Impact of Astrocyte Depletion upon Inflammation and Demyelination in a Murine Animal Model of Multiple Sclerosis
Abstract
1. Introduction
2. Results
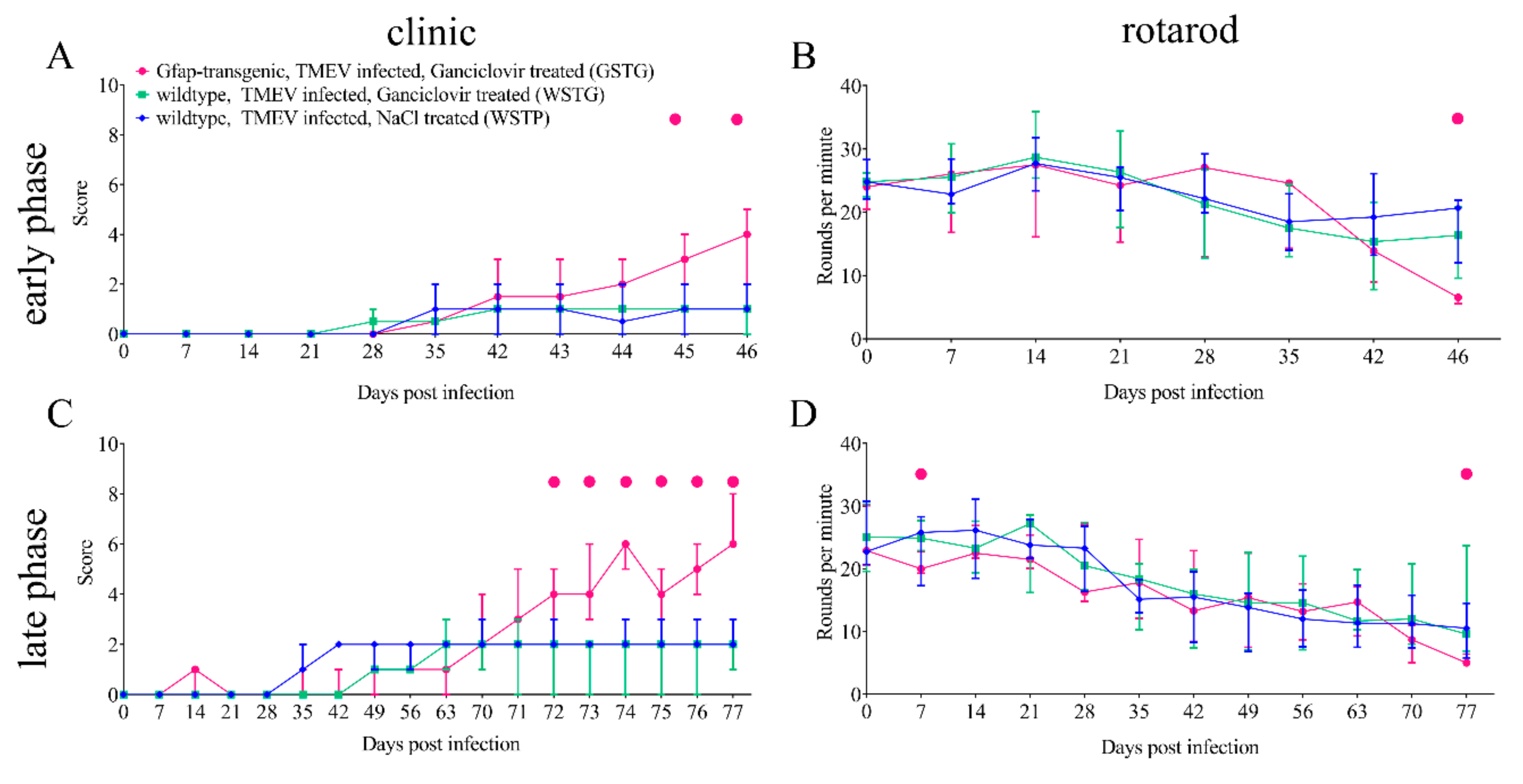
2.1. Clinical Investigation
2.2. Effects of Astrocyte Depletion upon TME
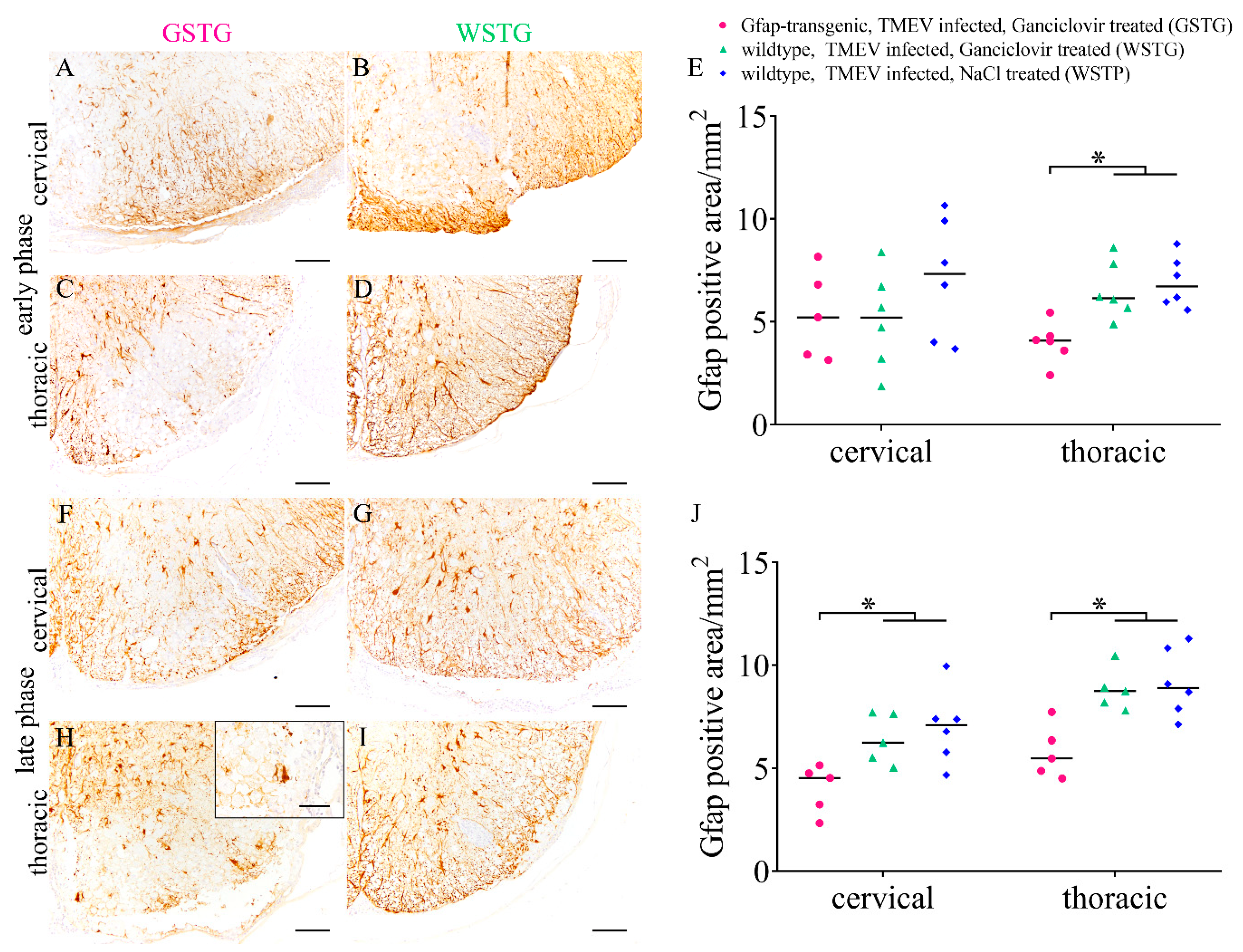
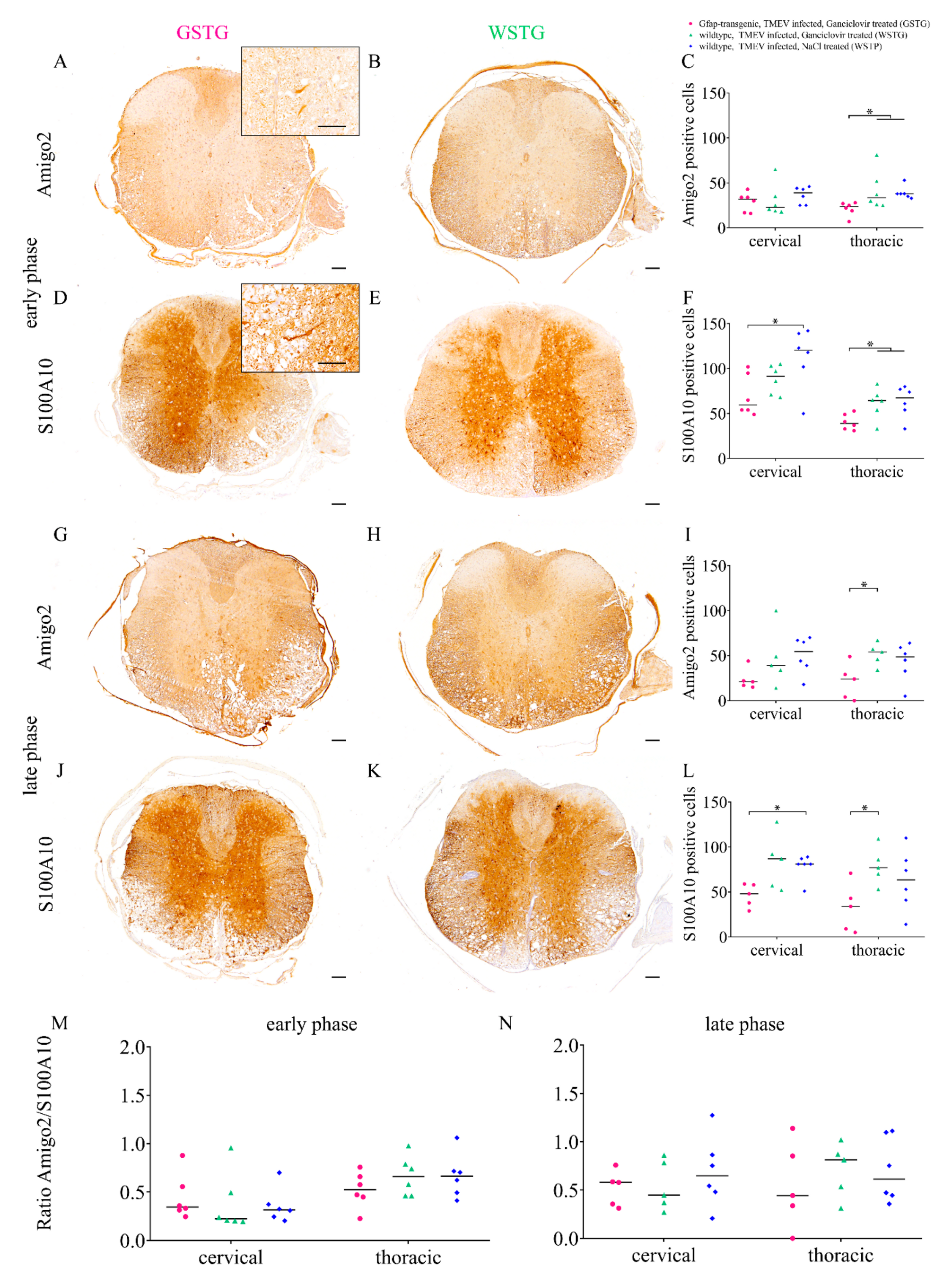
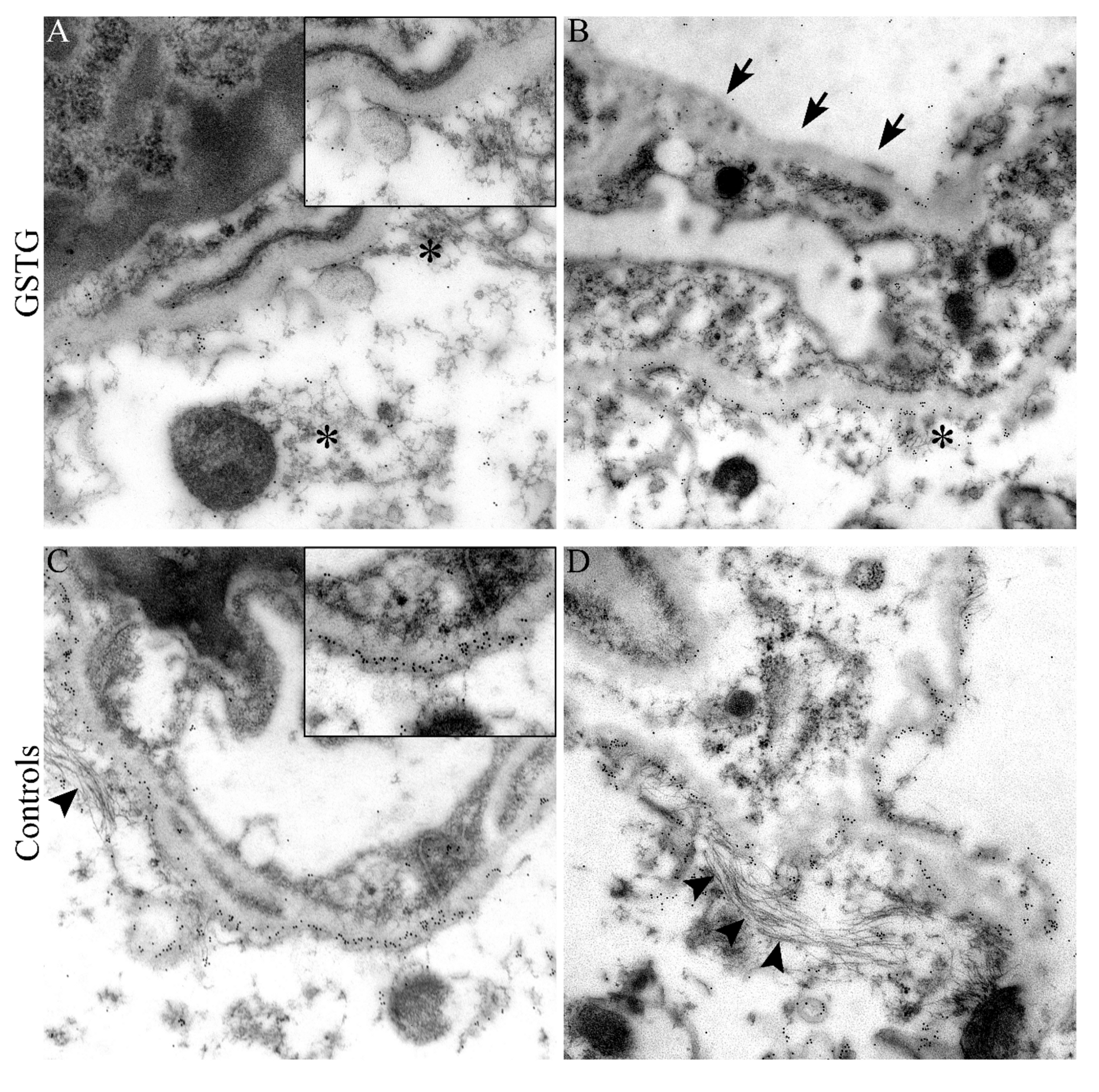
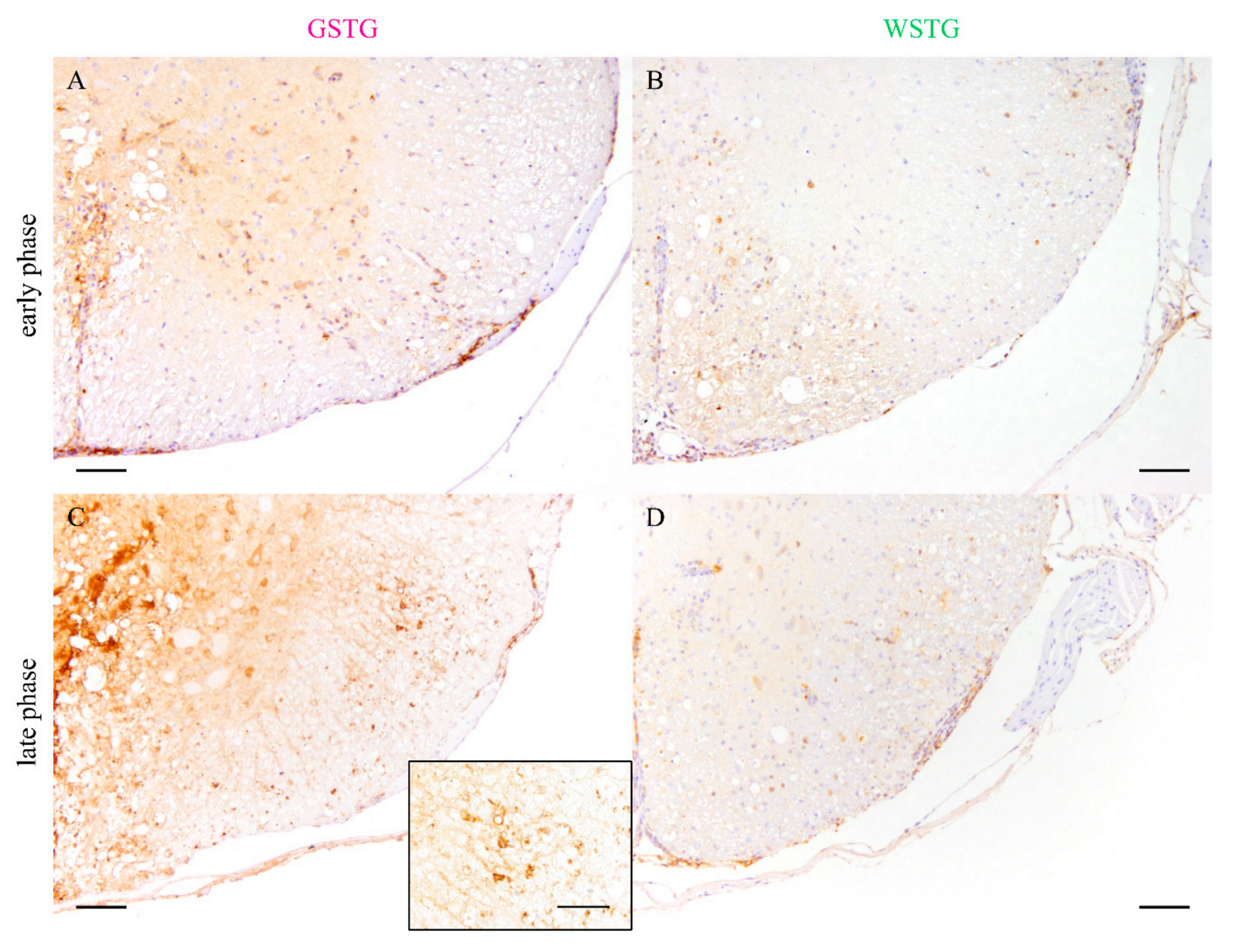
2.3. Astrocyte Phenotype
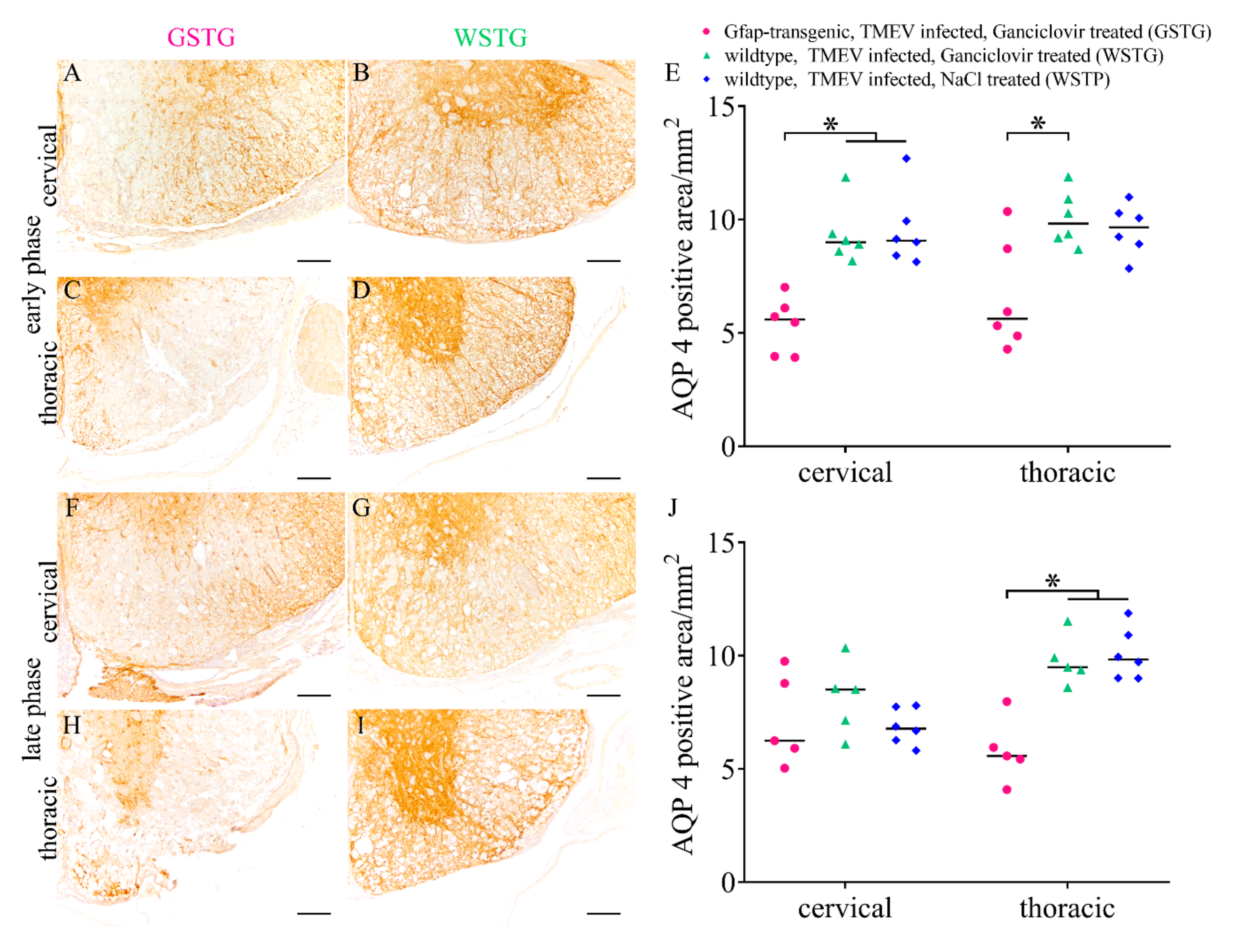
2.4. Quantification and Subcellular Localization of Aquaporin 4
2.5. Detection of Vascular Leakage
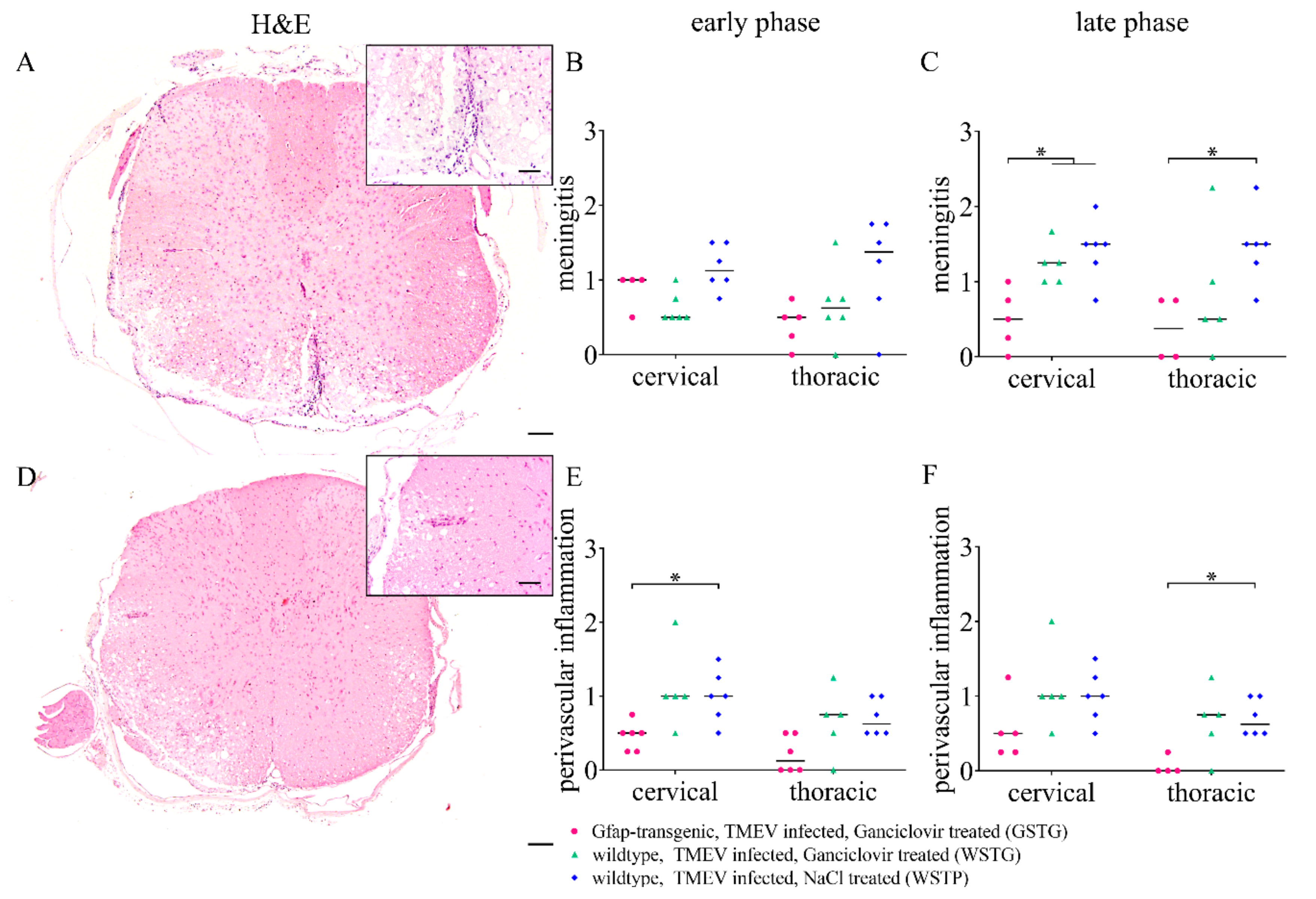
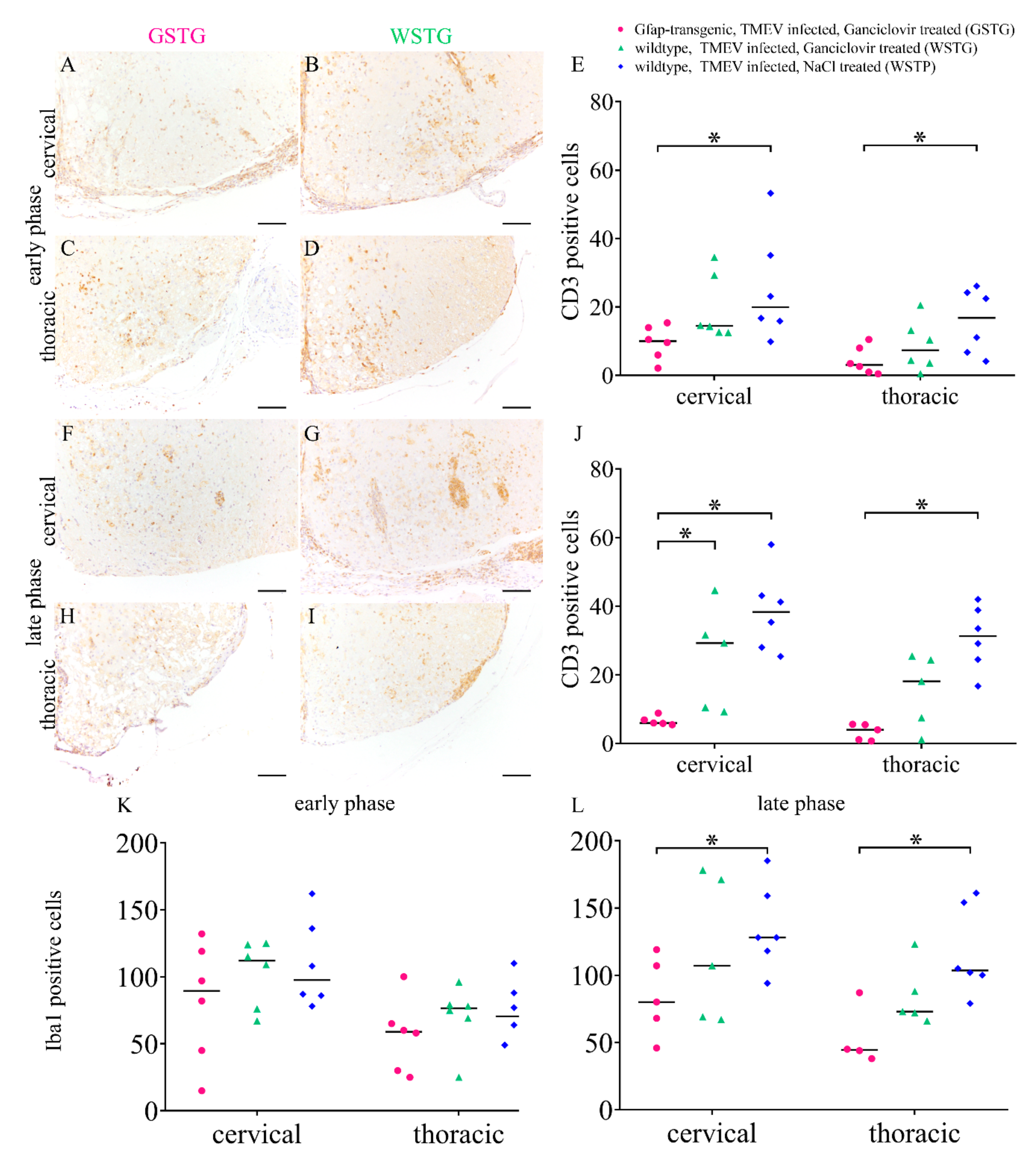
2.6. Quantification of Inflammation and Immunophenotyping of Inflammatory Cells
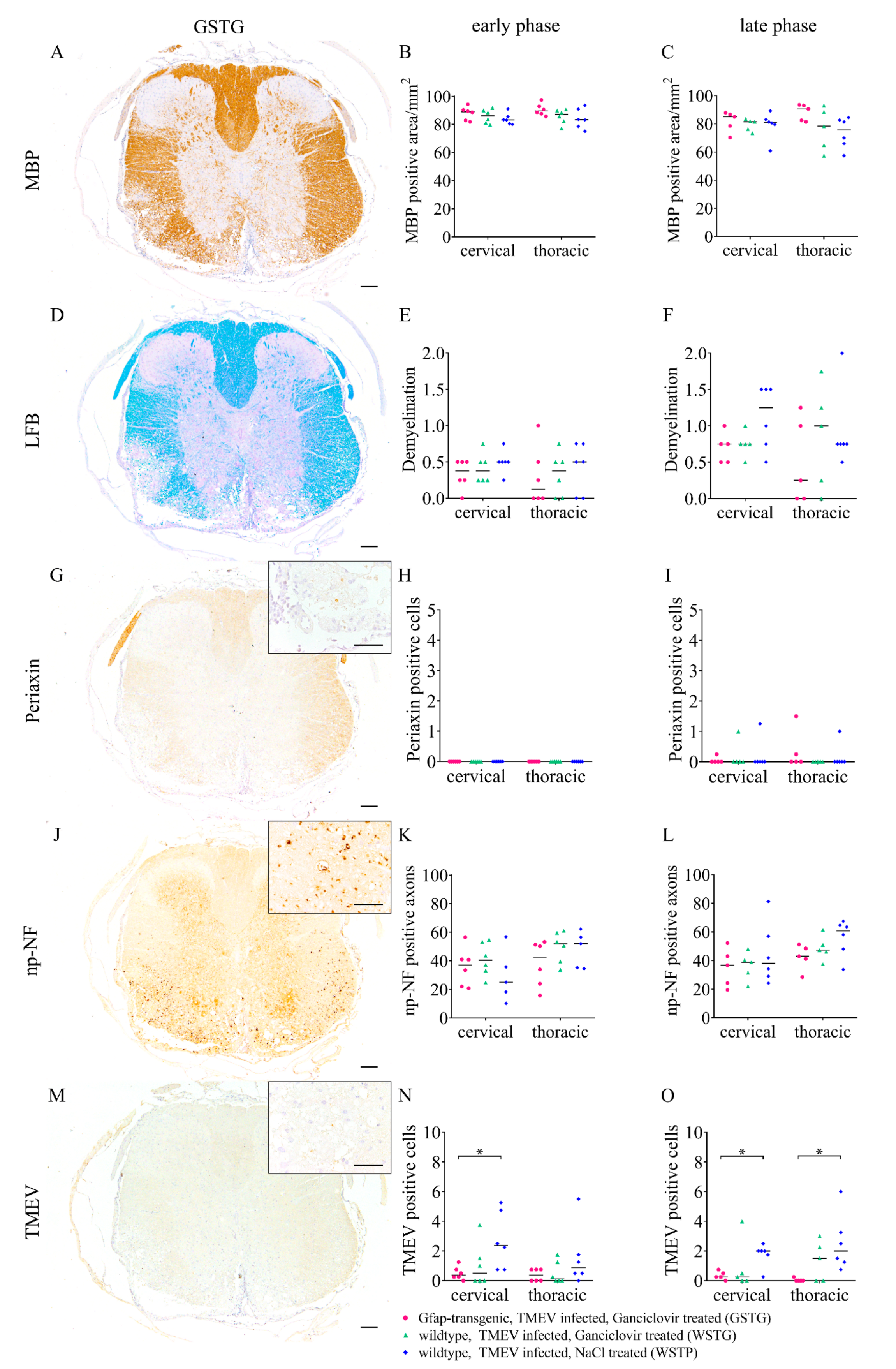
2.7. Impact of Astrocyte Depletion upon Demyelination, Schwann Cell Remyelination, Axonal Damage and Virus Protein
2.8. Effects of Ganciclovir Treatment in Mock Infected Mice
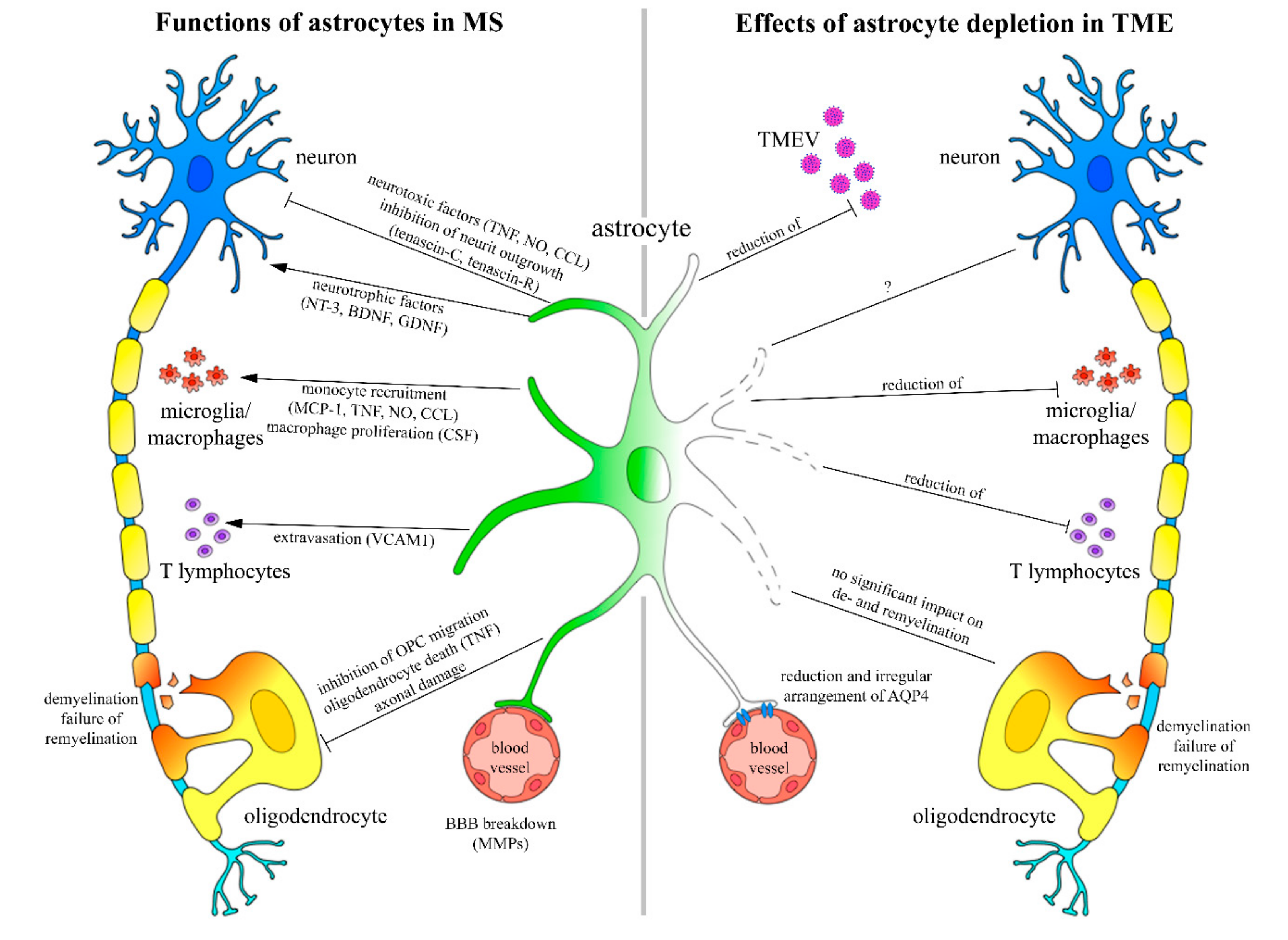
3. Discussion
4. Materials and Methods
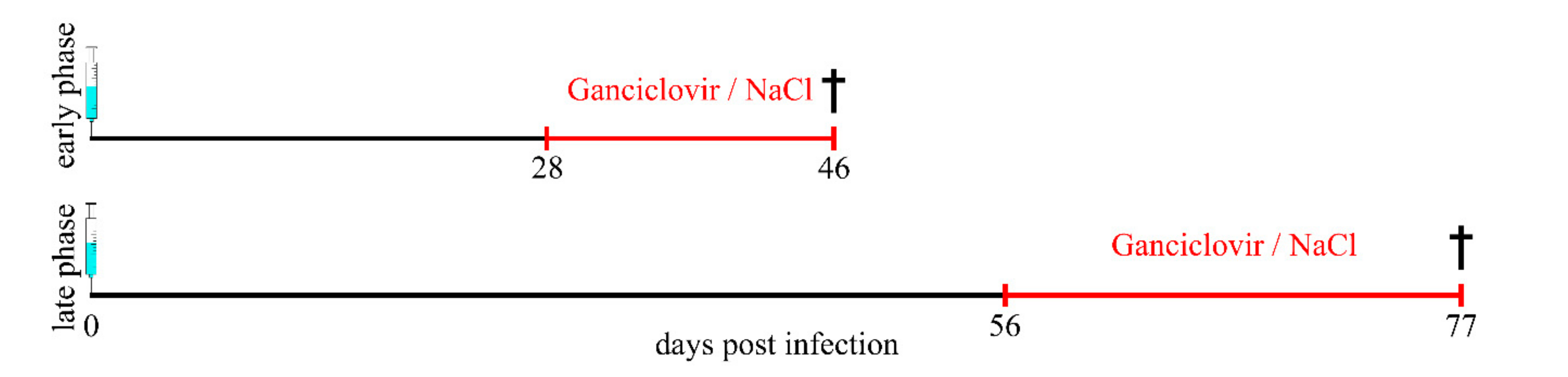
4.1. Study Design and Animals
4.2. Theiler Virus Infection and Ganciclovir Treatment
4.3. Clinical Examination and Rotarod Performance
4.4. Tissue Sampling and Preparation
4.5. Histochemistry and Immunohistochemistry
4.6. Immunoelectron Microscopy
4.7. Statistical Analysis
4.8. Ethics Statement
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Avidin-biotin-peroxidase complex method |
| AQP4 | Aquaporin 4 |
| BBB | Blood-brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BSCB | Blood-spinal cord barrier |
| CCL | Chemokine ligand |
| CNS | Central nervous system |
| CSF | Colony-stimulating factor |
| dpi | Days post infection |
| EAE | Experimental autoimmune encephalomyelitis |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| GSTG | Gfap-transgenic, TMEV infected, ganciclovir treated |
| H&E | Hematoxylin and eosin |
| Iba1 | Ionized calcium-binding adapter molecule 1 |
| IL | Interleukin |
| LFB | Luxol fast blue-cresyl violet |
| MBP | Myelin basic protein |
| MCP-1 | Monocyte chemotactic protein 1 |
| MHCII | Major histocompatibility complex class II |
