Molecular and Hormonal Aspects of Drought-Triggered Flower Shedding in Yellow Lupine
Abstract
1. Introduction
2. Results
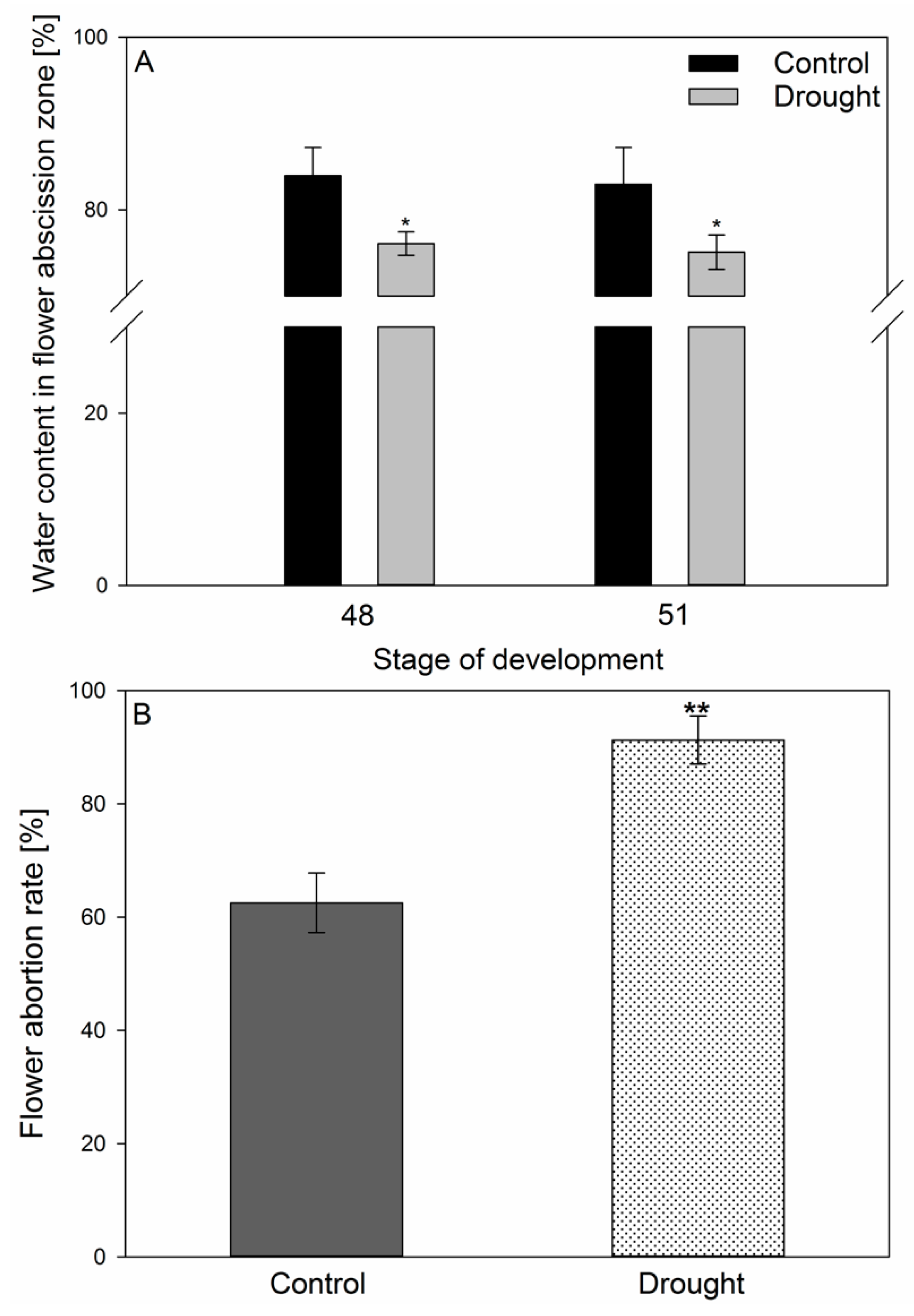
2.1. Lupine Biometric Parameters and Elements Composition Are Affected by Drought
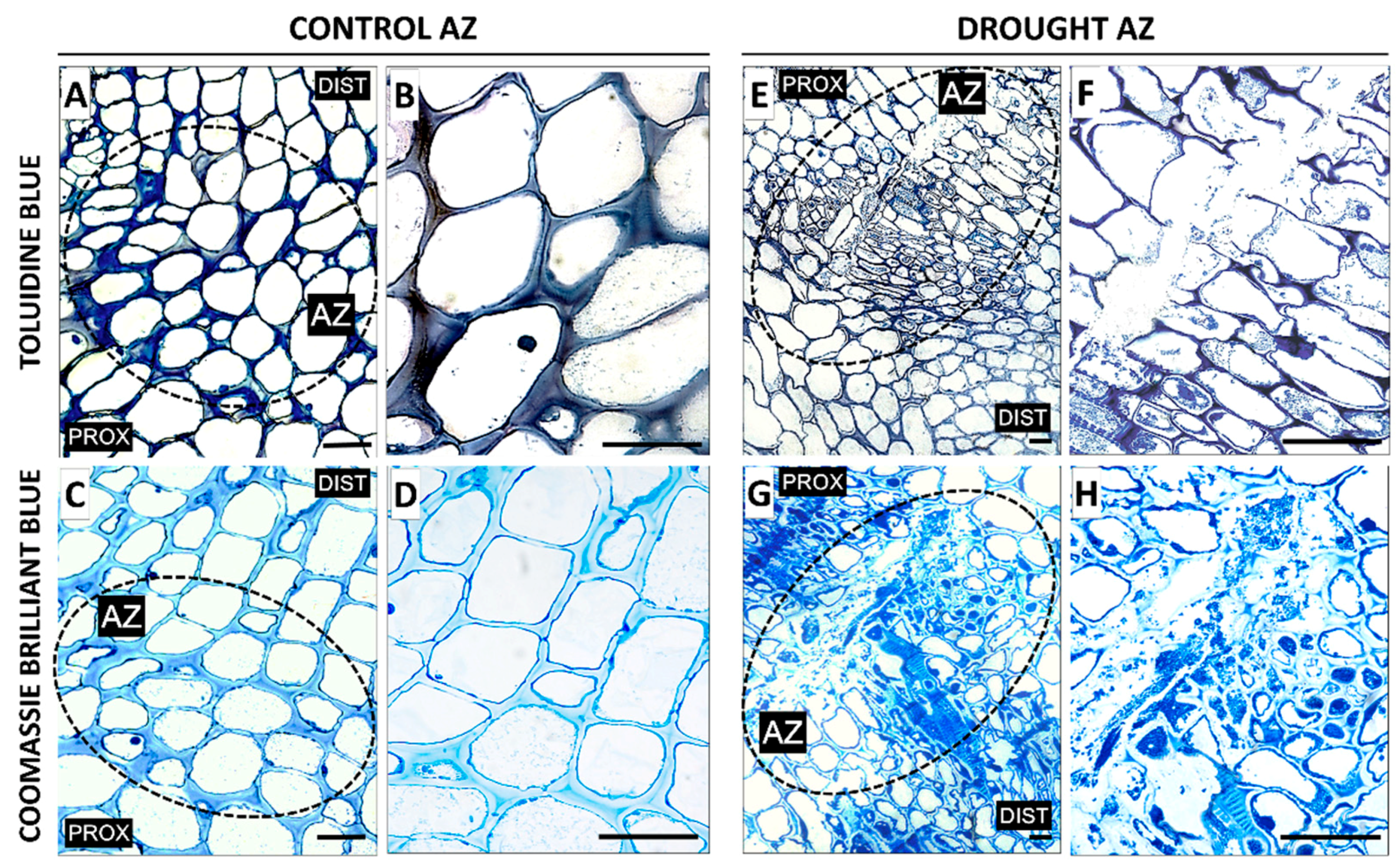
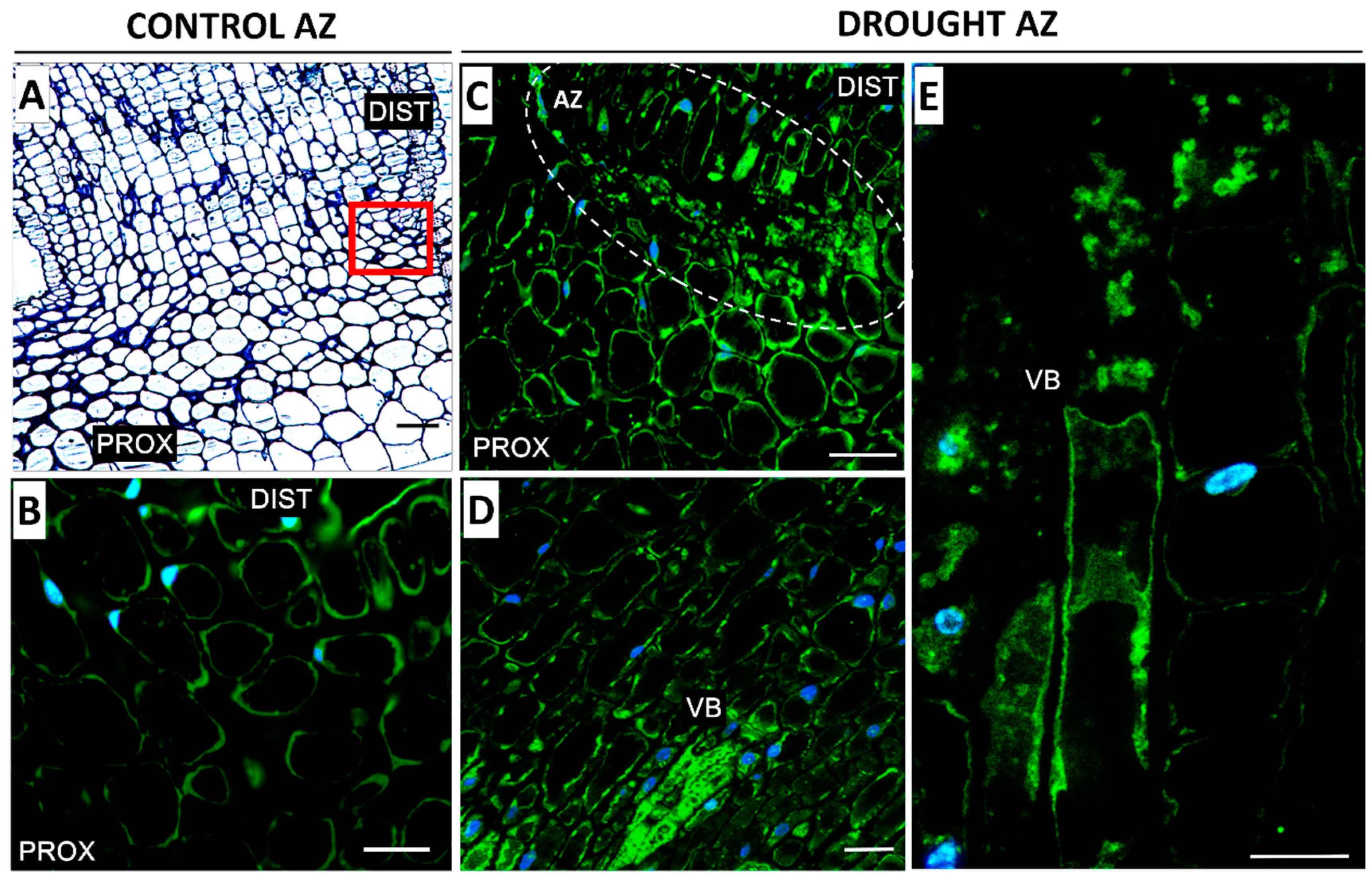
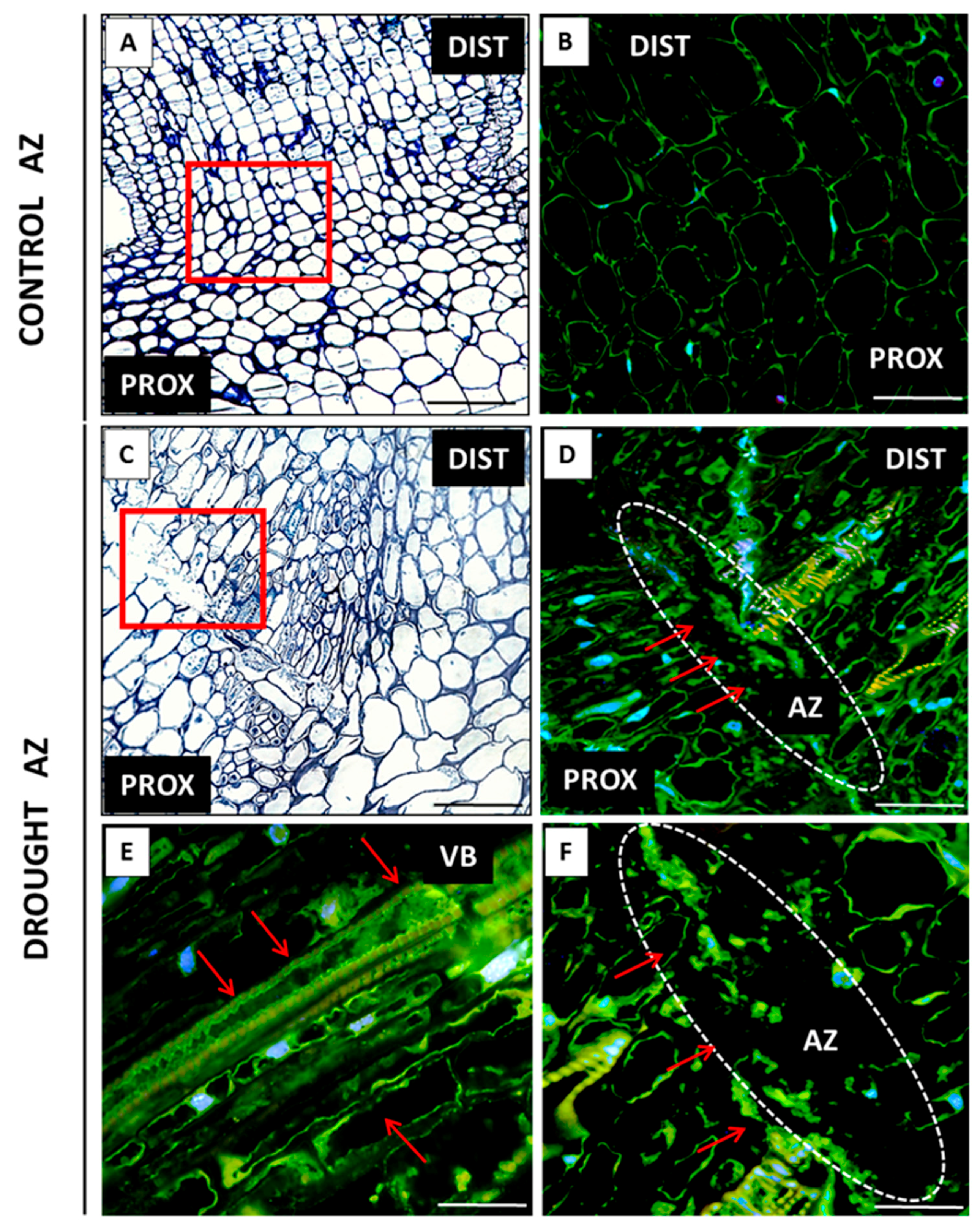
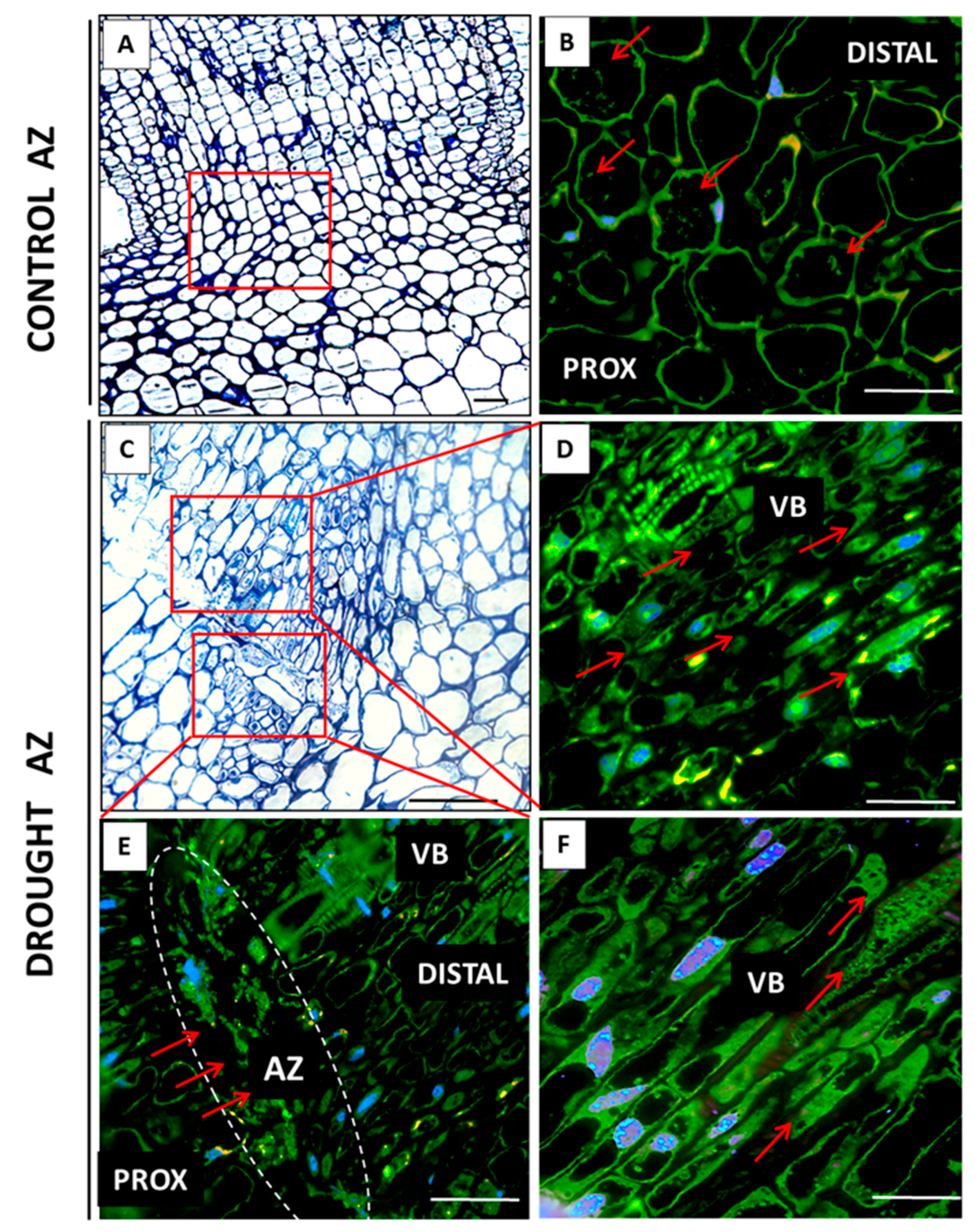
2.2. AZ Cells Exhibited Different Structure During Drought
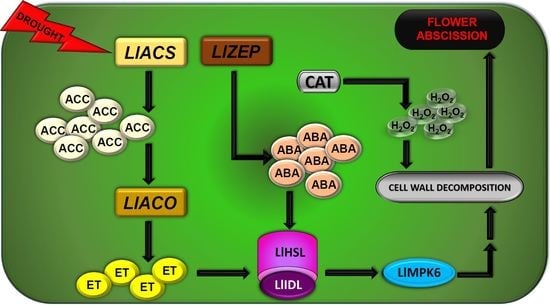
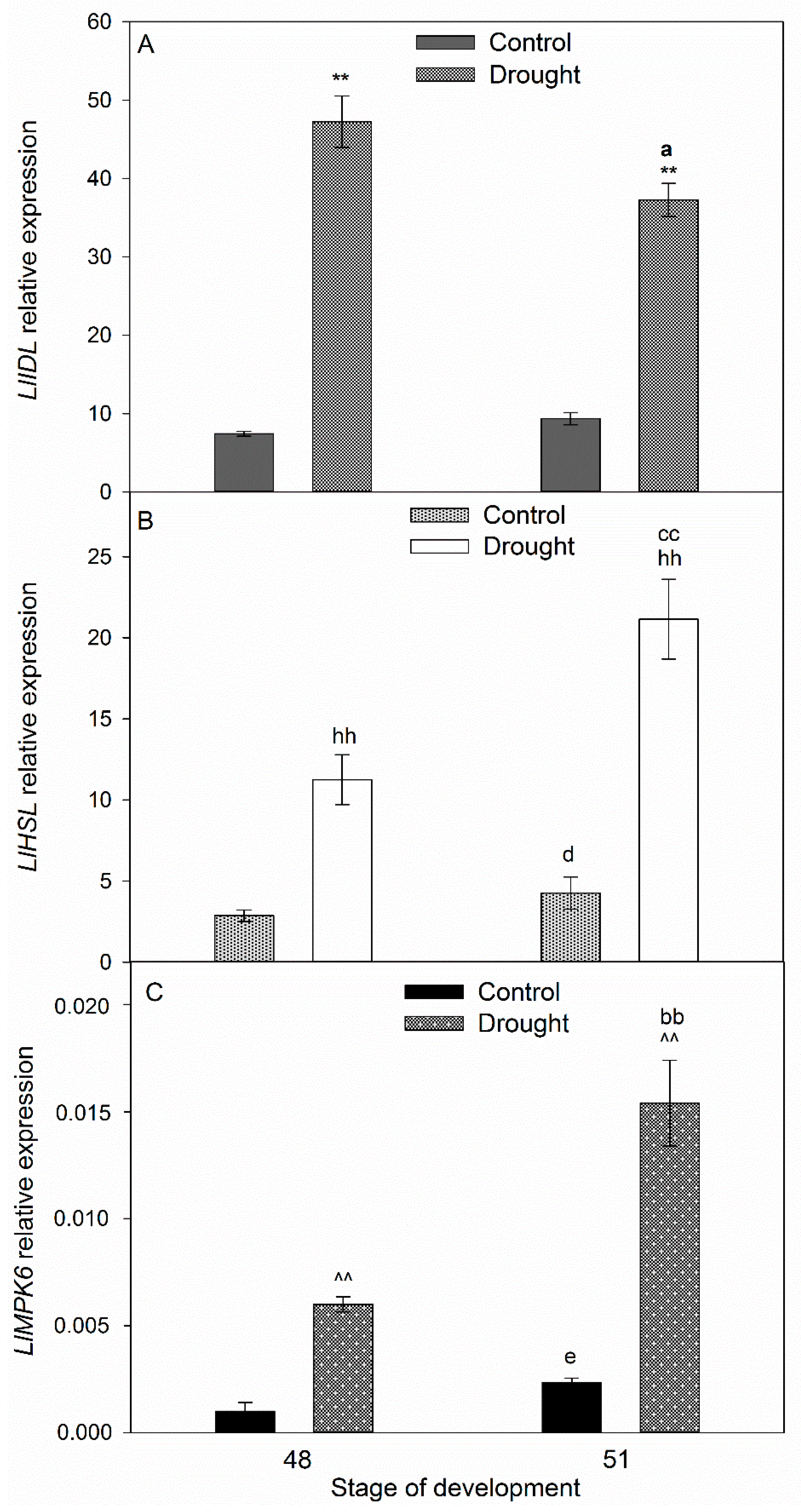
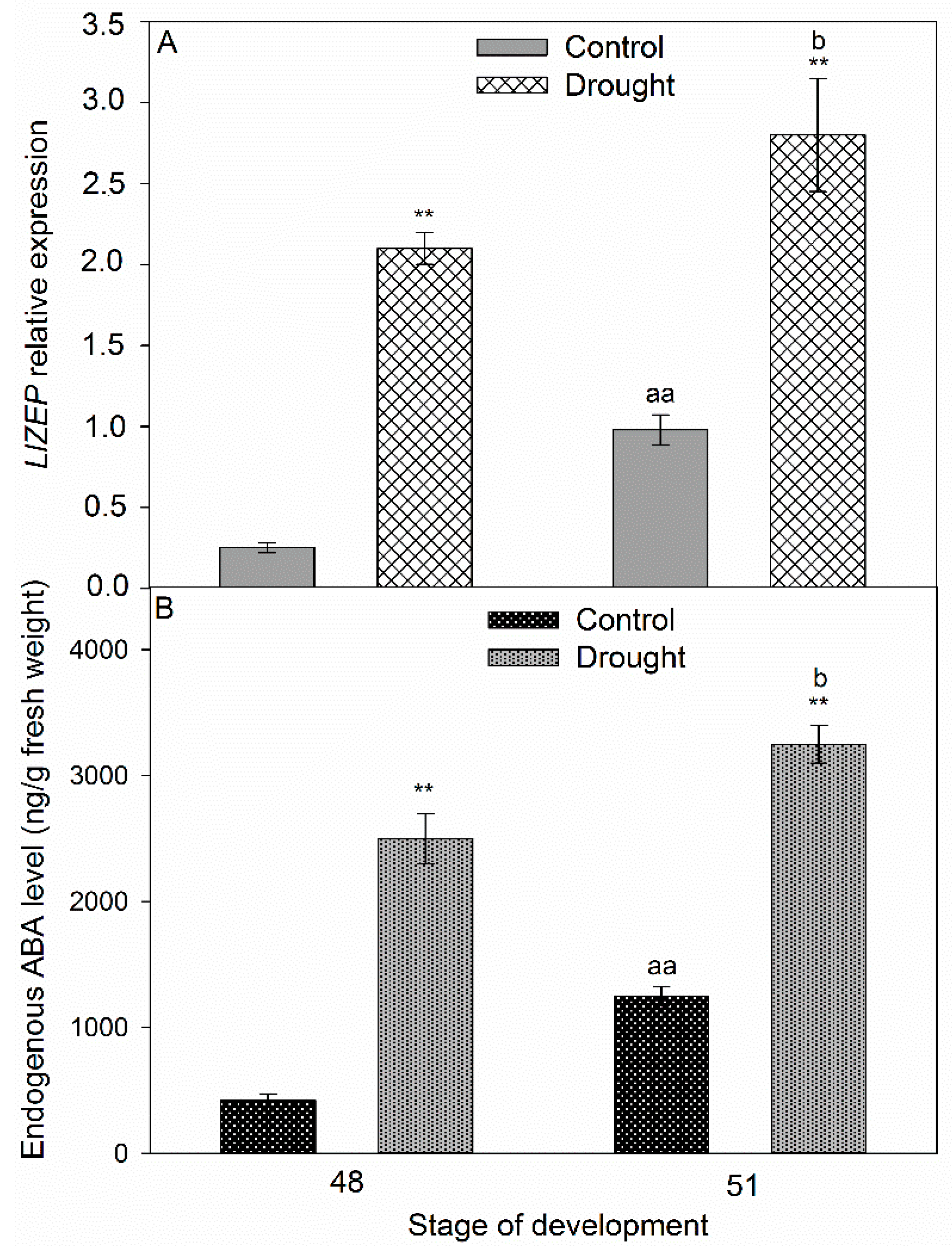
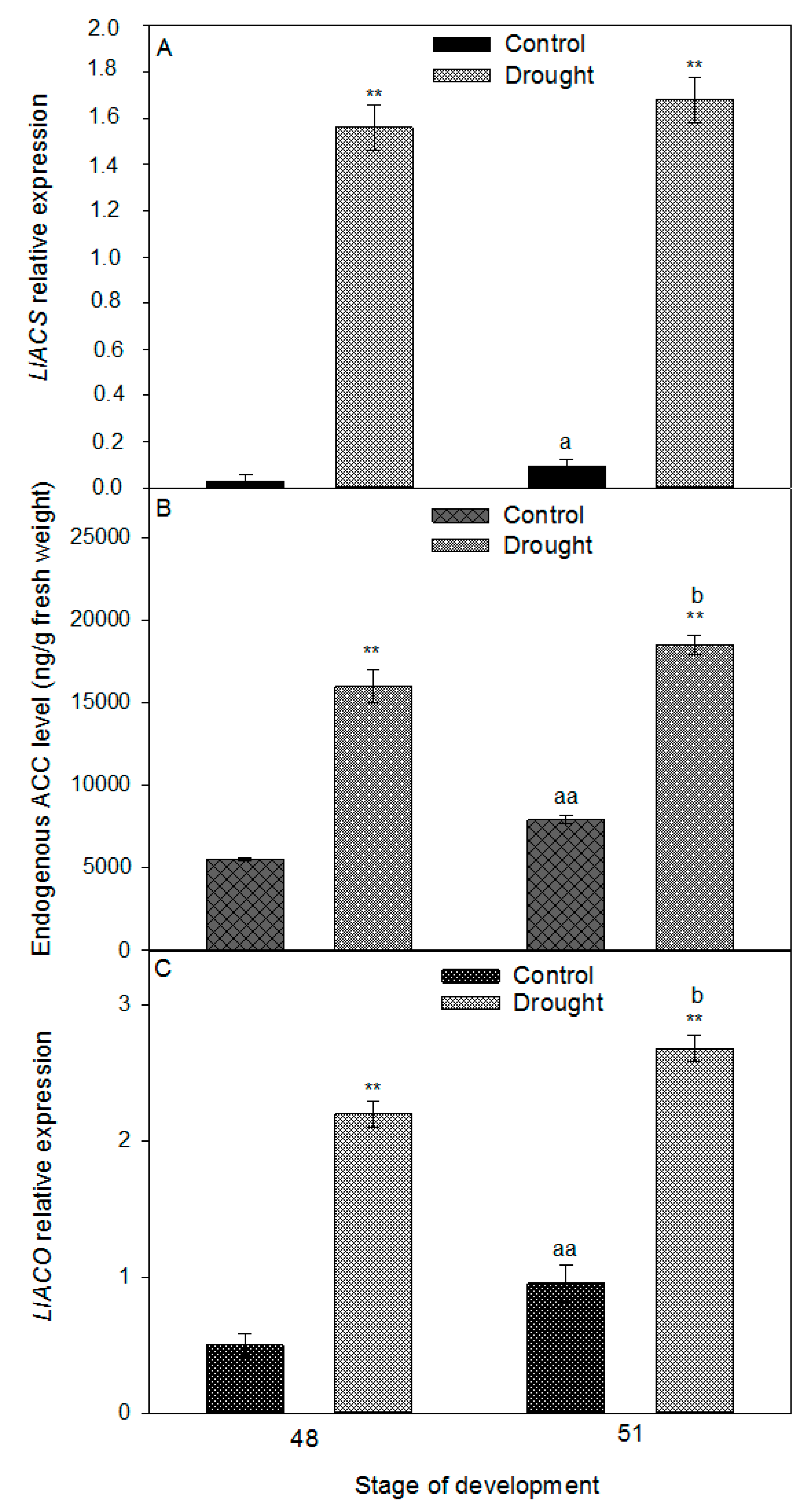
2.3. Drought Induces Expression of Components of IDA/HAE/MPK6 Pathway in AZ
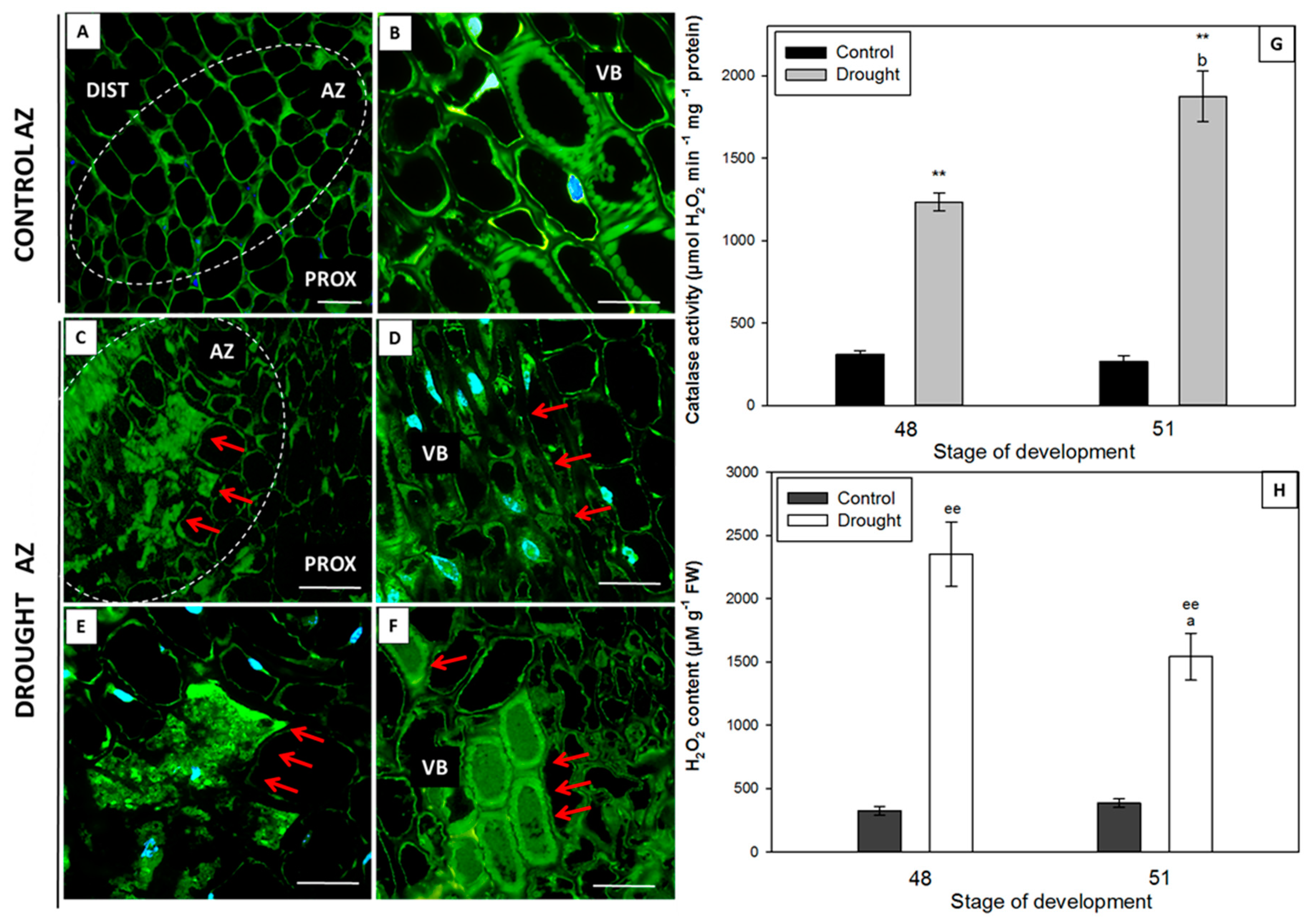
2.4. Water Deficit Influences H2O2 Level and Changes Localization and Activity of Catalase
2.5. Correlation Between Stress Phytohormones and Drought
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Sample Harvest
4.3. Evaluation of Stress Impact on Plants
4.4. Elements Determination
4.5. Preparation of AZ for Microscopic and Histological Studies
4.6. Immunolocalization of ABA, ACC, MPK6, and CAT
4.7. RNA Isolation and cDNA Synthesis
4.8. Isolation of LlZEP, LlHSL, and LlMPK6 cDNAs
4.9. RT-qPCR
4.10. ABA and ACC
4.11. Hydrogen Peroxide Detection and CAT Activity Measurements
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKeown, A.W.; Warland, J.; McDonald, M.R. Long-term climate and weather patterns in relation to crop yield: A mini review. Can. J. Bot. 2006, 84, 1031–1037. [Google Scholar] [CrossRef]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef]
- Ainouche, A.K.; Bayer, R.J.; Misset, M.T. Molecular phylogeny, diversification and character evolution in Lupinus (Fabaceae) with special attention to Mediterranean and African lupines. Plant Syst. Evol. 2004, 246, 211–222. [Google Scholar] [CrossRef]
- Estornell, L.H.; Agustí, J.; Merelo, P.; Talón, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199, 48–60. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Frankowski, K.; Kućko, A.; Świdziński, M.; de Dios Alché, J.; Nowakowska, A.; Kopcewicz, J. The influence of abscisic acid on the ethylene biosynthesis pathway in the functioning of the flower abscission zone in Lupinus luteus. J. Plant Physiol. 2016, 206, 49–58. [Google Scholar] [CrossRef]
- Kućko, A.; Wilmowicz, E.; Ostrowski, M. Spatio-temporal, IAA gradient is determined by interactions with ET and governs flower abscission. J. Plant Physiol. 2019, 236, 51–60. [Google Scholar] [CrossRef]
