Roles of Direct Photoreception and the Internal Circadian Oscillator in the Regulation of Melatonin Secretion in the Pineal Organ of the Domestic Turkey: A Novel In Vitro Clock and Calendar Model
Abstract
1. Introduction
2. Results
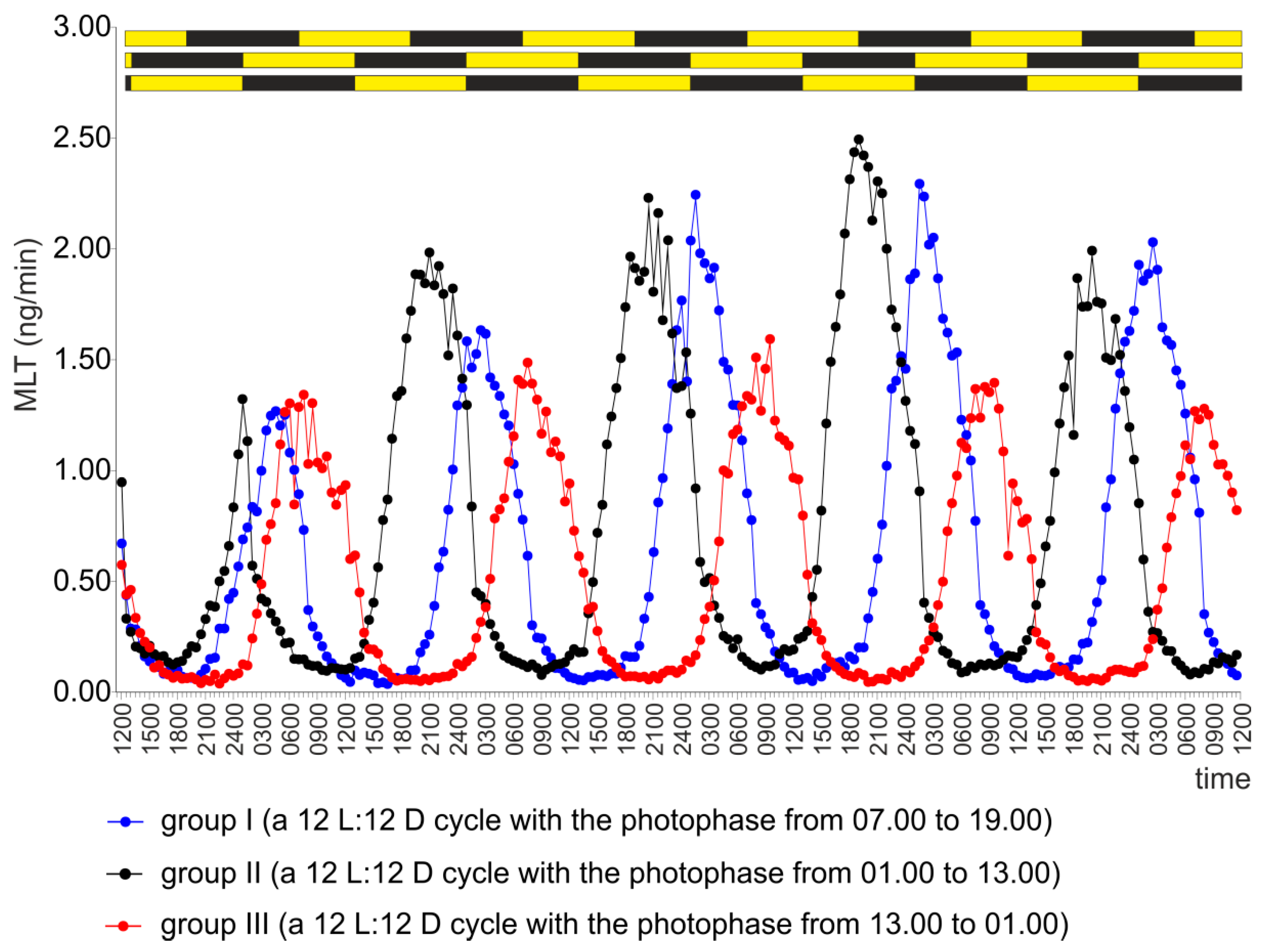
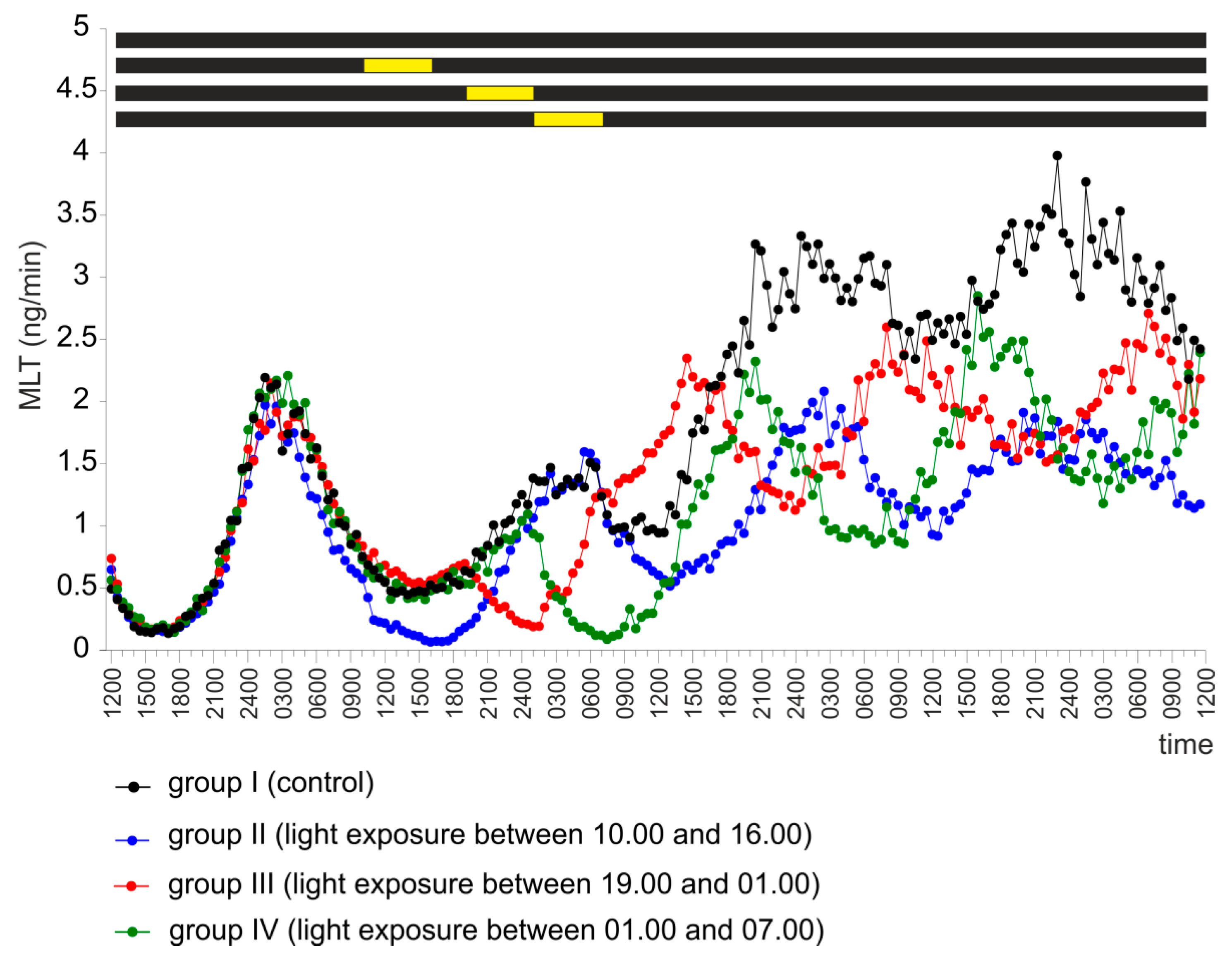
2.1. Experiment I
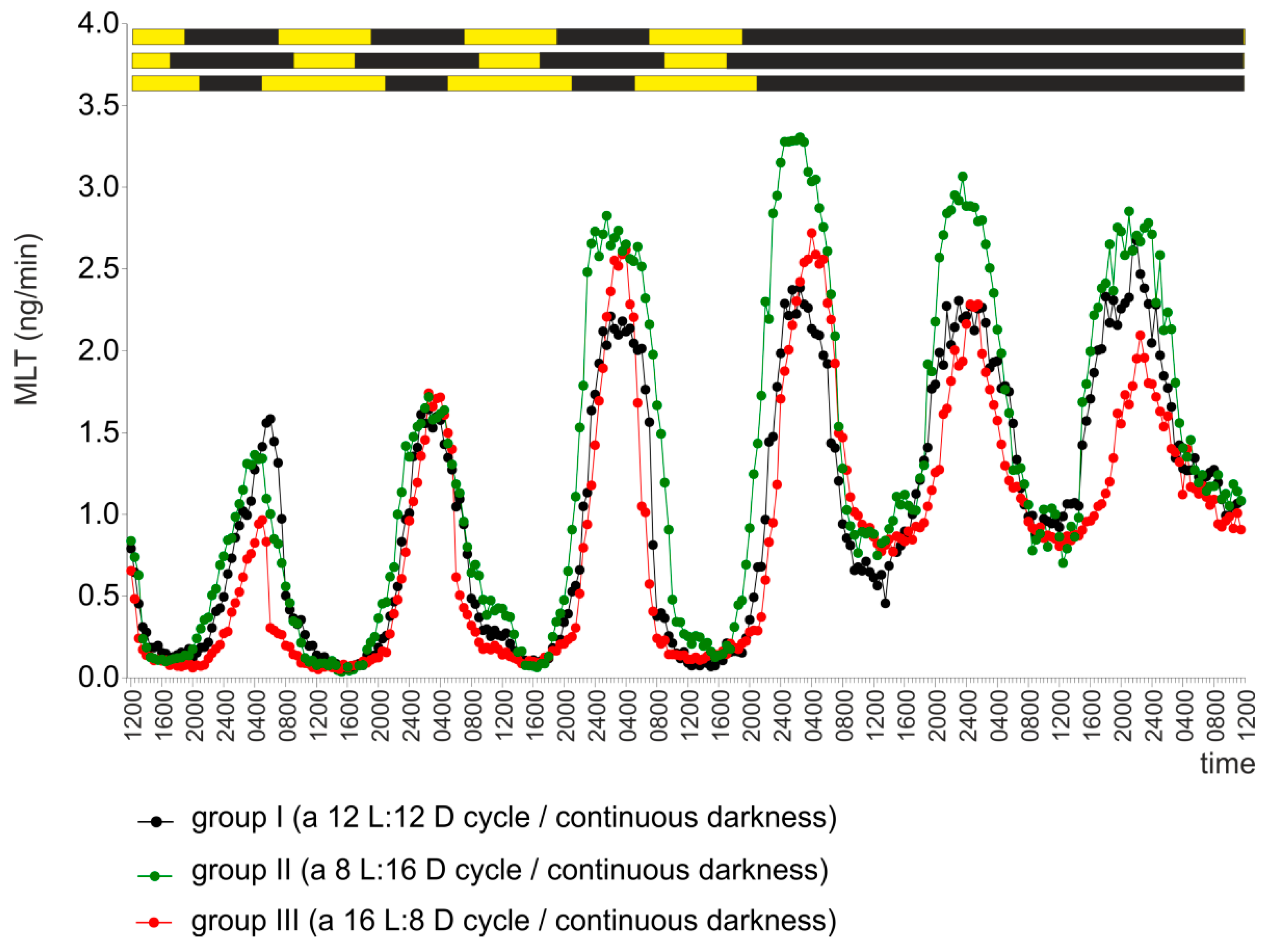
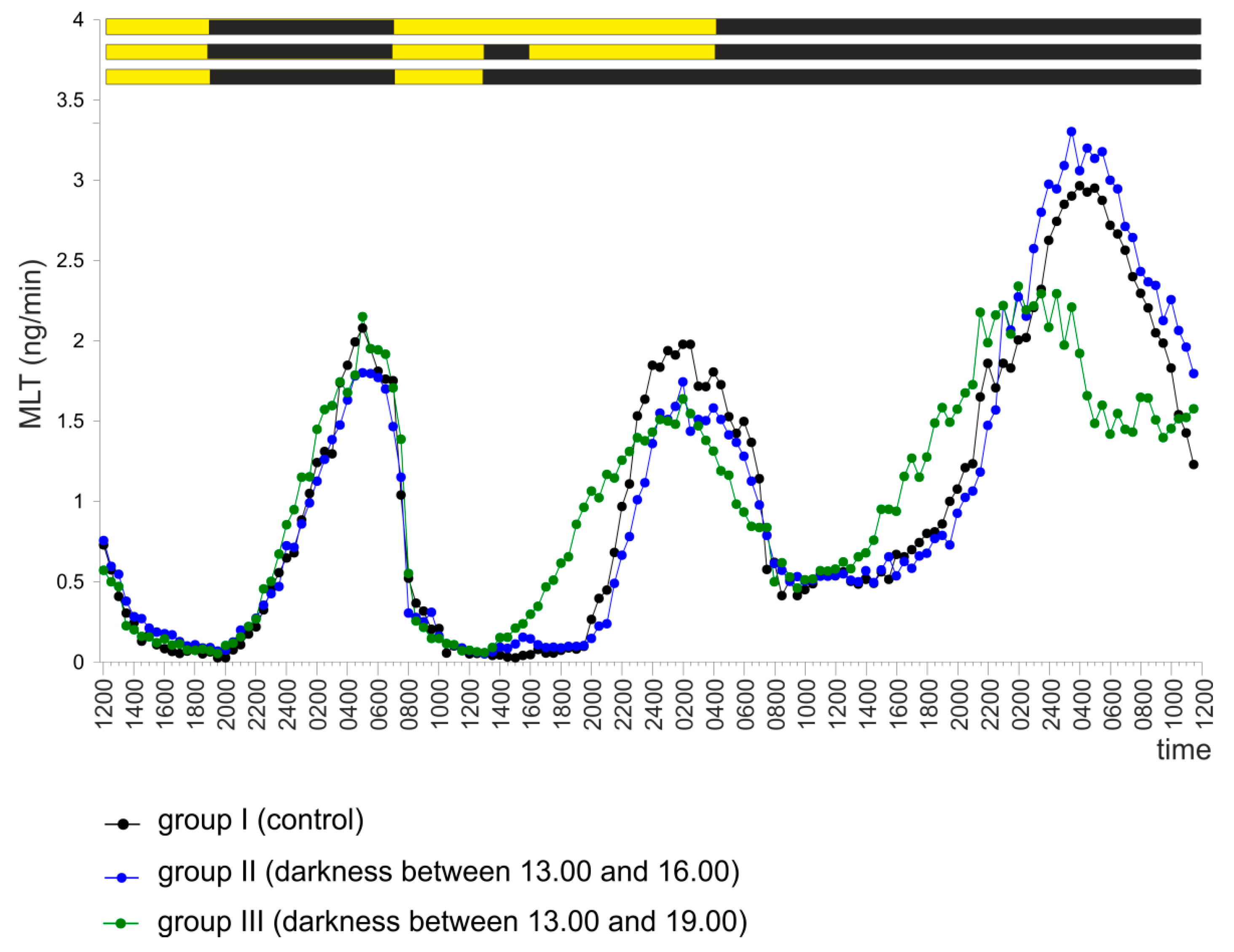
2.2. Experiment II
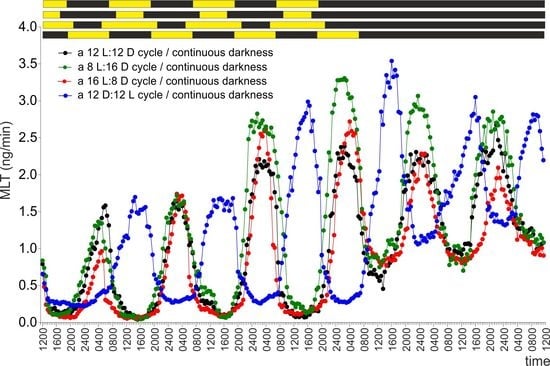
2.3. Experiment III
2.4. Experiment IV
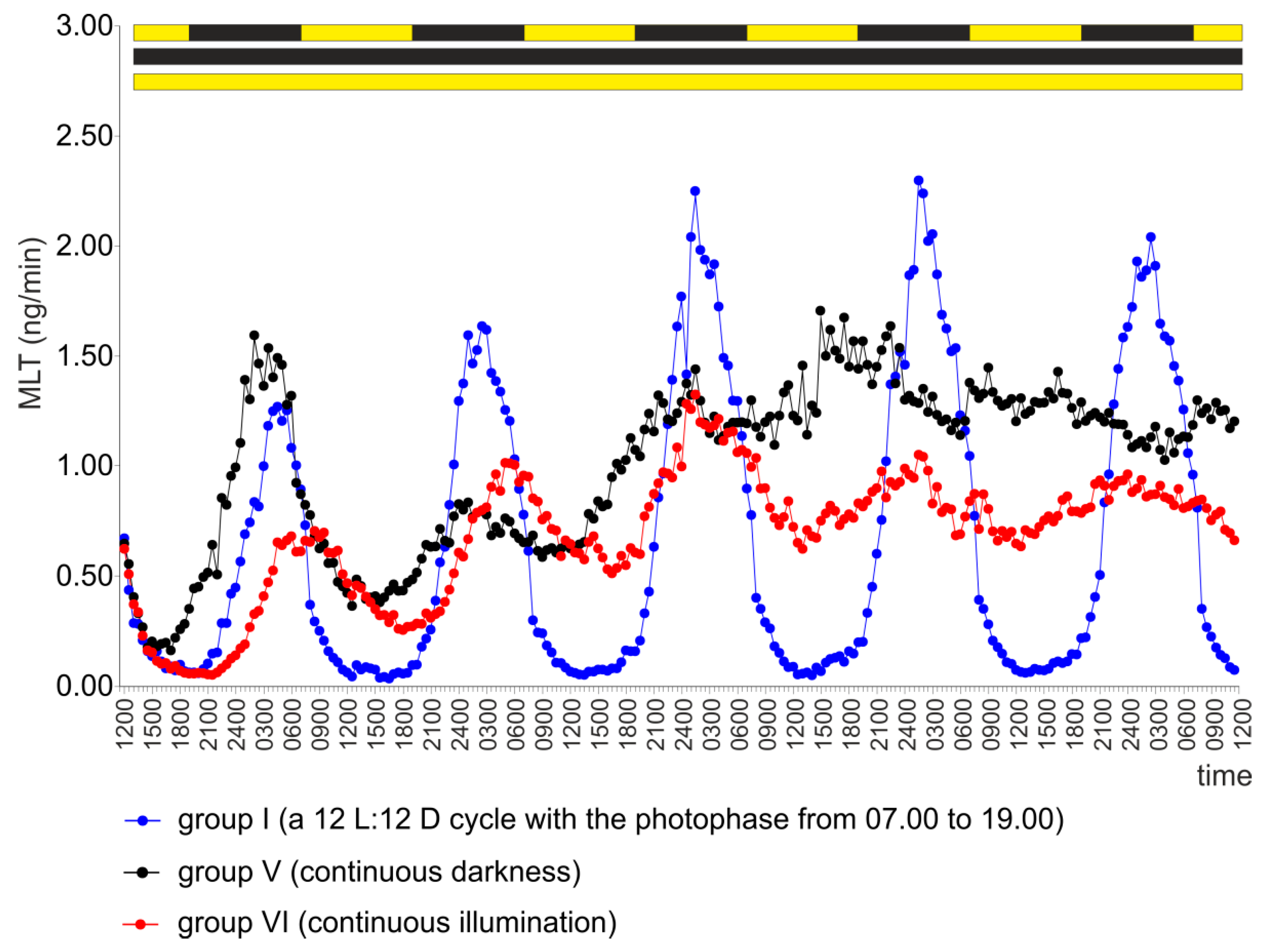
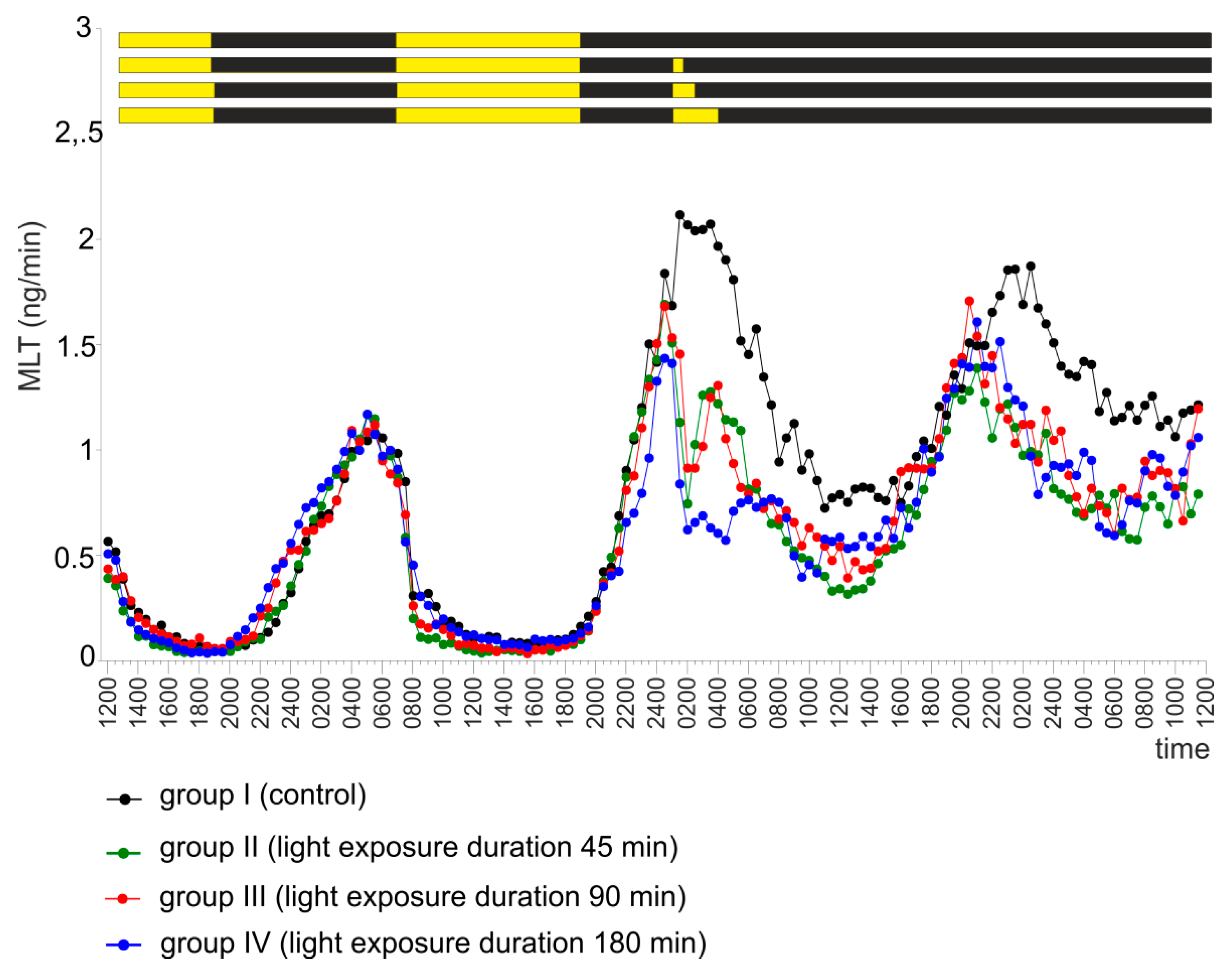
2.5. Experiment V
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Pineal Organs
4.3. Superfusion Culture
4.4. Experimental Procedures
4.4.1. Experiment I
4.4.2. Experiment II
4.4.3. Experiment III
4.4.4. Experiment IV
4.4.5. Experiment V
4.5. Melatonin Radioimmunoassay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pévet, P. Melatonin and biological rhythms. Biol. Signals Recept. 2000, 9, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Brandstätter, R. Encoding time of day and time of year by the avian circadian system. J. Neuroendocr. 2003, 15, 398–404. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front Endocrinol (Lausanne) 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Lorenc, A.; Berezińska, M.; Vivien-Roels, B.; Pévet, P.; Skene, D.J. Diurnal and circadian rhythms in melatonin synthesis in the turkey pineal gland and retina. Gen. Comp. Endocrinol. 2006, 145, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Lorenc, A.; Berezińska, M.; Vivien-Roels, B.; Pévet, P.; Skene, D.J. Photoperiod-dependent changes in melatonin synthesis in the turkey pineal gland and retina. Poult. Sci. 2007, 86, 1397–1405. [Google Scholar] [CrossRef]
- Kumar, V.; Wingfield, J.C.; Dawson, A.; Ramenofsky, M.; Rani, S.; Bartell, P. Biological clocks and regulation of seasonal reproduction and migration in birds. Physiol. Biochem. Zool. 2010, 83, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N. Studies on melatonin biosynthesis and mechanisms of its regulation in the pineal organ of the domestic goose. Ph.D. Thesis, University of Warmia and Mazury, Olsztyn, Poland, 27 November 2015. [Google Scholar]
- Brandstätter, R.; Kumar, V.; Van’t Hof, T.J.; Gwinner, E. Seasonal variations of in vivo and in vitro melatonin production in a passeriform bird, the house sparrow (Passer domesticus). J. Pineal Res. 2001, 31, 120–126. [Google Scholar] [CrossRef]
- Gwinner, E.; Brandstätter, R. Complex bird clocks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1801–1810. [Google Scholar] [CrossRef]
