Untargeted Metabolomics Reveals Molecular Effects of Ketogenic Diet on Healthy and Tumor Xenograft Mouse Models
Abstract
1. Introduction
2. Results and Discussion
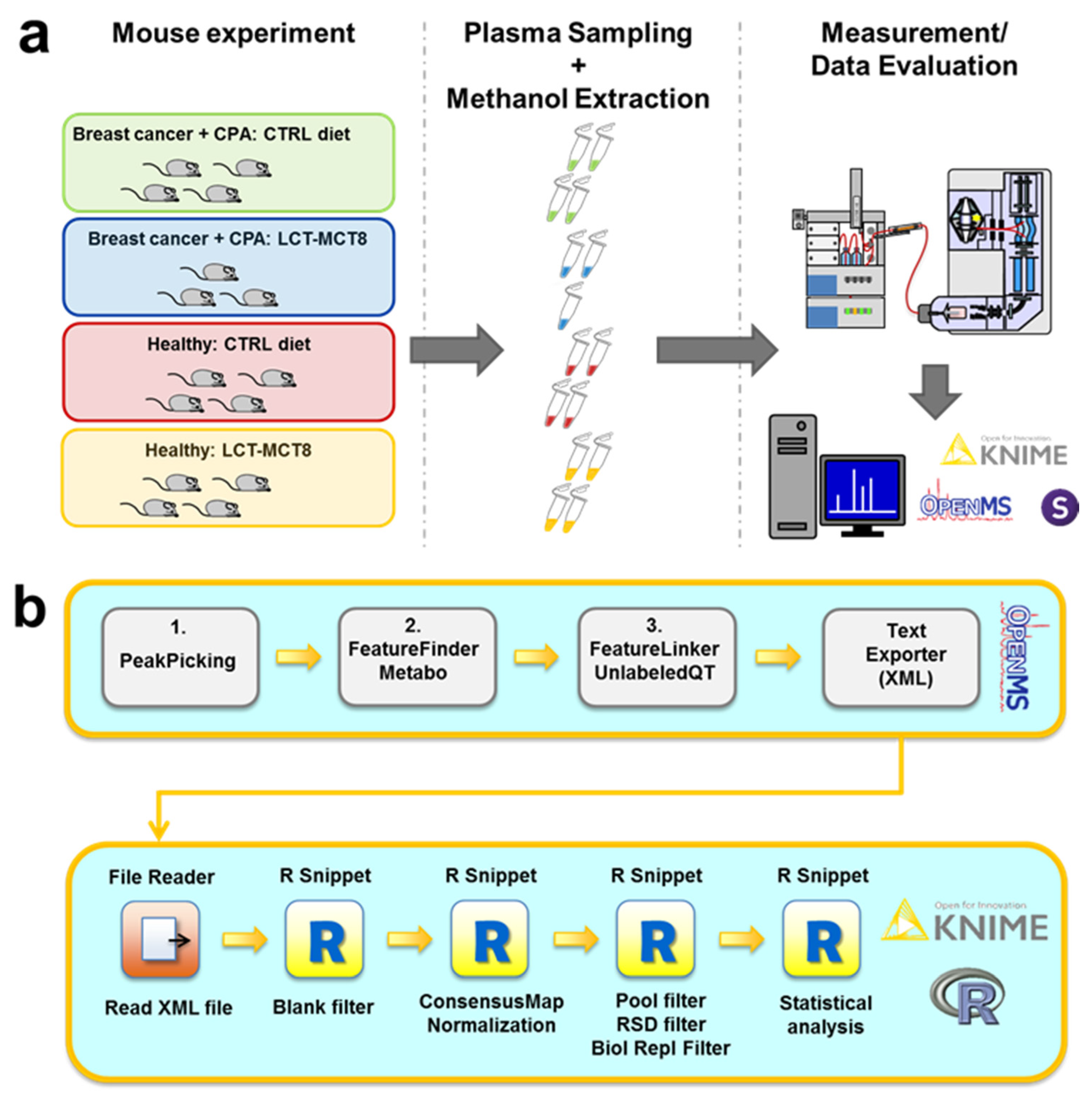
2.1. Study Design
2.2. Ketogenic Diet Induced Effects in MDA-MB-468 Tumor Bearing Mice
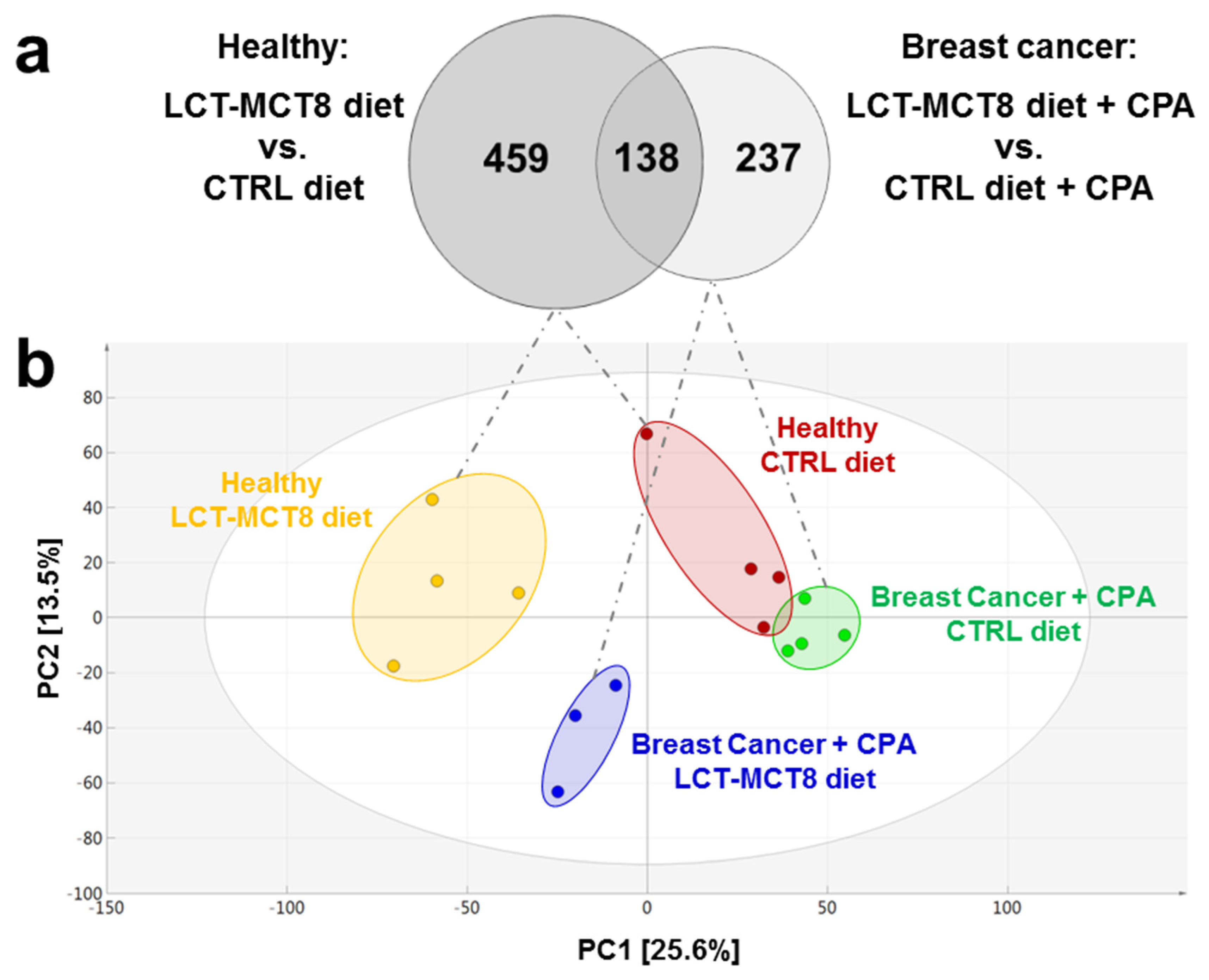
2.3. Results of Untargeted Metabolome Analysis
2.4. Regulated Metabolites upon KD Treatment
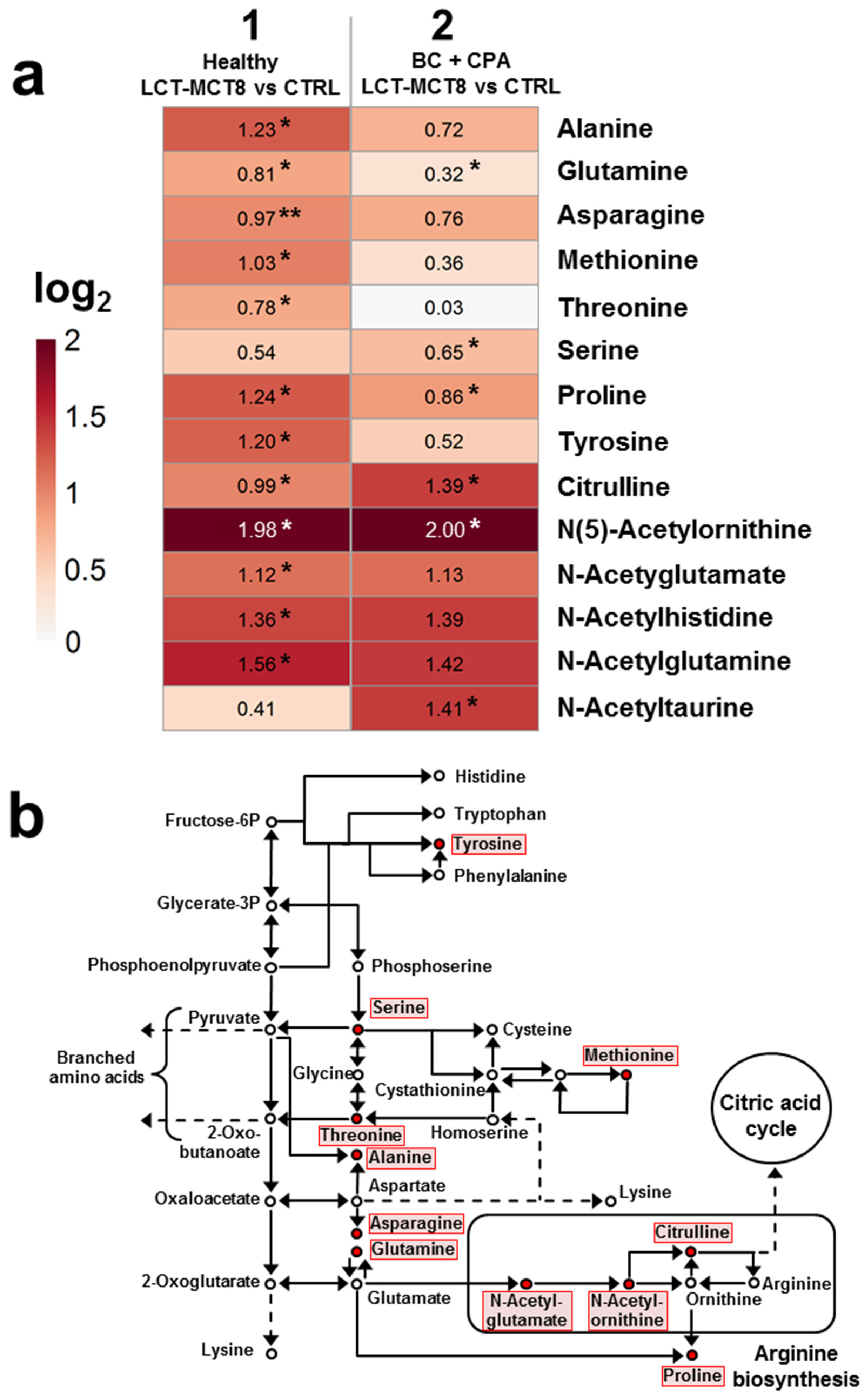
2.5. Regulations in Amino Acid Biosynthesis and N-Acetylation
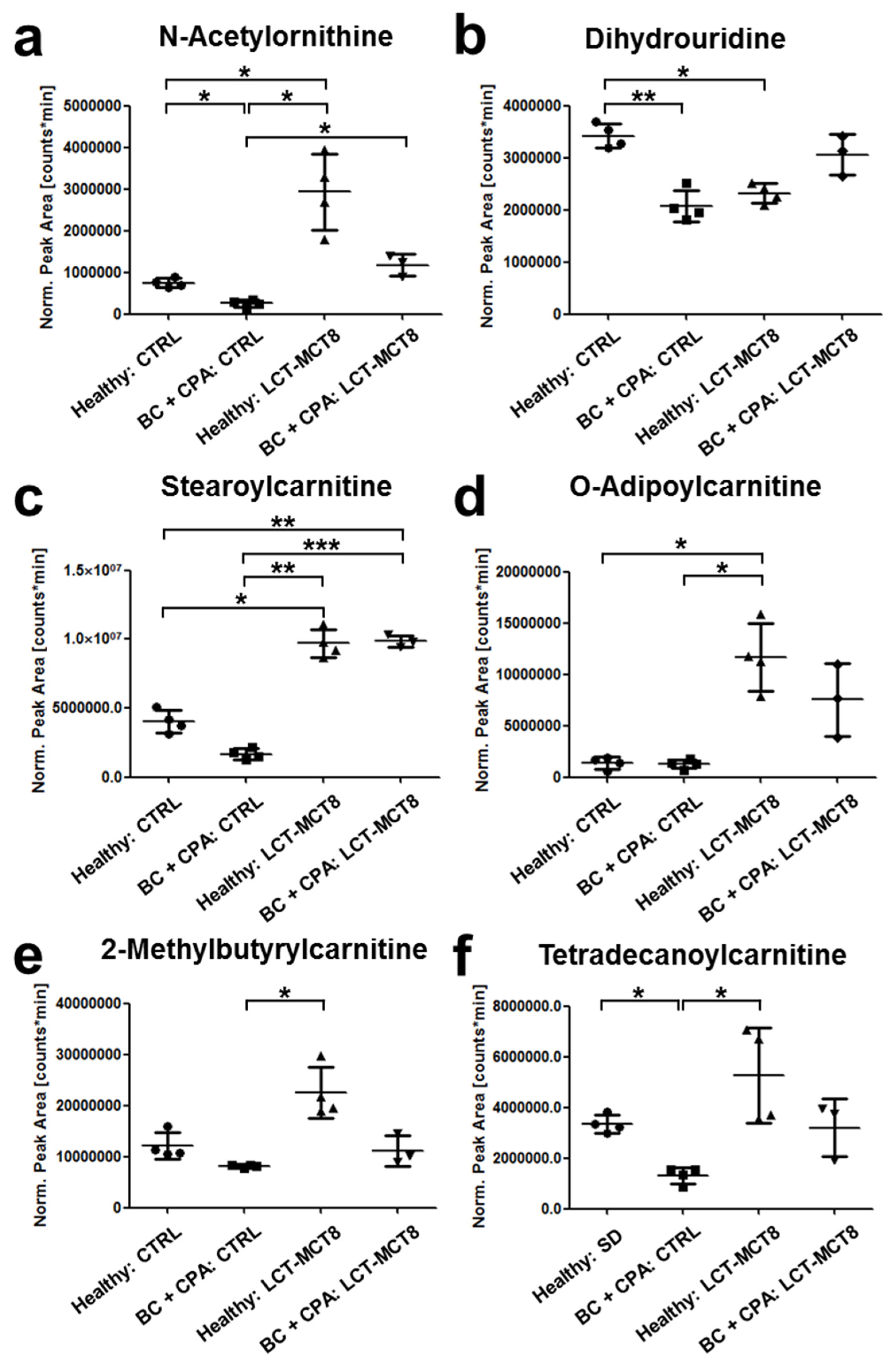
2.6. Regulation of N(5)-Acetylornithine and 5,6-Dihydrouridine
2.7. Fatty Acid Transport
3. Materials and Methods
3.1. Cell Culture
3.2. Animal Experiments
3.3. Preparation of Plasma Samples
3.4. HPLC-MS Measurements
3.5. Data Evaluation
3.6. Verification of Database Hits with Standards
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| KD | ketogenic diet |
| IGF-1 | insulin growth factor-1 |
| NF-E2 | nuclear factor erythroid-derived 2 |
| Nrf2 | nuclear factor erythroid-derived 2-related factor 2 |
| OXPHOS | oxidative phosphorylation |
| PI3K | phosphatidylinositol-3 kinase |
| MTX | metabolomics |
| TAMs | tumor-associated macrophages |
| TAFs | tumor-associated fibroblasts |
| CPA | cyclophosphamide |
| CTRL | control |
| MCT8 | medium-chain triglycerides |
| LCT | long-chain triglycerides |
| RP-HPLC | reversed-phase high-performance liquid chromatography |
| HILIC | hydrophilic interaction liquid chromatography |
| ESI-MS | electrospray ionization mass spectrometry |
| posESI | positive electrospray ionization |
| negESI | negative electrospray ionization |
| LIMMA | linear models for microarray data |
| HMDB | human metabolome database |
| mTOR | mammalian target of rapamycin |
| PCA | Principal Component Analysis |
| FXTAS | X-associated tremor/ataxia syndrome |
| UHPLC | ultra-high-performance liquid chromatography |
| KNIME | Konstanz Information Miner |