- Frankowski, K.; Wilmowicz, E.; Kućko, A.; Zienkiewicz, A.; Zienkiewicz, K.; Kopcewicz, J. Profiling the BLADE-ON-PETIOLE gene expression in the abscission zone of generative organs in Lupinus luteus. Acta Physiol. Plant. 2015, 37, 220. [Google Scholar] [CrossRef]
- Kućko, A.; Smoliński, D.; Wilmowicz, E.; Florkiewicz, A.; de Dios Alché, J. Spatio-temporal localization of LlBOP following early events of floral abscission in yellow lupine. Protoplasma. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Larue, C.T.; Chevalier, D.; Wang, H.; Jinn, T.L.; Zhang, S.; Walker, J.C. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. 2008, 105, 15629–15634. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.L.; Stenvik, G.E.; Vie, A.K.; Bones, A.M.; Pautot, V.; Proveniers, M.; Aalen, R.B.; Butenko, M.A. Arabidopsis class I KNOTTED-Like homeobox proteins act downstream in the IDA-HAE/HSL2 floral abscission signaling pathway. Plant Cell 2011, 23, 2553–2567. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Kućko, A.; Ostrowski, M.; Panek, K. INFLORESCENCE DEFICIENT IN ABSCISSION-like is an abscission-associated and phytohormone-regulated gene in flower separation of Lupinus luteus. Plant Growth Regul. 2018, 85, 91–100. [Google Scholar] [CrossRef]
- Vanoni, M.; Bugmann, H.; Nötzli, M.; Bigler, C. Quantifying the effects of drought on abrupt growth decreases of major tree species in Switzerland. Ecol. Evol. 2016, 6, 3555–3570. [Google Scholar] [CrossRef]
- Gossett, D.R.; Banks, S.W.; Millohollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in a control and an NaCl-tolerant cotton cell line grown in the presence of paraquate, buthinine sulfoximine, and endogenus glutathione. Plant Physiol. 1996, 112, 803–809. [Google Scholar] [CrossRef]
- Savoure, A.; Thorin, D.; Davey, M.; Hua, X.J.; Mauro, S.; Van Montagu, M.; Inze, D.; Verburuggen, N. NaCl and CuSO4 treatments trigger distinct oxidative defence mechanisms in Nicotiana plumbaginifolia L. Plant Cell Environ. 1999, 22, 387–396. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–57. [Google Scholar] [CrossRef]
- Liao, W.; Wang, G.; Li, Y.; Wang, B.; Zhang, P.; Peng, M. Reactive oxygen species regulate leaf pulvinus abscission zone cell separation in response to water-deficit stress in cassava. Sci. Rep. 2016, 6, 21542. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.C.H.; Saleem, M.F.; Man, C.H.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Rahdari, P.; Hosseini, S.M.; Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Stress Physiol. Biochem. 2012, 8, 182–193. [Google Scholar]
