Biochemistry of Copper Site Assembly in Heme-Copper Oxidases: A Theme with Variations
Abstract
1. Introduction
1.1. Transition Metal Ions in Nature
1.2. Copper is Essential for Terminal Oxidases
1.3. Assembly of COX Catalytic Core
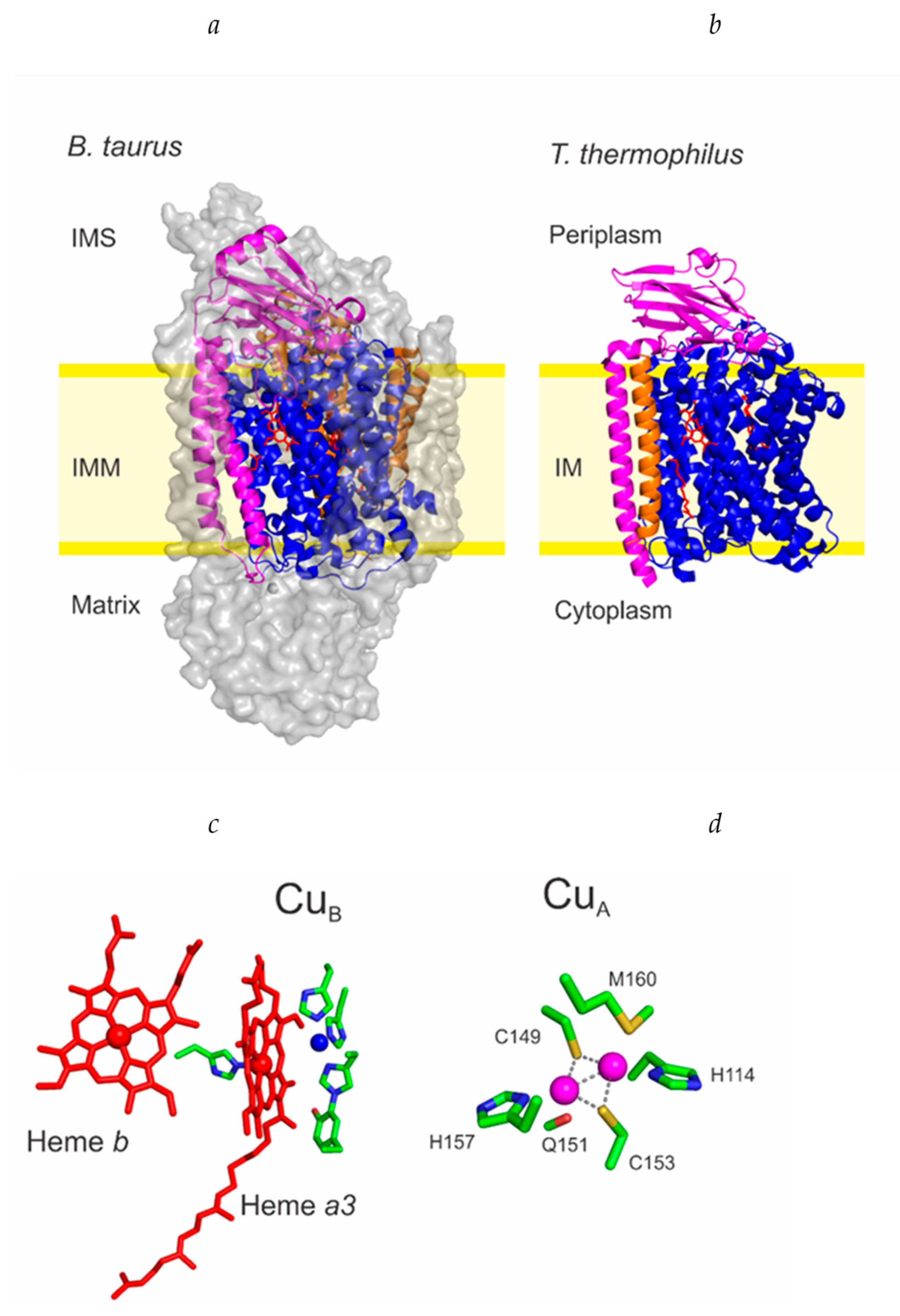
2. Maturation of COX I and the CuB Site
2.1. Translation and Membrane Insertion of Apo-COX I
2.2. Heme Insertion into COX I
2.3. Cu Insertion to COX I
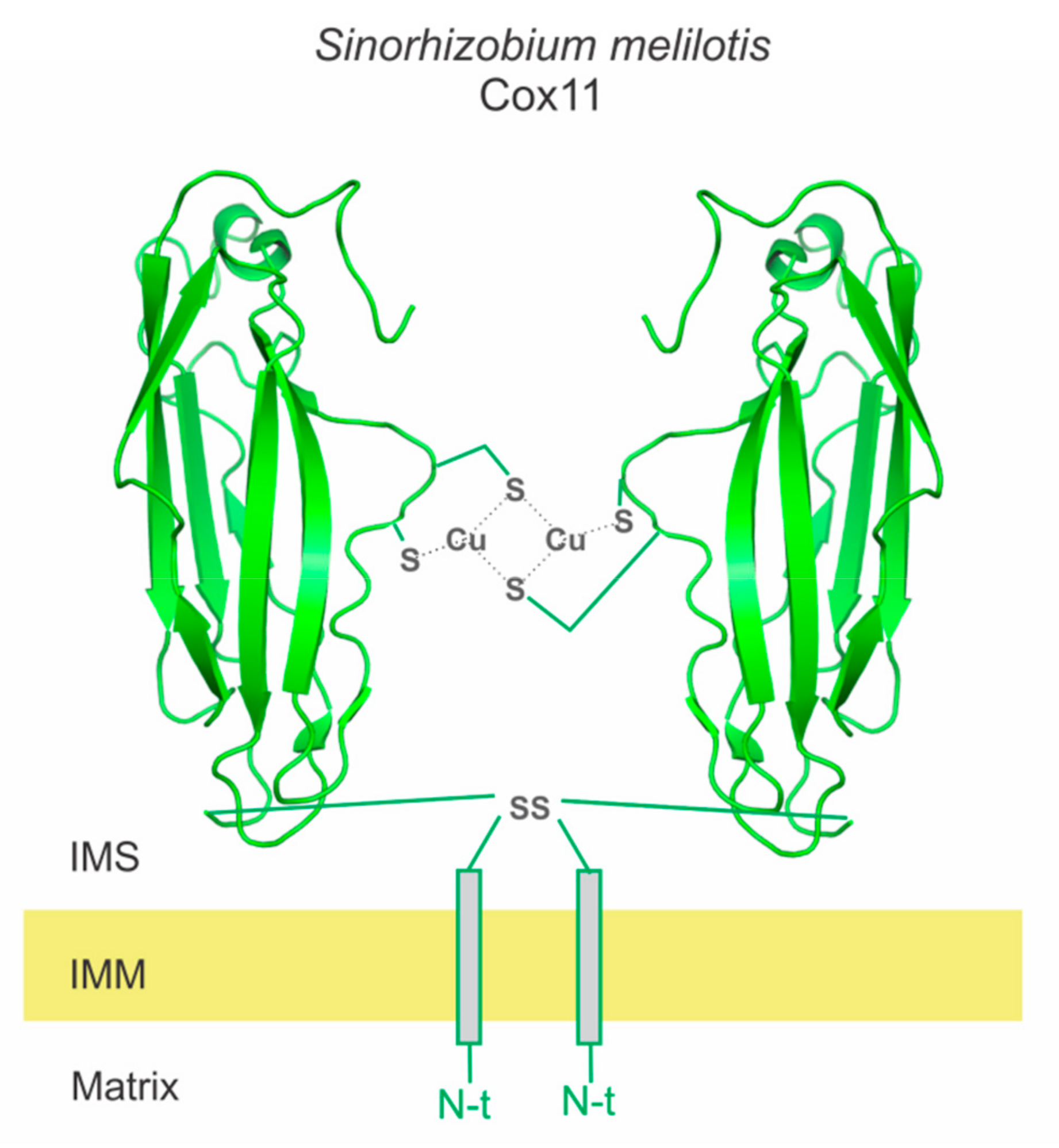
2.4. Sco1 as a Copper Donor
2.5. Heme Moieties and Protein Functionality
3. Maturation of COX II and the CuA Site
3.1. Translation and Membrane Insertion of Apo-COX II
3.2. The Metallochaperone Module and the Assembly of the CuA Site
3.3. CuA Assembly in Bacteria
3.4. CuA Assembly in Mitochondria
3.5. A Strategy for the Biochemical Analysis of the Mitochondrial Metallochaperone Module
4. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| COX | Cytochrome c Oxidase |
| IMM | Inner Mitochondrial Membrane |
| IMS | Inter-Membrane Space |
References
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. Metal ions in biological catalysis: From enzyme databases to general principles. JBIC J. Biol. Inorg. Chem. 2008, 13, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The stability of transition-metal complexes. J. Chem. Soc. 1953, 637, 3192–3210. [Google Scholar] [CrossRef]
- Brancaccio, D.; Gallo, A.; Piccioli, M.; Novellino, E.; Ciofi-Baffoni, S.; Banci, L. [4Fe-4S] cluster assembly in mitochondria and its impairment by copper. J. Am. Chem. Soc. 2017, 139, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, L.; Cotruvo, J.A.; Chan, J.; Kaluarachchi, H.; Muchenditsi, A.; Pendyala, V.S.; Jia, S.; Aron, A.T.; Ackerman, C.M.; Wal, M.N.; et al. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 2016, 12, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, 877–883. [Google Scholar] [CrossRef]
- Smith, A.D.; Logeman, B.L.; Thiele, D.J. Copper Acquisition and Utilization in Fungi. Annu. Rev. Microbiol. 2017, 71, 597–623. [Google Scholar] [CrossRef]
- Kim, B.-E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef]
- Neilands, J.B. Siderophores: Structure and Function of Microbial Iron Transport Compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J. Metal trafficking via siderophores in Gram-negative bacteria: Specificities and characteristics of the pyoverdine pathway. J. Inorg. Biochem. 2008, 102, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-I.; Henderson, J.P. Microbial Copper-binding Siderophores at the Host-Pathogen Interface. J. Biol. Chem. 2015, 290, 18967–18974. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.C.; Garcia-Herrero, A.; Johanson, T.H.; Krewulak, K.D.; Lau, C.K.; Peacock, R.S.; Slavinskaya, Z.; Vogel, H.J. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. BioMetals 2010, 23, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Jung, H.; Meloni, G. Copper metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.R.; Barton, J.K. DNA Protection by the Bacterial Ferritin Dps via DNA Charge Transport. J. Am. Chem. Soc. 2013, 135, 15726–15729. [Google Scholar] [CrossRef][Green Version]
- Smith, A.T.; Smith, K.P.; Rosenzweig, A.C. Diversity of the metal-transporting P1B-type ATPases. JBIC J. Biol. Inorg. Chem. 2014, 19, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.J.; Winge, D.R. Copper Metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef]
