A Novel Peptide Ameliorates LPS-Induced Intestinal Inflammation and Mucosal Barrier Damage via Its Antioxidant and Antiendotoxin Effects
Abstract
1. Introduction
2. Results
2.1. Peptide Design and Characterisation
2.2. Cytotoxicity on RAW264.7 Macrophage Cells
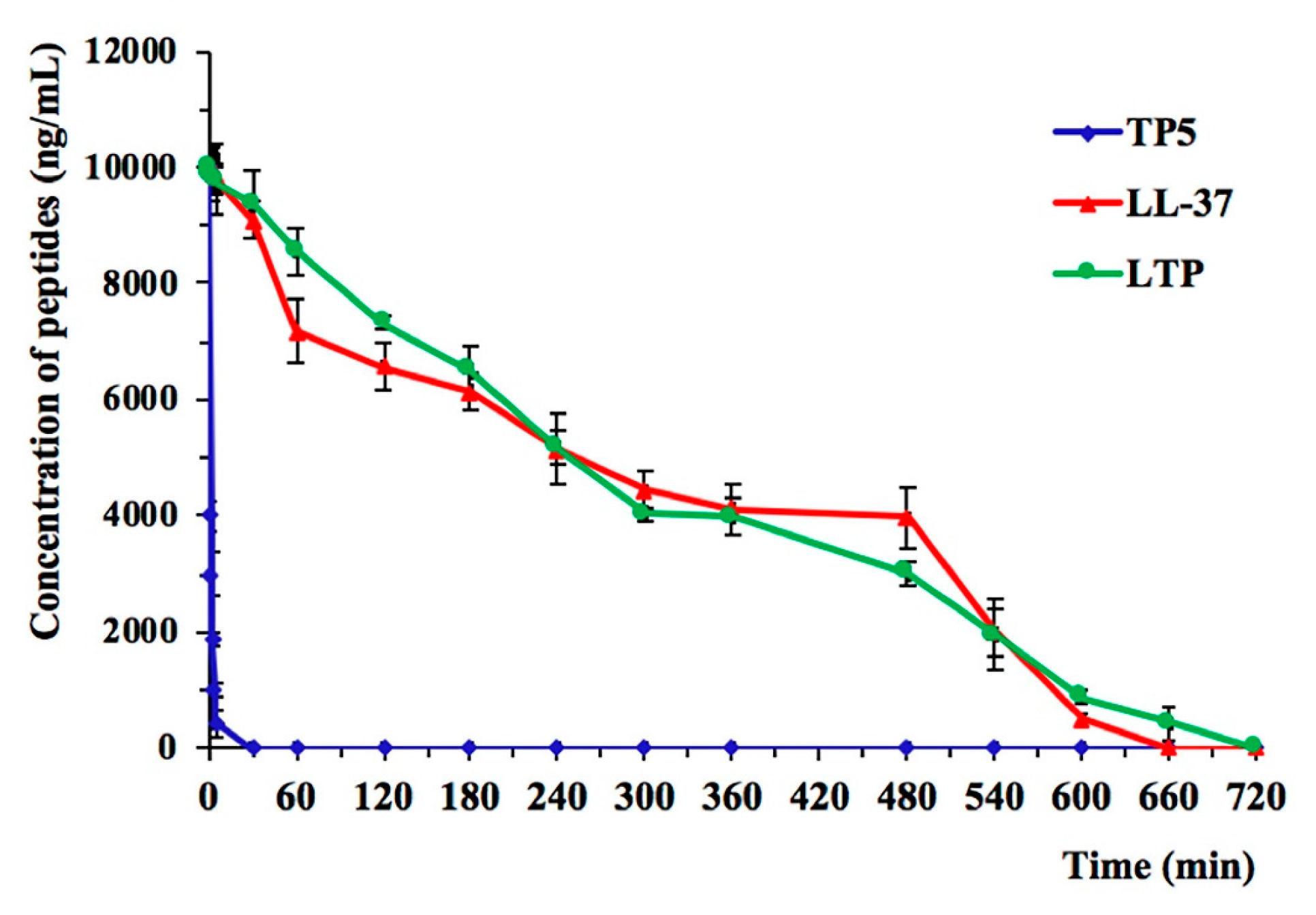
2.3. Ex Vivo Stability of LTP in Plasma
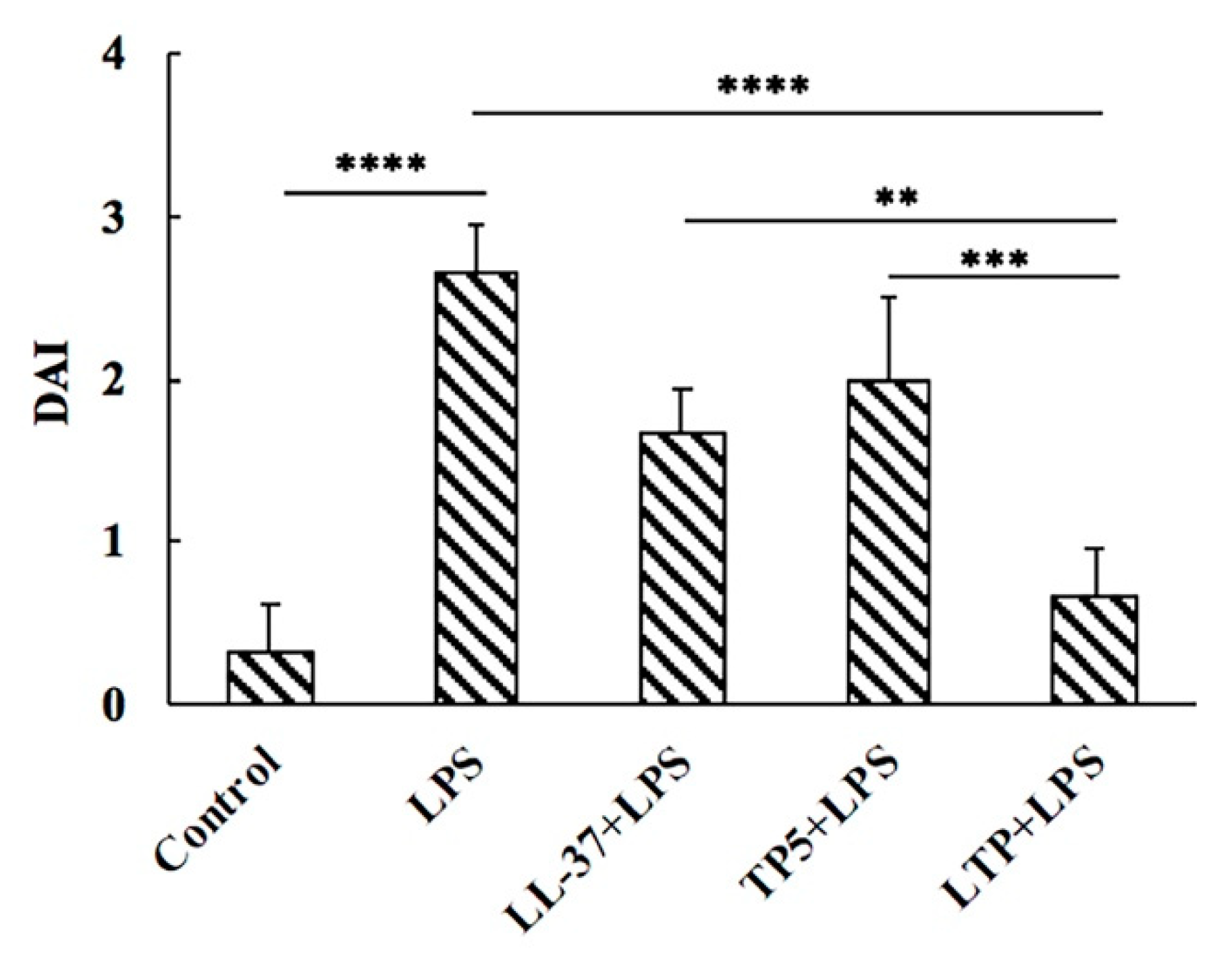
2.4. Effect of LTP on Body Weight and Disease Activity Index
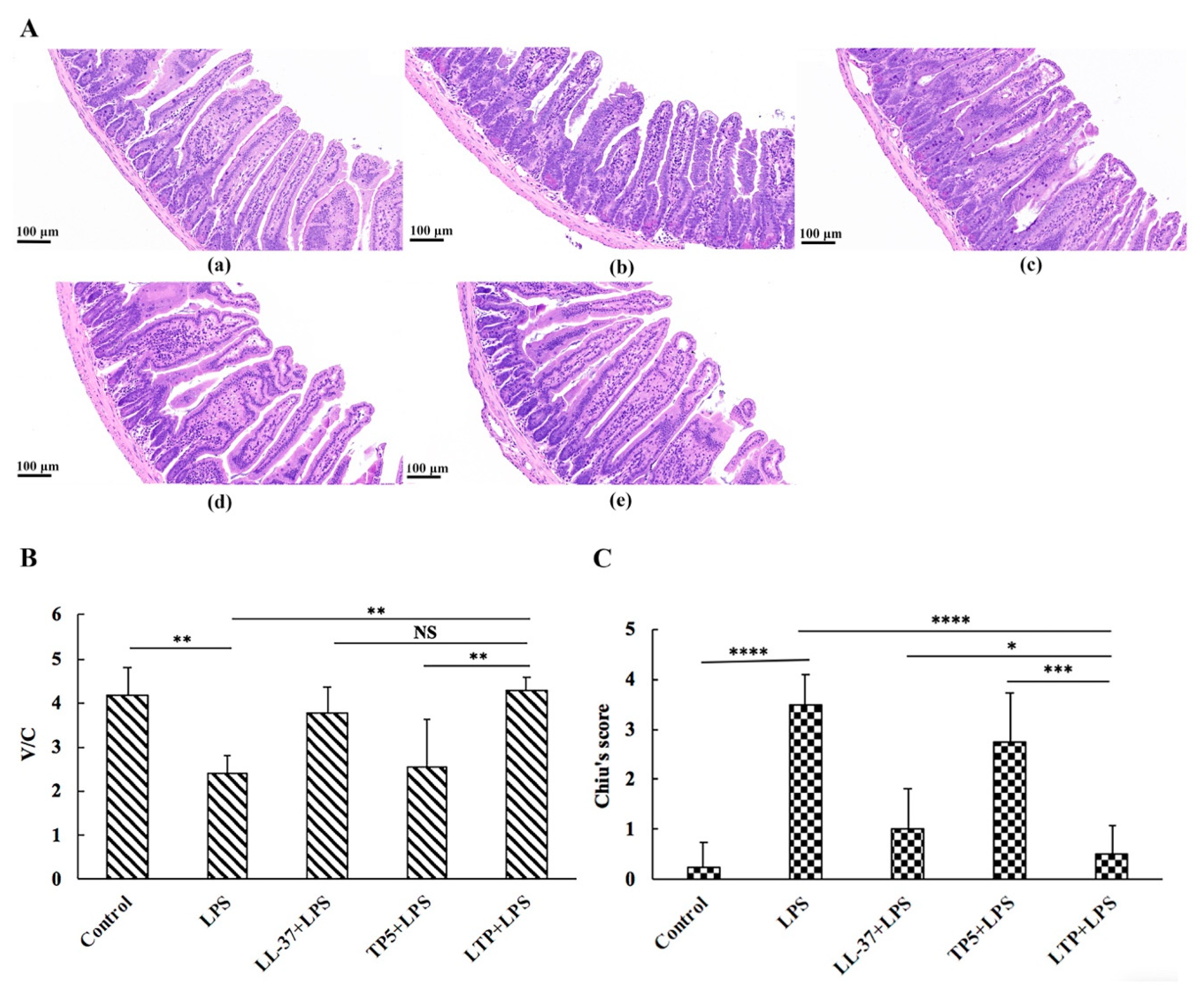
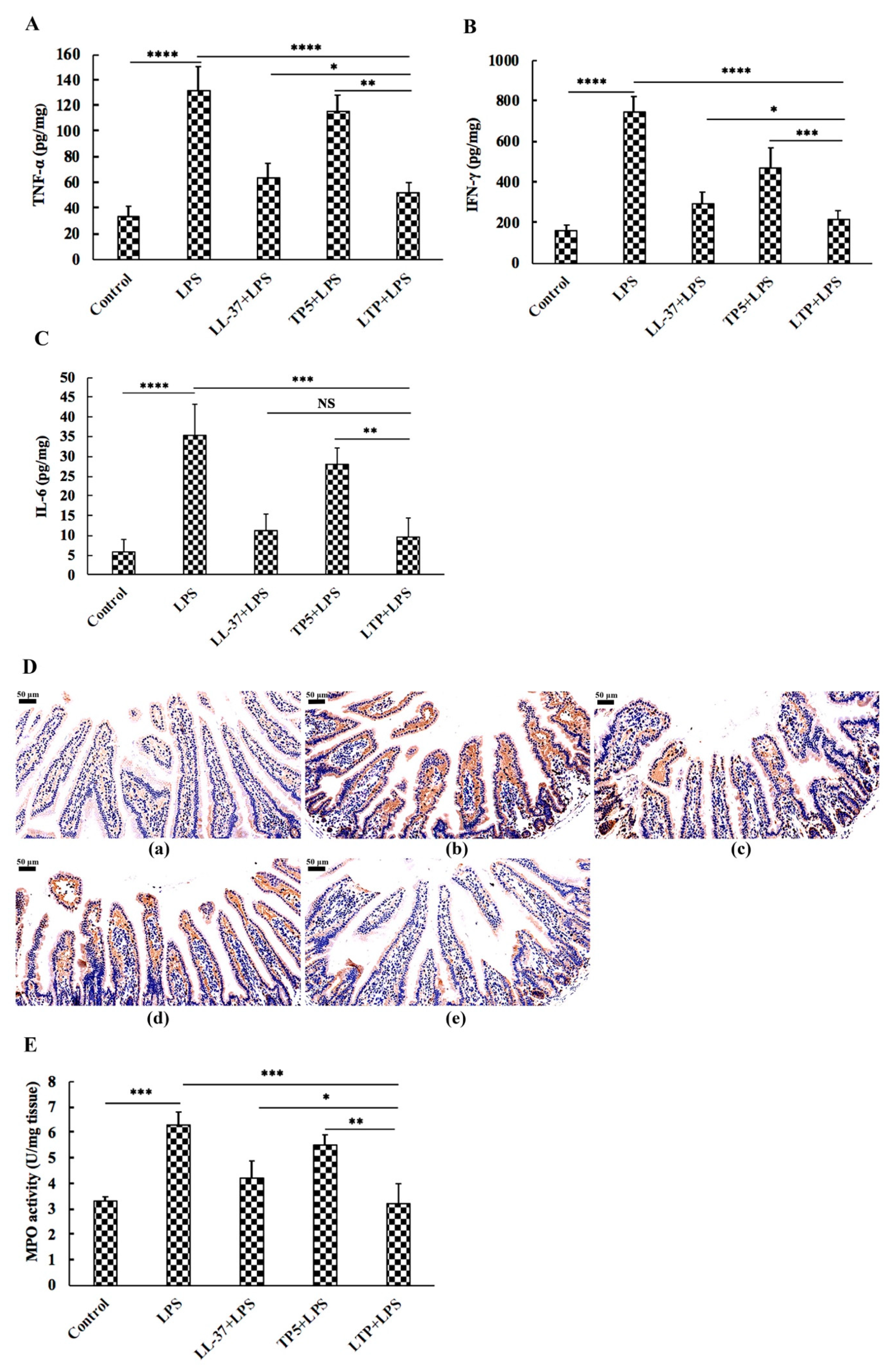
2.5. The Protective Effects of LTP Against LPS-Induced Damage in Jejunum Tissue
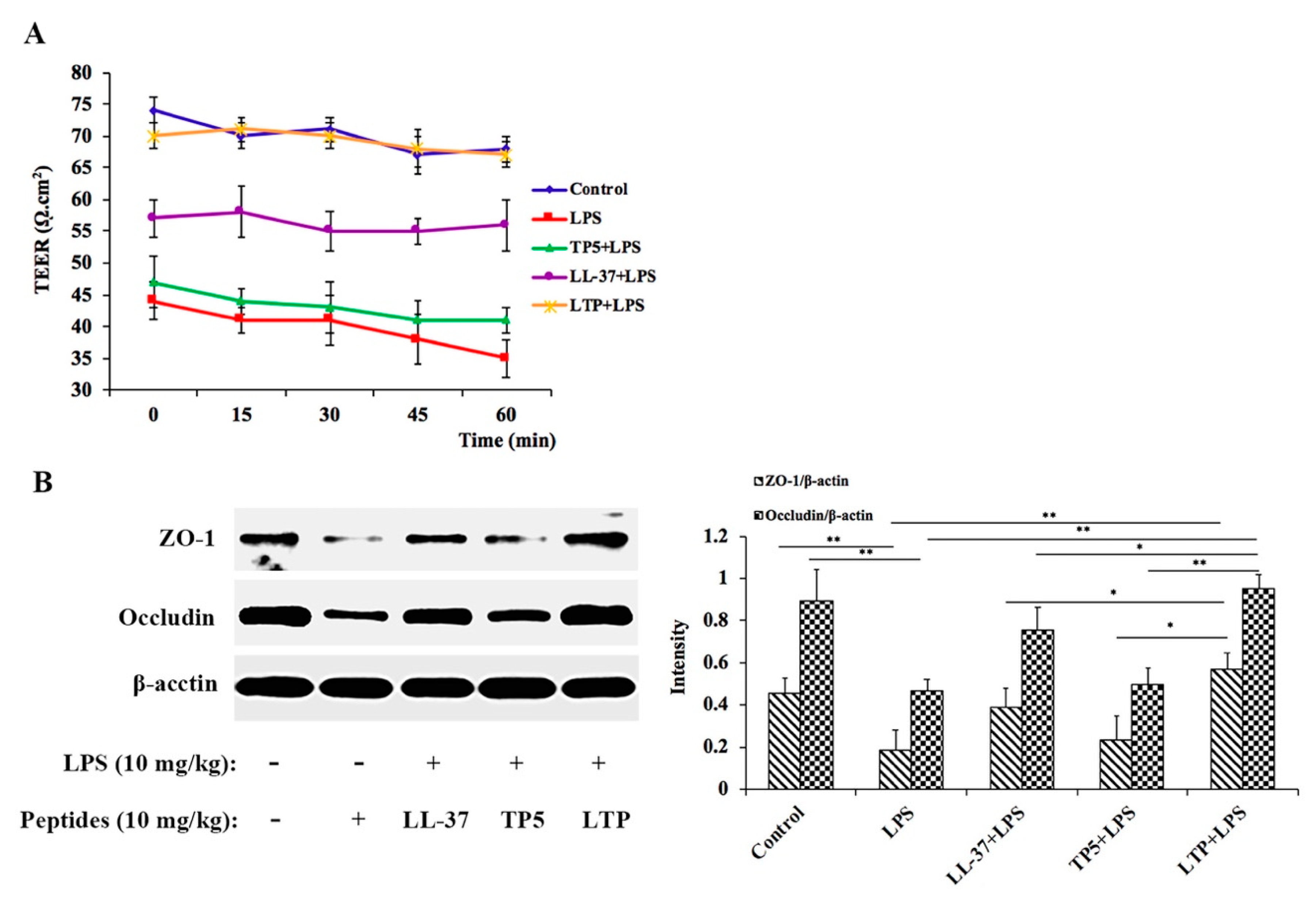
2.6. LTP Attenuated the LPS-Induced Disruption of Intestinal TJ Structure and Function
2.7. The Protective Effects of LTP Against LPS-Induced Oxidative Stress in Jejunum Tissue
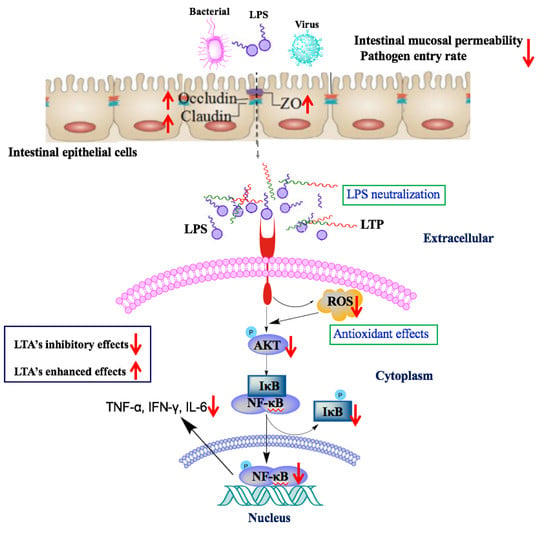
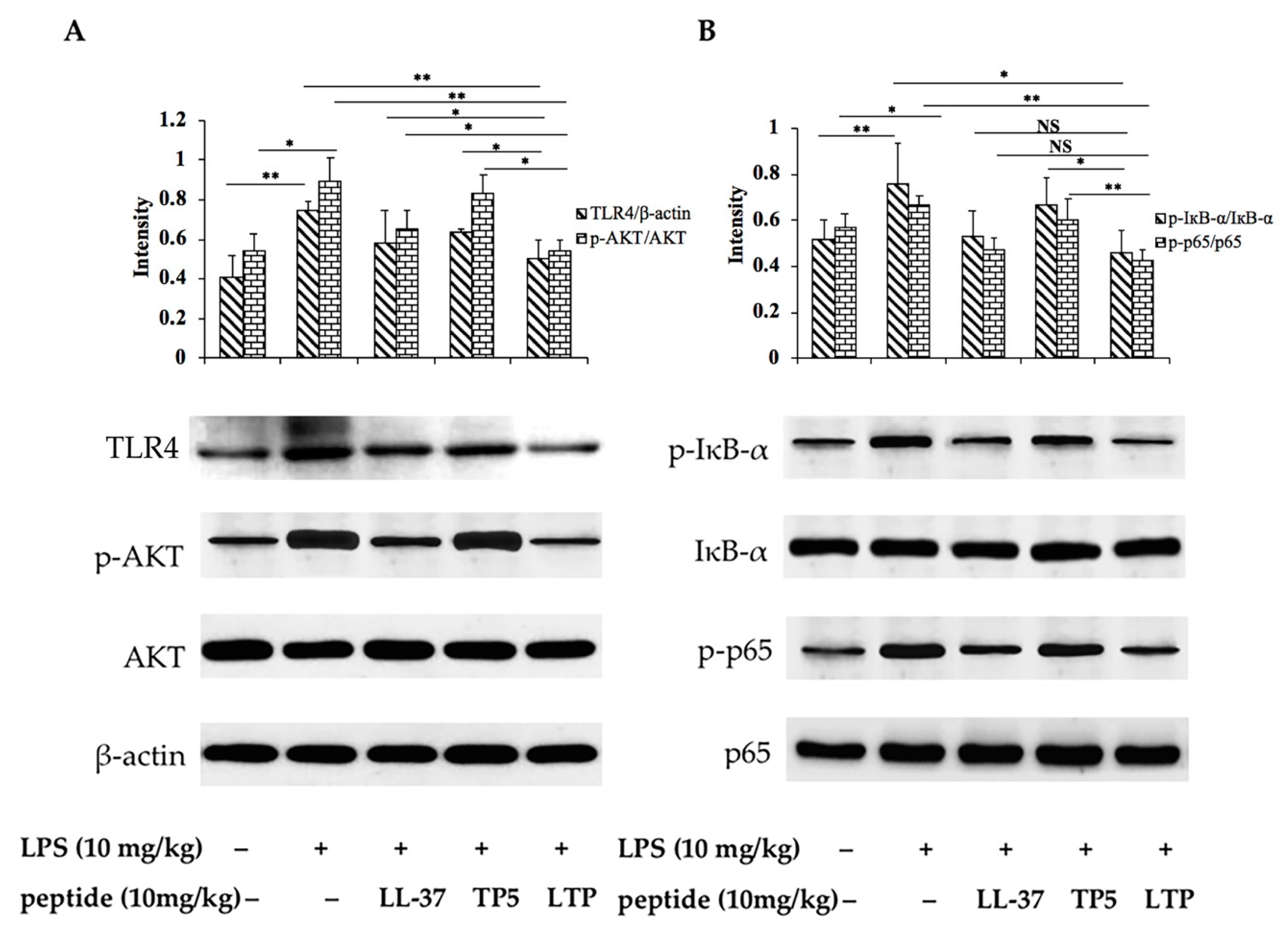
2.8. Effects of LTP on the NF-κB Signalling Pathway in LPS-Induced Mice
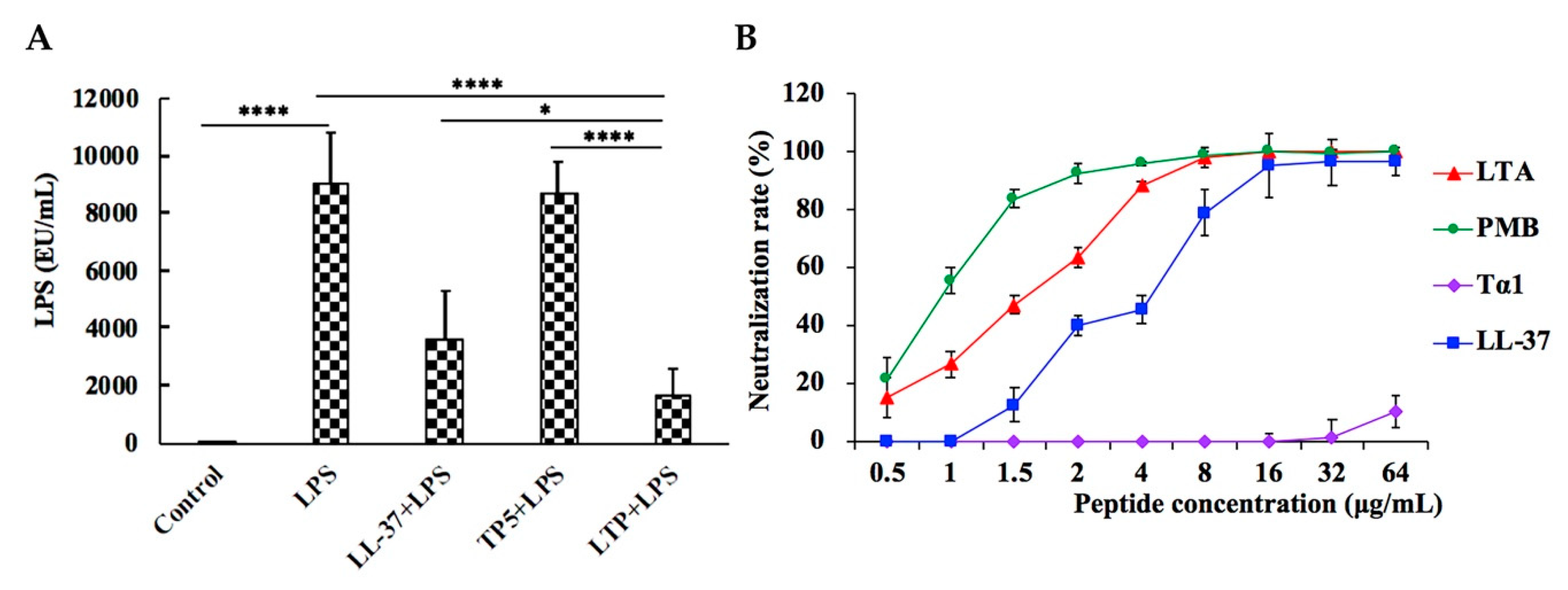
2.9. LPS Neutralization Activity of LTP in Vitro and in Vivo
3. Discussion
4. Materials and Methods
4.1. Hybrid Peptide Design
4.2. Sequence Analysis of the Hybrid Peptide
4.3. Peptide Synthesis
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Ex Vivo Stability of LTP in Plasma
4.7. Animal Model
4.8. Evaluation of Intestinal Inflammation
4.9. Histopathology and Immunohistochemistry
4.10. ELISA
4.11. Antioxidant Capacity and Antioxidant Enzymes
4.12. Western Blotting
4.13. Transmission Electron Microscopy (TEM)
4.14. Electrophysiology Measurements
4.15. Neutralization of LPS
4.16. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stromberg, Z.R.; Van Goor, A.; Redweik, G.A.J.; Brand, M.J.W.; Wannemuehler, M.J.; Mellata, M. Pathogenic and non-pathogenic Escherichia coli colonization and host inflammatory response in a defined microbiota mouse model. Dis. Model. Mech. 2018, 11, dmm035063. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Hu, W.Y.; Song, D.G.; Li, Z.; Du, H.H.; Lu, Z.Q.; Wang, Y.Z. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem. Pharmacol. 2016, 104, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Stoll, B.; Guan, X.F.; Cui, L.W.; Chang, X.Y.; Holst, J.J. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology 2005, 146, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Gillen, C.D.; Walmsley, R.S.; Prior, P.; Andrews, H.A.; Allan, R.N. Ulcerative colitis and Crohn’s disease: A comparison of the colorectal cancer risk in extensive colitis. Gut 1994, 35, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer risk in patients with inflammatory bowel disease—A population-based study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Torres-Rodriguez, L.; Garcia-Chavez, E.; Berhow, M.; de Mejia, E.G. Anti-inflammatory and anti-oxidant effect of Calea urticifolia lyophilized aqueous extract on lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2016, 188, 266–274. [Google Scholar] [CrossRef]
- Kornicka, K.; Al Naem, M.; Rocken, M.; Zmiertka, M.; Marycz, K. Osteochondritis Dissecans (OCD)-Derived Chondrocytes Display Increased Senescence, Oxidative Stress, Chaperone-Mediated Autophagy and, in Co-Culture with Adipose-Derived Stem Cells (ASCs), Enhanced Expression of MMP-13. J. Clin. Med. 2019, 8, 328. [Google Scholar] [CrossRef]
- Papada, E.; Forbes, A.; Amerikanou, C.; Torovic, L.; Kalogeropoulos, N.; Tzavara, C.; Triantafillidis, J.K.; Kaliora, A.C. Antioxidative Efficacy of a Pistacia Lentiscus Supplement and Its Effect on the Plasma Amino Acid Profile in Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1779. [Google Scholar] [CrossRef]
- Boumpas, D.T.; Chrousos, G.P.; Wilder, R.L.; Cupps, T.R.; Balow, J.E. Glucocorticoid Therapy for Immune-Mediated Diseases—Basic and Clinical Correlates. Ann. Intern. Med. 1993, 119, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Faubion, W.A.; Loftus, E.V.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology 2001, 121, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Castagliuolo, I.; Karalis, K.; Valenick, L.; Pasha, A.; Nikulasson, S.; Wlk, M.; Pothoulakis, C. Endogenous corticosteroids modulate Clostridium difficile toxin A-induced enteritis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G539–G545. [Google Scholar] [CrossRef] [PubMed]
- Smoak, K.A.; Cidlowski, J.A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech. Ageing Dev. 2004, 125, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Anstead, G.M. Steroids, retinoids, and wound healing. Adv. Wound Care 1998, 11, 277–285. [Google Scholar] [PubMed]