| MMPs | Matrix metalloproteinases |
| MS | Multiple sclerosis |
| NFĸB | Nuclear factor kappa light-chain-enhancer of activated B cells |
| np-NF | Non-phosphorylated-neurofilaments |
| NT-3 | Neurotrophin-3 |
| OPC | Oligodendrocyte precursor cell |
| PBS | Phosphate buffered saline |
| ROI | of interest |
| rpm | Rounds per minute |
| SJL | Swiss Jim Lambert |
| TME | Theiler’s murine encephalomyelitis |
| TMEV | Theiler’s murine encephalomyelitis virus |
| TNF | Tumor necrosis factor |
| VCAM1 | Vascular cell adhesion molecule 1 |
| WSTG | Wildtype, TMEV infected, ganciclovir treated |
| WSTP | Wildtype, TMEV infected, natrium chloride treated |
References
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Ludwin, S.K.; Rao, V.; Moore, C.S.; Antel, J.P. Astrocytes in multiple sclerosis. Mult. Scler. 2016, 22, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Dimou, L. Glial cells and their function in the adult brain: A journey through the history of their ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Nash, B.; Ioannidou, K.; Barnett, S.C. Astrocyte phenotypes and their relationship to myelination. J. Anat. 2011, 219, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front. Cell. Dev. Biol. 2016, 4, 71. [Google Scholar] [PubMed]
- Skripuletz, T.; Hackstette, D.; Bauer, K.; Gudi, V.; Pul, R.; Voss, E.; Berger, K.; Kipp, M.; Baumgärtner, W.; Stangel, M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 2013, 136, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Piaton, G.; Lubetzki, C. Astrocytes--friends or foes in multiple sclerosis? Glia 2007, 55, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Eilam, R.; Segal, M.; Malach, R.; Sela, M.; Arnon, R.; Aharoni, R. Astrocyte disruption of neurovascular communication is linked to cortical damage in an animal model of multiple sclerosis. Glia 2018, 66, 1098–1117. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.G.; Puvanachandra, N.; Horner, C.H.; Polito, A.; Ostenfeld, T.; Svendsen, C.N.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 1999, 23, 297–308. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Munch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces a1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Ottersen, O.P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 2013, 93, 1543–1562. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Chu, Y.; Namaka, M.; Deng, B.; Kong, J.; Bi, X. Astrocytes in oligodendrocyte lineage development and white matter pathology. Front. Cell. Neurosci. 2016, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in cns repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Benveniste, E.N. Immune function of astrocytes. Glia 2001, 36, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Cajal, R.Y. Histologie du système nerveux de l’homme & des vertébrés. Maloine 1909, 1. [Google Scholar]
- Herrmann, J.E.; Imura, T.; Song, B.; Qi, J.; Ao, Y.; Nguyen, T.K.; Korsak, R.A.; Takeda, K.; Akira, S.; Sofroniew, M.V. Stat3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008, 28, 7231–7243. [Google Scholar] [CrossRef] [PubMed]
- Monteiro de Castro, G.; Deja, N.A.; Ma, D.; Zhao, C.; Franklin, R.J. Astrocyte activation via stat3 signaling determines the balance of oligodendrocyte versus schwann cell remyelination. Am. J. Pathol. 2015, 185, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Dal Canto, M.C.; Kim, B.S.; Miller, S.D.; Melvold, R.W. Theiler’s murine encephalomyelitis virus (tmev)-induced demyelination: A model for human multiple sclerosis. Methods 1996, 10, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Haist, V.; Ulrich, R.; Kalkuhl, A.; Deschl, U.; Baumgärtner, W. Distinct spatio-temporal extracellular matrix accumulation within demyelinated spinal cord lesions in theiler’s murine encephalomyelitis. Brain Pathol. 2012, 22, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Oleszak, E.L.; Chang, J.R.; Friedman, H.; Katsetos, C.D.; Platsoucas, C.D. Theiler’s virus infection: A model for multiple sclerosis. Clin. Microbiol. Rev. 2004, 17, 174–207. [Google Scholar] [CrossRef] [PubMed]