- Polishchuk, R.; Lutsenko, S. Golgi in copper homeostasis: A view from the membrane trafficking field. Histochem. Cell Biol. 2013, 140, 285–295. [Google Scholar] [CrossRef]
- Vest, K.E.; Leary, S.C.; Winge, D.R.; Cobine, P.A. Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J. Biol. Chem. 2013, 288, 23884–23892. [Google Scholar] [CrossRef]
- Baker, Z.N.; Cobine, P.A.; Leary, S.C. The mitochondrion: a central architect of copper homeostasis. Metallomics 2017, 9, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Tapken, W.; Ravet, K.; Shahbaz, M.; Pilon, M. Regulation of Cu delivery to chloroplast proteins. Plant Signal. Behav. 2015, 10, e1046666. [Google Scholar] [PubMed]
- Bertini, I.; Cavallaro, G.; McGreevy, K.S. Cellular copper management-a draft user’s guide. Coord. Chem. Rev. 2010, 254, 506–524. [Google Scholar] [CrossRef]
- Fontanesi, F.; Soto, I.C.; Horn, D.; Barrientos, A. Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am. J. Physiol. Physiol. 2006, 291, 1129–1147. [Google Scholar] [CrossRef] [PubMed]
- Kroneck, P.M.H. Walking the seven lines: binuclear copper A in cytochrome c oxidase and nitrous oxide reductase. J. Biol. Inorg. Chem. 2018, 23, 27–39. [Google Scholar] [CrossRef]
- Soulimane, T.; Buse, G.; Bourenkov, G.P.; Bartunik, H.D.; Huber, R.; Than, M.E. Structure and mechanism of the aberrant ba3-cytochrome c oxidase from Thermus thermophilus. EMBO J. 2000, 19, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yamashita, E.; Inoue, N.; Yao, M.; Fei, M.J.; Libeu, C.P.; Mizushima, T.; et al. Redox-Coupled Crystal Structural Changes in Bovine Heart Cytochrome c Oxidase. Science 1998, 280, 1723–1729. [Google Scholar] [CrossRef]
- Leary, S.C.; Kaufman, B.A.; Pellecchia, G.; Guercin, G.H.; Mattman, A.; Jaksch, M.; Shoubridge, E.A. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum. Mol. Genet. 2004, 13, 1839–1848. [Google Scholar] [CrossRef]
- Hiser, L.; di Valentin, M.; Hamer, A.G.; Hosler, J.P. Cox11p is required for stable formation of the Cu(B) and magnesium centers of cytochrome c oxidase. J. Biol. Chem. 2000, 275, 619–623. [Google Scholar] [CrossRef]
- Hematian, S.; Garcia-Bosch, I.; Karlin, K.D. Synthetic Heme/Copper Assemblies: Toward an Understanding of Cytochrome c Oxidase Interactions with Dioxygen and Nitrogen Oxides. Acc. Chem. Res. 2015, 48, 2462–2474. [Google Scholar] [CrossRef]
- Pomowski, A.; Zumft, W.G.; Kroneck, P.M.H.; Einsle, O. N2O binding at a [4Cu:2S] copper–sulphur cluster in nitrous oxide reductase. Nature 2011, 477, 234–237. [Google Scholar] [CrossRef] [PubMed]
- McStay, G.P.; Su, C.H.; Tzagoloff, A. Modular assembly of yeast cytochrome oxidase. Mol. Biol. Cell 2013, 24, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Santana, M.; Teixeira, M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim. Biophys. Acta 2001, 1505, 185–208. [Google Scholar] [CrossRef]
- Jett, K.A.; Leary, S.C. Building the CuA site of cytochrome c oxidase: A complicated, redox-dependent process driven by a surprisingly large complement of accessory proteins. J. Biol. Chem. 2018, 293, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.C.; Fontanesi, F.; Liu, J.; Barrientos, A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim. Biophys. Acta 2012, 1817, 883–897. [Google Scholar] [CrossRef]