- Piesiewicz, A.; Kędzierska, U.; Podobas, E.; Adamska, I.; Zuzewicz, K.; Majewski, P.M. Season-dependent postembryonic maturation of the diurnal rhythm of melatonin biosynthesis in the chicken pineal gland. Chronobiol. Int. 2012, 29, 1227–1238. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Mishra, I.; Kumar, V. Temporal expression of genes coding for arylalkamine-N-acetyltransferase and melatonin receptors in circadian clock tissues: Circadian rhythm dependent role of melatonin in seasonal responses. Physiol Behav. 2019, 207, 167–178. [Google Scholar] [CrossRef]
- Okano, T.; Takanaka, Y.; Nakamura, A.; Hirunagi, K.; Adachi, A.; Ebihara, S.; Fukada, Y. Immunocytochemical identification of pinopsin in pineal glands of chicken and pigeon. Brain Res. Mol. Brain Res. 1997, 50, 190–196. [Google Scholar] [CrossRef]
- Okano, T.; Fukada, Y. Phototransduction cascade and circadian oscillator in chicken pineal gland. J. Pineal Res. 1997, 22, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Holthues, H.; Engel, L.; Spessert, R.; Vollrath, L. Circadian gene expression patterns of melanopsin and pinopsin in the chick pineal gland. Biochem. Biophys. Res. Commun. 2005, 326, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocr. 2014, 35, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Avian photoreceptors and their role in the regulation of daily and seasonal physiology. Gen. Comp. Endocrinol. 2015, 220, 13–22. [Google Scholar]
- Prusik, M. Avian central clocking system [In polish]. Med. Weter. 2018, 74, 233–242. [Google Scholar]
- Cassone, V.M.; Forsyth, A.M.; Woodlee, G.L. Hypothalamic regulation of circadian noradrenergic input to the chick pineal gland. J. Comp. Physiol. A. 1990, 167, 187–192. [Google Scholar] [CrossRef]
- Prusik, M.; Lewczuk, B. Regulation of melatonin secretion in the avian pineal gland [In polish]. Med. Weter. 2008, 64, 617–736. [Google Scholar]
- Okano, T.; Fukada, Y. Chicktacking pineal clock. J. Biochem. 2003, 134, 791–797. [Google Scholar] [CrossRef]
- Csernus, V.; Faluhelyi, N.; Nagy, A.D. Features of the circadian clock in the avian pineal gland. Ann. N. Y. Acad. Sci. 2005, 104, 281–287. [Google Scholar] [CrossRef]
- Adamska, I.; Marhelava, K.; Walkiewicz, D.; Kędzierska, U.; Markowska, M.; Majewski, P.M. All genes encoding enzymes participating in melatonin biosynthesis in the chicken pineal gland are transcribed rhythmically. J. Physiol. Pharm. 2016, 67, 521–530. [Google Scholar]
- Brandstätter, R.; Kumar, V.; Abraham, U.; Gwinner, E. Photoperiodic information acquired and stored in vivo is retained in vitro by a circadian oscillator, the avian pineal gland. Proc. Natl. Acad. Sci. USA 2000, 97, 12324–12328. [Google Scholar]
- Kumar, V.; Gwinner, E.; Van’t Hof, T.J. Circadian rhythms of melatonin in European starlings exposed to different lighting conditions: Relationship with locomotor and feeding rhythms. J. Comp. Physiol. A. 2000, 186, 205–215. [Google Scholar] [CrossRef]