- Leitzen, E.; Jin, W.; Herder, V.; Beineke, A.; Elmarabet, S.A.; Baumgärtner, W.; Hansmann, F. Comparison of reported spinal cord lesions in progressive multiple sclerosis with theiler’s murine encephalomyelitis virus induced demyelinating disease. Int. J. Mol. Sci. 2019, 20, 989. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, I.; Hansmann, F.; Ciurkiewicz, M.; Löscher, W.; Beineke, A. Facets of theiler’s murine encephalomyelitis virus-induced diseases: An update. Int. J. Mol. Sci. 2019, 20, 448. [Google Scholar] [CrossRef] [PubMed]
- Leitzen, E.; Raddatz, B.B.; Jin, W.; Goebbels, S.; Nave, K.A.; Baumgärtner, W.; Hansmann, F. Virus-triggered spinal cord demyelination is followed by a peripheral neuropathy resembling features of guillain-barre syndrome. Sci. Rep. 2019, 9, 4588. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.; Seeliger, F.; Kreutzer, M.; Germann, P.G.; Baumgärtner, W. Limited remyelination in theiler’s murine encephalomyelitis due to insufficient oligodendroglial differentiation of nerve/glial antigen 2 (ng2)-positive putative oligodendroglial progenitor cells. Neuropathol. Appl. Neurobiol. 2008, 34, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Haist, V.; Baumgärtner, W.; Wagner, S.; Stein, V.M.; Tipold, A.; Wendt, H.; Potschka, H. Impact of theiler’s virus infection on hippocampal neuronal progenitor cells: Differential effects in two mouse strains. Neuropathol. Appl. Neurobiol. 2012, 38, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Lipton, H.L. Theiler’s virus infection in mice: An unusual biphasic disease process leading to demyelination. Infect. Immun. 1975, 11, 1147–1155. [Google Scholar] [PubMed]
- Schneider, K.M.; Watson, N.B.; Minchenberg, S.B.; Massa, P.T. The influence of macrophage growth factors on theiler’s murine encephalomyelitis virus (tmev) infection and activation of macrophages. Cytokine 2018, 102, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Dal Canto, M.C. Effect of theiler’s murine encephalomyelitis virus and cytokines on cultured oligodendrocytes and astrocytes. J. Neurosci. Res. 1996, 45, 364–374. [Google Scholar] [CrossRef]