- Mick, D.U.; Fox, T.D.; Rehling, P. Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat. Rev. Mol. Cell Biol. 2011, 12, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, N.; Racca, S.; Gras, D.E.; Gonzalez, D.H.; Welchen, E. The complexity of mitochondrial complex iv: An update of cytochrome c oxidase biogenesis in plants. Int. J. Mol. Sci. 2018, 19, 662. [Google Scholar] [CrossRef]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef]
- Mattatall, N.R.; Jazairi, J.; Hill, B.C. Characterization of ypmQ, an accessory protein required for the expression of cytochrome c oxidase in Bacillus subtilis. J. Biol. Chem. 2000, 275, 28802–28809. [Google Scholar] [CrossRef]
- Badrick, A.C.; Hamilton, A.J.; Bernhardt, P.V.; Jones, C.E.; Kappler, U.; Jennings, M.P.; McEwan, A.G. PrrC, a Sco homologue from Rhodobacter sphaeroides, possesses thiol-disulfide oxidoreductase activity. FEBS Lett. 2007, 581, 4663–4667. [Google Scholar] [CrossRef][Green Version]
- Hennon, S.W.; Soman, R.; Zhu, L.; Dalbey, R.E. YidC/Alb3/Oxa1 Family of Insertases. J. Biol. Chem. 2015, 290, 14866–14874. [Google Scholar] [CrossRef] [PubMed]
- Mick, D.U.; Vukotic, M.; Piechura, H.; Meyer, H.E.; Warscheid, B.; Deckers, M.; Rehling, P. Coa3 and Cox14 are essential for negative feedback regulation of COX1 translation in mitochondria. J. Cell Biol. 2010, 191, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Colás, N.; Perea-García, A.; Mayo De Andrés, S.; Garcia-Molina, A.; Dorcey, E.; Rodríguez-Navarro, S.; Pérez-Amador, M.A.; Puig, S.; Peñarrubia, L. Comparison of global responses to mild deficiency and excess copper levels in Arabidopsis seedlings. Metallomics 2013, 5, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Hiser, L.; Hosler, J.P. Heme A is not essential for assembly of the subunits of cytochrome c oxidase of Rhodobacter sphaeroides. J. Biol. Chem. 2001, 276, 45403–45407. [Google Scholar] [CrossRef] [PubMed]
- Hederstedt, L. Heme A biosynthesis. Biochim. Biophys. Acta 2012, 1817, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Antonicka, H.; Mattman, A.; Carlson, C.G.; Glerum, D.M.; Hoffbuhr, K.C.; Leary, S.C.; Kennaway, N.G.; Shoubridge, E.A. Mutations in COX15 Produce a Defect in the Mitochondrial Heme Biosynthetic Pathway, Causing Early-Onset Fatal Hypertrophic Cardiomyopathy. Am. J. Hum. Genet. 2003, 72, 101–114. [Google Scholar] [CrossRef]
- Antonicka, H.; Leary, S.C.; Guercin, G.-H.; Agar, J.N.; Horvath, R.; Kennaway, N.G.; Harding, C.O.; Jaksch, M.; Shoubridge, E.A. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003, 12, 2693–2702. [Google Scholar] [CrossRef]
- Brown, B.M.; Wang, Z.; Brown, K.R.; Cricco, J.A.; Hegg, E.L. Heme O Synthase and Heme A Synthase from Bacillus subtilis and Rhodobacter sphaeroides Interact in Escherichia coli. Biochemistry 2004, 43, 13541–13548. [Google Scholar] [CrossRef]
- Barros, M.H.; Carlson, C.G.; Glerum, D.M.; Tzagoloff, A. Involvement of mitochondrial ferredoxin and Cox15p in hydroxylation of heme O. FEBS Lett. 2001, 492, 133–138. [Google Scholar] [CrossRef]
- Mashkevich, G.; Repetto, B.; Glerum, D.M.; Jin, C.; Tzagoloff, A. SHY1, the yeast homolog of the mammalian SURF-1 gene, encodes a mitochondrial protein required for respiration. J. Biol. Chem. 1997, 272, 14356–14364. [Google Scholar] [CrossRef]