- Prusik, M.; Lewczuk, B.; Ziółkowska, N.; Przybylska-Gornowicz, B. Regulation of melatonin secretion in the pineal organ of the domestic duck - an in vitro study. Pol. J. Vet. Sci. 2015, 18, 635–644. [Google Scholar] [CrossRef]
- Robertson, L.M.; Takahashi, J.S. Circadian clock in cell culture: I. Oscillation of melatonin release from dissociated chick pineal cells in flow-through microcarrier culture. J. Neurosci. 1988, 8, 12–21. [Google Scholar] [CrossRef]
- Robertson, L.M.; Takahashi, J.S. Circadian clock in cell culture: II. In vitro photic entrainment of melatonin oscillation from dissociated chick pineal cells. J. Neurosci. 1988, 8, 22–30. [Google Scholar]
- Zawilska, J.B.; Wawrocka, M. Chick retina and pineal gland differentially respond to constant light and darkness: In vivo studies on serotonin N-acetyltransferase (NAT) activity and melatonin content. Neurosci. Lett. 1993, 153, 21–24. [Google Scholar] [CrossRef]
- Zatz, M. Melatonin rhythms: Trekking toward the heart of darkness in the chick pineal. Cell Dev. Biol. 1996, 7, 811–820. [Google Scholar] [CrossRef]
- Nakahara, K.; Murakami, N.; Nasu, T.; Kuroda, H.; Murakami, T. Individual pineal cells in chick possess photoreceptive, circadian clock and melatonin-synthesizing capacities in vitro. Brain Res. 1997, 774, 242–245. [Google Scholar] [CrossRef]
- Csernus, V.; Ghosh, M.; Mess, B. Development and control of the circadian pacemaker for melatonin release in the chicken pineal gland. Gen. Comp. Endocrinol. 1998, 110, 19–28. [Google Scholar] [CrossRef]
- Csernus, V.; Becher, P.; Mess, B. Wavelength dependency of light-induced changes in rhythmic melatonin secretion from chicken pineal gland in vitro. Neuro Endocrinol. Lett. 1999, 20, 299–304. [Google Scholar]
- Zawilska, J.B.; Vivien-Roels, B.; Skene, D.J.; Pevet, P.; Nowak, J.Z. Phase-shifting effect of light on the circadian rhythm of 5-methoxytryptophol and melatonin in the chick pineal gland. J. Pineal Res. 2000, 29, 1–7. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Berezińska, M.; Lorenc, A.; Skene, D.J.; Nowak, J.Z. Retinal illumination phase shifts the circadian rhythm of serotonin Nacetyltransferase activity in the chicken pineal gland. Neurosci. Lett. 2004, 360, 153–156. [Google Scholar] [CrossRef]
- Turkowska, E.; Majewski, P.M.; Rai, S.; Skwarło-Sońta, K. Pineal oscillator functioning in the chicken - effect of photoperiod and melatonin. Chronobiol. Int. 2014, 31, 134–143. [Google Scholar] [CrossRef]
- Adamska, I.; Lewczuk, B.; Markowska, M.; Majewski, P.M. Daily profiles of melatonin synthesis-related indoles in the pineal glands of young chickens (Gallus gallus domesticus L.). J. Photochem. Photobiol. B Biol. 2016, 164, 335–343. [Google Scholar] [CrossRef]
- Korf, H.W.; Vigh-Teichmann, I. Sensory and central nervous elements in the avian pineal organ. Ophth. Res. 1984, 16, 96–101. [Google Scholar] [CrossRef]
