Durum Wheat Stress Tolerance Induced by Endophyte Pantoea agglomerans with Genes Contributing to Plant Functions and Secondary Metabolite Arsenal
Abstract
1. Introduction
2. Results
2.1. P. agglomerans Pa Strain Recovery
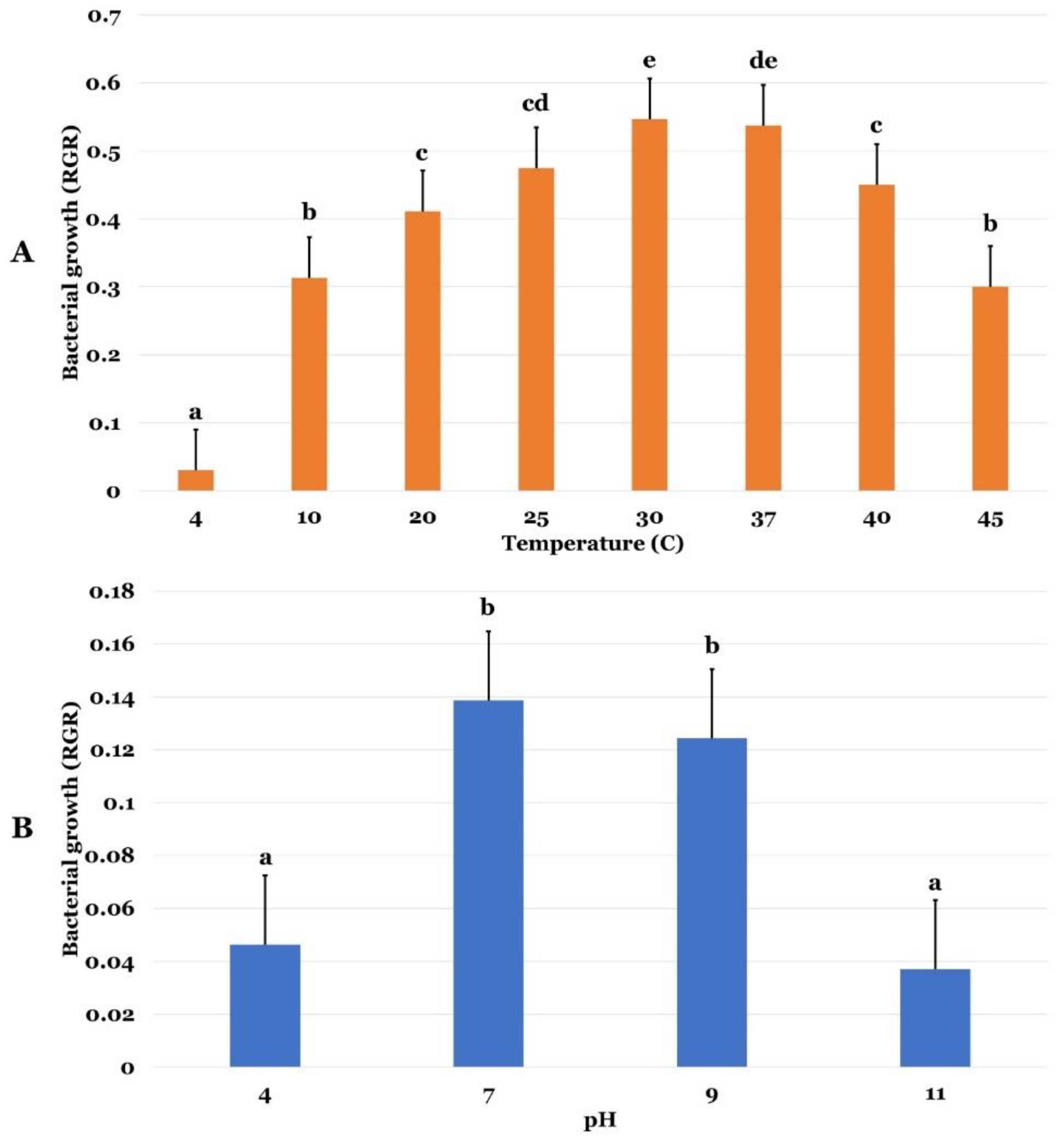
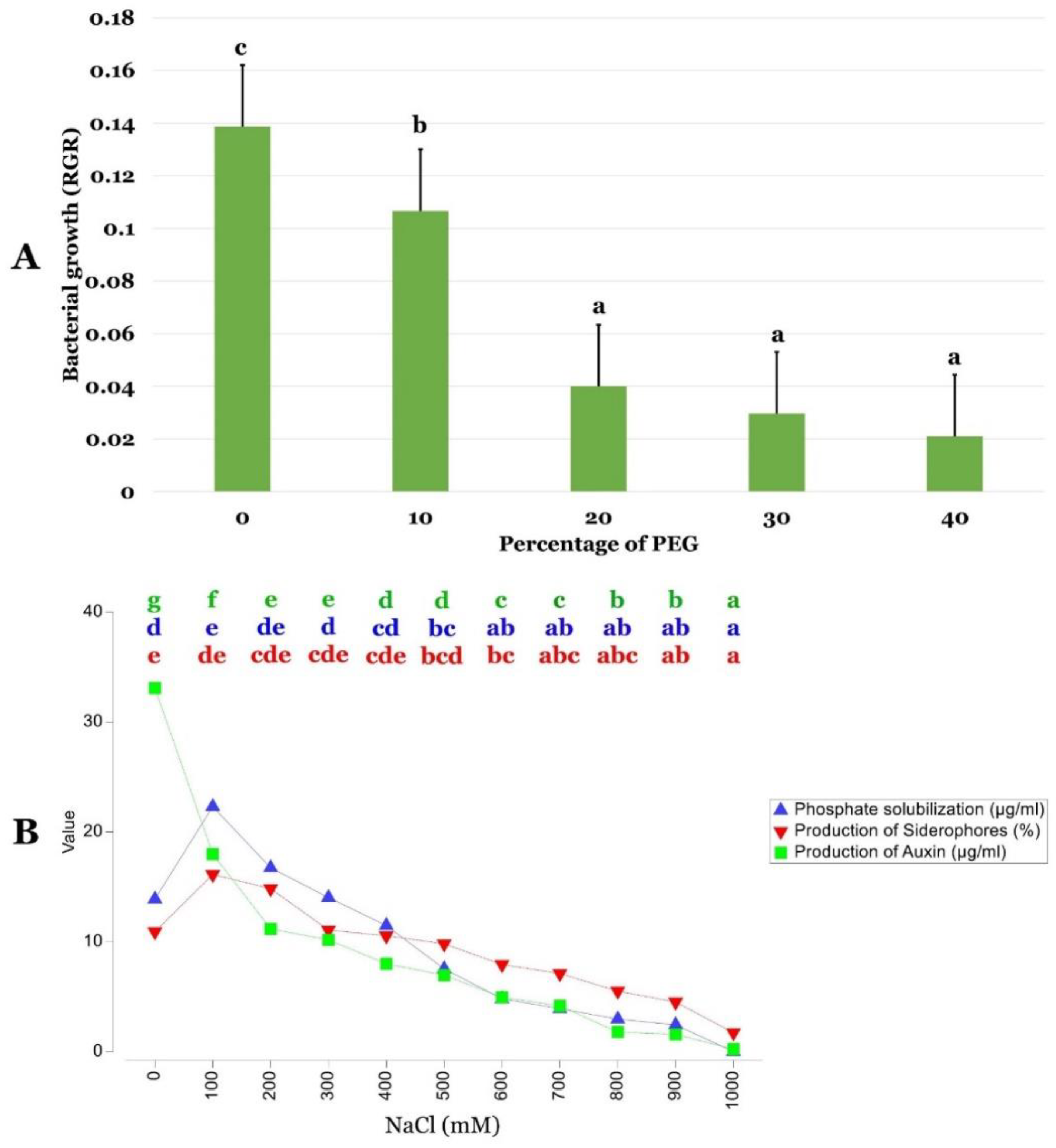
2.2. P. agglomerans Strain Pa Abiotic Stress Tolerance
2.3. P. agglomerans Strain Pa PGP Activities
2.4. NaCl Stress Effect on PGP Activities of P. agglomerans Strain Pa
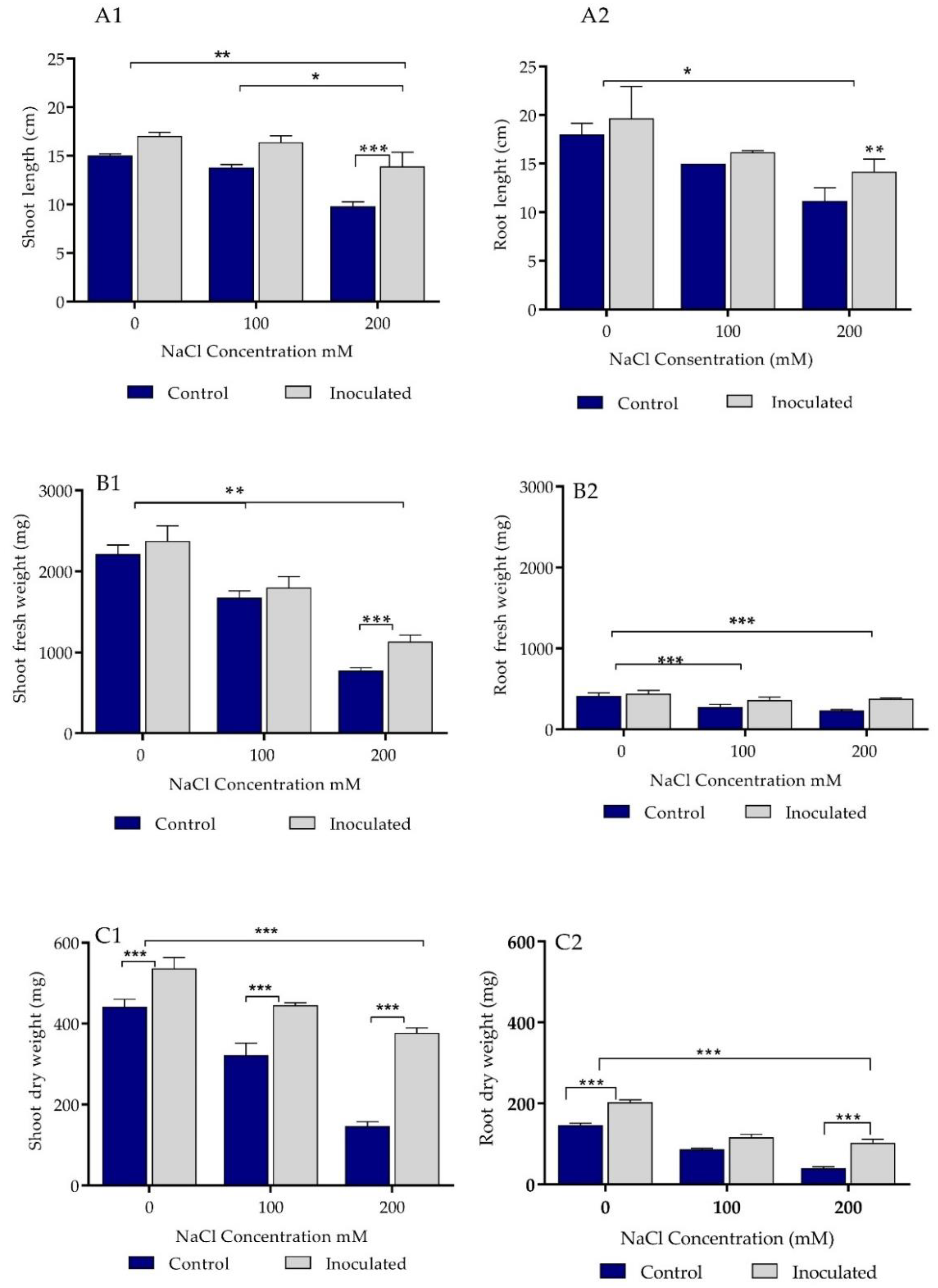
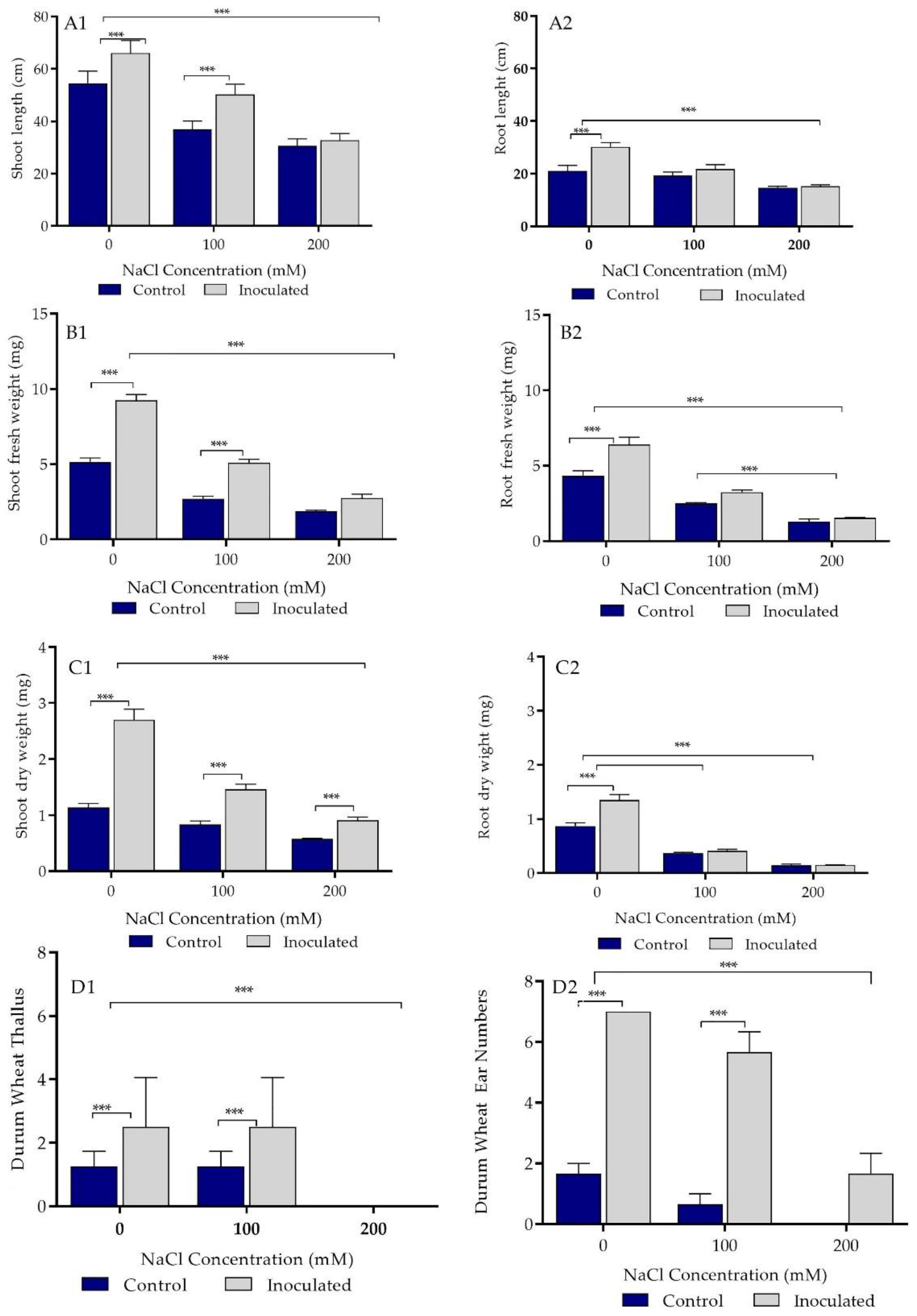
2.5. Growth Parameters Analysis of Control and Bacteria Inoculated Durum Wheat Plants Grown in Sand Pots
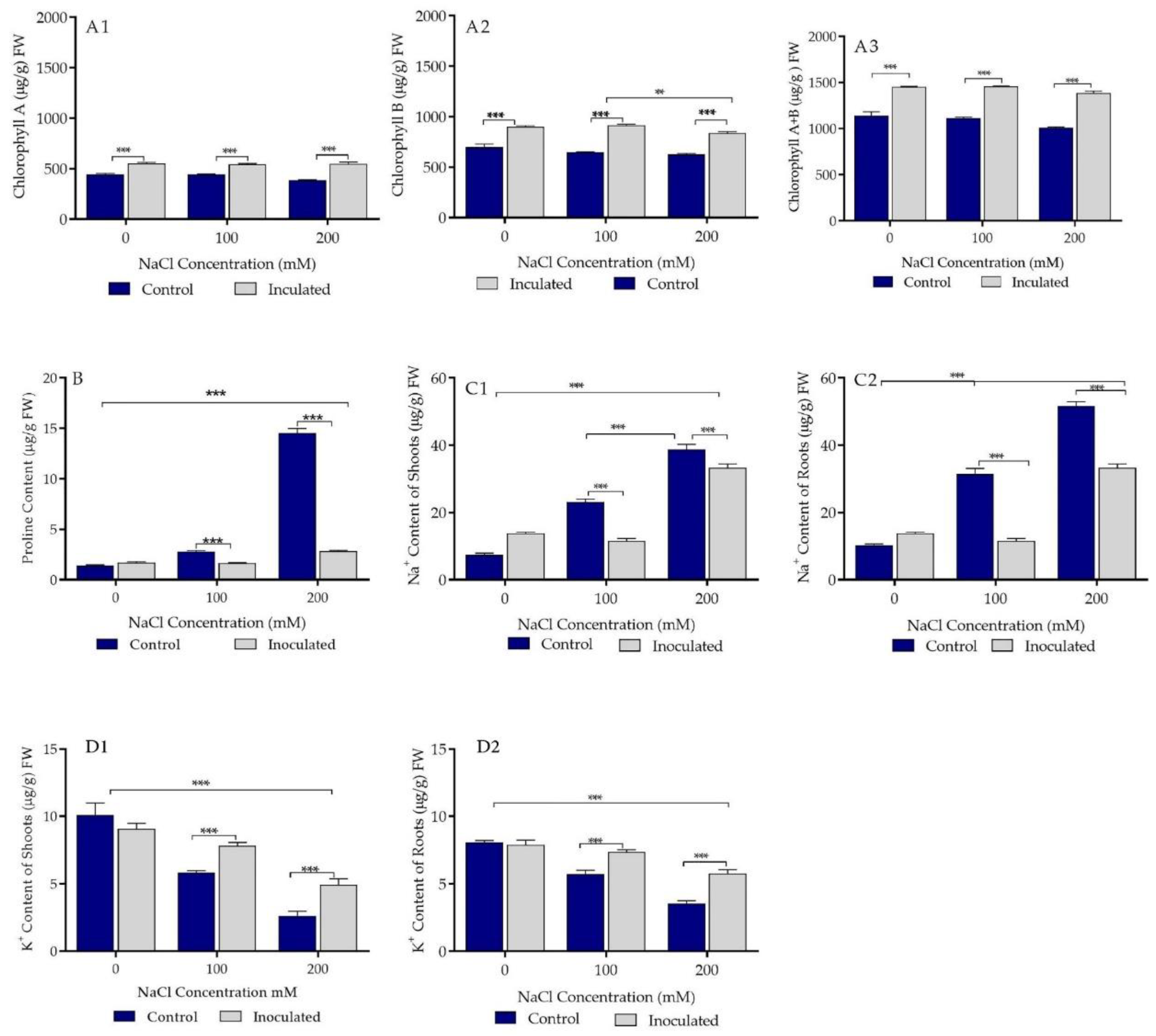
2.6. Biochemical Parameters of Control and Bacterial Inoculated Durum Wheat Plants Grown in Sand Pots
2.7. Growth Parameters Analysis of Control and Bacterial Inoculated Durum Wheat Plants Grown in Soil Pots
2.8. Biochemical Parameters of Control and Bacterial Inoculated Durum Wheat Plants Grown in Soil Pots
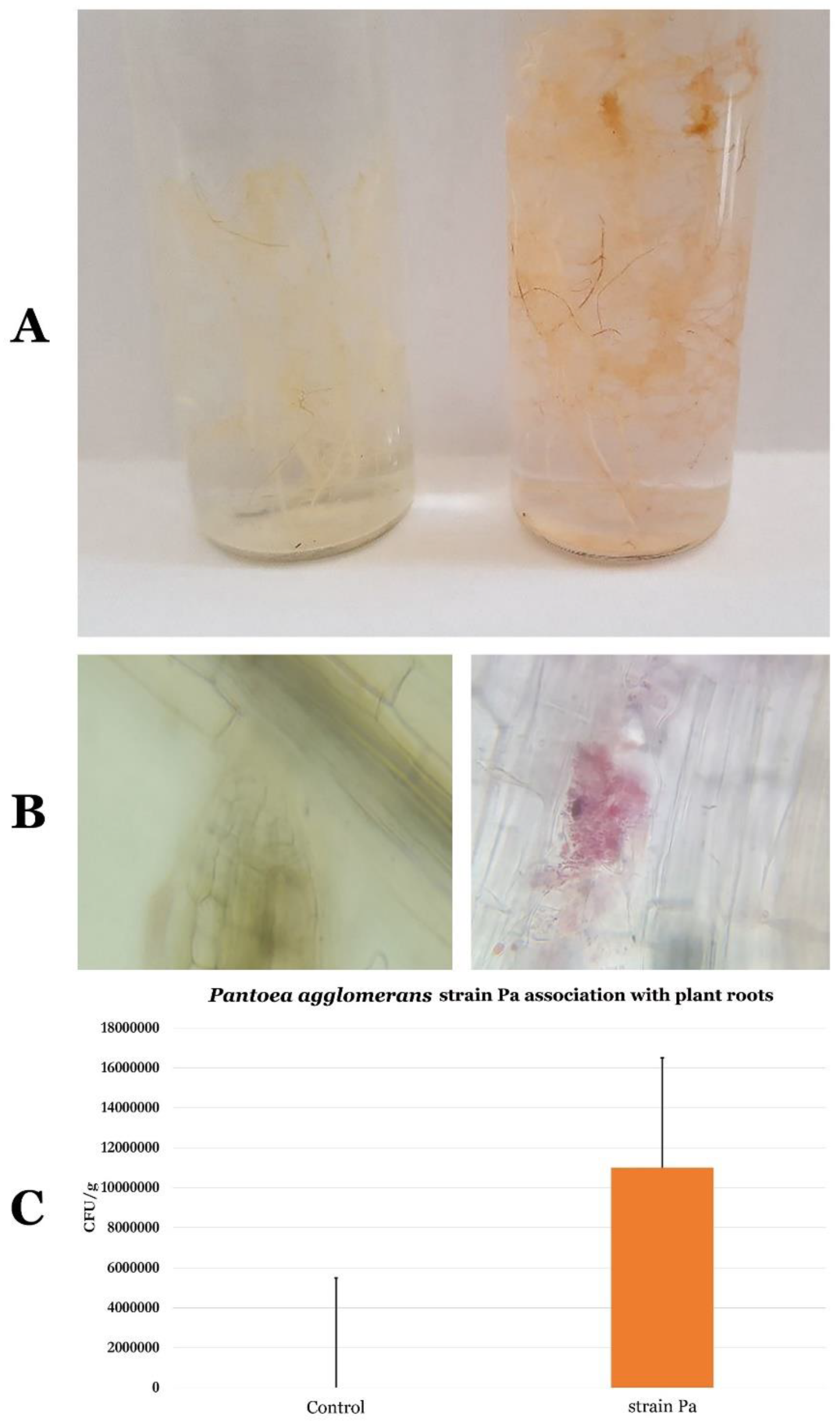
2.9. Life Style of P. agglomerans Strain Pa
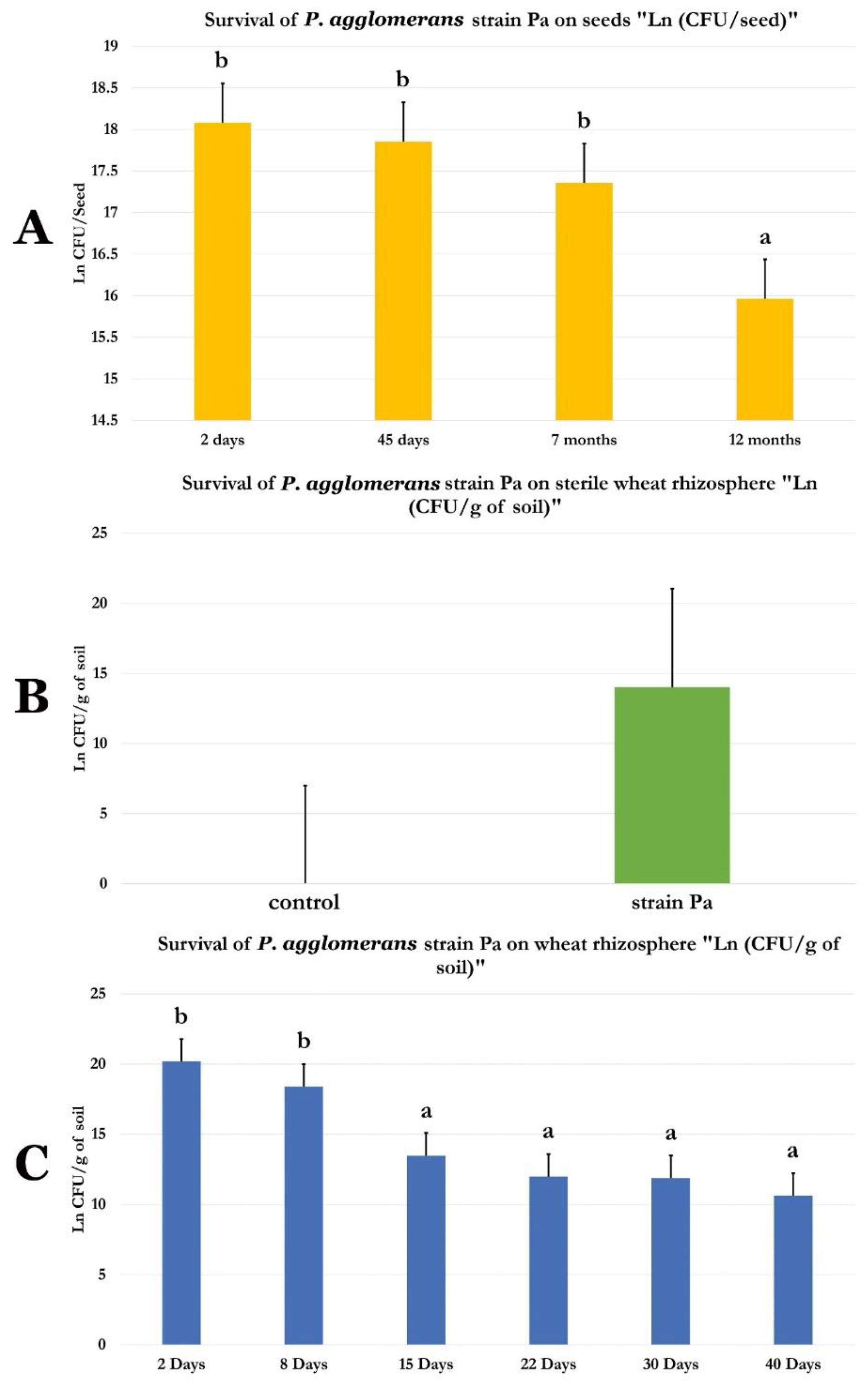
2.10. Survival of P. agglomerans Strain Pa in Wheat Seeds and in Non-Sterile and Sterile Wheat Rhizosphere
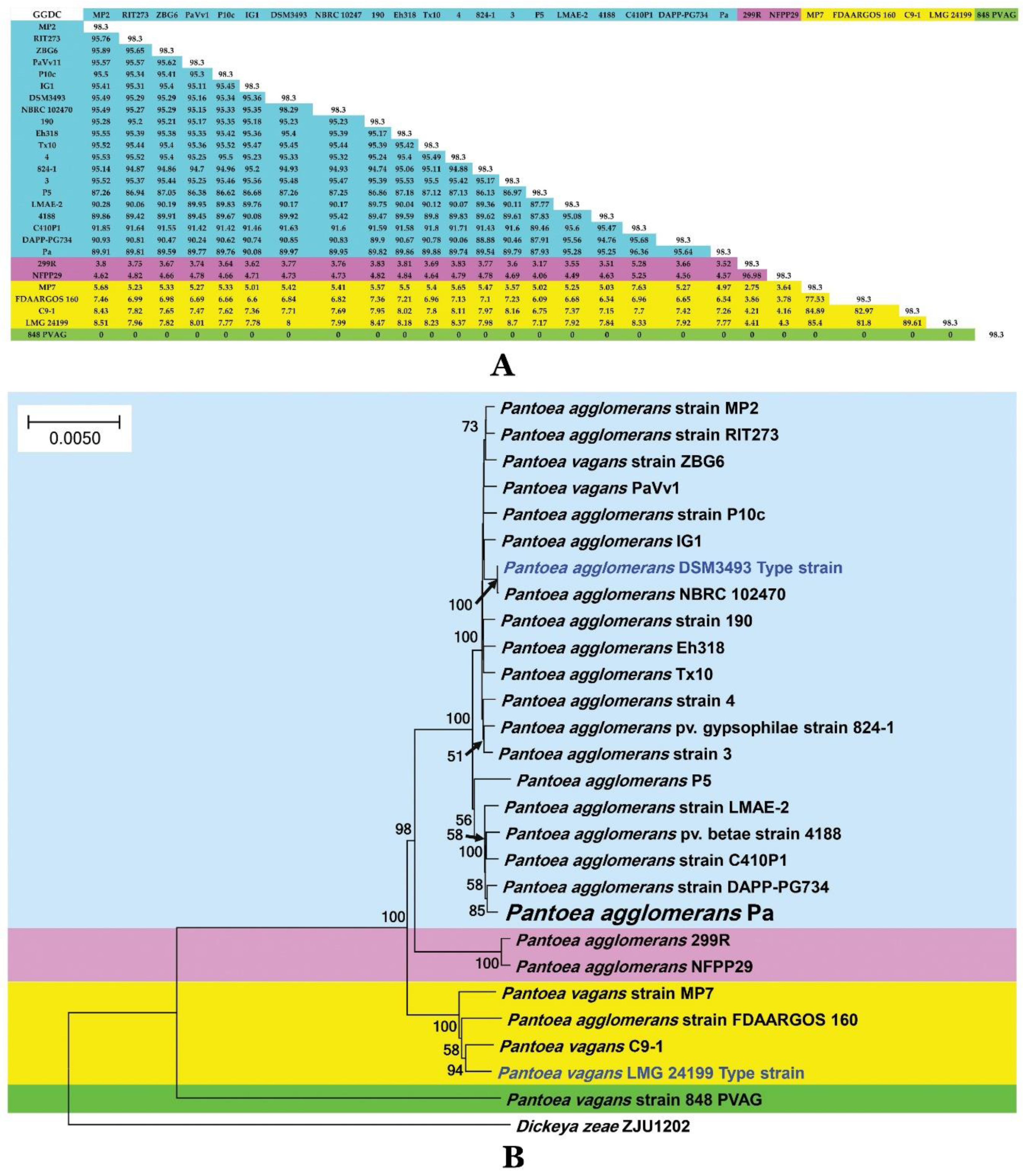
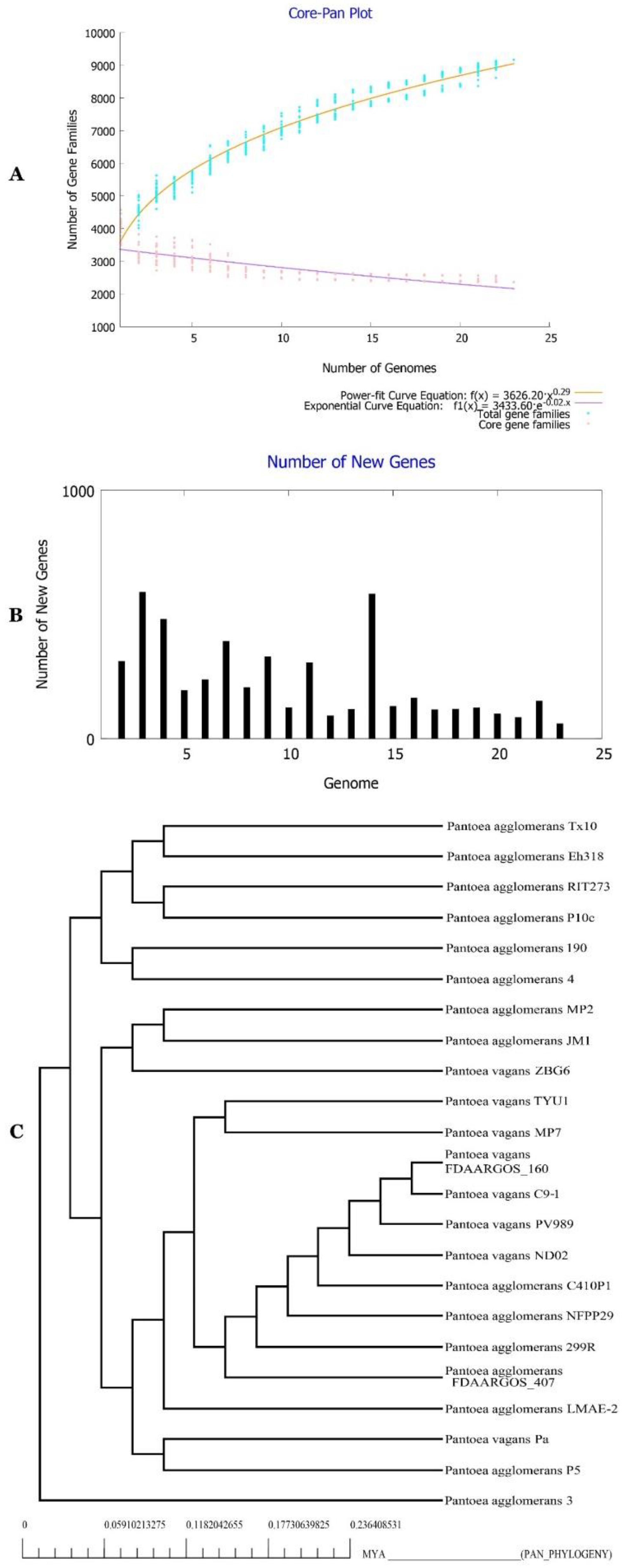
2.11. Phylogenetic Affinities of Strain Pa
2.12. P. vagans and P. agglomerans Genome Mining
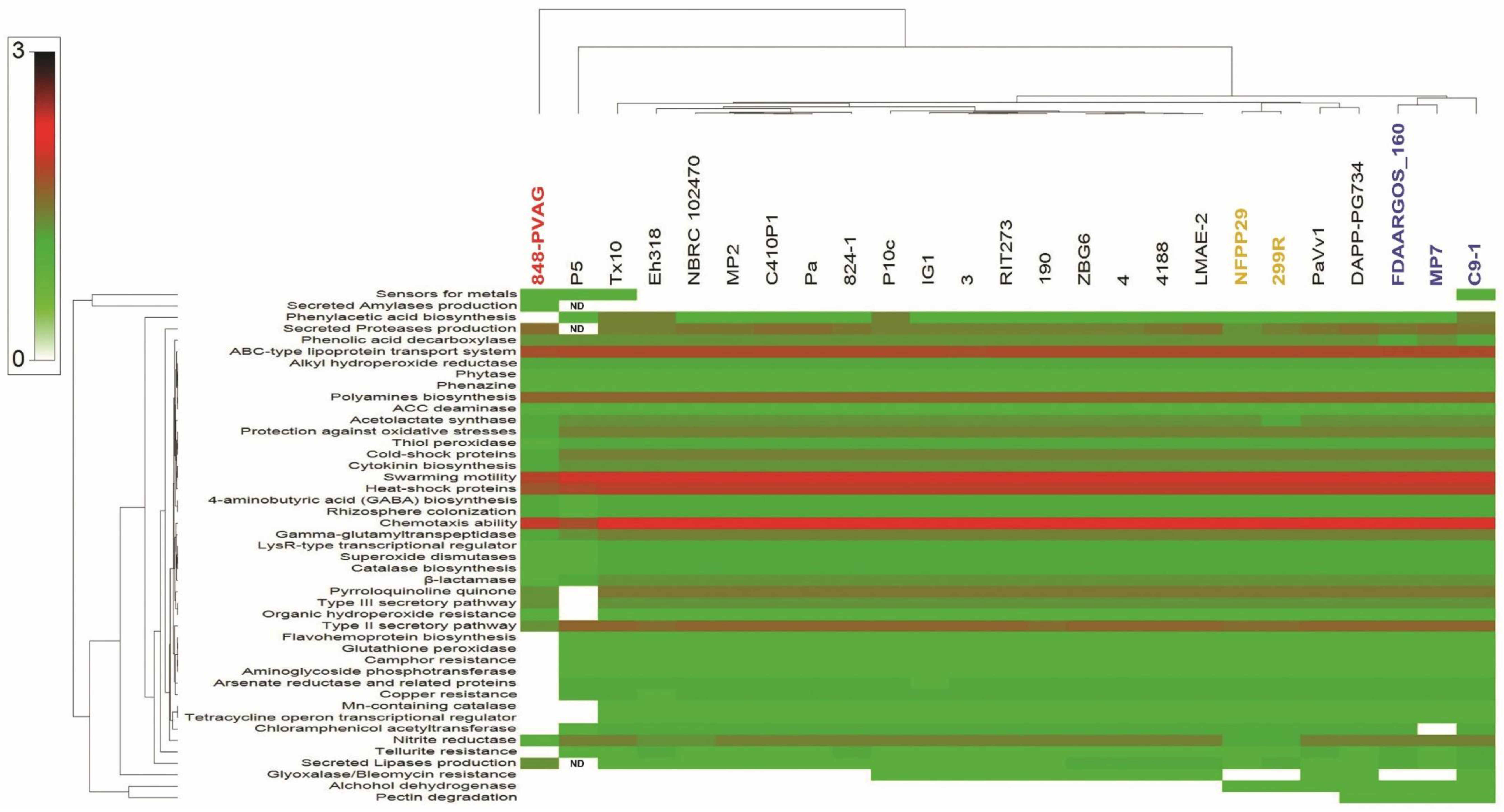
2.12.1. PGP Activities
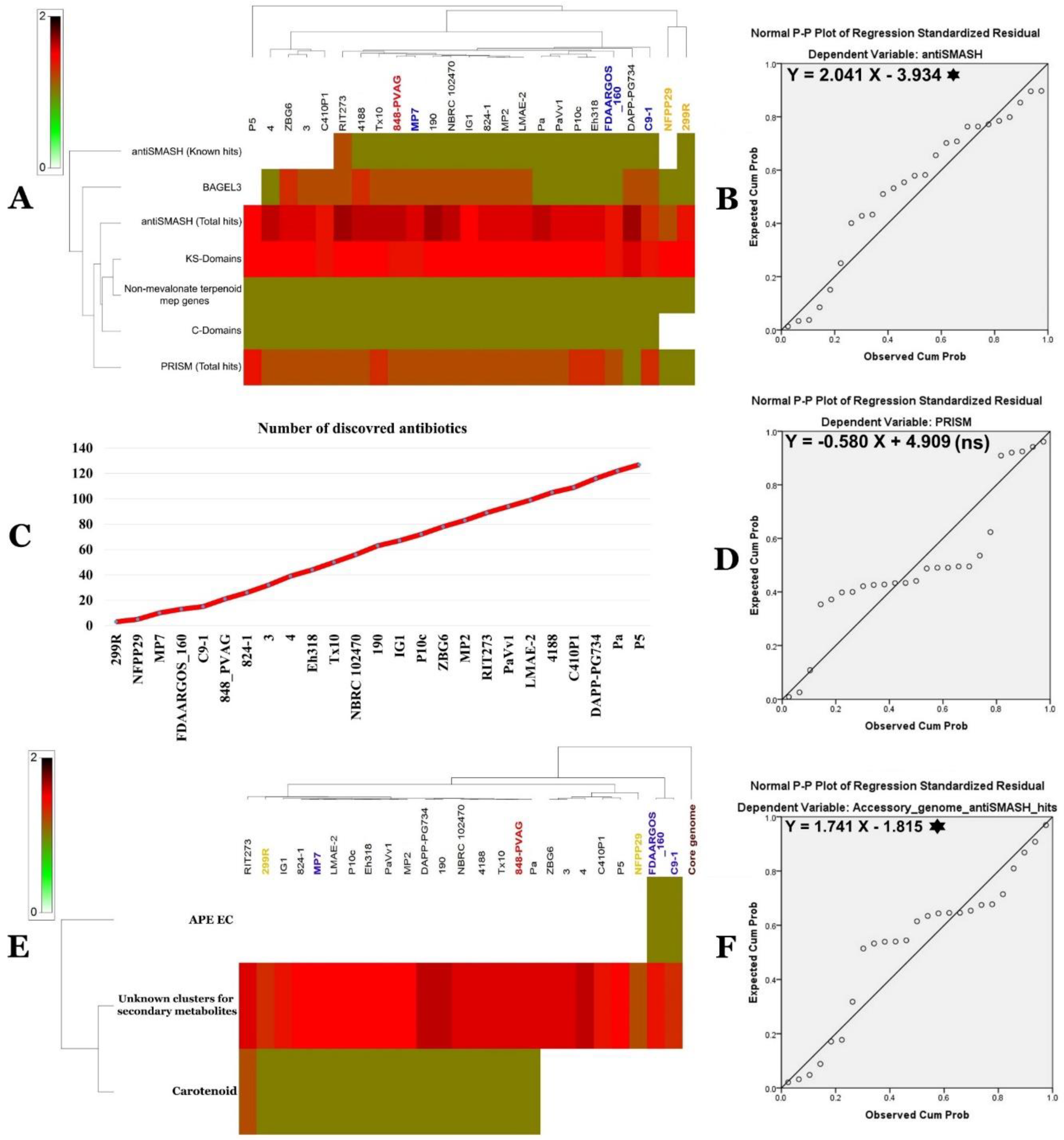
2.12.2. Secondary Metabolite Clusters
2.13. Predicted SM Clusters Location within Pantoea Genomes
2.14. Characterization of the Core and the Pan Genome of P. agglomerans Genomes
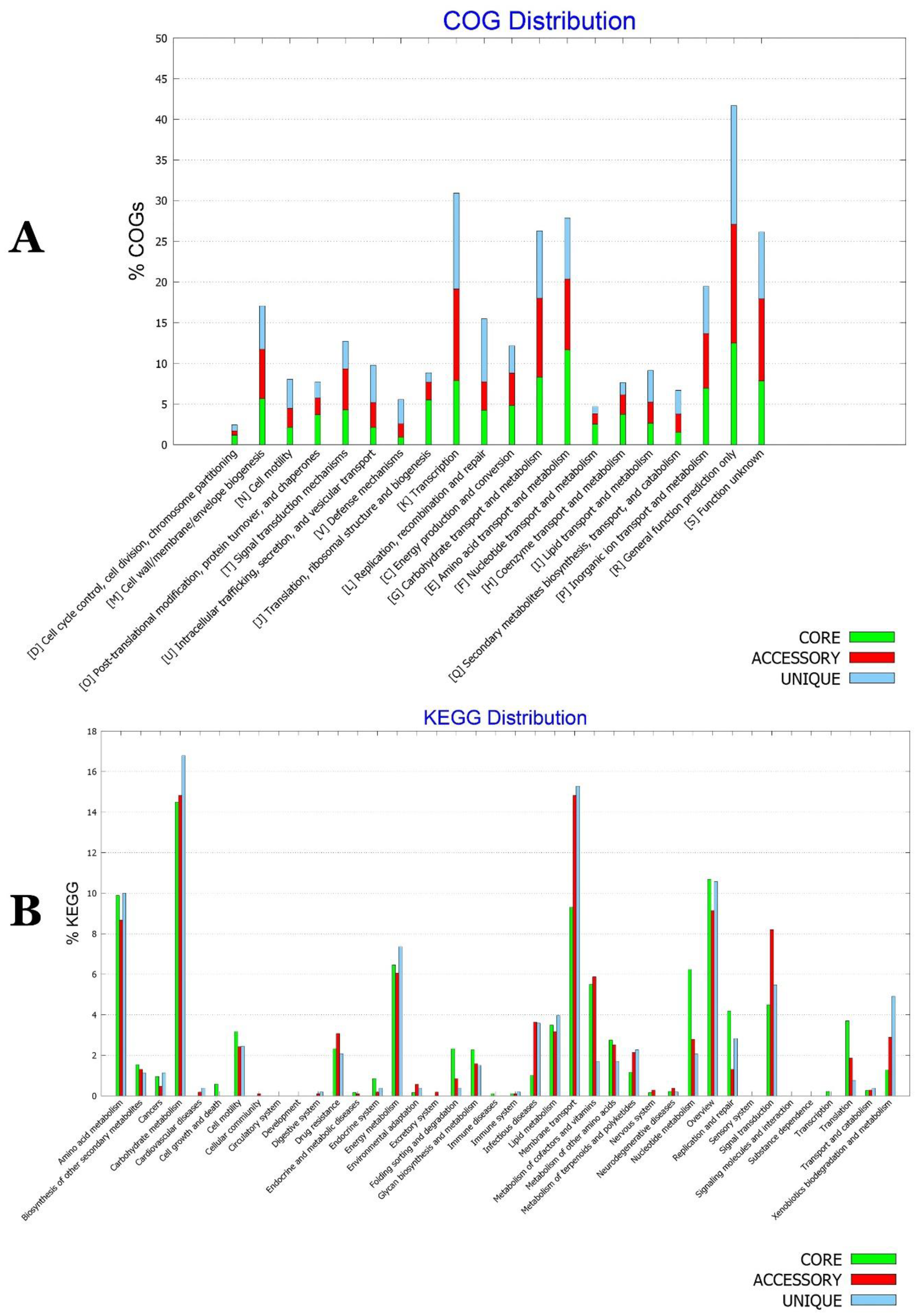
2.15. Functional Characterization of the Core, Accessory, and Unique Genomes of P. agglomerans–P. vagans Strains Collection
2.16. GC-MS Analysis of P. agglomerans Strain Pa
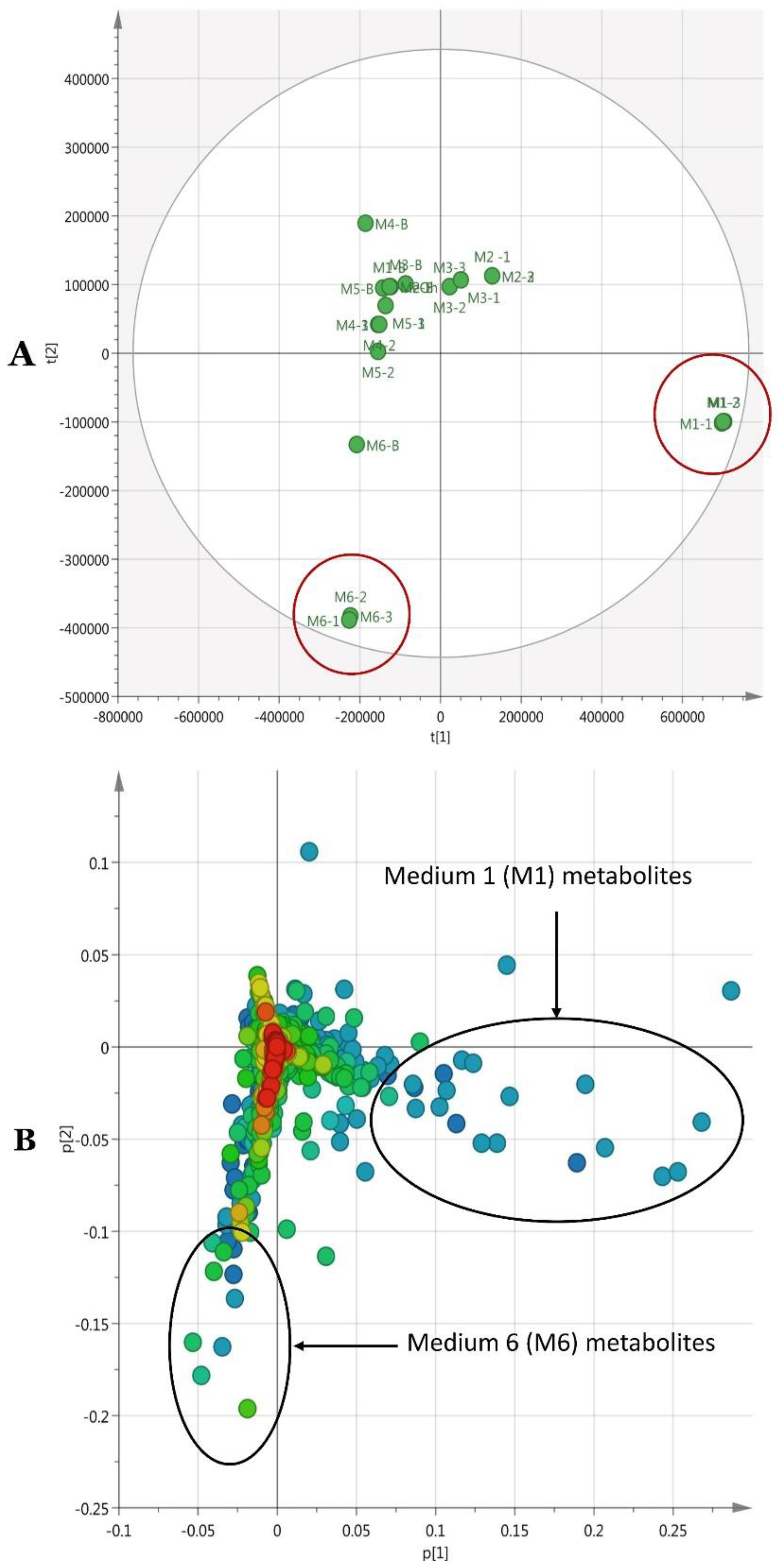
2.17. Metabolomics Profiling of P. agglomerans Strain Pa Under OSMAC Conditions
3. Discussion
4. Materials and Methods
4.1. Sampling Location and Soil Physicochemical Characterization
4.2. Bacterial Isolation from Rhizosphere of Healthy Durum Wheat
4.3. Effect of Temperature, PEG, and pH
4.4. Measurement of PGP Activities
4.4.1. Direct PGPR Activities
Growth on Nitrogen-Free Medium
Phosphate Solubilization
Siderophores Production
Indole Acetic Acid (IAA) Production
4.4.2. Indirect PGP Activities
Protease Production
Chitinase Production
ACC Deaminase Production
NH3 Production
Hydrogen Cyanide (HCN) Production
4.5. Bacterial DNA Extraction and Amplification
DNA Sequencing and Phylogenetic Analysis
4.6. Bacterial Genome Sequencing Assembly and Annotation
4.6.1. Selection of Genomes for Phylogenomic Analysis
4.6.2. Whole Genome Phylogeny
4.6.3. Average Nucleotide Identity Analysis
4.6.4. Genome-to-Genome Distance Calculator (GGDC) Analysis
4.7. P. agglomerans Pa Strain Location within Wheat Plant Root Tissues
4.8. P. agglomerans Strain Pa Survival on Wheat Seeds
4.9. P. agglomerans Strain Pa Survival on Sterilized and Non-Sterilized Wheat Rhizosphere
4.10. Plant Inoculation and NaCl Treatment
4.10.1. Durum Wheat Seeds Sterilization and Bacterial Inoculation
4.10.2. Durum Wheat Growth in Pots Filled with Sand