References
- WILDER, R.M. The Effects of Ketonemia on the Course of Epilepsy. Mayo Clin. Proc. 1921, 2, 307–308. [Google Scholar]
- VanItallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of alzheimer’s disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Gasior, M.; Rogawski, M.A.; Hartman, A.L. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 2006, 17, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Grimaldi, K.; Toniolo, L.; Canato, M.; Bianco, A.; Fratter, A. Nutrition and acne: Therapeutic potential of ketogenic diets. Skin Pharmacol. Physiol. 2012, 25, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S.; Foy, M.; Chalecki, A.M.; Vernon, M.C.; Westman, E.C. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Urbain, P.; Strom, L.; Morawski, L.; Wehrle, A.; Deibert, P.; Bertz, H. Impact of a 6-week non-energy-restricted ketogenic diet on physical fitness, body composition and biochemical parameters in healthy adults. Nutr. Metab. 2017, 14, 17. [Google Scholar] [CrossRef]
- Kasprzak, A.; Kwasniewski, W.; Adamek, A.; Gozdzicka-Jozefiak, A. Insulin-like growth factor (igf) axis in cancerogenesis. Mutat. Res. 2017, 772, 78–104. [Google Scholar] [CrossRef]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: New perspectives for neuroprotection in alzheimer’s disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef]
- Vidali, S.; Aminzadeh, S.; Lambert, B.; Rutherford, T.; Sperl, W.; Kofler, B.; Feichtinger, R.G. Mitochondria: The ketogenic diet—A metabolism-based therapy. Int. J. Biochem. Cell Biol. 2015, 63, 55–59. [Google Scholar] [CrossRef]
- Allen, B.G.; Bhatia, S.K.; Buatti, J.M.; Brandt, K.E.; Lindholm, K.E.; Button, A.M.; Szweda, L.I.; Smith, B.J.; Spitz, D.R.; Fath, M.A. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin. Cancer Res. 2013, 19, 3905–3913. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J. The emerging role of ketogenic diets in cancer treatment. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the international ketogenic diet study group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Page, K.A.; Williamson, A.; Yu, N.; McNay, E.C.; Dzuira, J.; McCrimmon, R.J.; Sherwin, R.S. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes 2009, 58, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Shao, S.X.; Jiang, H.P.; Cao, Y.W.; Wang, Y.H.; Yang, X.C.; Wang, Y.L.; Wang, X.S.; Niu, H.T. Warburg effect or reverse warburg effect? A review of cancer metabolism. Oncol. Res. Treat. 2015, 38, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Douris, N.; Melman, T.; Pecherer, J.M.; Pissios, P.; Flier, J.S.; Cantley, L.C.; Locasale, J.W.; Maratos-Flier, E. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Mukherjee, P.; Kiebish, M.A.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. 2007, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Morscher, R.J.; Aminzadeh-Gohari, S.; Feichtinger, R.G.; Mayr, J.A.; Lang, R.; Neureiter, D.; Sperl, W.; Kofler, B. Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a cd1-nu mouse model. PLoS ONE 2015, 10, e0129802. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh-Gohari, S.; Feichtinger, R.G.; Vidali, S.; Locker, F.; Rutherford, T.; O’Donnel, M.; Stöger-Kleiber, A.; Mayr, J.A.; Sperl, W.; Kofler, B. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a cd1-nu mouse model. Oncotarget 2017, 8, 64728–64744. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Pauli, C.; Du, X.; Wang, D.G.; Li, X.; Wu, D.; Amadiume, S.C.; Goncalves, M.D.; Hodakoski, C.; Lundquist, M.R. Suppression of insulin feedback enhances the efficacy of pi3k inhibitors. Nature 2018, 560, 499. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Chan, D.K.; Haugrud, A.B.; Miskimins, W.K. Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS ONE 2014, 9, e108444. [Google Scholar] [CrossRef] [PubMed]