- Stanbury, R.M.; Graham, E.M. Systemic corticosteroid therapy—Side effects and their management. Br. J. Ophthalmol. 1998, 82, 704–708. [Google Scholar] [CrossRef]
- Akiyama, T.; Niyonsaba, F.; Kiatsurayanon, C.; Nguyen, T.T.; Ushio, H.; Fujimura, T.; Ueno, T.; Okumura, K.; Ogawa, H.; Ikeda, S. The Human Cathelicidin LL-37 Host Defense Peptide Upregulates Tight Junction-Related Proteins and Increases Human Epidermal Keratinocyte Barrier Function. J. Innate Immun. 2014, 6, 739–753. [Google Scholar] [CrossRef]
- Yu, J.; Mookherjee, N.; Wee, K.; Bowdish, D.M.E.; Pistolic, J.; Li, Y.X.; Rehaume, L.; Hancock, R.E.W. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1 beta, augments immune responses by multiple pathways. J. Immunol. 2007, 179, 7684–7691. [Google Scholar] [CrossRef]
- Barlow, P.G.; Li, Y.X.; Wilkinson, T.S.; Bowdish, D.M.E.; Lau, Y.E.; Cosseau, C.; Haslett, C.; Simpson, A.J.; Hancock, R.E.W.; Davidson, D.J. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 2006, 80, 509–520. [Google Scholar] [CrossRef]
- Jonsson, D.; Nilsson, B.O. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J. Periodontal Res. 2012, 47, 330–335. [Google Scholar] [CrossRef]
- Taniguchi, M.; Noda, Y.; Aida, R.; Saito, K.; Ochiai, A.; Saitoh, E.; Tanaka, T. Cationic peptides from enzymatic hydrolysates of soybean proteins exhibit LPS-neutralizing and angiogenic activities. J. Biosci. Bioeng. 2019, 127, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, G.; Scheid, M.P.; Boyse, E.A.; Schlesinger, D.H.; Vanwauwe, J. Synthetic Pentapeptide with Biological-Activity Characteristic of the Thymic Hormone Thymopoietin. Science 1979, 204, 1309–1310. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Biswas, S.; Mathur, K.B.; Haq, W.; Garg, S.K.; Agarwal, S.S. Thymopentin and splenopentin as immunomodulators—Current status. Immunol. Res. 1998, 17, 345–368. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, E.G.; Lunin, S.M.; Khrenov, M.O.; Novoselova, T.V.; Fesenko, E.E. Involvement of NF-kappaB transcription factor in the antiinflammatory activity of thymic peptides. Dokl. Biol. Sci. 2009, 428, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J. Thymulin and zinc (Zn2+)-mediated inhibition of endotoxin-induced production of proinflammatory cytokines and NF-kappaB nuclear translocation and activation in the alveolar epithelium: Unraveling the molecular immunomodulatory, anti-inflammatory effect of thymulin/Zn2+ in vitro. Mol. Immunol. 2009, 47, 205–214. [Google Scholar]