4.10.3. Durum Wheat Growth in Pots Filled with Soil of Bou-Saâda Durum Wheat Fields
4.10.4. Growth Parameters Analysis of Control and Bacterial Inoculated Durum Wheat
4.10.5. Biochemical Parameters of Control and Bacterial Inoculated Durum Wheat
4.11. Secondary Metabolites Profiling and Dereplication Using Metabolomics Analysis
4.11.1. Small Scale Fermentation Under OSMAC Conditions and Metabolites Extraction
4.11.2. Preparation of Samples for LC-HRMS Analysis
4.11.3. LC-MS Analysis: HPLC-HRMS Instrumentation and Conditions
4.11.4. Raw Mass Data Analysis Using MZmine 2.3.7
4.11.5. Dereplication Process to Identify Known and New Hits
4.11.6. Multivariate Analysis for Media Optimization
4.11.7. GC-MS Analysis
4.12. Homology-Based Identification of Genes Contributing to Plant-Beneficial Functions
4.13. SM Clusters Identification
4.14. Identification of Core Genome and Accessory Genomes of the Strain Collection
4.15. Comparative Genomics Analysis of P. agglomerans Strains
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Cereal crop proteomics: Systemic analysis of crop drought stress responses towards marker-assisted selection breeding. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Wichelns, D.; Qadir, M. Achieving sustainable irrigation requires effective management of salts, soil salinity, and shallow groundwater. Agric. Water Manag. 2017, 157, 31–38. [Google Scholar] [CrossRef]
- Ritzema, H.P. Drain for gain: Managing salinity in irrigated lands—A review. Agric. Water Manag. 2016, 176, 18–28. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Rekik, I.; Chaabane, Z.; Missaoui, A.; Chenari Bouket, A.; Luptakova, L.; Elleuch, A.; Belbahri, L. Effects of untreated and treated wastewater at the morphological, physiological and biochemical levels on seed germination and development of sorghum (Sorghum bicolor (L.) Moench), alfalfa (Medicago sativa L.) and fescue (Festuca arundinacea Schreb). J. Hazard. Mater. 2017, 326, 165–176. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.D.A.; Greer, C.; Espino, L.; Doty, S.L.; Rodriguez, R.J. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE 2010, 6, e14823. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhang, Y.J.; Yuan, B.; Xu, P.Y.; Xing, K.; Wang, J.; Jiang, J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Soares, M.A.; Li, H.Y.; Kowalski, K.P.; Bergen, M.; Torres, M.S.; White, J.F. Evaluation of the functional roles of fungal endophytes of Phragmites australis from high saline and low saline habitats. Biol. Invasions 2016, 18, 2689–2702. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.Y.; Yuan, Z.L. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Cherrad, S.; Charnay, A.; Hernandez, C.; Steva, H.; Belbahri, L.; Vacher, S. Emergence of boscalid-resistant strains of Erysiphe necator in French vineyards. Microbiol. Res. 2018, 216, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth-promoting rhizobacteria. J. Plant Growth Reg. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Slama, H.B.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for Fusarium antagonistic bacteria from contrasting niches designated the endophyte Bacillus halotolerans as plant warden against Fusarium. Front. Microbiol. 2019, 9, 3236. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Rekik, I.; Chenari Bouket, A.; Luptakova, L.; Weitz, H.J.; Rateb, M.E.; Jaspars, M.; Woodward, S.; Belbahri, L. Increased biological activity of Aneurinibacillus migulanus strains correlates with the production of new gramicidin secondary metabolites. Front. Microbiol. 2017, 8, 517. [Google Scholar] [CrossRef]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Fraser, S.; Belka, M.; Dogmuş, T.H.; Heckova, Z.; Oskay, F.; Belbahri, L.; Woodward, S. Biological control of Dothistroma needle blight on pine with Aneurinibacillus migulanus. For. Pathol. 2016, 46, 555–558. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Rekik, I.; Bełka, M.; Ibrahim, A.F.; Luptakova, L.; Jaspars, M.; Woodward, S.; Belbahri, L. Strain-level diversity of secondary metabolism in the biocontrol species Aneurinibacillus migulanus. Microbiol. Res. 2016, 182, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Belbahri, L.; Chenari Bouket, A.; Rekik, I.; Alenezi, F.N.; Vallat, A.; Luptakova, L.; Oszako, T.; Cherrad, S.; Vacher, S.; Rateb, M.E. Comparative genomics of Bacillus amyloliquefaciens strains reveals a core genome with traits for adaptation to plant-associated habitats and a dynamic accessory genome with wealth of unsuspected new and unlocked secondary metabolites arsenal. Front. Microbil. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Mefteh, F.B.; Daoud, A.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Rateb, M.E.; Kadri, A.; Gharsallah, N.; Belbahri, L. Fungal root microbiome from healthy and brittle leaf diseased date palm trees (Phoenix dactylifera L.) reveals a hidden untapped arsenal of antibacterial and broad spectrum antifungal secondary metabolites. Front. Microbiol. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, F.N.; Weitz, H.J.; Belbahri, L.; Nidhal, J.; Luptakova, L.; Jaspars, M.; Woodward, S. Draft genome sequence of Aneurinibacillus migulanus NCTC 7096. Genome Announc. 2015, 3, e00234-15. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, F.N.; Weitz, H.J.; Belbahri, L.; Ben Rebah, H.; Luptakova, L.; Jaspars, M.; Woodward, S. Draft genome sequence of Aneurinibacillus migulanus strain Nagano. Genome Announc. 2015, 3, e00232-15. [Google Scholar] [CrossRef] [PubMed]
- Belbahri, L.; Alenezi, F.N.; Luptakova, L.; Rateb, M.E.; Woodward, S. Complete genome sequence of Aneurinibacillus migulanus E1, a Gramicidin S- and D-phenylalanyl-l-propyl diketopiperazine-deficient mutant. Genome Announc. 2015, 3, e01441-15. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinf. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.N.; Kirzinger, W.B.; Stavrindes, J. Draft genome sequence of the antibiotic-producing cystic fibrosis isolates Pantoea agglomerans Tx10. Genome Announc. 2013, 1, e00904-13. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.A.I.; Zumoff, C.H.; Schneider, L.; Beer, S.V. Pantoea agglomerans strain Eh318 produces two antibiotics that inhibit Erwinia amylovora in vitro. Appl. Environ. Microbiol. 2001, 67, 284–292. [Google Scholar] [CrossRef]
- Lim, J.A.; Lee, D.H.; Kim, B.Y.; Heu, S. Draft genome sequence of Pantoe aagglomerans R190, a producer of antibiotics against phytopathogens and food borne pathogens. J. Biotechnol. 2014, 188, 7–8. [Google Scholar] [CrossRef]