- Gluschnaider, U.; Hertz, R.; Ohayon, S.; Smeir, E.; Smets, M.; Pikarsky, E.; Bar-Tana, J. Long-chain fatty acid analogues suppress breast tumorigenesis and progression. Cancer Res. 2014, 74, 6991–7002. [Google Scholar] [CrossRef] [PubMed]
- İyikesici, M.S.; Slocum, A.K.; Slocum, A.; Berkarda, F.B.; Kalamian, M.; Seyfried, T.N. Efficacy of metabolically supported chemotherapy combined with ketogenic diet, hyperthermia, and hyperbaric oxygen therapy for stage iv triple-negative breast cancer. Cureus 2017, 9, e1445. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.; Pacini, S.; Ruggiero, M. Effects of pre-surgical vitamin d supplementation and ketogenic diet in a patient with recurrent breast cancer. Anticancer Res. 2015, 35, 5525–5532. [Google Scholar] [PubMed]
- Klement, R.J.; Sweeney, R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res. Notes 2016, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Balasse, E.O.; Féry, F. Ketone body production and disposal: Effects of fasting, diabetes, and exercise. Diabetes Metab. Rev. 1989, 5, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Ranninger, C.; Rurik, M.; Limonciel, A.; Ruzek, S.; Reischl, R.; Wilmes, A.; Jennings, P.; Hewitt, P.; Dekant, W.; Kohlbacher, O. Nephron toxicity profiling via untargeted metabolome analysis employing a high performance liquid chromatography-mass spectrometry-based experimental and computational pipeline. J. Biol. Chem. 2015, 290, 19121–19132. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, K.M.; Pountain, A.W.; Regnault, C.; Achcar, F.; Vincent, I.M.; Goodyear, C.S.; Barrett, M.P. Metabolomic profiling of macrophages determines the discrete metabolomic signature and metabolomic interactome triggered by polarising immune stimuli. PLoS ONE 2018, 13, e0194126. [Google Scholar] [CrossRef] [PubMed]
- León, Z.; García-Cañaveras, J.C.; Donato, M.T.; Lahoz, A. Mammalian cell metabolomics: Experimental design and sample preparation. Electrophoresis 2013, 34, 2762–2775. [Google Scholar] [CrossRef]