- Ohshima, K.; Matsuo, S. Functional morphology of the pineal gland in young chickens. Anat. Anz. 1984, 156, 407–418. [Google Scholar]
- Sato, T.; Wake, K. Regressive post-hatching development of acetylcholinesterase-positive neurons in the pineal organs of Coturnix coturnix japonica and Gallus gallus. Cell Tissue Res. 1984, 237, 269–275. [Google Scholar] [CrossRef]
- Ohshima, K.; Hiramatsu, K. Ultrastructural study of post-hatching development in the pineal gland of the Japanese quail. J. Vet. Med. Sci. 1993, 55, 945–950. [Google Scholar] [CrossRef][Green Version]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Nowicki, M. Post-hatching development of the turkey pineal organ: Histological and immunohistochemical studies. Neuro Endocrinol. Lett. 2005, 26, 383–392. [Google Scholar]
- Prusik, M.; Lewczuk, B.; Nowicki, M.; Przybylska-Gornowicz, B. Histology and ultrastructure of the pineal gland of the domestic goose. Histol. Histopathol. 2006, 21, 1075–1090. [Google Scholar]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Bulc, M. Pineal concretions in Turkey (Meleagris gallopavo) as a results of collagen mediated calcification. Histol. Histopathol. 2009, 24, 407–415. [Google Scholar]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Kalicki, M.; Ziółkowska, N. Morphological studies on the pineal gland in the Common gull (Larus canus) reveal uncommon features of pinealocytes. Anat. Rec. (Hoboken). 2012, 295, 673–685. [Google Scholar] [CrossRef]
- Petrusewicz-Kosińska, M.; Przybylska-Gornowicz, B.; Ziółkowska, N.; Martyniuk, K.; Lewczuk, B. Developmental morphology of the turkey pineal organ. Immunocytochemical and ultrastructural studies. Micron. 2019, 122, 8–20. [Google Scholar] [CrossRef]
- Prusik, M.; Lewczuk, B. Structure of the avian pineal gland [In polish]. Med. Weter. 2008, 64, 734–848. [Google Scholar]
- Singh, N.S.; Dixit, A.S. Morphology and ultrastructural studies of pineal organ of the tree sparrow (Passer montanus). Micron 2014, 58, 9–14. [Google Scholar] [CrossRef]
- Kwiecińska, K.; Hanuszewska, M.; Petrusewicz-Kosińska, M. Pineal organ of the Muscovy duck. Med. Weter. 2017, 73, 549–555. [Google Scholar] [CrossRef]
- Siopes, T.D.; Underwood, H.A. Pineal gland and ocular influences on turkey breeder hens. 1. Reproductive performance. Poult. Sci. 1987, 66, 521–527. [Google Scholar] [CrossRef]
- Siopes, T.D. Pineal gland and ocular influences on turkey breeder hens. 2. Body weight, feed intake, and egg characteristics. Poult. Sci. 1987, 66, 528–534. [Google Scholar] [CrossRef]
- Siopes, T.D. Initiation of egg production by turkey breeder hens: Sexual maturation and age at lighting. Poult. Sci. 2010, 89, 1490–1496. [Google Scholar] [CrossRef]