- Hannappel, A.; Bundschuh, F.A.; Ludwig, B. Role of Surf1 in heme recruitment for bacterial COX biogenesis. Biochim. Biophys. Acta 2012, 1817, 928–937. [Google Scholar] [CrossRef]
- Poyau, A.; Buchet, K.; Bouzidi, M.F.; Zabot, M.T.; Echenne, B.; Yao, J.; Shoubridge, E.A.; Godinot, C. Missense mutations in SURF1 associated with deficient cytochrome c oxidase assembly in Leigh syndrome patients. Hum. Genet. 2000, 106, 194–205. [Google Scholar] [CrossRef]
- Barrientos, A. Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh’s syndrome. EMBO J. 2002, 21, 43–52. [Google Scholar] [CrossRef]
- Radin, I.; Mansilla, N.; Rödel, G.; Steinebrunner, I. The Arabidopsis COX11 Homolog is Essential for Cytochrome c Oxidase Activity. Front. Plant Sci. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Thompson, A.K.; Smith, D.; Gray, J.; Carr, H.S.; Liu, A.; Winge, D.R.; Hosler, J.P. Mutagenic Analysis of Cox11 of Rhodobacter sphaeroides: Insights into the Assembly of CuB of Cytochrome c Oxidase. Biochemistry 2010, 49, 5651–5661. [Google Scholar] [CrossRef][Green Version]
- Tzagoloff, A.; Capitanio, N.; Nobrega, M.P.; Gatti, D. Cytochrome oxidase assembly in yeast requires the product of COX11, a homolog of the P. denitrificans protein encoded by ORF3. EMBO J. 1990, 9, 2759–2764. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cantini, F.; Ciofi-Baffoni, S.; Gonnelli, L.; Mangani, S. Solution Structure of Cox11, a Novel Type of β-Immunoglobulin-like Fold Involved in CuB Site Formation of Cytochrome c Oxidase. J. Biol. Chem. 2004, 279, 34833–34839. [Google Scholar] [CrossRef]
- Carr, H.S.; Maxfield, A.B.; Horng, Y.-C.; Winge, D.R. Functional Analysis of the Domains in Cox11. J. Biol. Chem. 2005, 280, 22664–22669. [Google Scholar] [CrossRef]
- Carr, H.S.; George, G.N.; Winge, D.R. Yeast Cox11, a Protein Essential for Cytochrome c Oxidase Assembly, Is a Cu(I)-binding Protein. J. Biol. Chem. 2002, 277, 31237–31242. [Google Scholar] [CrossRef]
- Frangipani, E.; Haas, D. Copper acquisition by the SenC protein regulates aerobic respiration in Pseudomonas aeruginosa PAO1. FEMS Microbiol. Lett. 2009, 298, 234–240. [Google Scholar] [CrossRef]
- Lohmeyer, E.; Schröder, S.; Pawlik, G.; Trasnea, P.-I.; Peters, A.; Daldal, F.; Koch, H.-G. The ScoI homologue SenC is a copper binding protein that interacts directly with the cbb3-type cytochrome oxidase in Rhodobacter capsulatus. Biochim. Biophys. Acta 2012, 1817, 2005–2015. [Google Scholar] [CrossRef]
- Bonnefoy, N.; Fiumera, H.L.; Dujardin, G.; Fox, T.D. Roles of Oxa1-related inner-membrane translocases in assembly of respiratory chain complexes. Biochim. Biophys. Acta 2009, 1793, 60–70. [Google Scholar] [CrossRef]
- Kiefer, D.; Kuhn, A. YidC-mediated membrane insertion. FEMS Microbiol. Lett. 2018, 365, 106. [Google Scholar] [CrossRef]
- Kolli, R.; Soll, J.; Carrie, C. OXA2b is crucial for proper membrane insertion of COX2 during biogenesis of complex IV in plant mitochondria. Plant Physiol. 2019, 179, 601–615. [Google Scholar] [CrossRef]
