Extracellular Vesicles Released by Colorectal Cancer Cell Lines Modulate Innate Immune Response in Zebrafish Model: The Possible Role of Human Endogenous Retroviruses
Abstract
1. Introduction
2. Results
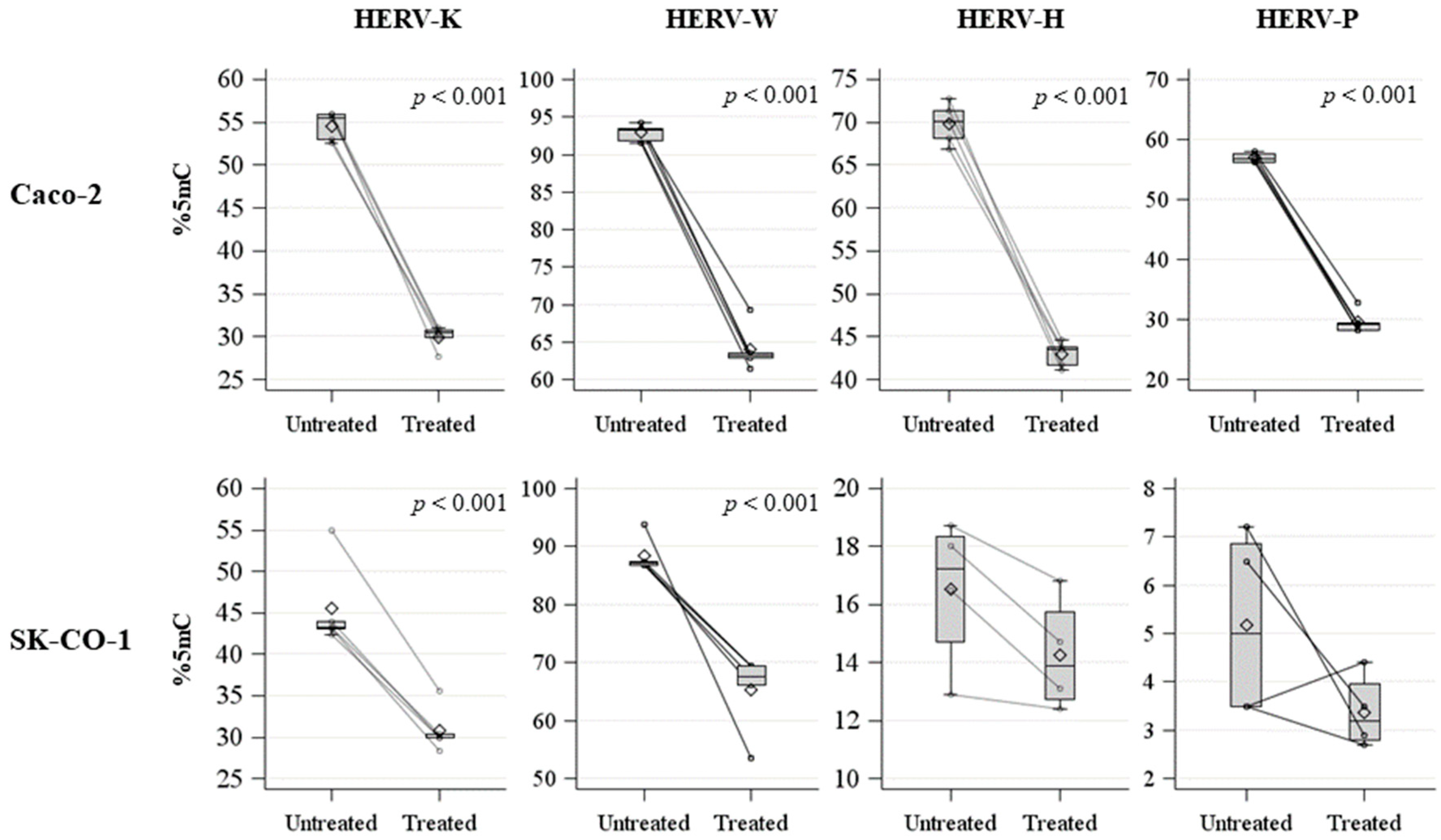
2.1. HERV Methylation Analysis
2.2. Evaluation and Characterization of EVs
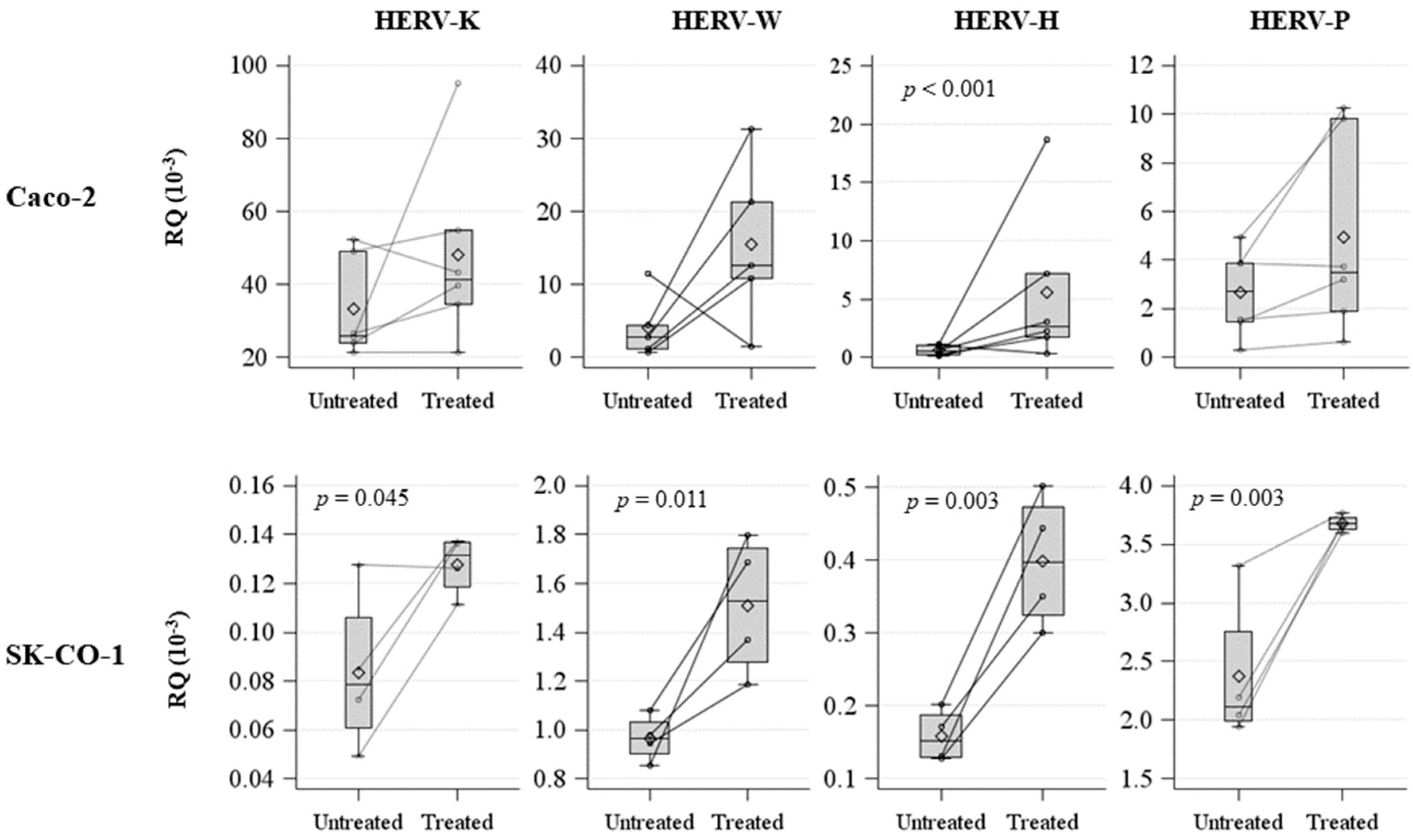
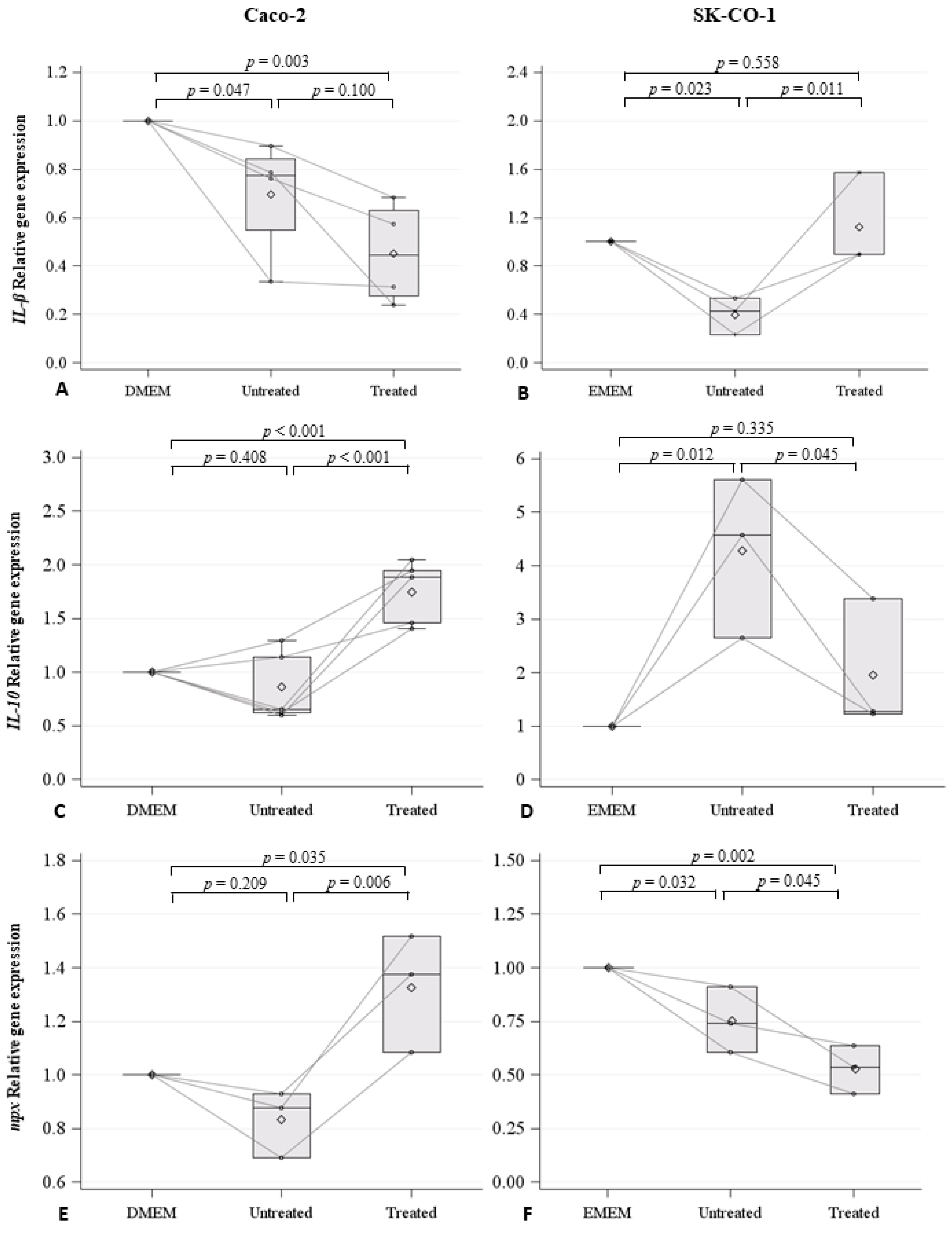
2.3. Effects on Innate Immune Response in Zebrafish Embryos
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Treatments
4.2. EVs Analysis
4.3. Isolation and Purification of EVs
4.4. Nanoparticle Tracking Analysis (NTA) of EVs
4.5. High Resolution Flow Cytometry on EVs
4.6. Methylation Analysis
4.7. RNA Extraction form Caco-2 and SK-CO-1 Cells, Reverse Transcription-PCR, and Real-Time Quantitative-PCR Assays (RT-qPCR)
4.8. Animals
4.9. EVs Microinjections
4.10. RNA Extraction form Zebrafish Embryos, Reverse Transcription-PCR, and RT-qPCR