- Rajasekaran, L.R.; Blake, T.J. New plant growth regulators protect photosynthesis and enhance growth under drought of jack pine seedlings. J. Plant Growth Regul. 1999, 18, 175–181. [Google Scholar] [CrossRef]
- Hellin, E.; Hernandez-Cortez, J.A.; Piqueras, A.; Olmos, E.; Sevilla, F. The influence of the iron content on the superoxide dismutase activity and chloroplast ultrastructure of Citrus limon in iron nutrition in soil and plants. J. Plant Nutr. 1995, 5, 211–229. [Google Scholar]
- Brumbarova, T.; Matros, A.; Mock, H.P.; Bauer, P. A proteomic study showing differential regulation of stress, redox regulation and peroxidase proteins by iron supply and the transcription factor FER. Plant J. 2008, 54, 321–334. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Cadmium stress tolerance in crop plants probing the role of sulfur. Plant Signal. Behav. 2011, 6, 215–222. [Google Scholar] [CrossRef]
- Kim, S.A.; Guerinot, M.L. Mining iron: Iron uptake and transport in plants. FEBS Lett. 2007, 581, 2273–2280. [Google Scholar] [CrossRef]
- Lan, P.; Li, W.; Wen, T.N.; Shiau, J.Y.; Wu, Y.C.; Lin, W.; Schmidt, W. iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiol. 2011, 155, 821–834. [Google Scholar] [CrossRef]
- Arif, M.; Chohan, M.A.; Ali, S.; Gul, R.; Khan, S. Response of wheat to foliar application of nutrients. J. Agric. Biol. Sci. 2006, 1, 30–34. [Google Scholar]
- Kobraee, S.; Shamsi, K.; Rasekhi, B. Effect of micronutrients application on yield and yield components of soybean. Annu. Biol. Res. 2011, 2, 476–482. [Google Scholar]
- Afshar, R.M.; Hadi, H.; Pirzad, A. Effect of nano-iron on the yield and yield component of cowpea (Vignaunguiculata) under end season water deficit. Int. J. Agric. Res. Rev. 2013, 3, 27–34. [Google Scholar]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Sun, X.; Song, D.; Chen, W.; Zhang, A.; et al. Phosphorous application improves drought tolerance of Phoebezhennan. Front. Plant Sci. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Abuelsoud, W.; Hirschmann, F.; Papenbrock, J. Sulfur metabolism and drought stress tolerance in plants. Drought Stress Toler. Plants 2016, 1, 227–249. [Google Scholar]
- Frankowski, K.; Wilmowicz, E.; Kućko, A.; Zienkiewicz, A.; Zienkiewicz, K.; Kopcewicz, J. Molecular cloning of the BLADE-ON-PETIOLE gene and expression analyses during nodule development in Lupinus luteus. J. Plant Physiol. 2015, 179, 35–39. [Google Scholar] [CrossRef]