- Tamotsu, S.; Oishi, T.; Nakao, K.; Fukada, Y.; Shichida, Y.; Yoshizawa, T.; Morita, Y. Localization of iodopsin and rod-opsin immunoreactivity in the retina and pineal complex of the river lamprey, Lampetra japonica. Cell Tissue Res. 1994, 278, 1–10. [Google Scholar] [CrossRef]
- Kawano-Yamashita, E.; Koyanagi, M.; Wada, S.; Tsukamoto, H.; Nagata, T.; Terakita, A. Activation of transducin by bistable pigment parapinopsin in the pineal organ of lower vertebrates. PLoS ONE 2015, 10, e0141280. [Google Scholar] [CrossRef]
- Peirson, S.N.; Halford, S.; Foster, R.G. The evolution of irradiance detection: Melanopsin and the non-visual opsins. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009, 364, 2849–2865. [Google Scholar] [CrossRef]
- Eilertsen, M.; Drivenes, O.; Edvardsen, R.B.; Bradley, C.A.; Ebbesson, L.O.; Helvik, J.V.J. Exorhodopsin and melanopsin systems in the pineal complex and brain at early developmental stages of Atlantic halibut (Hippoglossus hippoglossus). Comp. Neurol. 2014, 522, 4003–4022. [Google Scholar] [CrossRef]
- Kang, S.W.; Leclerc, B.; Kosonsiriluk, S.; Mauro, L.J.; Iwasawa, A.E.; Halawani, M.E. Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience 2010, 170, 200–213. [Google Scholar] [CrossRef]
- Deguchi, T. A circadian oscillator in cultured cells of chicken pineal gland. Nature 1979, 282, 94–96. [Google Scholar] [CrossRef]
- Murakami, N.; Nakamura, H.; Nishi, R.; Marumoto, N.; Nasu, T. Comparison of circadian oscillation of melatonin release in pineal cells of house sparrow, pigeons and Japanese quail, using cell perfusion system. Brain Res. 1994, 651, 209–214. [Google Scholar] [CrossRef]
- Steele, C.T.; Zivkovic, B.D.; Siopes, T.; Underwood, H. Ocular clocks are tightly coupled and act as pacemakers in the circadian system of Japanese quail. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R208–R218. [Google Scholar] [CrossRef][Green Version]
- Lorenc-Duda, A.; Berezińska, M.; Bothorel, B.; Pévet, P.; Zawilska, J.B. Turkey retina and pineal gland differentially respond to constant environment. J. Comp. Physiol. A. 2008, 194, 907–913. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Prusik, M. Diurnal and circadian variations in indole contents in the goose pineal gland. Chronobiol. Int. 2018, 25, 1–16. [Google Scholar] [CrossRef]
- Binkley, S.; Muller, G.; Hernandez, T. Circadian rhythm in pineal N-acetyltransferase activity: Phase shifting by light pulses (I). J. Neurochem. 1981, 37, 798–800. [Google Scholar]
- Binkley, S. Circadian rhythm in pineal N-acetyltransferase activity: Phase shifting by light pulses (II). J. Neurochem. 1983, 41, 273–276. [Google Scholar] [CrossRef]
- Binkley, S.; Mosher, K.; White, B.H. Circadian rhythm in pineal N-acetyltransferase activity: Phase shifting by dark pulses (III). J. Neurochem. 1985, 45, 875–878. [Google Scholar] [CrossRef]