- Sato, F.; Tanaka, H.; Hasanovic, F.; Tsunoda, I. Theiler’s virus infection: Pathophysiology of demyelination and neurodegeneration. Pathophysiology 2011, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Herder, V.; Iskandar, C.D.; Kegler, K.; Hansmann, F.; Elmarabet, S.A.; Khan, M.A.; Kalkuhl, A.; Deschl, U.; Baumgärtner, W.; Ulrich, R.; et al. Dynamic changes of microglia/macrophage m1 and m2 polarization in theiler’s murine encephalomyelitis. Brain Pathol. 2015, 25, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.A.; Lee, H.G.; Kang, B.S.; Kang, H.K.; Kim, B.S. Capsid-specific cytotoxic t lymphocytes recognize three distinct h-2d(b)-restricted regions of the bean strain of theiler’s virus and exhibit different cytokine profiles. J. Virol. 2002, 76, 3125–3134. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, I.; Hansmann, F.; Puff, C.; Kumnok, J.; Schaudien, D.; Wewetzer, K.; Baumgärtner, W. Theiler’s murine encephalomyelitis virus induced phenotype switch of microglia in vitro. J. Neuroimmunol. 2012, 252, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, B.B.; Sun, W.; Brogden, G.; Sun, Y.; Kammeyer, P.; Kalkuhl, A.; Colbatzky, F.; Deschl, U.; Naim, H.Y.; Baumgärtner, W.; et al. Central nervous system demyelination and remyelination is independent from systemic cholesterol level in theiler’s murine encephalomyelitis. Brain Pathol. 2016, 26, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Peterson, R.S.; Song, B.; Ao, Y.; Morales, L.B.; Tiwari-Woodruff, S.; Sofroniew, M.V. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the cns. J. Neurosci. 2009, 29, 11511–11522. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Begovic-Kupresanin, V.; Pekovic, S.; Lavrnja, I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2018, 96, 1021–1042. [Google Scholar] [CrossRef] [PubMed]
- Toft-Hansen, H.; Fuchtbauer, L.; Owens, T. Inhibition of reactive astrocytosis in established experimental autoimmune encephalomyelitis favors infiltration by myeloid cells over t cells and enhances severity of disease. Glia 2011, 59, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Shiow, L.R.; Favrais, G.; Schirmer, L.; Schang, A.L.; Cipriani, S.; Andres, C.; Wright, J.N.; Nobuta, H.; Fleiss, B.; Gressens, P.; et al. Reactive astrocyte cox2-pge2 production inhibits oligodendrocyte maturation in neonatal white matter injury. Glia 2017, 65, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, P.; Hahn, A.; Soulika, A.; Gallo, V.; Pleasure, D. Astrogliosis in eae spinal cord: Derivation from radial glia, and relationships to oligodendroglia. Glia 2007, 55, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Schwab, M.E.; Popovich, P.G. Central nervous system regenerative failure: Role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 2014, 7, a020602. [Google Scholar] [CrossRef] [PubMed]
- Minagar, A.; Shapshak, P.; Fujimura, R.; Ownby, R.; Heyes, M.; Eisdorfer, C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: Hiv-associated dementia, alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 2002, 202, 13–23. [Google Scholar] [CrossRef]
- Morcos, Y.; Lee, S.M.; Levin, M.C. A role for hypertrophic astrocytes and astrocyte precursors in a case of rapidly progressive multiple sclerosis. Mult Scler 2003, 9, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.A.; Quintana, F.J. Regulation of astrocyte functions in multiple sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029009. [Google Scholar] [CrossRef] [PubMed]
- Zamvil, S.S.; Steinman, L. Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron 2003, 38, 685–688. [Google Scholar] [CrossRef]
- Skripuletz, T.; Salinas Tejedor, L.; Prajeeth, C.K.; Hansmann, F.; Chhatbar, C.; Kucman, V.; Zhang, N.; Raddatz, B.B.; Detje, C.N.; Suhs, K.W.; et al. The antiviral drug ganciclovir does not inhibit microglial proliferation and activation. Sci. Rep. 2015, 5, 14935. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Veruki, M.L.; Torp, R.; Haug, F.M.; Laake, J.H.; Nielsen, S.; Agre, P.; Ottersen, O.P. Aquaporin-4 water channel protein in the rat retina and optic nerve: Polarized expression in muller cells and fibrous astrocytes. J. Neurosci. 1998, 18, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Ikeshima-Kataoka, H.; Abe, Y.; Yasui, M. Aquaporin 4-dependent expression of glial fibrillary acidic protein and tenascin-c in activated astrocytes in stab wound mouse brain and in primary culture. J. Neurosci. Res. 2015, 93, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. Igg marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Verkman, A.S. Greatly attenuated experimental autoimmune encephalomyelitis in aquaporin-4 knockout mice. BMC Neurosci 2009, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Varrin-Doyer, M.; Zamvil, S.S.; Verkman, A.S. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J. 2011, 25, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Srinivas, M.; Li, W.; Brosnan, C.F.; Frigeri, A.; Spray, D.C. New possible roles for aquaporin-4 in astrocytes: Cell cytoskeleton and functional relationship with connexin43. FASEB J. 2005, 19, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Frigeri, A.; Liuzzi, G.M.; Svelto, M. Inhibition of aquaporin-4 expression in astrocytes by rnai determines alteration in cell morphology, growth, and water transport and induces changes in ischemia-related genes. FASEB J. 2003, 17, 1508–1510. [Google Scholar] [CrossRef] [PubMed]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and clinical implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Sykova, E.; Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef] [PubMed]
- Tatar, I.; Chou, P.C.; Desouki, M.M.; El Sayed, H.; Bilgen, M. Evaluating regional blood spinal cord barrier dysfunction following spinal cord injury using longitudinal dynamic contrast-enhanced mri. BMC Med. Imaging 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, F.; Herder, V.; Kalkuhl, A.; Haist, V.; Zhang, N.; Schaudien, D.; Deschl, U.; Baumgärtner, W.; Ulrich, R. Matrix metalloproteinase-12 deficiency ameliorates the clinical course and demyelination in theiler’s murine encephalomyelitis. Acta Neuropathol. 2012, 124, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Kummerfeld, M.; Meens, J.; Haas, L.; Baumgärtner, W.; Beineke, A. Generation and characterization of a polyclonal antibody for the detection of theiler’s murine encephalomyelitis virus by light and electron microscopy. J. Virol. Methods 2009, 160, 185–188. [Google Scholar] [CrossRef] [PubMed]
| Antigen | Pretreatment | Dilution | Clonality | Supplier | Catalog Number |
|---|---|---|---|---|---|
| Amigo2 | 0.5% triton | 1:200 | polyclonal rabbit | Bioss Antibodies Inc., Woburn, Massachusetts, USA | ABIN138654 |