- Homuth, G.; Teumer, A.; Völker, U.; Nauck, M. A description of large-scale metabolomics studies: Increasing value by combining metabolomics with genome-wide snp genotyping and transcriptional profiling. J. Endocrinol. 2012, 215, 17–28. [Google Scholar] [CrossRef]
- Boyles, M.S.P.; Ranninger, C.; Reischl, R.; Rurik, M.; Tessadri, R.; Kohlbacher, O.; Duschl, A.; Huber, C.G. Copper oxide nanoparticle toxicity profiling using untargeted metabolomics. Part. Fibre Toxicol. 2016, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Roig, B.; Rodríguez-Balada, M.; Samino, S.; Lam, E.W.-F.; Guaita-Esteruelas, S.; Gomes, A.R.; Correig, X.; Borràs, J.; Yanes, O.; Gumà, J. Metabolomics reveals novel blood plasma biomarkers associated to the brca1-mutated phenotype of human breast cancer. Sci. Rep. 2017, 7, 17831. [Google Scholar] [CrossRef] [PubMed]
- Röst, H.L.; Sachsenberg, T.; Aiche, S.; Bielow, C.; Weisser, H.; Aicheler, F.; Andreotti, S.; Ehrlich, H.-C.; Gutenbrunner, P.; Kenar, E.; et al. Openms: A flexible open-source software platform for mass spectrometry data analysis. Nat. Methods 2016, 13, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Böcker, S.; Letzel, M.C.; Lipták, Z.; Pervukhin, A. Sirius: Decomposing isotope patterns for metabolite identification. Bioinformatics 2008, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Böcker, S.; Dührkop, K. Fragmentation trees reloaded. J. Cheminform. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using csi: Fingerid. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. Metlin: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Morishima, K.; Tanabe, M.; Furumichi, M.; Kanehisa, M.; Sato, Y. Kegg: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2016, 45, D353–D361. [Google Scholar]
- Morishima, K.; Tanabe, M.; Furumichi, M.; Kanehisa, M.; Sato, Y. New approach for understanding genome variations in kegg. Nucleic Acids Res. 2018, 47, D590–D595. [Google Scholar]