- Binkley, S.; Mosher, K. Prior light alters the circadian clock in the chick pineal gland. J. Exp. Zool. 1984, 232, 551–556. [Google Scholar] [CrossRef]
- Bernard, M.; Iuvone, P.M.; Cassone, V.M.; Roseboom, P.H.; Coon, S.L.; Klein, D.C. Avian melatonin synthesis: Photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J. Neurochem. 1997, 68, 213–224. [Google Scholar] [CrossRef]
- Illnerova, H.; Sumova, A. Photic entrainment of the mammalian rhythm in melatonin production. J. Biol. Rhythm. 1997, 12, 547–555. [Google Scholar] [CrossRef]
- Fraser, S.; Cowen, P.; Franklin, M.; Franey, C.; Arendt, J. Direct radioimmunoassay for melatonin in plasma. Clin. Chem. 1983, 29, 396–397. [Google Scholar]
- Paterson, A.M.; Martin, G.B.; Foldes, A.; Maxwell, C.A.; Pearce, G.P. Concentrations of plasma melatonin and luteinizing hormone in domestic gilts reared under artificial long and short days. J. Reprod. Fertil. 1992, 94, 85–95. [Google Scholar] [CrossRef][Green Version]
- Lewczuk, B.; Ziółkowska, N.; Prusik, M.; Przybylska-Gornowicz, B. Diurnal profiles of melatonin synthesis-related indoles, catecholamines and their metabolites in the duck pineal organ. Int. J. Mol. Sci. 2014, 15, 12604–12630. [Google Scholar] [CrossRef]
- Lewczuk, B.; Prusik, M.; Ziółkowska, N.; Dąbrowski, M.; Martniuk, K.; Hanuszewska, M.; Zielonka, Ł. Effects of Streptozotocin-Induced Diabetes on the Pineal Gland in the Domestic Pig. Int. J. Mol. Sci. 2018, 19, 3077. [Google Scholar] [CrossRef]


| Groups | Minimum | Maximum | I50 | I75 | M | ||
|---|---|---|---|---|---|---|---|
| Clock Time | Secretion Level | Clock Time | Secretion Level | ||||
| Day I | |||||||
| Group I 12 L:12 D | 18.15 a ( ± 25.1) | 0.06 a ( ± 0.01) | 04.32 a ( ± 20.2) | 1.27 a ( ± 0.25) | 00.37 a ( ± 10.7) | 03.08 a ( ± 21.7) | 04.32 a ( ± 11.4) |
| Group II 12 L:12 D 1 | 18.07 a ( ± 37.5) | 0.13 a b ( ± 0.05) | 01.04 d ( ± 5.7) | 1.31 a ( ± 0.12) | 23.30 d ( ± 25.63) | 00.08 d ( ± 15.7) | n.d. |
| Group III 12 L:12 D 2 | 22.17 b ( ± 32.7) | 0.04 a ( ± 0.01) | 07.00 c ( ± 15.2) | 1.37 a ( ± 0.07) | 03.37 e ( ± 32.5) | 05.09 c ( ± 42.5) | 07.10 c ( ± 15.5) |
| Group IV 12 D:12 L | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Group V 0 L:24 D | 17.07 a ( ± 34.5) | 0.16 b ( ± 0.02) | 02.08 b ( ± 32.7) | 1.58 a ( ± 0.23) | 21.32 b ( ± 21.4) | 01.14 b ( ± 17.3) | 03.30 b ( ± 12.4) |
| Group VI 24 L:0 D | 21.28 b ( ± 21.5) | 0.05 a ( ± 0.01) | 06.17 c ( ± 41.2) | 0.68 b ( ± 0.09) | 02.07 c ( ± 32.1) | 04.10 c ( ± 21.4) | 07.00 c ( ± 10.2) |
| Day II | |||||||
| Group I 12 L:12 D | 12.08 a ( ± 40.8) | 0.04 a ( ± 0.01) | 02.37 a ( ± 19.0) | 1.63 a ( ± 0.24) | 23.08 a ( ± 19.7) | 00.17 a ( ± 22.7) | 02.38 a ( ± 23.4) |
| Group II 12 L:12 D 1 | 10.32 c ( ± 55.8) | 0.09 a ( ± 0.01) | 21.10 c ( ± 35.5) | 1.98 a ( ± 0.21) | 16.40 c ( ± 42.5) | 18.15 c ( ± 33.7) | 21.12 d ( ± 26.7) |