- Palmer, M.; de Maayer, P.; Poulsen, M.; Steenkamp, E.T.; van Zyl, E.; Coutinho, T.A.; Venter, S.N. Draft genome sequences of Pantoea agglomerans and Pantoea vagans isolates associated with termites. Stand. Genom. Sci. 2016, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Mori, K.; Kadowaki, T.; Shimada, M.; Tashiro, K.; Kuhara, S.; Inagawa, H.; Soma, G.I.; Takegawa, K. Genome sequence of Pantoea agglomerans strain IG1. Genome Announc. 2012, 194, 1258–1259. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.; Cortese, C.; da Silva, D.P.; Venturi, V.; Torelli, E.; Firrao, G. Draft Genome sequence of a hypersensitive reaction-inducing Pantoea agglomerans strain isolated from olive knots caused by Pseudomonas savastanoi pv. savastanoi Roberto Buonaurioa. Genome Announc. 2014, 2, e00774-14. [Google Scholar] [CrossRef] [PubMed]
- Corsini, G.; Valdes, N.; Pradel, P.; Tello, M.; Cottet, L.; Muiño, L.; Karahanian, E.; Castillo, A.; Gonzalez, A.R. Draft genome sequence of a copper-resistant marine bacterium, Pantoea agglomerans strain LMAE-2, a bacterial strain with potential use in bioremediation. Genome Announc. 2016, 4, e00525-16. [Google Scholar] [CrossRef] [PubMed]
- Smits, T.H.M.; Rezzonico, F.; Blom, J.; Goesmann, A.; Abelli, A.; Morelli, R.K.; Vanneste, J.L.; Duffy, B. Draft genome sequence of the commercial biocontrol strain Pantoea agglomerans P10c. Genome Announc. 2015, 3, e01448-15. [Google Scholar] [CrossRef] [PubMed]
- Smits, T.H.M.; Rezzonico, F.; Kamber, T.; Goesmann, A.; Ishimaru, C.A.; Virginia, O.; Stockwell, V.O.; Frey, J.E.; Duffy, B. Genome sequence of the biocontrol agent Pantoea vagans strain C9-1. J. Bacteriol. 2010, 192, 6486–6487. [Google Scholar] [CrossRef] [PubMed]
- Remus-Emsermann, M.N.P.; Kim, E.B.; Marco, M.L.; Tecon, R.; Leveau, J.H.J. Draft genome sequence of the phyllosphere model bacterium Pantoea agglomerans 299R. Genome Announc. 2013, 1, e00036-13. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Müller, R.; Wohlleben, W.; et al. antiSMASH 3.0—A comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Dejong, C.A.; Rees, P.N.; Johnston, C.W.; Li, H.; Webster, A.L.H.; Wyatt, M.A.; Magarvey, N.A. Genomes to natural products prediction informatics for secondary metabolomes (PRISM). Nucleic Acids Res. 2015, 43, 9645–9662. [Google Scholar] [CrossRef]
- Ziemert, N.; Podell, S.; Penn, K.; Badger, J.H.; Allen, E.; Jensen, P.R. The natural product domain seeker NaPDoS: A phylogeny based bioinformatic tool to classify secondary metabolite gene diversity. PLoS ONE 2012, 7, e34064. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.T.; Ung, P.M.U.; Zajkowski, J.; Garneau-Tsodikova, S.; Sherman, D.H. Automated genome mining for natural products. BMC Bioinf. 2009, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, A.J.; de Jong, A.; Montalban-Lopez, M.; Kok, J.; Kuipers, O.P. BAGEL3: Automated identification of genes encoding bacteriocins and (non-)bactericidal post translationally modified peptides. Nucleic Acids Res. 2013, 41, W448–W453. [Google Scholar] [CrossRef] [PubMed]