- Vidali, S.; Aminzadeh-Gohari, S.; Feichtinger, R.G.; Vatrinet, R.; Koller, A.; Locker, F.; Rutherford, T.; O’Donnell, M.; Stöger-Kleiber, A.; Lambert, B.; et al. The ketogenic diet is not feasible as a therapy in a cd-1 nu/nu mouse model of renal cell carcinoma with features of stauffer’s syndrome. Oncotarget 2017, 8, 57201–57215. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E. Hmdb 3.0—The human metabolome database in 2013. Nucleic Acids Res. 2012, 41, D801–D807. [Google Scholar] [CrossRef]
- Ni, F.-F.; Li, C.-R.; Liao, J.-X.; Wang, G.-B.; Lin, S.-F.; Xia, Y.; Wen, J.-L. The effects of ketogenic diet on the th17/treg cells imbalance in patients with intractable childhood epilepsy. Seizure 2016, 38, 17–22. [Google Scholar] [CrossRef]
- Lussier, D.M.; Woolf, E.C.; Johnson, J.L.; Brooks, K.S.; Blattman, J.N.; Scheck, A.C. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 2016, 16, 310. [Google Scholar] [CrossRef]
- Fomin, D.A.; McDaniel, B.; Crane, J. The promising potential role of ketones in inflammatory dermatologic disease: A new frontier in treatment research. J. Dermatol. Treat. 2017, 28, 484–487. [Google Scholar] [CrossRef]
- Weichhart, T.; Hengstschläger, M.; Linke, M. Regulation of innate immune cell function by mtor. Nat. Rev. Immunol. 2015, 15, 599–614. [Google Scholar] [CrossRef]
- Suhre, K.; Shin, S.-Y.; Petersen, A.-K.; Mohney, R.P.; Meredith, D.; Wägele, B.; Altmaier, E.; CardioGram; Deloukas, P.; Erdmann, J.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef]
- Armstrong, M.D. N-delta-acetylornithine and s-methylcysteine in blood plasma. Biochim. Biophys. Acta 1979, 587, 638–642. [Google Scholar] [CrossRef]
