Zeng, H.; Yang, Y.; Bian, Z.; Xu, B. Anti-inflammatory activity of polysaccharide from Schizophyllum commune as affected by ultrasonication. Int. J. Biol. Macromol. 2016, 91, 100–105. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Sun, L.; Chu, J.; Sun, Z.; Chen, L. Physicochemical properties, immunomodulation and antitumor activities of polysaccharide from Pavlova viridis. Life Sci. 2016, 144, 156–161. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C. Cryogelation of cereal β-glucans: Structure and molecular size effects. Food Hydrocoll. 2004, 18, 933–947. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G.; Izydorczyk, M.S. Molecular size effects on rheological properties of oat β-glucans in solution and gels. Food Hydrocoll. 2003, 17, 693–712. [Google Scholar] [CrossRef]
- Vaikousi, H.; Biliaderis, C.G.; Izydorczyk, M.S. Solution flow behavior and gelling properties of water-soluble barley (1→3,1→4)-β-glucans varying in molecular size. J. Cereal Sci. 2004, 39, 119–137. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. In vitro bile-acid binding and fermentation of high, medium, and low molecular weight β-glucan. J. Agric. Food Chem. 2010, 58, 628–634. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. Optimizing the molecular weight of oat β-glucan for in vitro bile acid binding and fermentation. J. Agric. Food Chem. 2011, 59, 10322–10328. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. Molecular weight of β-glucan affects physical characteristics, in vitro bile acid binding, and fermentation of muffins. Cereal Chem. J. 2011, 88, 64–71. [Google Scholar] [CrossRef]
- Bae, I.Y.; Lee, S.; Kim, S.M.; Lee, H.G. Effect of partially hydrolyzed oat β-glucan on the weight gain and lipid profile of mice. Food Hydrocoll. 2009, 23, 2016–2021. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Zhang, L. Structure and chain conformation of β-glucan isolated from Auricularia auricula-judae. Biopolymers 2008, 89, 614–622. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, F.; Xu, X.; Zhang, L. Extended chain conformation of β-glucan and its effect on antitumor activity. J. Mater. Chem. B 2017, 5, 5623–5631. [Google Scholar] [CrossRef]
- Burkus, Z.; Temelli, F. Determination of the molecular weight of barley β-glucan using intrinsic viscosity measurements. Carbohydr. Polym. 2003, 54, 51–57. [Google Scholar] [CrossRef]
- Cui, W.; Wood, P.J. Relationships between structural features, molecular weight and rheological properties of cereal β-d-glucans. Hydrocolloids 2000, 159–168. [Google Scholar]
- Zhang, M.; Zhang, L.; Wang, Y.; Cheung, P.C.K. Chain conformation of sulfated derivatives of β-glucan from sclerotia of Pleurotus tuber-regium. Carbohydr. Res. 2003, 338, 2863–2870. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L. Chain conformation of carboxymethylated derivatives of (1 → 3)-β-d-glucan from Poria cocos sclerotium. Carbohydr. Polym. 2006, 65, 504–509. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Q.; Zhang, J.; Xia, Y.; Yang, Y.; Wu, D.; Fan, H.; Cui, S.W. Triple helix conformation of β-d-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int. J. Biol. Macromol. 2018, 114, 1064–1070. [Google Scholar] [CrossRef]
- Du, B.; Yang, Y.; Bian, Z.; Xu, B. Characterization and anti-inflammatory potential of an exopolysaccharide from submerged mycelial culture of Schizophyllum commune. Front. Pharmacol. 2017, 8, 252. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Q.; Chen, J.; Zeng, F. Solution properties of antitumor sulfated derivative of α-(1→3)-d-glucan from Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2000, 64, 2172–2178. [Google Scholar] [CrossRef]