- Bergey, D.R.; Ryan, C.A. Wound-and systemin-inducible calmodulin gene expression in tomato leaves. Plant Mol. Biol. 1999, 40, 815–823. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–340. [Google Scholar] [CrossRef]
- Sawicki, M.; AïtBarka, E.; Clément, C.; Vaillant-Gaveau, N.; Jacquard, C. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J. Exp. Bot. 2015, 66, 1707–1719. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Stead, A.D. Abscission of flowers and floral parts. J. Exp. Bot. 1997, 48, 821–837. [Google Scholar] [CrossRef]
- Evensen, K.B.; Page, A.M.; Stead, A.D. Anatomy of ethylene induced petal abscission in Pelargonium × hortorum. Ann. Bot. 1993, 71, 559–566. [Google Scholar] [CrossRef]
- Butenko, M.A.; Patterson, S.E.; Grini, P.E.; Stenvik, G.E.; Amundsen, S.S.; Mandal, A.; Aalen, R.B. INFLORESCENCE DEFICIENT IN ABSCISSION controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell 2003, 15, 2296–2307. [Google Scholar] [CrossRef]
- Stenvik, G.E.; Butenko, M.A.; Aalen, R.B. Identification of a putative receptor ligand pair controlling cell separation in plants. Plant Signal Behav. 2008, 3, 1109–1110. [Google Scholar] [CrossRef]
- Burr, C.A.; Leslie, M.E.; Orlowski, S.K.; Chen, I.; Wright, C.E.; Daniels, M.J.; Liljegren, S.J. CAST AWAY, a membrane-associated receptor-like kinase, inhibits organ abscission in Arabidopsis. Plant Physiol. 2011, 156, 1837–1850. [Google Scholar] [CrossRef]
- Estornell, L.H.; Wildhagen, M.; Pérez-Amador, M.A.; Talón, M.; Tadeo, F.R.; Butenko, M.A. The IDA peptide controls abscission in Arabidopsis and Citrus. Front. Plant Sci. 2015, 6, 1003. [Google Scholar] [CrossRef]
- Ying, P.; Li, C.; Liu, X.; Xia, R.; Zhao, M.; Li, J. Identification and molecular characterization of an IDA-like gene from litchi, LcIDL1, whose ectopic expression promotes floral organ abscission in Arabidopsis. Sci. Rep. 2016, 6, 37135. [Google Scholar] [CrossRef]
- Stenvik, G.E.; Butenko, M.A.; Urbanowicz, B.R.; Rose, J.K.; Aalen, R.B. Overexpression of INFLORESCENCE DEFICIENT IN ABSCISSION activates cell separation in vestigial abscission zones in Arabidopsis. Plant Cell 2006, 18, 1467–1476. [Google Scholar] [CrossRef]
- Santiago, J.; Brandt, B.; Wildhagen, M.; Hohmann, U.; Hothorn, L.A.; Butenko, M.A.; Hothorn, M. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. Elife 2016, 5, e15075. [Google Scholar] [CrossRef]
- Stenvik, G.E.; Tandstad, N.M.; Guo, Y.; Shi, C.L.; Kristiansen, W.; Holmgren, A.; Clark, S.E.; Aalen, R.B.; Butenko, M.A. The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in Arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell 2008, 20, 1805–1817. [Google Scholar] [CrossRef]