- Sun, J.; Jiao, C.; Ma, Y.; Chen, J.; Wu, W.; Liu, S. Anti-ageing effect of red ginseng revealed by urinary metabonomics using rrlc-q-tof-ms. Phytochem. Anal. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- McClay, J.L.; Vunck, S.A.; Batman, A.M.; Crowley, J.J.; Vann, R.E.; Beardsley, P.M.; van den Oord, E.J. Neurochemical metabolomics reveals disruption to sphingolipid metabolism following chronic haloperidol administration. J. Neuroimmune Pharmacol. 2015, 10, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Schneider, A.; Wang, J.Y.; Trivedi, A.; Carrillo, N.R.; Tassone, F.; Rogawski, M.; Hagerman, R.J.; Giulivi, C. Allopregnanolone treatment improves plasma metabolomic profile associated with gaba metabolism in fragile x-associated tremor/ataxia syndrome: A pilot study. Mol. Neurobiol. 2019, 56, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Dalluge, J.J.; Hashizume, T.; Sopchik, A.E.; McCloskey, J.A.; Davis, D.R. Conformational flexibility in rna: The role of dihydrouridine. Nucleic Acids Res. 1996, 24, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.; Ramani, P.; Patankar, S. Serum metabolomics in oral leukoplakia and oral squamous cell carcinoma. J. Cancer Res. Ther. 2017, 13, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Y.; Li, Z.; Liu, X. Untargeted metabolomics profiles delineate metabolic alterations in mouse plasma during lung carcinoma development using uplc-qtof/ms in mse mode. R. Soc. Open Sci. 2018, 5, 181143. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B. Disorders of the carnitine cycle and detection by newborn screening. Ann. Acad. Med. Singap. 2008, 37, 71–73. [Google Scholar]
- Berry-Kravis, E.; Booth, G.; Sanchez, A.C.; Woodbury-Kolb, J. Carnitine levels and the ketogenic diet. Epilepsia 2001, 42, 1445–1451. [Google Scholar] [CrossRef]
- Haug, K.; Salek, R.M.; Conesa, P.; Hastings, J.; de Matos, P.; Rijnbeek, M.; Mahendraker, T.; Williams, M.; Neumann, S.; Rocca-Serra, P. Metabolights—An open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 2012, 41, D781–D786. [Google Scholar] [CrossRef]