- Mikkelsen, M.S.; Jespersen, B.M.; Larsen, F.H.; Blennow, A.; Engelsen, S.B. Molecular structure of large-scale extracted β-glucan from barley and oat: Identification of a significantly changed block structure in a high β-glucan barley mutant. Food Chem. 2013, 136, 130–138. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.; Micha-Screttas, M.; Steele, B. A comparative study on structure–function relations of mixed-linkage (1→3), (1→4) linear β-d-glucans. Food Hydrocoll. 2004, 18, 837–855. [Google Scholar] [CrossRef]
- Storsley, J.M.; Izydorczyk, M.S.; You, S.; Biliaderis, C.G.; Rossnagel, B. Structure and physicochemical properties of β-glucans and arabinoxylans isolated from hull-less barley. Food Hydrocoll. 2003, 17, 831–844. [Google Scholar] [CrossRef]
- Skendi, A.; Biliaderis, C.G.; Lazaridou, A.; Izydorczyk, M.S. Structure and rheological properties of water soluble β-glucans from oat cultivars of Avena sativa and Avena bysantina. J. Cereal Sci. 2003, 38, 15–31. [Google Scholar] [CrossRef]
- Brummer, Y.; Defelice, C.; Wu, Y.; Kwong, M.; Wood, P.J.; Tosh, S.M. Textural and rheological properties of oat β-glucan gels with varying molecular weight composition. J. Agric. Food Chem. 2014, 62, 3160–3167. [Google Scholar] [CrossRef]
- Tosh, S.M.; Wood, P.J.; Wang, Q.; Weisz, J. Structural characteristics and rheological properties of partially hydrolyzed oat β-glucan: The effects of molecular weight and hydrolysis method. Carbohydr. Polym. 2004, 55, 425–436. [Google Scholar] [CrossRef]
- Liu, R.; Wang, N.; Li, Q.; Zhang, M. Comparative studies on physicochemical properties of raw and hydrolyzed oat β-glucan and their application in low-fat meatballs. Food Hydrocoll. 2015, 51, 424–431. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between modification, conformation and functional activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef]
- Goodwin, D.J.; Picout, D.R.; Ross-Murphy, S.B.; Holland, S.J.; Martini, L.G.; Lawrence, M.J. Ultrasonic degradation for molecular weight reduction of pharmaceutical cellulose ethers. Carbohydr. Polym. 2011, 83, 843–851. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. Interactional effects of β-glucan, starch, and protein in heated oat slurries on viscosity and in vitro bile acid binding. J. Agric. Food Chem. 2012, 60, 6217–6222. [Google Scholar] [CrossRef]
- Doublier, J.L.; Wood, P.J. Rheological properties of aqueous solutions of (l →3), (1 →4) β-d-glucan from oats (Avena sativa L.). Cereal Chem. 1995, 72, 335–340. [Google Scholar]
- Tiwari, U.; Cummins, E. Factors influencing β-glucan levels and molecular weight in cereal-based products. Cereal Chem. J. 2009, 86, 290–301. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. Impact of the molecular weight, viscosity, and solubility of β-glucan on in vitro oat starch digestibility. J. Agric. Food Chem. 2013, 61, 3270–3277. [Google Scholar] [CrossRef]
- Li, W.; Cui, S.W.; Kakuda, Y. Extraction, fractionation, structural and physical characterization of wheat β-d-glucans. Carbohydr. Polym. 2006, 63, 408–416. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Yada, R.Y. Studies of aggregation behaviours of cereal β-glucans in dilute aqueous solutions by light scattering: Part I. Structure effects. Food Hydrocoll. 2011, 25, 189–195. [Google Scholar] [CrossRef]