- Tettelin, H.; Riley, D.; Cattuto, C.; Medini, D. Comparative genomics: The bacterial pan-genome. Curr. Opin. Microbiol. 2008, 11, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Wang, H.; Shao, H.B. Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front. Plant Sci. 2015, 6, 792. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ortega, C.O.; Ochoa-Alfaro, A.E.; Reyes-Agüero, J.A.; Aguado-Santacruz, G.A.; Jiménez-Bremont, J.F. Salt stress increases the expression of p5cs gene and induces proline accumulation in cactus pear. Plant Phys. Biochem. 2008, 46, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.; Cherif-Silini, H.; Yahiaoui, B. Growing varieties durum wheat (Triticum durum) in response to the effect of osmolytes and inoculation by Azotobacter chroococcum under salt stress. Afr. J. Microbiol. Res. 2016, 10, 387–399. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galvan, A.; Pardo-Diaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Gulati, A.; Nayyar, H. Indigenous salt-tolerant rhizobacterium Pantoea dispersa (PSB3) reduces sodium uptake and mitigates the effects of salt stress on growth and yield of chickpea. Acta Physiol. Plant 2016, 38, 278. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Nayyar, H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp improve seed yield of Mungbean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol. Mol. Biol. Plants 2016, 22, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Páliz, K.I.; Caviedes, M.A.; Doukkali, B.; Mateos-Naranjo, E.; Rodríguez-Llorente, I.D.; Pajuelo, E. Screening beneficial rhizobacteria from Spartina maritima for phytoremediation of metal polluted salt marshes: Comparison of gram-positive and gram-negative strains. Environ. Sci. Pollut. Res. Int. 2016, 23, 19825–19837. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Páliz, K.I.; Pajuelo, E.; Doukkali, B.; Caviedes, M.Á.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Bacterial inoculants for enhanced seed germination of Spartina densiflora: Implications for restoration of metal polluted areas. Mar. Pollut. Bull. 2016, 110, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, X.; Xie, R.; Fu, L.; Jiang, J.; Gao, L.; Sun, J. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol. Biofuels 2016, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Walitang, D.I.; Kim, C.G.; Kim, K.; Kang, Y.; Kim, Y.K.; Sa, T. The influence of host genotype and salt stress on the seed endophytic community of salt-sensitive and salt-tolerant rice cultivars. BMC Plant Biol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Suresh, T. Isolation and Characterization of Bioactive Compounds from Marine Bacteria. Ph.D. Thesis, Animal and Fisheries Sciences University, Bidar, Degan Sultan, 2009. [Google Scholar]
- De Carvalho, M.P.; Abraham, W.R. Antimicrobial and biofilm inhibiting diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef]
- Degrassi, G.; Aguilar, C.; Bosco, M.; Zahariev, S.; Pongor, S.; Venturi, V. Plant growth-promoting Pseudomonas putida WCS358 produces and secretes four cyclic dipeptides: Cross-Talk with quorum sensing bacterial sensors. Curr. Microbiol. 2002, 45, 250–254. [Google Scholar] [CrossRef]
- Mefteh, B.F.; Daoud, A.; Chenari Bouket, A.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date palm trees root-derived endophytes as fungal cell factories for diverse bioactive metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef]
- Li, L.; Hou, X.; Tsuge, T.; Ding, M.; Aoyama, T.; Oka, A.; Gu, H.; Zhao, Y.; Qu, L. The possible action mechanisms of indole-3-acetic acid methyl ester in Arabidopsis. Plant Cell Rep. 2008, 27, 575–584. [Google Scholar] [CrossRef]