- Berthold, M.R.; Cebron, N.; Dill, F.; Gabriel, T.R.; Kötter, T.; Meinl, T.; Ohl, P.; Thiel, K.; Wiswedel, B. Knime-the konstanz information miner: Version 2.0 and beyond. SIGKDD Explor. 2009, 11, 26–31. [Google Scholar] [CrossRef]
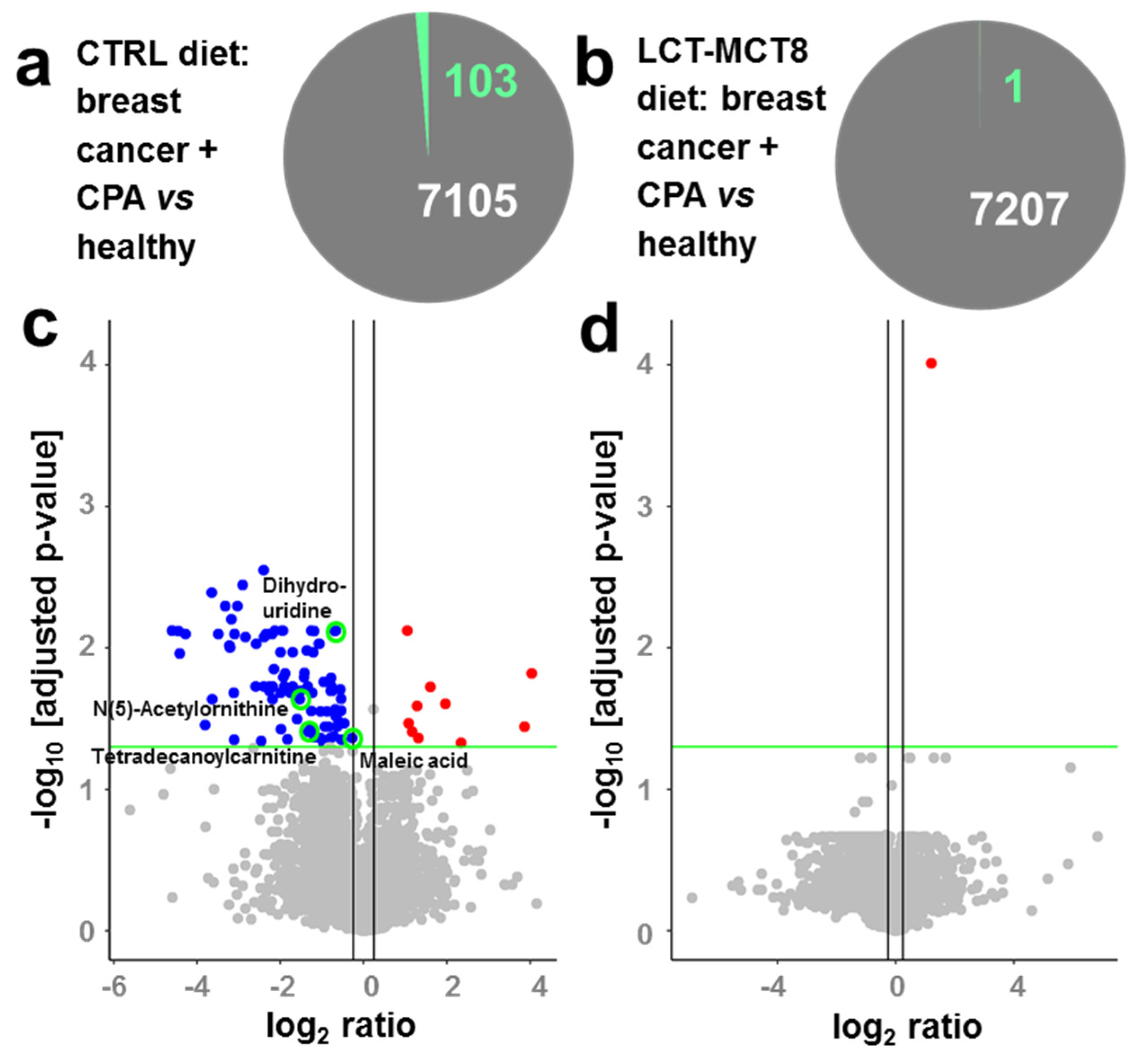

| Number of Significantly Regulated Features | |||
|---|---|---|---|
| Treatment Group | BC a + CPA b + LCT-MCT8 c | Healthy + CTRL d | Healthy + LCT-MCT8 |
| BC + CPA + CTRL | 375 | 103 | 1340 |
| BC + CPA + LCT-MCT8 | - | 371 | 1 |
| Healthy + CTRL | - | - | 597 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licha, D.; Vidali, S.; Aminzadeh-Gohari, S.; Alka, O.; Breitkreuz, L.; Kohlbacher, O.; Reischl, R.J.; Feichtinger, R.G.; Kofler, B.; Huber, C.G. Untargeted Metabolomics Reveals Molecular Effects of Ketogenic Diet on Healthy and Tumor Xenograft Mouse Models. Int. J. Mol. Sci. 2019, 20, 3873. https://doi.org/10.3390/ijms20163873
Licha D, Vidali S, Aminzadeh-Gohari S, Alka O, Breitkreuz L, Kohlbacher O, Reischl RJ, Feichtinger RG, Kofler B, Huber CG. Untargeted Metabolomics Reveals Molecular Effects of Ketogenic Diet on Healthy and Tumor Xenograft Mouse Models. International Journal of Molecular Sciences. 2019; 20(16):3873. https://doi.org/10.3390/ijms20163873
Chicago/Turabian StyleLicha, David, Silvia Vidali, Sepideh Aminzadeh-Gohari, Oliver Alka, Leander Breitkreuz, Oliver Kohlbacher, Roland J. Reischl, René G. Feichtinger, Barbara Kofler, and Christian G. Huber. 2019. "Untargeted Metabolomics Reveals Molecular Effects of Ketogenic Diet on Healthy and Tumor Xenograft Mouse Models" International Journal of Molecular Sciences 20, no. 16: 3873. https://doi.org/10.3390/ijms20163873
APA StyleLicha, D., Vidali, S., Aminzadeh-Gohari, S., Alka, O., Breitkreuz, L., Kohlbacher, O., Reischl, R. J., Feichtinger, R. G., Kofler, B., & Huber, C. G. (2019). Untargeted Metabolomics Reveals Molecular Effects of Ketogenic Diet on Healthy and Tumor Xenograft Mouse Models. International Journal of Molecular Sciences, 20(16), 3873. https://doi.org/10.3390/ijms20163873