- Baxter, S.; Zivanovic, S.; Weiss, J. Molecular weight and degree of acetylation of high-intensity ultrasonicated chitosan. Food Hydrocoll. 2005, 19, 821–830. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Smith, G.E.; Shao, Q. In vitro binding of bile acids by kidney bean (Phaseolus vulgaris), black gram (Vigna mungo), bengal gram (Cicer arietinum) and moth bean (Phaseolus aconitifolins). Food Chem. 2005, 90, 241–246. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and solubility. In Solubility of polysaccharides; Xu, Z., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.; Liu, D.; Ye, X.; Ke, F. Impact of ultrasonic treatment on properties of starch film-forming dispersion and the resulting films. Carbohydr. Polym. 2010, 81, 707–711. [Google Scholar] [CrossRef]
- Pérez-Quirce, S.; Lazaridou, A.; Biliaderis, C.G.; Ronda, F. Effect of β-glucan molecular weight on rice flour dough rheology, quality parameters of breads and in vitro starch digestibility. LWT 2017, 82, 446–453. [Google Scholar] [CrossRef]
- Gómez, C.; Navarro, A.; Manzanares, P.; Horta, A.; Carbonell, J.V. Physical and structural properties of barley (1 → 3),(1 → 4)-β-d-glucan. Part II. Viscosity, chain stiffness and macromolecular dimensions. Carbohydr. Polym. 1997, 32, 17–22. [Google Scholar] [CrossRef]
- Kontogiorgos, V.; Biliaderis, C.; Kiosseoglou, V.; Doxastakis, G. Stability and rheology of egg-yolk-stabilized concentrated emulsions containing cereal β-glucans of varying molecular size. Food Hydrocoll. 2004, 18, 987–998. [Google Scholar] [CrossRef]
- Agbenorhevi, J.K.; Kontogiorgos, V.; Kirby, A.R.; Morris, V.J.; Tosh, S.M. Rheological and microstructural investigation of oat β-glucan isolates varying in molecular weight. Int. J. Biol. Macromol. 2011, 49, 369–377. [Google Scholar] [CrossRef]
- Sayar, S.; Jannink, J.-L.; White, P.J. Textural and bile acid-binding properties of muffins impacted by oat β-glucan with different molecular weights. Cereal Chem. J. 2011, 88, 564–569. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Shah, A.; Masoodi, F.A.; Hussain, P.R.; Wani, I.A.; Khanday, F.A. Effect of γ-irradiation on structural, functional and antioxidant properties of β-glucan extracted from button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 31, 123–130. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yang, Z.; Zhu, Y.; Wu, Y.; Huang, J.; Mao, J. Oxidation of β-glucan extracted from Poria cocos and its physiological activities. Carbohydr. Polym. 2011, 85, 798–802. [Google Scholar] [CrossRef]
- de Moura, F.A.; Pereira, J.M.; da Silva, D.O.; Eda R, Z.; da Silveira Moreira, A.; Helbig, E.; Dias, A.R.G. Effects of oxidative treatment on the physicochemical, rheological and functional properties of oat β-glucan. Food Chem. 2011, 128, 982–987. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Mao, J. Carboxymethylated β-glucan derived from poria cocos with biological activities. J. Agric. Food Chem. 2009, 57, 10913–10915. [Google Scholar] [CrossRef]