- Tucker, M.L.; Yang, R. IDA-like gene expression in soybean and tomato leaf abscission and requirement for a diffusible stelar abscission signal. AoB Plants 2012, pls035. [Google Scholar] [CrossRef]
- Stø, I.M.; Orr, R.J.; Fooyontphanich, K.; Jin, X.; Knutsen, J.M.; Fischer, U.; Tranbarger, T.J.; Nordal, I.; Aalen, R.B. Conservation of the abscission signaling peptide IDA during Angiosperm evolution: Withstanding genome duplications and gain and loss of the receptors HAE/HSL2. Front Plant Sci. 2015, 6, 931. [Google Scholar] [CrossRef]
- Vie, A.K.; Najafi, J.; Liu, B.; Winge, P.; Butenko, M.A.; Hornslien, K.S.; Kumpf, R.; Aalen, R.B.; Bones, A.M.; Brembu, T. The IDA/IDA-LIKE and PIP/PIP-LIKE gene families in Arabidopsis: Phylogenetic relationship, expression patterns, and transcriptional effect of the PIPL3 peptide. J. Exp. Bot. 2015, 66, 5351–5365. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Floral organ abscission is regulated by a positive feedback loop. Proc. Natl. Acad. Sci. USA 2015, 112, 2906–2911. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Core mechanisms regulating developmentally timed and environmentally triggered abscission. Plant Physiol. 2016, 172, 510–520. [Google Scholar] [CrossRef]
- Li, Y.; Pi, L.; Huang, H.; Xu, L. ATH1 and KNAT2 proteins act together in regulation of plant inflorescence architecture. J. Exp. Bot. 2012, 63, 1423–1433. [Google Scholar] [CrossRef]
- Ehlers, K.; van Bel, A.J. Dynamics of plasmodesmal connectivity in successive interfaces of the cambial zone. Planta 2010, 231, 371–385. [Google Scholar] [CrossRef]
- Xing, Y.; Jia, W.; Zhang, J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008, 54, 440–451. [Google Scholar] [CrossRef]
- Wang, N.; Fang, W.; Han, H.; Sui, N.; Li, B.; Meng, Q.W. Overexpression of zeaxanthin epoxidase gene enhances the sensitivity of tomato PSII photoinhibition to high light and chilling stress. Physiol. Plant. 2008, 132, 384–396. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Chang, L.; Zhang, T.; An, J.; Liu, Y.; Cao, Y.; Zhao, X.; Sha, X.; Hu, T.; et al. MsZEP, a novel zeaxanthin epoxidase gene from alfalfa (Medicago sativa), confers drought and salt tolerance in transgenic tobacco. Plant Cell Rep. 2016, 35, 439–453. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Tuteja, N. Review abscisic acid and abiotic stress signaling. Plant Signal Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.J.; Jung, H.J.; Maruyama, K.; Suzuki, N.; Kang, H. Overexpression of microRNA395c or 395e affects differently the seed germination of Arabidopsis thaliana under stress conditions. Planta 2010, 232, 1447–1454. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Borel, C.; Simonneau, T.; This, D.; Tardieu, F. Stomatal conductance and ABA concentration in the xylem sap of barley lines of contrasting genetic origins. Funct. Plant Biol. 1997, 24, 607–615. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.Q.; Watson, M.B.; Assmann, S.M. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 2000, 287, 300–303. [Google Scholar] [CrossRef]
- Vernieri, P.; Lenzi, A.; Figaro, M.; Tognoni, F.; Pardossi, A. How the roots contribute to the ability of Phaseolus vulgaris L. to cope with chilling-induced water stress. J. Exp. Bot. 2001, 52, 2199–2206. [Google Scholar] [CrossRef]
- Pospíšilová, J. Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol. Plant. 2003, 46, 491–506. [Google Scholar] [CrossRef]
- Wakrim, R.; Wahbi, S.; Tahi, H.; Aganchich, B.; Serraj, R. Comparative effects of partial root drying (PRD) and regulated deficit irrigation (RDI) on water relations and water use efficiency in common bean (Phaseolus vulgaris L.). Agric. Ecosyst. Environ. 2005, 106, 275–287. [Google Scholar] [CrossRef]
- Marino, G.; Salvatore, L.C.; Brunetti, C.; De Carlo, A.; Avola, G.; Riggi, E.; Loreto, F.; Centritto, M. Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundodonax L.). Environ. Exp. Bot. 2018, 147, 116–124. [Google Scholar]
- Bochicchio, A.; Vazzana, C.; Velasco, R.; Singh, M.; Bartels, D. Exogenous ABA induces desiccation tolerance and leads to the synthesis of specific gene transcripts in immature embryos of maize. Maydica 1991, 36, 11–16. [Google Scholar]
- Franks, P.J.; Farquhar, G.D. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 2001, 125, 935–942. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ. 2002, 25, 195–210. [Google Scholar] [CrossRef]
- Audran, C.; Borel, C.; Frey, A.; Sotta, B.; Meyer, C.; Simonneau, T.; Marion-Poll, A. Expression studies of the zeaxanthin epoxidase gene in Nicotiana plumbaginifolia. Plant Physiol. 1998, 118, 1021–1028. [Google Scholar] [CrossRef]
- Schwarz, N.; Armbruster, U.; Iven, T.; Brückle, L.; Melzer, M.; Feussner, I.; Jahns, P. Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol. 2015, 56, 346–357. [Google Scholar] [CrossRef]
- Thompson, A.J.; Jackson, A.C.; Parker, R.A.; Morpeth, D.R.; Burbidge, A.; Taylor, I.B. Abscisic acid biosynthesis in tomato: Regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Mol. Biol. 2000, 42, 833–845. [Google Scholar] [CrossRef]