- Lorenzi, I.; Oeljeklaus, S.; Ronsör, C.; Bareth, B.; Warscheid, B.; Rehling, P.; Dennerlein, S. Ribosome-Associated Mba1 Escorts Cox2 from Insertion Machinery to Maturing Assembly Intermediates. Mol. Cell. Biol. 2016, 36, 2782–2793. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cavallaro, G.; Ciofi-Baffoni, S. Seeking the determinants of the elusive functions of Sco proteins. FEBS J. 2011, 278, 2244–2262. [Google Scholar] [CrossRef]
- Siluvai, G.S.; Mayfield, M.; Nilges, M.J.; Debeer George, S.; Blackburn, N.J. Anatomy of a red copper center: Spectroscopic identification and reactivity of the copper centers of bacillus subtilis Sco and its Cys-to-ala variants. J. Am. Chem. Soc. 2010, 132, 5215–5226. [Google Scholar] [CrossRef]
- Balatri, E.; Banci, L.; Bertini, I.; Cantini, F.; Ciofi-Baffoni, S. Solution structure of Sco1: A thioredoxin-like protein involved in cytochrome c oxidase assembly. Structure 2003, 11, 1431–1443. [Google Scholar] [CrossRef]
- Ye, Q.; Imriskova-Sosova, I.; Hill, B.C.; Jia, Z. Identification of a disulfide switch in BsSco, a member of the Sco family of cytochrome c oxidase assembly proteins. Biochemistry 2005, 44, 2934–2942. [Google Scholar] [CrossRef]
- Andruzzi, L.; Nakano, M.; Nilges, M.J.; Blackburn, N.J. Spectroscopic studies of metal binding and metal selectivity in Bacillus subtilis BSco, a homologue of the yeast mitochondrial protein Sco1p. J. Am. Chem. Soc. 2005, 127, 16548–16558. [Google Scholar] [CrossRef]
- Hill, B.C.; Andrews, D. Differential affinity of BsSCO for Cu(II) and Cu(I) suggests a redox role in copper transfer to the CuA center of cytochrome c oxidase. Biochim. Biophys. Acta 2012, 1817, 948–954. [Google Scholar] [CrossRef][Green Version]
- Blundell, K.L.I.M.; Wilson, M.T.; Svistunenko, D.A.; Vijgenboom, E.; Worrall, J.A.R. Morphological development and cytochrome c oxidase activity in Streptomyces lividans are dependent on the action of a copper bound Sco protein. Open Biol. 2013, 3, 120163. [Google Scholar] [CrossRef][Green Version]
- Blundell, K.L.I.M.; Hough, M.A.; Vijgenboom, E.; Worrall, J.A.R. Structural and mechanistic insights into an extracytoplasmic copper trafficking pathway in Streptomyces lividans. Biochem. J. 2014, 459, 525–538. [Google Scholar] [CrossRef]
- Abriata, L.A.; Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Gkazonis, P.; Spyroulias, G.A.; Vila, A.J.; Wang, S. Mechanism of CuA assembly. Nat. Chem. Biol. 2008, 4, 599. [Google Scholar] [CrossRef]
- Llases, M.-E.; Lisa, M.-N.; Morgada, M.N.; Giannini, E.; Alzari, P.M.; Vila, A.J. Arabidopsis thaliana Hcc1 is a Sco-like metallochaperone for CuA assembly in Cytochrome c Oxidase. FEBS J. 2019, in press. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Janicka, A.; Martinelli, M.; Kozlowski, H.; Palumaa, P. A structural-dynamical characterization of human Cox17. J. Biol. Chem. 2008, 283, 7912–7920. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Katsari, E.; Katsaros, N.; Kubicek, K.; Mangani, S. A copper(I) protein possibly involved in the assembly of CuA center of bacterial cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2005, 102, 3994–3999. [Google Scholar] [CrossRef]
- Dash, B.P.; Alles, M.; Bundschuh, F.A.; Richter, O.M.H.; Ludwig, B. Protein chaperones mediating copper insertion into the CuAsite of the aa3-type cytochrome c oxidase of Paracoccus denitrificans. Biochim. Biophys. Acta 2015, 1847, 202–211. [Google Scholar] [CrossRef][Green Version]