- Shin, M.S.; Lee, S.; Lee, K.Y.; Lee, H.G. Structural and biological characterization of aminated-derivatized oat β-glucan. J. Agric. Food Chem. 2005, 53, 5554–5558. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, I.Y.; Lee, S.; Lee, H.G. Physicochemical and hypocholesterolemic characterization of oxidized oat β-glucan. J. Agric. Food Chem. 2009, 57, 439–443. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, W. Microwave-assisted solubilization and solution properties of hyperbranched polysaccharide. Carbohydr. Res. 2008, 343, 3071–3078. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Zhang, L.; Zeng, F. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos. Carbohydr. Polym. 2009, 78, 581–587. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L. Preparation, chain conformation and anti-tumor activities of water-soluble phosphated (1→3)-α-d-glucan from Poria cocos mycelia. Carbohydr. Polym. 2011, 83, 1363–1369. [Google Scholar] [CrossRef]
- Roubroeks, J.P.; Andersson, R.; Mastromauro, D.I.; Christensen, B.E.; Åman, P. Molecular weight, structure and shape of oat (1→3),(1→4)-β-d-glucan fractions obtained by enzymatic degradation with (1→4)-β-d-glucan 4-glucanohydrolase from Trichoderma reesei. Carbohydr. Polym. 2001, 46, 275–285. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of β-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Sharafbafi, N.; Tosh, S.M.; Alexander, M.; Corredig, M. Phase behaviour, rheological properties, and microstructure of oat β-glucan-milk mixtures. Food Hydrocoll. 2014, 41, 274–280. [Google Scholar] [CrossRef]
- Rinaldi, L.; Rioux, L.-E.; Britten, M.; Turgeon, S.L. In vitro bioaccessibility of peptides and amino acids from yogurt made with starch, pectin, or β-glucan. Int. Dairy J. 2015, 46, 39–45. [Google Scholar] [CrossRef]
- Amini Sarteshnizi, R.; Hosseini, H.; Bondarianzadeh, D.; Colmenero, F.J.; khaksar, R. Optimization of prebiotic sausage formulation: Effect of using β-glucan and resistant starch by D-optimal mixture design approach. LWT 2015, 62, 704–710. [Google Scholar] [CrossRef]
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Integration of β-glucan fibre rich fractions from barley and mushrooms to form healthy extruded snacks. Plant Foods Hum. Nutr. 2013, 68, 78–82. [Google Scholar] [CrossRef]
- Lumaga, R.B.; Azzali, D.; Fogliano, V.; Scalfi, L.; Vitaglione, P. Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a β-glucan-enriched beverage. Food Funct. 2012, 3, 67–75. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.M.; Bae, I.Y.; Park, H.-G.; Gyu Lee, H.; Lee, S. (1-3)(1-6)-β-Glucan-enriched materials from Lentinus edodes mushroom as a high-fibre and low-calorie flour substitute for baked foods. J. Sci. Food Agric. 2011, 91, 1915–1919. [Google Scholar] [CrossRef]
- Kittisuban, P.; Ritthiruangdej, P.; Suphantharika, M. Optimization of hydroxypropylmethylcellulose, yeast β-glucan, and whey protein levels based on physical properties of gluten-free rice bread using response surface methodology. LWT - Food Sci. Technol. 2014, 57, 738–748. [Google Scholar] [CrossRef]
- Lazaridou, A.; Marinopoulou, A.; Matsoukas, N.P.; Biliaderis, C.G. Impact of flour particle size and autoclaving on β-glucan physicochemical properties and starch digestibility of barley rusks as assessed by in vitro assays. Bioact. Carbohydrates Diet. Fibre 2014, 4, 58–73. [Google Scholar] [CrossRef]
- Kofuji, K.; Huang, Y.; Tsubaki, K.; Kokido, F.; Nishikawa, K.; Isobe, T.; Murata, Y. Preparation and evaluation of a novel wound dressing sheet comprised of β-glucan–chitosan complex. React. Funct. Polym. 2010, 70, 784–789. [Google Scholar] [CrossRef]
- Berdal, M.; Appelbom, H.I.; Eikrem, J.H.; Lund, Å.; Zykova, S.; Busund, L.-T.; Seljelid, R.; Jenssen, T. Aminated β-1,3-d-glucan improves wound healing in diabetic db/db mice. Wound Repair Regen. 2007, 15, 825–832. [Google Scholar] [CrossRef]