| AQP4 | / | 1:200 | polyclonal rabbit | Merck Millipore, Danvers, Massachusetts, USA | AB 3594 |
| CD3 | microwave/ citrate buffer | 1:250 | polyclonal rabbit | DakoCytomation GmbH, Hamburg, Germany | A0452 |
| CD34 | microwave/ citrate buffer | 1:50 | monoclonal mouse | BDBioscience, Heidelberg, Germany | 550390 |
| GFAP | / | 1:1000 | polyclonal rabbit | DakoCytomation GmbH, Hamburg, Germany | Z0334 |
| Iba1 | / | 1:1000 | polyclonal rabbit | Wako Chemicals Inc., Richmond, VA, USA | PN 019-19741 |
| MBP | / | 1:500 | polyclonal rabbit | Chemicon, Temecula, CA, USA | AB980 |
| np-NF | microwave/ citrate buffer | 1:20000 | polyclonal rabbit | Covance, Los Angeles, CA, USA | SMI-311R |
| Periaxin | microwave/ citrate buffer | 1:5000 | polyclonal rabbit | Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany | HPA001868 |
| S100A10 | / | 1:100 | monoclonal rabbit | Bio-Techne GmbH, Wiesbaden, Germany | Ab JF0987 |
| TMEV | / | 1:2000 | polyclonal rabbit | Kummerfeld et al. [64] | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allnoch, L.; Baumgärtner, W.; Hansmann, F. Impact of Astrocyte Depletion upon Inflammation and Demyelination in a Murine Animal Model of Multiple Sclerosis. Int. J. Mol. Sci. 2019, 20, 3922. https://doi.org/10.3390/ijms20163922
Allnoch L, Baumgärtner W, Hansmann F. Impact of Astrocyte Depletion upon Inflammation and Demyelination in a Murine Animal Model of Multiple Sclerosis. International Journal of Molecular Sciences. 2019; 20(16):3922. https://doi.org/10.3390/ijms20163922
Chicago/Turabian StyleAllnoch, Lisa, Wolfgang Baumgärtner, and Florian Hansmann. 2019. "Impact of Astrocyte Depletion upon Inflammation and Demyelination in a Murine Animal Model of Multiple Sclerosis" International Journal of Molecular Sciences 20, no. 16: 3922. https://doi.org/10.3390/ijms20163922
APA StyleAllnoch, L., Baumgärtner, W., & Hansmann, F. (2019). Impact of Astrocyte Depletion upon Inflammation and Demyelination in a Murine Animal Model of Multiple Sclerosis. International Journal of Molecular Sciences, 20(16), 3922. https://doi.org/10.3390/ijms20163922