- Xiong, L.; Lee, H.; Ishitani, M.; Zhu, J.K. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J. Biol. Chem. 2002, 277, 8588–8596. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol. 2000, 123, 553–562. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Huang, X.; Li, J.; Wang, H.; Li, J. An improved fruit transcriptome and the identification of the candidate genes involved in fruit abscission induced by carbohydrate stress in litchi. Front. Plant Sci. 2015, 6, 439. [Google Scholar] [CrossRef]
- HensonI, E.; Mahalakshmi, V.; Bidinger, F.R.; Alagarswamy, G. Genotypic variation in pearl millet (Pennisetumamericanum (L.) Leeke), in the ability to accumulate abscisic acid in response to water stress. J. Expt. Bot. 1981, 32, 899–910. [Google Scholar] [CrossRef]
- Harris, M.J.; Outlaw, W.H. Rapid adjustment of guard-cell abscisic acid levels to current leaf-water status. Plant Physiol. 1991, 95, 171–173. [Google Scholar] [CrossRef]
- Tamminen, I.; Makela, P.; Heino, P.; Palva, E.T. Ectopic expression of ABI3 gene enhances freezing tolerance in response to abscisic acid and low temperature in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2001, 25, 1–8. [Google Scholar] [CrossRef]
- Aroca, R.; Ferrante, A.; Vernieri, P.; Chrispeels, M.J. Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann. Bot. 2006, 98, 1301–1310. [Google Scholar] [CrossRef]
- Saruhashi, M.; Kumar, G.T.; Arai, K.; Ishizaki, Y.; Hagiwara, K.; Komatsu, K.; Shiwa, Y.; Izumikawa, K.; Yoshikawa, H.; Umezawa, T.; et al. Plant Raf-like kinase integrates abscisic acid and hyperosmotic stress signaling upstream of SNF1-related protein kinase2. Proc. Natl. Acad. Sci. 2015, 112, 6388–6396. [Google Scholar] [CrossRef]
- Kim, J.; Sundaresan, S.; Philosoph-Hadas, S.; Yang, R.; Meir, S.; Tucker, M.L. Examination of the abscission-associated transcriptomes for soybean, tomato, and Arabidopsis highlights the conserved biosynthesis of an extensible extracellular matrix and boundary layer. Front. Plant Sci. 2015, 6, 1109. [Google Scholar] [CrossRef]
- Roberts, J.A.; Whitelaw, C.A.; Gonzalez-Carranza, Z.H.; McManus, M.T. Cell separation processes in plants: Models, mechanisms and manipulation. Ann. Bot. 2000, 86, 223–235. [Google Scholar] [CrossRef]
- Zhu, H.; Yuan, R.; Greene, D.W.; Beers, E.P. Effects of 1-methylcyclopropene and napththaleneacetic acid on fruit set and expression of genes related to ethylene biosynthesis and perception and cell wall degradation in apple. J. Am. Soc. Hort. Sci. 2010, 135, 402–409. [Google Scholar] [CrossRef]