- Delatte, S.J.; Evans, J.; Hebra, A.; Adamson, W.; Othersen, H.B.; Tagge, E.P. Effectiveness of β-glucan collagen for treatment of partial-thickness burns in children. J. Pediatr. Surg. 2001, 36, 113–118. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Şener, G.; Jahovic, N.; Uslu, B.; Arbak, S.; Yeğen, B.Ç. β-glucan protects against burn-induced oxidative organ damage in rats. Int. Immunopharmacol. 2006, 6, 156–169. [Google Scholar] [CrossRef]
- Kim, H.-L.; Lee, J.-H.; Lee, M.H.; Kwon, B.J.; Park, J.-C. Evaluation of electrospun (1,3)-(1,6)-β-d-glucans/biodegradable polymer as artificial skin for full-thickness wound healing. Tissue Eng. Part A 2012, 18, 2315–2322. [Google Scholar] [CrossRef]
- Belcarz, A.; Ginalska, G.; Pycka, T.; Zima, A.; Ślósarczyk, A.; Polkowska, I.; Paszkiewicz, Z.; Piekarczyk, W. Application of β-1,3-glucan in production of ceramics-based elastic composite for bone repair. Open Life Sci. 2013, 8, 534–548. [Google Scholar] [CrossRef]
- Anusuya, S.; Sathiyabama, M. Preparation of β-d-glucan nanoparticles and its antifungal activity. Int. J. Biol. Macromol. 2014, 70, 440–443. [Google Scholar] [CrossRef]
- Kogan, G.; Staško, A.; Bauerová, K.; Polovka, M.; Šoltés, L.; Brezová, V.; Navarová, J.; Mihalová, D. Antioxidant properties of yeast (1→3)-β-d-glucan studied by electron paramagnetic resonance spectroscopy and its activity in the adjuvant arthritis. Carbohydr. Polym. 2005, 61, 18–28. [Google Scholar] [CrossRef]
- Camelini, C.M.; Maraschin, M.; de Mendonça, M.M.; Zucco, C.; Ferreira, A.G.; Tavares, L.A. Structural characterization of β-glucans of Agaricus brasiliensis in different stages of fruiting body maturity and their use in nutraceutical products. Biotechnol. Lett. 2005, 27, 1295–1299. [Google Scholar] [CrossRef]
- Rieder, A.; Samuelsen, A.B. Do cereal mixed-linked β-glucans possess immune-modulating activities? Mol. Nutr. Food Res. 2012, 56, 536–547. [Google Scholar] [CrossRef]
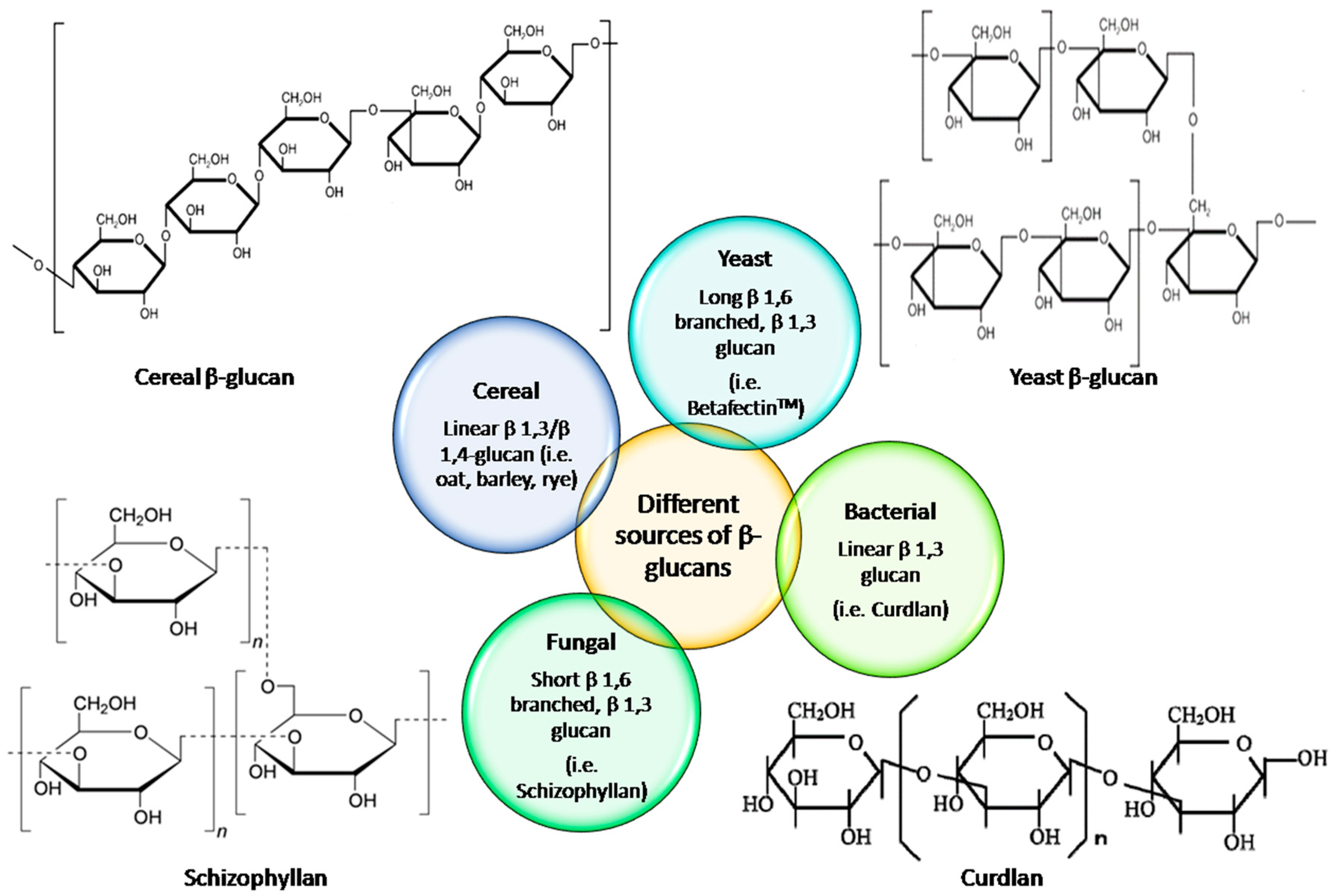
| Source of β-Glucan | Determination Methods | Chromatographic Conditions | Standard Used | Molecular Weight (g/mol) | References | |
|---|---|---|---|---|---|---|
| Mobile Phase | Column | |||||
| Schizophyllum commune Fr. ACCC51174 | HPLC-MALLS-RI | 0.1 M NaNO3 at 0.5 mL/min | OCpak SD-822 M ZQ | -- | 808,000–240,4000 Pd: 1.18–1.86 | [16] |
| Lentinan | SEC–MALLS-DRI | 0.15 M aq NaCl at 0.50 mL/min | TSK-GEL G4000 PWXL and G6000 PWXL at 25 °C | -- | 14.6 × 10−4–163.5 × 10−4 | [17] |
| Saccharomyces cereviseae | SEC-RI | -- | -- | Dextrans | 279,00–175,000 | [19] |
| Oat | SEC-RI | Distilled water at 3.5 mL/min | JAIGEL-W254, JAIGEL-W-253, JAIGELW252 | -- | 68,000–130,000 | [20] |
| Schizophyllum commune | HPLC-RI | 0.1 M NaNO3 at 0.8 mL/min | PL aquageloh MIXED-H | -- | 197,000–290,0000 | [23] |
| Oat, wheat, barley | HPSEC-RI | 0.15 M NaNO3 containing 0.02% NaN3 at 0.5 mL/min | TSK G5000 PW-SEC at 25 °C | β-glucan standards from Megazyme | 65 × 103–200 × 103 | [26] |
| Oat | HPSEC-RI | 0.15 M NaNO3 containing 0.02% NaN3 at 0.5 mL/min | TSK G5000 PW-SEC at 25 °C | β-glucan from Megazyme | 35 × 10−3–250 × 10−3 | [27] |
| Barley | HPSEC-RI | 0.15 M NaNO3, containing 0.02% NaN3 at 0.5 mL/min | TSK G5000 PW-SEC at 25 °C | (1 → 3, 1 → 4)-β-glucan from Megazyme | 40 × 103–250 × 103 | [28] |
| Oat | SE-HPLC-RI | Milli-Q water with 0.02% sodium azide at 0.5 mL/min | Ohpak SB-806 HQ, Ohpak SB-805 HQ and Ohpak SB-804 HQ at 40 °C | β-glucan from Megazyme | 1.56 × 105–6.87 × 105 | [29,30] |