- Henriques-Coelho, T.; Oliveira, S.M.; Moura, R.S.; Roncon-Albuquerque, R.; Neves, A.L.; Santos, M.; Nogueira-Silva, C.; Carvalho, F.L.F.; Brandao-Nogueira, A.; Correia-Pinto, J.; et al. Thymulin inhibits monocrotaline-induced pulmonary hypertension modulating interleukin-6 expression and suppressing p38 pathway. Endocrinology 2008, 149, 4367–4373. [Google Scholar] [CrossRef][Green Version]
- Mendling, W.; Koldovsky, U. Investigations by cell-mediated immunologic tests and therapeutic trials with thymopentin in vaginal mycoses. Infect. Dis. Obstet. Gynecol. 1996, 4, 225–231. [Google Scholar] [CrossRef]
- London, W.B.; Castel, V.; Monclair, T.; Ambros, P.F.; Pearson, A.D.J.; Cohn, S.L.; Berthold, F.; Nakagawara, A.; Ladenstein, R.L.; Iehara, T.; et al. Clinical and Biologic Features Predictive of Survival After Relapse of Neuroblastoma: A Report From the International Neuroblastoma Risk Group Project. J. Clin. Oncol. 2011, 29, 3286–3292. [Google Scholar] [CrossRef]
- Anders, E.; Dahl, S.; Svensson, D.; Nilsson, B.O. LL-37-induced human osteoblast cytotoxicity and permeability occurs independently of cellular LL-37 uptake through clathrin-mediated endocytosis. Biochem. Biophys. Res. Commun. 2018, 501, 280–285. [Google Scholar] [CrossRef]
- Gonser, S.; Crompton, N.E.A.; Folkers, G.; Weber, E. Increased radiation toxicity by enhanced apoptotic clearance of HL-60 cells in the presence of the pentapeptide thymopentin, which selectively binds to apoptotic cells. Mutat. Res.-Genet. Toxicol. Environ. 2004, 558, 19–26. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, M.; Wang, Y.J.; Wang, Y.N.; Zhao, S.R.; Wu, J.H.; Li, X.M.; Peng, S.Q. Tetrahydro-beta-carboline-3-carboxyl-thymopentin: A nano-conjugate for releasing pharmacophores to treat tumor and complications. J. Mater. Chem. B 2016, 4, 1384–1397. [Google Scholar] [CrossRef]
- Li, Y.Q.; Smith, C.; Wu, H.F.; Teng, P.; Shi, Y.; Padhee, S.; Jones, T.; Nguyen, A.M.; Cao, C.H.; Yin, H.; et al. Short Antimicrobial Lipo-alpha/gamma-AA Hybrid Peptides. ChemBioChem 2014, 15, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.B.; Wu, R.J.; Zhang, L.L.; Ahmad, B.; Si, D.Y.; Zhang, R.J. Expression, Purification, and Characterization of a Novel Hybrid Peptide with Potent Antibacterial Activity. Molecules 2018, 23, 1491. [Google Scholar] [CrossRef] [PubMed]