- Schimo, S.; Wittig, I.; Pos, K.M.; Ludwig, B. Cytochrome c oxidase biogenesis and metallochaperone interactions: Steps in the assembly pathway of a bacterial complex. PLoS ONE 2017, 12, e0170037. [Google Scholar] [CrossRef]
- Serventi, F.; Youard, Z.A.; Murset, V.; Huwiler, S.; Bühler, D.; Richter, M.; Luchsinger, R.; Fischer, H.M.; Brogioli, R.; Niederer, M.; et al. Copper starvation-inducible protein for cytochrome oxidase biogenesis in Bradyrhizobium japonicum. J. Biol. Chem. 2012, 287, 38812–38823. [Google Scholar] [CrossRef]
- Hennecke, H. Expression, purification and functional properties of a soluble form of Bradyrhizobium japonicum TlpA, a thioredoxin-like protein. Eur. J. Biochem. 1994, 344, 339–344. [Google Scholar]
- Capitani, G.; Rossmann, R.; Sargent, D.F.; Gru, M.G.; Richmond, T.J.; Hennecke, H.; Institut, B.; Zu, C. Structure of the Soluble Domain of a Membrane-anchored Thioredoxin-like Protein from Bradyrhizobium japonicum Reveals Unusual Properties. J. Mol. Biol. 2001, 311, 1037–1048. [Google Scholar] [CrossRef]
- Mohorko, E.; Abicht, H.K.; Bühler, D.; Glockshuber, R.; Hennecke, H.; Fischer, H.M. Thioredoxin-like protein TlpA from Bradyrhizobium japonicum is a reductant for the copper metallochaperone ScoI. FEBS Lett. 2012, 586, 4094–4099. [Google Scholar] [CrossRef]
- Abicht, H.K.; Schärer, M.A.; Quade, N.; Ledermann, R.; Mohorko, E.; Capitani, G.; Hennecke, H.; Glockshuber, R. How periplasmic thioredoxin TlpA reduces bacterial copper chaperone ScoI and cytochrome oxidase subunit II (CoxB) prior to metallation. J. Biol. Chem. 2014, 289, 32431–32444. [Google Scholar] [CrossRef]
- Bühler, D.; Rossmann, R.; Landolt, S.; Balsiger, S.; Fischer, H.M.; Hennecke, H. Disparate pathways for the biogenesis of cytochrome oxidases in Bradyrhizobium japonicum. J. Biol. Chem. 2010, 285, 15704–15713. [Google Scholar] [CrossRef]
- Arnesano, F.; Balatri, E.; Banci, L.; Bertini, I.; Winge, D.R.; Sacconi, V.L. Folding Studies of Cox17 Reveal an Important Interplay of Cysteine Oxidation and Copper Binding. Structure 2005, 13, 713–722. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kako, K.; Kashiwabara, S.-I.; Takehara, A.; Inada, Y.; Arai, H.; Nakada, K.; Kodama, H.; Hayashi, J.-I.; Baba, T.; et al. Mammalian Copper Chaperone Cox17p Has an Essential Role in Activation of Cytochrome c Oxidase and Embryonic Development. Mol. Cell. Biol. 2002, 22, 7614–7621. [Google Scholar] [CrossRef]
- Garcia, L.; Welchen, E.; Gey, U.; Arce, A.L.; Steinebrunner, I.; Gonzalez, D.H. The cytochrome c oxidase biogenesis factor AtCOX17 modulates stress responses in Arabidopsis. Plant Cell Environ. 2016, 39, 628–644. [Google Scholar] [CrossRef]
- Abajian, C.; Yatsunyk, L.A.; Ramirez, B.E.; Rosenzweig, A.C. Yeast Cox17 solution structure and copper(I) binding. J. Biol. Chem. 2004, 279, 53584–53592. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Hadjiloi, T.; Martinelli, M.; Palumaa, P. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 6803–6808. [Google Scholar] [CrossRef]
- Chojnacka, M.; Gornicka, A.; Oeljeklaus, S.; Warscheid, B.; Chacinska, A. Cox17 protein is an auxiliary factor involved in the control of the mitochondrial contact site and cristae organizing system. J. Biol. Chem. 2015, 290, 15304–15312. [Google Scholar] [CrossRef]