| Oat | SEC | Deionized water at 3.5 mL/min | JAIGEL-W254–255 at 25 °C | Dextran | 370 × 103-1450 × 103 | [32] |
| Lentinus edodes | SEC-MALLS-DRI | 0.9% aqueous NaCl and Me2SO at 1.00 mL/min | TSK-GEL G6000 PWXL, G4000 PWXL, G4000-H8, G3000H8 at 25 °C | No standard sample was employed | 1.87 × 10−5–28.3 × 10−5 | [33] |
| Pleurotus tuber-regium | SEC-LLS and interferometric refractometer | PBS at 1.0 mL/min | PSW5000 and PSW3000 at 37 °C | -- | 5.76 × 104–77.4 × 104 Pd: 1.55–1.83 | [38] |
| Poria cocos | SEC-LLS-DRI | 0.2 M NaCl at 1.0 mL/min | TSK-GEL G5000 and G3000 PWXL at 25 °C | -- | 6.1 × 10−4–45.4 × 10−4 Pd: 1.3–1.7 | [39] |
| Ganoderma lucidum | HPSEC-MALLS-RI-VS | 0.15 M NaNO3, 0.05 M NaH2PO4, and 0.02% NaN3 at 0.5 mL/min | TSK G6000 PWxl, TSK G4000 PWxl at 30 °C | -- | 24.2 × 105–2.9 × 105 Pd: 1.22–1.83 | [40] |
| Schizophyllum commune | HPLC-RI | 0.05mol/L phosphate buffer (pH 6.7) containing 0.05% NaN3 at 0.5 mL/min | TOSOHTSK-GEL G3000 SW XL at 35 °C | Dextran | 2,900,000 | [41] |
| Ganoderma lucidum | SEC-LLS-RI | 0.2 M NaCl at 1.0 mL/min | TSK-GEL G4000 PWXL at 25 °C | -- | 5.7 × 10−4–44.5 × 10−4 Pd: 1.8–2.2 | [42] |
| Barley and oat | HPSEC-RI | 0.05 M NaCl at 0.5 mL/min | Ultrahydrogel 1000 and 2000 at 60 °C | β-glucan from Megazyme | 130,000–390,000 and 190,000–410,000 | [43] |
| Oat and barley | HPSEC-RI | 0.15 M NaNO3 containing 0.02% NaN3 at 0.5 mL/min | TSK G5000 PW-SEC at 25 °C | β-glucan from Megazyme | 105 × 10−3–213 × 10−3 | [44] |
| Barley | HPSEC-MALLS-RI-UV detector | 0.15 M NaNO3 containing 0.02% NaN3 | TSK G5000 PW at 25 °C | -- | 0.22 × 10−6–2.45 × 10−6 Pd: 2.0–7.0 | [45] |
| Oat | HPSEC-MALLS-RI | 0.15 M NaNO3 containing 0.02% NaN3 at 0.4 mL/min | TSK G5000 PW at 25 °C | Pullulan | 0.18 × 10−6–0.85 × 10−6 Pd: 1.50–2.39. | [46] |
| Wheat | HPSEC-RALLS-DV-RI | 0.1 M NaNO3 containing 0.03% (w/w) NaN3 at 0.6 mL/min | Shodex Ohpak KB-806M and Ultrahydrogel linear at 40 °C | -- | 0.43 × 105–7.58 × 105 Pd: 1.03–1.26 | [37] |
| Oat | HPSEC-RI-DP-LLS | 0.1 M NaNO3 with 5 mM NaN3 at 0.6 mL/min | Ultrahydrogel linear column, and Shodex OHpak Kb-806M at 40 °C | Pullulan | 31,200–1,190,500 Pd: 1.20–1.27 | [47] |
| Oat | SEC with Viscotek triple detector | 100 mM NaNO3 containing 5 mM NaN3 at 0.6 mL/min | Shodex Ohpak Kb-806M at 40 °C | Pullulan | 30,800–1,190,500 Pd: 1.20–1.73 | [48] |
| Oat | HPSEC system with refractive index detector | Ultrapure water with 5 mM NaN3 at 0.8 mL/min | OHpak SB-804HQ at 30 °C | Dextran | 0.06 × 103–9.4 × 108 Pd: 1.1–11.4 | [49] |
| Source | Conformation | Branching Degree | References |
|---|---|---|---|
| Barley | -- | Linear chains of β-d-glucopyranosyl units linked via (1 → 3) and (1 → 4) linkages. | [10,28,43] |
| Schizophyllan from S. commune Fr. ACCC51174 | -- | Linear chain of β-d-(1 → 3)-glucopyranosyl groups and β-d-(1 → 6)-glucopyranosyl groups | [16] |
| Lentinan from Lentinus edodes | Triple helix in 0.15 M aq NaCl sulfated derivative exists as single semi-stiff chains in 0.15 M aq NaCl | β-(1 → 3)-d-glucan bearing β-(1 → 6)-d-glucopyranosyl branches | [17,33,35] |
| β-glucan from Saccharomyces cereviseae | -- | Linearly linked β-d-glucopyranosyl units with (1 → 3) and (1 → 6) linkages | [19] |
| Oat | -- | Unbranched polymers composed of (1 → 3)-and (1 → 4)-β-d-glucose units with (1 → 4) β-linkage predominating. | [20,27,29,30,43,47,53,55] |
| Oat, barley, and wheat | Rigid, rod-like conformation | Mixed-linkage linear (1 → 3), (1 → 4)-β-d-glucan | [26,44,56,57] |