- Lehmann, T.; Hoffmann, M.; Hentrich, M.; Pollmann, S. Indole-3-acetamide-dependent auxin biosynthesis: A widely distributed way of indole-3-acetic acid production? Eur. J. Cell Biol. 2010, 89, 895–905. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Ghoul, M.; Yadav, S. Isolation and characterization of plant growth promoting traits of a rhizobacteria: Pantoea agglomerans lma2. Pak. J. Biol. Sci. 2012, 15, 267–276. [Google Scholar] [CrossRef]
- Dworkin, M.; Foster, J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [PubMed]
- Gaur, A.C. Physiological functions of phosphate solubilizing micro-organisms. In Phosphate Solubilizing Micro-Organisms as Biofertilizers; Gaur, A.C., Ed.; Omega Scientific Publisher: New Delhi, India, 1990; pp. 16–72. [Google Scholar]
- Olsen, R.S.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, 2nd ed.; Part 2: Chemical and microbiological properties; Pages, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Mefteh, B.F.; Frikha, F.; Daoud, A.; Chenari Bouket, A.; Luptakova, L.; Alenezi, F.N.; Al-Anzi, B.S.; Oszako, T.; Gharsallah, N.; Belbahri, L. Response surface methodology optimization of an acidic protease produced by Penicillium bilaiae isolate TDPEF30, a newly recovered endophytic fungus from healthy roots of date palm trees (Phoenix dactylifera L.). Microorganisms 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Jacobson, C.B.; Schwarze, M.M.K.; Pasternak, J.J. 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR12-2 do not stimulate canola root elongation. Can. J. Microbiol. 1994, 40, 911–915. [Google Scholar] [CrossRef]
- Mlaik, N.; Bakonyi, J.; Borsodi, A.; Woodward, S.; Belbahri, L.; Mechichi, T. Microbial diversity in tanning wastewaters treatment reactors. Environ. Prog. Sustain. Energy 2015, 34, 401–410. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Lane, D. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bertels, F.; Silander, O.K.; Pachkov, M.; Rainey, P.B.; van Nimwegen, E. Automated reconstruction of whole-genome phylogenies from short-sequence reads. Mol. Biol. Evol. 2014, 31, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, D.G.; Dobereiner, J. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Can. J. Microbiol. 1978, 24, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Shweta, B.; Maheshwari, D.K.; Dubey, R.C.; Arora, D.S.; Bajpai, V.K.; Kang, S.C. Beneficial effects of fluorescent pseudomonads on seed germination, growth promotion, and suppression of charcoal rot in groundnut (Arachis hypogea L.). J. Microbiol. Biotechnol. 2008, 18, 1578–1583. [Google Scholar] [PubMed]
- Naidu, B.P.; Cameron, D.F.; Konduri, S.V. Improving drought tolerance of cotton by glycine betaine application and selection. In Proceedings of the 9th Australian Agronomy Conference, Wagga Wagga, Australia, 20–23 July 1998. [Google Scholar]
- Singh, T.N.; Aspinall, D.; Paleg, L.G.; Bogges, S.F. Stress metabolism. II. Changes in proline concentration in excised plant tissues. Aust. J. Biol. Sci. 1973, 26, 57–63. [Google Scholar] [CrossRef]