- Leary, S.C.; Cobine, P.A.; Kaufman, B.A.; Guercin, G.H.; Mattman, A.; Palaty, J.; Lockitch, G.; Winge, D.R.; Rustin, P.; Horvath, R.; et al. The Human Cytochrome c Oxidase Assembly Factors SCO1 and SCO2 Have Regulatory Roles in the Maintenance of Cellular Copper Homeostasis. Cell Metab. 2007, 5, 9–20. [Google Scholar] [CrossRef]
- Glerum, D.M.; Shtanko, A.; Tzagoloff, A. Characterization of C O X 1 7, a Yeast Gene Involved in Copper Metabolism and Assembly of Cytochrome Oxidase. J. Biol. Chem. 1996, 24, 14504–14509. [Google Scholar] [CrossRef]
- Rigby, K.; Cobine, P.A.; Khalimonchuk, O.; Winge, D.R. Mapping the functional interaction of Sco1 and Cox2 in cytochrome oxidase biogenesis. J. Biol. Chem. 2008, 283, 15015–15022. [Google Scholar] [CrossRef]
- Ghosh, A.; Pratt, A.T.; Soma, S.; Theriault, S.G.; Griffin, A.T.; Trivedi, P.P.; Gohil, V.M. Mitochondrial disease genes COA6, COX6B and SCO2 have overlapping roles in COX2 biogenesis. Hum. Mol. Genet. 2016, 25, 660–671. [Google Scholar] [CrossRef]
- Jaksch, M. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000, 9, 795–801. [Google Scholar] [CrossRef]
- Valnot, I.; Osmond, S.; Gigarel, N.; Mehaye, B.; Amiel, J.; Cormier-Daire, V.; Munnich, A.; Bonnefont, J.; Rustin, P.; Rotig, A. Mutations of the SCO1 Gene in Mitochondrial Cytochrome c Oxidase Deficiency with Neonatal-Onset Hepatic Failure and Encephalopathy. Am. J. Hum. Genet. 2000, 67, 1104–1109. [Google Scholar] [CrossRef]
- Papadopoulou, L.C.; Sue, C.M.; Davidson, M.M.; Tanji, K.; Nishino, I.; Sadlock, J.E.; Krishna, S.; Walker, W.; Selby, J.; Glerum, D.M.; et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 1999, 23, 333–337. [Google Scholar] [CrossRef]
- Morgada, M.N.; Abriata, L.A.; Cefaro, C.; Gajda, K.; Banci, L.; Vila, A.J. Loop recognition and copper-mediated disulfide reduction underpin metal site assembly of CuA in human cytochrome oxidase. Proc. Natl. Acad. Sci. USA 2015, 112, 11771–11776. [Google Scholar] [CrossRef]
- Stroud, D.A.; Maher, M.J.; Lindau, C.; Vögtle, F.N.; Frazier, A.E.; Surgenor, E.; Mountford, H.; Singh, A.P.; Bonas, M.; Oeljeklaus, S.; et al. COA6 is a mitochondrial complex IV assembly factor critical for biogenesis of mtDNA-encoded COX2. Hum. Mol. Genet. 2015, 24, 5404–5415. [Google Scholar] [CrossRef]
- Pacheu-Grau, D.; Bareth, B.; Dudek, J.; Juris, L.; Vögtle, F.N.; Wissel, M.; Leary, S.C.; Dennerlein, S.; Rehling, P.; Deckers, M. Cooperation between COA6 and SCO2 in COX2 maturation during cytochrome c oxidase assembly links two mitochondrial cardiomyopathies. Cell Metab. 2015, 21, 823–833. [Google Scholar] [CrossRef]
- Cerqua, C.; Morbidoni, V.; Desbats, M.A.; Doimo, M.; Frasson, C.; Sacconi, S.; Baldoin, M.C.; Sartori, G.; Basso, G.; Salviati, L.; et al. COX16 is required for assembly of cytochrome c oxidase in human cells and is involved in copper delivery to COX2. Biochim. Biophys. Acta 2018, 1859, 244–252. [Google Scholar] [CrossRef]
- Aich, A.; Wang, C.; Chowdhury, A.; Ronsör, C.; Pacheu-Grau, D.; Richter-Dennerlein, R.; Dennerlein, S.; Rehling, P. COX16 promotes COX2 metallation and assembly during respiratory complex IV biogenesis. Elife 2018, 7, 1–18. [Google Scholar] [CrossRef]
- Carlson, C.G.; Barrientos, A.; Tzagoloff, A.; Glerum, D.M. COX16 encodes a novel protein required for the assembly of cytochrome oxidase in Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 3770–3775. [Google Scholar] [CrossRef]