| β-glucan from Auricularia auricular-judae | Semi-stiff conformation | (1 → 4)-linked d-glucopyranosyl with branching points at O-6 of (1 → 6)-linked d-glucopyranosyl residues | [34] |
| β-glucan from Pleurotus tuber-regium | Expanded flexible chain in PBS | Main chain of (1 → 3)-β-d-glucopyranosyl units with every third unit having on average a (1 → 6)-β-d-glucopyranosyl branch. | [38] |
| β-glucan from Poria cocos | Extended flexible chain in 0.2 M NaCl | (1 → 3)-β-d-glucan | [39] |
| β-glucan from Ganoderma lucidum | Triple-helical conformation with high rigidity | β-(1 → 3)-d-glucan with β-(1 → 6) branches | [40,42] |
| Chitosan | -- | (1 → 4)-2-amino-2-deoxy-β-d-glucan | [58] |
| Oat | More extended and stiffer conformation for the low-Mw β-glucans | Unbranched polymers composed of (1 → 3)-and (1 → 4)-β-d-glucose units with (1 → 4) β-linkage predominating. | [46] |
| Functional Properties | Source of β-Glucan | Inferences | References |
|---|---|---|---|
| Bile acid-binding capacity | Cereal | Low-Mw β-glucan bound more bile acid than did the high-Mw β-glucan (p < 0.05). | [29] |
| Agaricus bisporus | Enhanced bile acid-binding was observed in low-Mw β-glucan obtained after γ-irradiation | [68] | |
| Poria cocos | Improved solubility of β-glucan after oxidation led to improved bile acid-binding capacity | [69] | |
| Oat | Oxidative treatment with hydrogen peroxide enhanced the sums of carbonyl and carboxyl contents of the β-glucan and also lead to an improvement in bile acid-binding | [70] | |
| Oat | The decrease in the molecular weight of oat β-glucan exhibited higher bile acid-binding capacity | [32] | |
| Oat | Acetylation of β-glucan enhanced the bile acid-binding ability | [21] | |
| Poria cocos | Carboxymethylation of β-glucan enhanced the in vitro bile acid-binding capacity | [71] | |
| Oat | Sulfation of β-glucan reduced the in vitro bile acid-binding capacity due to the decrease in the molecular weight of β-glucan | [20] | |
| Oat | Aminated-derivatized β-glucan exhibited enhanced bile acid-binding activity | [72] | |
| Oat | Enhanced bile acid-binding capacity was observed in oxidized β-glucan | [73] | |
| Oat | β-Glucan fractions with Mw 2.42 × 105 and 1.61 × 105 g/mol bound the greatest amounts of bile acid | [30] | |
| Oat | Oat slurries treated with proteinase or proteinase and α-amylase exhibit improved bile acid binding | [52] | |
| Oat | Bile acid binding capacities of low-Mw (157,000) and medium-Mw β-glucan fractions (277,000) tended to be greater than that of the high-Mw fraction (560,000). | [67] | |
| Solubility | Pleurotus tuber-regium | Globular molecular structure of β-glucan in 0.02% NaN3 after microwave heating exhibit high solubility | [74] |
| Poria cocos | Extended flexible chains of phosphorylated β-glucan exhibit enhanced solubility in 0.15 M NaCl | [75,76] | |
| Oat | β-Glucanase treated β-glucan exhibit semi-flexible chain to an extended random coil conformation and enhanced water solubility | [77] | |
| Trichoderma strain LE02 | β-Glucanase treatment of β-glucan lead to reduced Mw and improved solubility | [50] | |
| Poria cocos | Introduction of carboxyl groups due to the oxidation of β-glucan improved its water solubility | [69] | |
| Poria cocos | Introduction of carboxymethyl groups in β-glucan improved its water solubility | [71] | |
| Oat | Sulfation increased numbers of small fragments of β-glucan that lead to an improvement in solubility | [20] | |