| Group III 12 L:12 D 2 | 20.07 d ( ± 25.7) | 0.05 a ( ± 0.01) | 07.28 d ( ± 32.5) | 1.49 a ( ± 0.04) | 04.09 d ( ± 25.4) | 06.10 d ( ± 35.7) | 08.00 e ( ± 14.7) |
| Group IV 12 D:12 L | 01.32 e ( ± 41.2) | 0.14 a ( ± 0.01) | 14.02 e ( ± 17.8) | 1.70 a ( ± 0.23) | 08.31 e ( ± 23.5) | 10.14 e ( ± 44.2) | 13.12 f ( ± 55.4) |
| Group V 0 L:24 D | 12.29 a ( ± 21.4) | 0.36 b ( ± 12.7) | 01.02 a ( ± 24.9) | 0.83 b ( ± 11.2) | 18.07 b ( ± 11.4) | 20.08 b ( ± 24.3) | 01.05 b ( ± 17.8) |
| Group VI 24 L:0 D | 18.19 b ( ± 25.8) | 0.27 b ( ± 0.03) | 05.12 b ( ± 31.2) | 1.01 b ( ± 0.02) | 23.39 a ( ± 19.8) | 01.29 a ( ± 37.8) | 05.10 c ( ± 24.9) |
| Day III | |||||||
| Group I 12 L:12 D | 13.11 a ( ± 19.7) | 0.05 a ( ± 0.01) | 01.37 a ( ± 28.4) | 2.23 a ( ± 0.42) | 22.37 a ( ± 39.3) | 23.34 a ( ± 29.5) | 02.28 a ( ± 38.4) |
| Group II 12 L:12 D 1 | 09.07 b ( ± 27.1) | 0.08 a ( ± 0.01) | 20.27 b ( ± 41.2) | 2.19 a ( ± 0.21) | 16.10 c ( ± 21.4) | 17.35 b ( ± 25.7) | 21.00 b ( ± 32.78) |
| Group III 12 L:12 D 2 | 20.32 d ( ± 24.5) | 0.06 a ( ± 0.01) | 08.07 c ( ± 48.7) | 1.49 a b ( ± 0.41) | 04.10 d ( ± 15.2) | 05.40 c ( ± 22.3) | 07.45 c ( ± 18.7) |
| Group IV 12 D:12 L | 01.21 e ( ± 25.7) | 0.12 a ( ± 0.02) | 14.48 d (±24.9) | 2.87 a ( ± 0.12) | 09.27 e ( ± 14.7) | 11.07 d ( ± 24.7) | 14.05 d ( ± 19.8) |
| Group V 0 L:24 D | 09.05 b ( ± 22.5) | 0.59 b ( ± 0.09) | 01.30 a ( ± 19.8) | 1.44 b ( ± 12.5) | 13.55 b ( ± 22.3) | 17.05 b ( ± 12.9) | 22.30 b ( ± 42.5) |
| Group VI 24 L:0 D | 16.29 c ( ± 25.6) | 0.52 b ( ± 0.04) | 01.35 a ( ± 25.5) | 1.32 b ( ± 0.13) | 19.37 a ( ± 42.3) | 22.14 a ( ± 31.89) | 03.08 a ( ± 28.1) |
| Day IV | |||||||
| Group I 12 L:12 D | 13.07 a ( ± 27.4) | 0.05 a ( ± 0.02) | 01.35 a ( ± 17.8) | 2.29 a ( ± 0.22) | 22.37 a ( ± 36.2) | 00.07 a ( ± 25.6) | 02.28 a ( ± 22.2) |
| Group II 12 L:12 D 1 | 09.08 b ( ± 21.4) | 0.11 a ( ± 0.04) | 19.02 c ( ± 25.7) | 2.52 a ( ± 0.21) | 15.07 c ( ± 31.2) | 17.05 d ( ± 14.5) | 20.01 b ( ± 25.7) |
| Group III 12 L:12 D 2 | 21.08 d ( ± 21.4) | 0.05 a ( ± 0.01) | 08.29 d ( ± 32.8) | 1.38 a b ( ± 0.31) | 04.32 d ( ± 23.5) | 05.17 e ( ± 42.5) | 08.32 d ( ± 32.5) |
| Group IV 12 D:12 L | 03.28 e ( ± 27.8) | 0.11 a ( ± 0.05) | 15.05 e ( ± 42.5) | 2.79 a ( ± 0.07) | 10.07 e ( ± 23.5) | 11.25 f ( ± 17.5) | 14.17 e ( ± 25.7) |
| Group V 0 L:24 D | 09.55 b ( ± 42.5) | 1.09 b ( ± 0.23) | 17.24 b ( ± 19.8) | 1.65 b ( ± 0.17) | n.d. | 15.04 b ( ± 27.8) | 20.03 b ( ± 19.8) |
| Group VI 24 L:0 D | 13.21 c ( ± 21.7) | 0.65 b ( ± 0.19) | 02.10 a ( ± 32.5) | 1.04 b ( ± 0.2) | 18.32 b ( ± 32.5) | 21.14 c ( ± 22.7) | 23.51 c ( ± 23.2) |
| Day V | |||||||
| Group I 12 L:12 D | 13.28 a ( ± 21.2) | 0.06 a ( ± 0.01) | 02.57 a ( ± 18.4) | 2.03 a ( ± 0.17) | 22.00 a ( ± 24.2) | 23.32 a ( ± 14.7) | 02.41 a ( ± 19.7) |