- Macintyre, L.; Zhang, T.; Viegelmann, C.; Martinez, I.J.; Cheng, C.; Dowdells, C.; Abdelmohsen, U.R.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R. Metabolomic tools for secondary metabolite discovery from marine microbial symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef] [PubMed]
- Ozer, E.A.; Allen, J.P.; Hauser, A.R. Characterization of the core and accessory genomes of Pseudomonas aeruginosa using bioinformatic tools Spine and AGEnt. BMC Genomics 2014, 15, 737. [Google Scholar] [CrossRef]
- Chaudhari, N.M.; Gupta, V.K.; Dutta, C. BPGA—An ultra-fast pan-genome analysis pipeline. Sci. Rep. 2016, 6, 24373. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [PubMed]

| Species | Strain | Genome Size (Mb) | Plasmid | Description/References | GenBank Accession | |
|---|---|---|---|---|---|---|
| Pantoea agglomerans | 1 | C410P1 (Complete) | 5.11524 | Y | Isolated from lettuce in China (Anhui province); Biocontrol agent | CP016889.1 |
| 2 | FDAARGOS_160 (Complete) | 4.80881 | Y | Isolated from wound swab of human (Homo sapiens); Children’s National Hospital, the United States | CP014129.1 | |
| 3 | Tx10 (Scaffold) | 4.85699 | N | Isolated from sputum from a cystic fibrosis patient (Homo sapiens) in Canada; produces multiple antibiotics that are effective against Erwinia amylovora, Staphylococcus aureus, S. epidermidis, and Escherichia coli; biocontrol agent [28] | ASJI00000000.1 | |
| 4 | Eh318 (Scaffold) | 5.03584 | N | Isolated from the stem of an asymptomatic apple (NY, the United States); produces two antibiotics, named pantocin A and B, that are effective against Erwinia amylovora [29] | AXOF00000000.1 | |
| 5 | 190 (Scaffold) | 5.00257 | N | Isolated from soil in South Korea [30] | JNGC00000000.1 | |
| 6 | MP2 (Scaffold) | 4.73383 | N | Isolated from fungus-growing termites (Macrotermes natalensis) in South Africa [31] | JPKQ00000000.1 | |
| 7 | IG1 (Contig) | 4.82958 | N | Isolated from Japan; grows symbiotically with various plants; the lipopolysaccharides derived from P. agglomerans IG1 were effective in the prevention of various diseases, such as bacterial or viral infection, lifestyle-related diseases [32] | BAEF00000000.1 | |
| 8 | NBRC 102470 (contig) | 4.65204 | N | Isolated from Japan | BCZA00000000.1 | |
| 9 | DAPP-PG734 (Contig) | 5.36593 | N | A hypersensitive reaction-inducing Pantoea agglomerans strain isolated from olive knots caused by Pseudomonas savastanoi pv. savastanoi [33] | JNVA00000000.1 | |
| 10 | 4 (Scaffold) | 4.82789 | N | Isolated from wheat (Triticum aestivum) seed in Canada | JPOT00000000.2 | |
| 11 | 3 (Scaffold) | 4.81358 | N | Isolated from wheat (Triticum aestivum) seed in Canada | LVHW00000000.1 | |
| 12 | 824-1 (Scaffold) | 4.98907 | N | Elicits galls on Gypsophila paniculata and hypersensitive response (HR) on beet; isolated from NY (the United States) | LXSX00000000.1 | |
| 13 | 4188 (Scaffold) | 5.002 | N | Isolated from Beta vulgaris in NY (the United States); causes galls on beet and gypsophila | LXSW00000000.1 | |
| 14 | P5 (Scaffold) | 3.52764 | N | Isolated from soil (desert) in Iran | NGNU00000000.1 | |
| 15 | NFPP29(Scaffold) | 4.10462 | N | - | FUWI00000000.1 | |
| Pantoea agglomerans | 16 | LMAE-2 (Contig) | 4.98116 | N | Isolated from seabed sediment moderately contaminated with Cu2+ in Puerto Montt (Chile); a copper-resistant marine bacterium with potential use in bioremediation [34] | JWLQ00000000.1 |