| Oat | Increase in the Mw of β-glucan led to a decrease in the solubility | [55] | |
| Oat | Oxidized β-glucan exhibit enhanced water solubility | [73] | |
| Viscosity | Oat | Increase in the Mw of β-glucan lead to enhanced viscosity | [55] |
| Agaricus bisporus | The decrease in the degree of polymerization of β-glucan due to γ-irradiation decrease in the viscosity | [68] | |
| Oat | Final viscosity of the β-glucan gel decreased with intense oxidation treatment | [70] | |
| Oat | The decrease in the molecular weight of β-glucan followed by enzymatic hydrolysis lead to reduced viscosity | [32] | |
| Oat | Acetylated β-glucan was less viscous due to lower swelling power | [21] | |
| Oat | The decrease in the molecular weight of β-glucan followed by sulfation lead to a decrease in the viscosity. | [20] | |
| Oat | Enzymatic and heat treatment reduced the peak and final viscosities of oat slurries | [52] | |
| Schizophyllan | The higher viscosity of schizophyllan was observed at higher Mw | [16] | |
| Swelling power | Agaricus bisporus | The decrease in the swelling power of β-glucan with an increase in γ-irradiation dose due to structural disintegration | [68] |
| Oat | Low-intensity oxidative treatment of β-glucan enhanced its swelling power. However, the more intense treatment led to structural disintegration and reduced swelling power | [70] | |
| Oat | Acetylation of β-glucan enhanced its swelling power | [21] | |
| Fat binding capacity | Agaricus bisporus | An increase in γ-irradiation dose of β-glucan leads to enhanced fat binding capacity | [68] |
| Oat | Oxidative treatment of β-glucan did not affect its fat binding capacity | [70] | |
| Oat | The decrease in the molecular weight of β-glucan exhibit higher fat binding capacity | [32] | |
| Oat | acetylation of β-glucan resulted in a reduction of fat binding ability | [21] | |
| Textural properties | Oat | Lower Mw of β-glucan exhibited less impact on the batter firmness | [67] |
| Oat | Gels formed by oxidized β-glucan exhibit a decrease in hardness, adhesiveness, gumminess. No significant impact was observed in gel cohesiveness | [70] | |
| Oat | Acetylation of β-glucan lead to reduced hardness, increased cohesiveness, springiness, gumminess, and no adhesiveness of β-glucan gels | [21] | |
| Oat | The mix of 50% high-Mw (1,190,500) and 50% low-Mw (31,200) β-glucan produced the hardest but the most elastic, gels. | [47] | |
| Oat, barley, wheat | An increase in strength of cereal β-glucan cryogels was observed with increase in its molecular size | [26] | |
| Oat | An increase in strength and decrease in the brittleness of oat β-glucan gels was observed with increasing in its Mw | [27] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. https://doi.org/10.3390/ijms20164032
Du B, Meenu M, Liu H, Xu B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. International Journal of Molecular Sciences. 2019; 20(16):4032. https://doi.org/10.3390/ijms20164032
Chicago/Turabian StyleDu, Bin, Maninder Meenu, Hongzhi Liu, and Baojun Xu. 2019. "A Concise Review on the Molecular Structure and Function Relationship of β-Glucan" International Journal of Molecular Sciences 20, no. 16: 4032. https://doi.org/10.3390/ijms20164032
APA StyleDu, B., Meenu, M., Liu, H., & Xu, B. (2019). A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. International Journal of Molecular Sciences, 20(16), 4032. https://doi.org/10.3390/ijms20164032