- Kim, H.J.; Khalimonchuk, O.; Smith, P.M.; Winge, D.R. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim. Biophys. Acta 2012, 1823, 1604–1616. [Google Scholar] [CrossRef]
- Su, C.H.; Tzagoloff, A. Cox16 protein is physically associated with Cox1p assembly intermediates and with cytochrome oxidase. J. Biol. Chem. 2017, 292, 16277–16283. [Google Scholar] [CrossRef]
- Kocabey, A.E.; Kost, L.; Gehlhar, M.; Rödel, G.; Gey, U. Mitochondrial Sco proteins are involved in oxidative stress defense. Redox Biol. 2018, 21, 101079. [Google Scholar] [CrossRef]
- Hartley, A.M.; Lukoyanova, N.; Zhang, Y.; Cabrera-Orefice, A.; Arnold, S.; Meunier, B.; Pinotsis, N.; Maréchal, A. Structure of yeast cytochrome c oxidase in a supercomplex with cytochrome bc1. Nat. Struct. Mol. Biol. 2019, 26, 78. [Google Scholar] [CrossRef]
- Guo, R.; Zong, S.; Wu, M.; Gu, J.; Yang, M. Architecture of Human Mitochondrial Respiratory Megacomplex I2III2IV2. Cell 2017, 170, 1247–1257. [Google Scholar] [CrossRef]
- Morgada, M.N.; Abriata, L.A.; Zitare, U.; Alvarez-Paggi, D.; Murgida, D.H.; Vila, A.J. Control of the electronic ground state on an electron-transfer copper site by second-sphere perturbations. Angew. Chem. Int. Ed. 2014, 53, 6188–6192. [Google Scholar] [CrossRef]
- Zaballa, M.-E.; Abriata, L.A.; Donaire, A.; Vila, A.J. Flexibility of the metal-binding region in apo-cupredoxins. Proc. Natl. Acad. Sci. USA 2012, 109, 9254–9259. [Google Scholar] [CrossRef]
- Abriata, L.A. Structural Models And Considerations On The COA6, COX18 And COX20 Factors That Assist Assembly Of Human Cytochrome C Oxidase Subunit II. bioRxiv 2017. [Google Scholar] [CrossRef]
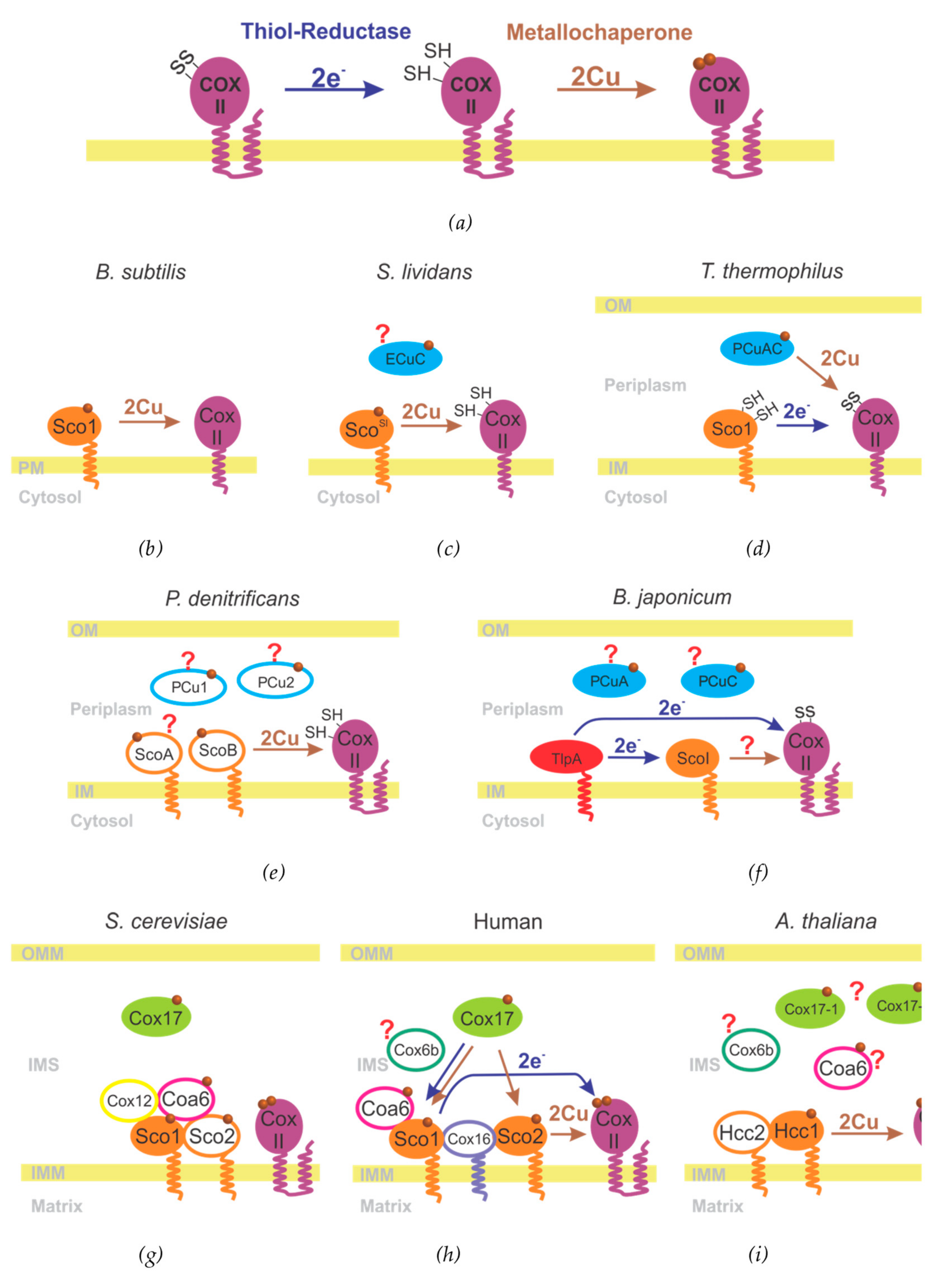

| Organism | pCuAC Family | Cox17 Family | Sco Family | ||
|---|---|---|---|---|---|
| Prokaryotes | Gram+ | B. subtilis | -- | -- | Sco (YpmQ) 1 |
| S. lividans | ECuC | -- | Sco | ||
| Gram- | T. thermophilus | pCuAC | -- | Sco | |
| Alpha- proteobacteria | P. denitrificans | pCu1 pCu2 | -- | ScoA ScoB 2 | |
| B. japonicum | pCuA 1 PCuC 1 | -- | Sco1 | ||
| Eukaryotes | S. cerevisiae | -- | Cox17 | Sco1 1 Sco2 2 | |
| Human | -- | Cox17 1 | Sco1 1 Sco2 1 | ||
| A. thaliana | -- | Cox17a 1 Cox17b 1 | Sco1 (Hcc1) 1 Sco2 (Hcc2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llases, M.-E.; Morgada, M.N.; Vila, A.J. Biochemistry of Copper Site Assembly in Heme-Copper Oxidases: A Theme with Variations. Int. J. Mol. Sci. 2019, 20, 3830. https://doi.org/10.3390/ijms20153830
Llases M-E, Morgada MN, Vila AJ. Biochemistry of Copper Site Assembly in Heme-Copper Oxidases: A Theme with Variations. International Journal of Molecular Sciences. 2019; 20(15):3830. https://doi.org/10.3390/ijms20153830
Chicago/Turabian StyleLlases, María-Eugenia, Marcos N. Morgada, and Alejandro J. Vila. 2019. "Biochemistry of Copper Site Assembly in Heme-Copper Oxidases: A Theme with Variations" International Journal of Molecular Sciences 20, no. 15: 3830. https://doi.org/10.3390/ijms20153830
APA StyleLlases, M.-E., Morgada, M. N., & Vila, A. J. (2019). Biochemistry of Copper Site Assembly in Heme-Copper Oxidases: A Theme with Variations. International Journal of Molecular Sciences, 20(15), 3830. https://doi.org/10.3390/ijms20153830