| Group II 12 L:12 D 1 | 06.07 c ( ± 25.8) | 0.09 a ( ± 0.01) | 20.04 d ( ± 34.7) | 1.97 a ( ± 21.4) | 16.10 b ( ± 29.8) | 17.17 d ( ± 17.8) | 20.01 c ( ± 20.7) |
| Group III 12 L:12 D 2 | 21.04 d ( ± 55.2) | 0.05 a ( ± 0.01) | 08.30 b ( ± 32.5) | 1.25 a b ( ± 0.42) | 04.10 c ( ± 24.8) | 05.30 e ( ± 32.1) | 07.38 d ( ± 35.5) |
| Group IV 12 D:12 L | 02.30 e ( ± 24.9) | 0.17 a ( ± 0.03) | 15.01 e ( ± 12.5) | 2.08 a ( ± 0.21) | 10.05 d ( ± 32.5) | 10.55 f ( ± 42.5) | 14.02 b ( ± 32.5) |
| Group V 0 L:24 D | 04.09 b ( ± 23.5) | 1.02 b ( ± 012) | 09.01 b ( ± 42.3) | 1.44 a b ( ± 15.7) | n.d. | 07.08 b ( ± 33.5) | 13.10 b ( ± 25.8) |
| Group VI 24 L:0 D | 12.35 a ( ± 32.5) | 0.63 b ( ± 0.04) | 00.14 c ( ± 23.5) | 0.96 b ( ± 28.9) | n.d. | 19.05 c ( ± 19.8) | 01.07 a ( ± 26.7) |
| Groups | Day I | Day II | Day III | Day IV | Day V | Day VI |
|---|---|---|---|---|---|---|
| Group I 12 L:12 D | 13.6 A ( ± 23.2) | 13.5 A ( ± 18.6) | 13.6 A ( ± 14.8) | 13.9 A ( ± 16.1) | 13.5 A ( ± 14.9) | 13.1 A ( ± 19.2) |
| Group II 16 L:8 D | 10.1 B ( ± 24.9) | 11.2 B ( ± 14.9) | 10.4 B ( ± 15.6) | 11.7 B ( ± 14. 1) | 11.1 B ( ± 15.7) | 11.5 B ( ± 18.9) |
| Group III 8 L:16 D | 13.6 A ( ± 20.4) | 15.1 C ( ± 21.8) | 15.5 C ( ± 21.7) | 14.7 C ( ± 15.1) | 15.2 C ( ± 19.2) | 13.2 A ( ± 22.5) |
| Group IV 12 D:12 L | n.d. | 13.5 A ( ± 19.5) | 13.7 A ( ± 15.7) | 14.1 A ( ± 14.8) | 13.5 A ( ± 14.9) | 14.2 A ( ± 20.4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prusik, M.; Lewczuk, B. Roles of Direct Photoreception and the Internal Circadian Oscillator in the Regulation of Melatonin Secretion in the Pineal Organ of the Domestic Turkey: A Novel In Vitro Clock and Calendar Model. Int. J. Mol. Sci. 2019, 20, 4022. https://doi.org/10.3390/ijms20164022
Prusik M, Lewczuk B. Roles of Direct Photoreception and the Internal Circadian Oscillator in the Regulation of Melatonin Secretion in the Pineal Organ of the Domestic Turkey: A Novel In Vitro Clock and Calendar Model. International Journal of Molecular Sciences. 2019; 20(16):4022. https://doi.org/10.3390/ijms20164022
Chicago/Turabian StylePrusik, Magdalena, and Bogdan Lewczuk. 2019. "Roles of Direct Photoreception and the Internal Circadian Oscillator in the Regulation of Melatonin Secretion in the Pineal Organ of the Domestic Turkey: A Novel In Vitro Clock and Calendar Model" International Journal of Molecular Sciences 20, no. 16: 4022. https://doi.org/10.3390/ijms20164022
APA StylePrusik, M., & Lewczuk, B. (2019). Roles of Direct Photoreception and the Internal Circadian Oscillator in the Regulation of Melatonin Secretion in the Pineal Organ of the Domestic Turkey: A Novel In Vitro Clock and Calendar Model. International Journal of Molecular Sciences, 20(16), 4022. https://doi.org/10.3390/ijms20164022