- Nell, M.J.; Tjabringa, G.S.; Wafelman, A.R.; Verrijk, R.; Hiemstra, P.S.; Drijfhout, J.W.; Grote, J.J. Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 2006, 27, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.H.; Bang, J.K.; Jacob, B.; Park, I.S.; Shin, S.Y. Prokaryotic selectivity and LPS-neutralizing activity of short antimicrobial peptides designed from the human antimicrobial peptide LL-37. Peptides 2012, 35, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Tischio, J.P.; Patrick, J.E.; Weintraub, H.S.; Chasin, M.; Goldstein, G. Short in vitro half-life of thymopoietin32--36 pentapeptide in human plasma. Int. J. Pept. Protein Res. 1979, 14, 479–484. [Google Scholar] [CrossRef]
- Shi, M.Y.; Yang, Y.; Zhou, X.T.; Cai, L.L.; Fang, C.X.; Wang, C.; Sun, H.P.; Sun, Y.T.; Gao, Y.; Gu, J.K.; et al. Determination of thymopentin in beagle dog blood by liquid chromatography with tandem mass spectrometry and its application to a preclinical pharmacokinetic study. J. Sep. Sci. 2015, 38, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.C.; de Araujo, A.A.; Araujo, D.F.D.; Lima, M.C.J.S.; Vasconcelos, R.C.; de Araujo, R.F.; Langasnner, S.M.Z.; Pedrosa, M.D.F.; de Medeiros, C.A.C.X.; Guerra, G.C.B. Intestinal Anti-Inflammatory Activity of the Aqueous Extract from Ipomoea asarifolia in DNBS-Induced Colitis in Rats. Int. J. Mol. Sci. 2018, 19, 4016. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar]
- Semple, F.; MacPherson, H.; Webb, S.; Cox, S.L.; Mallin, L.J.; Tyrrell, C.; Grimes, G.R.; Semple, C.A.; Nix, M.A.; Millhauser, G.L.; et al. Human beta-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur. J. Immunol. 2011, 41, 3291–3300. [Google Scholar] [CrossRef]
- Durand, N.; Russell, A.; Zubair, A.C. Effect of Comedications and Endotoxins on Mesenchymal Stem Cell Secretomes, Migratory and Immunomodulatory Capacity. J. Clin. Med. 2019, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Ishida, W.; Harada, Y.; Fukuda, K.; Fukushima, A. Inhibition by the Antimicrobial Peptide LL37 of Lipopolysaccharide-Induced Innate Immune Responses in Human Corneal Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2016, 57, 30–39. [Google Scholar]