- Tudela, D.; Primo-Millo, E. 1-Aminocyclopropane-1-carboxylic acid transported from roots to shoots promotes leaf abscission in Cleopatra mandarin (Citrus reshni Hort. ex Tan.) seedlings rehydrated after water stress. Plant Physiol. 1992, 100, 131–137. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Tadeo, F.R.; Talon, M.; Primo-Millo, E. Leaf abscission induced by ethylene in water-stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol. 1996, 112, 401–408. [Google Scholar] [CrossRef]
- Saftner, R.A.; Martin, M.N. Transport of 1-aminocyclopropane-1-carboxylic acid into isolated maize mesophyll vacuoles. Physiol. Plant. 1993, 87, 535–543. [Google Scholar] [CrossRef]
- Bradford, K.J.; Hsiao, T.C.; Yang, S.F. Inhibition of ethylene synthesis in tomato plants subjected to anaerobic root stress. Plant Physiol. 1982, 70, 1503–1507. [Google Scholar] [CrossRef]
- Morris, D.; Larcombe, N.J. Phloem transport and conjugation of foliar-applied 1-aminocyclopropane-1-carboxylic acid in cotton (Gossypiumhirsutum L.). J. Plant Physiol. 1995, 146, 429–436. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Growth and reproduction of junglerice (Echinochloa colona) in response to water stress. Weed Sci. 2010, 58, 132–135. [Google Scholar] [CrossRef]
- Krause, G.H. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Weng, J.H. Underestimate of PS2 efficiency in the field due to high temperature resulting from leaf clipping and its amendment. Photosynthetica 2006, 44, 467–470. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilmowicz, E.; Kućko, A.; Burchardt, S.; Przywieczerski, T. Molecular and Hormonal Aspects of Drought-Triggered Flower Shedding in Yellow Lupine. Int. J. Mol. Sci. 2019, 20, 3731. https://doi.org/10.3390/ijms20153731
Wilmowicz E, Kućko A, Burchardt S, Przywieczerski T. Molecular and Hormonal Aspects of Drought-Triggered Flower Shedding in Yellow Lupine. International Journal of Molecular Sciences. 2019; 20(15):3731. https://doi.org/10.3390/ijms20153731
Chicago/Turabian StyleWilmowicz, Emilia, Agata Kućko, Sebastian Burchardt, and Tomasz Przywieczerski. 2019. "Molecular and Hormonal Aspects of Drought-Triggered Flower Shedding in Yellow Lupine" International Journal of Molecular Sciences 20, no. 15: 3731. https://doi.org/10.3390/ijms20153731
APA StyleWilmowicz, E., Kućko, A., Burchardt, S., & Przywieczerski, T. (2019). Molecular and Hormonal Aspects of Drought-Triggered Flower Shedding in Yellow Lupine. International Journal of Molecular Sciences, 20(15), 3731. https://doi.org/10.3390/ijms20153731