| 17 | P10c (Contig) | 4.77592 | Y | Isolated from pear blossoms in New Zealand; ability to rapidly colonize apple and pear flowers and to suppress fire blight disease; developed as a commercial biocontrol agent [35] | LIME00000000.1 | |
| 18 | DSM 3493 (contig) | 4.65938 | N | Type strain of Pantoea agglomerans | FYAZ00000000.1 | |
| 19 | Pa (Contig) | 4.79516 | N | Isolated from rhizosphere of durum wheat in arid environment Bousaada (Algeria) | MUJJ00000000.1 | |
| Pantoea vagans | 20 | ZBG6 (Contig) | 4.729 | N | Isolated from soil in Zellenberg (France) | LFQL00000000.1 |
| 21 | PaVv11 (Scaffold) | 4.85077 | N | - | CEFP00000000.1 | |
| 22 | C9-1 (Complete) | 4.88834 | Y | Commercially registered for biological control of fire blight, a disease of pear and apple trees caused by Erwinia amylovora [36] | CP002206.1 | |
| 23 | MP7 (Scaffold) | 4.5987 | N | Isolated from fungus-growing termites (Macrotermes natalensis) in South Africa [31] | JPKP00000000.1 | |
| 24 | RIT273 (Contig) | 5.36534 | N | Isolated from Willow (Salix sp.) in NY (the United States) | JFOK00000000.1 | |
| 25 | 299R (Contig) | 4.58148 | N | A spontaneous rifampin-resistant derivative of the phyllosphere model bacterium isolate 299, a pigmented bacterium that was recovered from healthy leaves of a Bartlett pear tree near Healdsburg, CA [37] | ANKX00000000.1 | |
| 26 | 848_PVAG (Contig) | 4.99032 | N | Isolated from human (Homo sapiens) in WA (the United States) | JUQR00000000.1 | |
| 27 | LMG 24199 (Complete) | 4.79033 | 2 | Type strain of Pantoea vagans | CP038853.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherif-Silini, H.; Thissera, B.; Bouket, A.C.; Saadaoui, N.; Silini, A.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Luptakova, L.; Yahiaoui, B.; et al. Durum Wheat Stress Tolerance Induced by Endophyte Pantoea agglomerans with Genes Contributing to Plant Functions and Secondary Metabolite Arsenal. Int. J. Mol. Sci. 2019, 20, 3989. https://doi.org/10.3390/ijms20163989
Cherif-Silini H, Thissera B, Bouket AC, Saadaoui N, Silini A, Eshelli M, Alenezi FN, Vallat A, Luptakova L, Yahiaoui B, et al. Durum Wheat Stress Tolerance Induced by Endophyte Pantoea agglomerans with Genes Contributing to Plant Functions and Secondary Metabolite Arsenal. International Journal of Molecular Sciences. 2019; 20(16):3989. https://doi.org/10.3390/ijms20163989
Chicago/Turabian StyleCherif-Silini, Hafsa, Bathini Thissera, Ali Chenari Bouket, Nora Saadaoui, Allaoua Silini, Manal Eshelli, Faizah N. Alenezi, Armelle Vallat, Lenka Luptakova, Bilal Yahiaoui, and et al. 2019. "Durum Wheat Stress Tolerance Induced by Endophyte Pantoea agglomerans with Genes Contributing to Plant Functions and Secondary Metabolite Arsenal" International Journal of Molecular Sciences 20, no. 16: 3989. https://doi.org/10.3390/ijms20163989
APA StyleCherif-Silini, H., Thissera, B., Bouket, A. C., Saadaoui, N., Silini, A., Eshelli, M., Alenezi, F. N., Vallat, A., Luptakova, L., Yahiaoui, B., Cherrad, S., Vacher, S., Rateb, M. E., & Belbahri, L. (2019). Durum Wheat Stress Tolerance Induced by Endophyte Pantoea agglomerans with Genes Contributing to Plant Functions and Secondary Metabolite Arsenal. International Journal of Molecular Sciences, 20(16), 3989. https://doi.org/10.3390/ijms20163989