- DeGraw, J.I.; Almquist, R.G.; Hiebert, C.K.; Colwell, W.T.; Crase, J.; Hayano, T.; Judd, A.K.; Dousman, L.; Smith, R.L.; Waud, W.R.; et al. Stabilized analogs of thymopentin. 1. 4,5-ketomethylene pseudopeptides. J. Med. Chem. 1997, 40, 2386–2397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Xia, X.; Xu, L.; Wang, Y.Z. Design of hybrid beta-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials 2013, 34, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wei, D.D.; Yan, P.; Zhu, X.; Shan, A.S.; Bi, Z.P. Characterization of cell selectivity, physiological stability and endotoxin neutralization capabilities of alpha-helix-based peptide amphiphiles. Biomaterials 2015, 52, 517–530. [Google Scholar] [CrossRef]
- Sun, Y.; Shang, D.J. Inhibitory Effects of Antimicrobial Peptides on Lipopolysaccharide-Induced Inflammation. Mediat. Inflamm. 2015. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Cui, F.C.; Chen, H.Q.; Zhang, T.S.; Yang, K.C.; Wang, Y.B.; Jiang, Z.Y.; Rice, K.C.; Watkins, L.R.; Hutchinson, M.R.; et al. Dissecting the Innate Immune Recognition of Opioid Inactive Isomer (+)-Naltrexone Derived Toll-like Receptor 4 (TLR4) Antagonists. J. Chem. Inf. Model. 2018, 58, 816–825. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Derakhshan, M.H.; Goodson, N.J.; Packham, J.; Sengupta, R.; Molto, A.; Marzo-Ortega, H.; Siebert, S.; BRITSpA; ASAS-COMOSPA Investigators. Association of Diverticulitis with Prolonged Spondyloarthritis: An Analysis of the ASAS-COMOSPA International Cohort. J. Clin. Med. 2019, 8, 281. [Google Scholar] [CrossRef]
- Buell, M.G.; Berin, M.C. Neutrophil-Independence of the Initiation of Colonic Injury—Comparison of Results from 3 Models of Experimental Colitis in the Rat. Dig. Dis. Sci. 1994, 39, 2575–2588. [Google Scholar] [CrossRef]
- Wera, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, J.L.; Guo, L.; Zou, Y.; Wang, F.; Shao, H.; Li, J.B.; Deng, X.M. Cholecystokinin protects mouse liver against ischemia and reperfusion injury. Int. Immunopharmacol. 2017, 48, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.W.; Pan, C.; Zheng, S.Y.; Alhamdi, Y.; Sun, B.W.; Shi, Q.K.; Wang, X.; Sun, Z.W.; Toh, C.; Wang, G.Z. Protective Effects of Carbon Monoxide-Releasing Molecule-2 on the Barrier Function of Intestinal Epithelial Cells. PLoS ONE 2014, 9, e104032. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, Z.L.; Ji, Y.; Sun, K.J.; Dai, Z.L.; Wu, G.Y. L-Glutamine Enhances Tight Junction Integrity by Activating CaMK Kinase 2-AMP-Activated Protein Kinase Signaling in Intestinal Porcine Epithelial Cells. J. Nutr. 2016, 146, 501–508. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- McCall, I.C.; Betanzos, A.; Weber, D.A.; Nava, P.; Miller, G.W.; Parkos, C.A. Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol. Appl. Pharm. 2009, 241, 61–70. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhang, Z.W.; Hang, X.M.; Jiang, Y.Q. L. plantarum prevents Enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009, 9. [Google Scholar] [CrossRef]
- Fessler, M.B.; Malcolm, K.C.; Duncan, M.W.; Worthen, G.S. A genomic and proteomic analysis of activation of the human neutrophil by lipopolysaccharide and its mediation by p38 mitogen-activated protein kinase. J. Biol. Chem. 2002, 277, 31291–31302. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.S.; Wang, X.X.; Zhu, Y.F.; Zhang, J.J.; Liu, H.; Jia, L. Characterization, anti-oxidation and anti-inflammation of polysaccharides by Hypsizygus marmoreus against LPS-induced toxicity on lung. Int. J. Biol. Macromol. 2018, 111, 121–128. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, L.; Huang, X.; Shi, W.; Xiang, J.; Gan, H. Tetrandrine ameliorates dextran-sulfate-sodium-induced colitis in mice through inhibition of nuclear factor-kappa B activation. Int. J. Colorectal Dis. 2009, 24, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappa B. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Zhang, J.J.; Huang, X.J.; Huang, L.N.; Li, S.T.; Wang, Z.P. Magnesium sulfate inhibits the secretion of high mobility group box 1 from lipopolysaccharide- activated RAW264.7 macrophages in vitro. J. Surg. Res. 2013, 179, E189–E195. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.M.; Zhang, X.L.; Zhang, J.; Cao, J.C.; Wang, F.S. Expression of Thymosin alpha 1-thymopentin Fusion Peptide in Pichia pastoris and its Characterization. Arch. Pharm. Res. 2008, 31, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Han, F.F.; Zhang, H.W.; Xia, X.; Xiong, H.T.; Song, D.G.; Zong, X.; Wang, Y.Z. Porcine beta-Defensin 2 Attenuates Inflammation and Mucosal Lesions in Dextran Sodium Sulfate-Induced Colitis. J. Immunol. 2015, 194, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, F.; Huang, X.; Rong, Y.; Yi, H.; Wang, Y. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: A comparative study. J. Anim. Sci. 2013, 91, 5614–5625. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; Scott, H.J.; Gurd, F.N. Intestinal Mucosal Lesion in Low-Flow States. II. Protective Effect of Intraluminal Glucose as Energy Substrate. Arch. Surg.-Chic. 1970, 101, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, Y.H.; Fang, Y.; Djukic, Z.; Yamamoto, M.; Shaheen, N.J.; Orlando, R.C.; Chen, X.X. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut 2014, 63, 711–719. [Google Scholar] [CrossRef] [PubMed]



| Peptides | Sequence | H 1 | Net Charge |
|---|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | −0.724 | +6 |
| TP5 | RKDVY | −1.8 | +1 |
| LTP | IGKEFKRIVQRIKDFLRNLVPRTERKDVY | −0.821 | +5 |
| Peptide | LL-37 | TP5 | LTP |
|---|---|---|---|
| t1/2 (min) | 242.47 ± 45.09 a | 1.32 ± 0.24 b | 240.03 ± 55.14 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wei, X.; Zhang, R.; Si, D.; Petitte, J.N.; Ahmad, B.; Zhang, M. A Novel Peptide Ameliorates LPS-Induced Intestinal Inflammation and Mucosal Barrier Damage via Its Antioxidant and Antiendotoxin Effects. Int. J. Mol. Sci. 2019, 20, 3974. https://doi.org/10.3390/ijms20163974
Zhang L, Wei X, Zhang R, Si D, Petitte JN, Ahmad B, Zhang M. A Novel Peptide Ameliorates LPS-Induced Intestinal Inflammation and Mucosal Barrier Damage via Its Antioxidant and Antiendotoxin Effects. International Journal of Molecular Sciences. 2019; 20(16):3974. https://doi.org/10.3390/ijms20163974
Chicago/Turabian StyleZhang, Lulu, Xubiao Wei, Rijun Zhang, Dayong Si, James N. Petitte, Baseer Ahmad, and Manyi Zhang. 2019. "A Novel Peptide Ameliorates LPS-Induced Intestinal Inflammation and Mucosal Barrier Damage via Its Antioxidant and Antiendotoxin Effects" International Journal of Molecular Sciences 20, no. 16: 3974. https://doi.org/10.3390/ijms20163974
APA StyleZhang, L., Wei, X., Zhang, R., Si, D., Petitte, J. N., Ahmad, B., & Zhang, M. (2019). A Novel Peptide Ameliorates LPS-Induced Intestinal Inflammation and Mucosal Barrier Damage via Its Antioxidant and Antiendotoxin Effects. International Journal of Molecular Sciences, 20(16), 3974. https://doi.org/10.3390/ijms20163974