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Parcesepe, P.; Giordano, G.; Laudanna, C.; Febbraro, A.; Pancione, M. Cancer-Associated Immune Resistance and Evasion of Immune Surveillance in Colorectal Cancer. Gastroenterol. Res. Pract. 2016, 2016, 6261721. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Qu, S.; Chen, X.; Wu, Q.; Shi, M. Promising Targets for Cancer Immunotherapy: TLRs, RLRs, and STING-Mediated Innate Immune Pathways. Int. J. Mol. Sci. 2017, 18, 404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef]
- Zha, Q.B.; Yao, Y.F.; Ren, Z.J.; Li, X.J.; Tang, J.H. Extracellular vesicles: An overview of biogenesis, function, and role in breast cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317691182. [Google Scholar] [CrossRef]
- Greening, D.W.; Gopal, S.K.; Xu, R.; Simpson, R.J.; Chen, W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Ogorevc, E.; Kralj-Iglic, V.; Veranic, P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol. Oncol. 2013, 47, 197–205. [Google Scholar] [CrossRef]
- Hummel, J.; Kammerer, U.; Muller, N.; Avota, E.; Schneider-Schaulies, S. Human endogenous retrovirus envelope proteins target dendritic cells to suppress T-cell activation. Eur. J. Immunol. 2015, 45, 1748–1759. [Google Scholar] [CrossRef]
- Kudo-Saito, C.; Yura, M.; Yamamoto, R.; Kawakami, Y. Induction of immunoregulatory CD271+ cells by metastatic tumor cells that express human endogenous retrovirus H. Cancer Res. 2014, 74, 1361–1370. [Google Scholar] [CrossRef]
- Jones, P.A.; Ohtani, H.; Chakravarthy, A.; De Carvalho, D.D. Epigenetic therapy in immune-oncology. Nat. Rev. Cancer 2019, 19, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Foroozesh, M.; Qin, Z. Transactivation of human endogenous retroviruses by tumor viruses and their functions in virus-associated malignancies. Oncogenesis 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Nakagawa, S.; Kusama, K. Placental Development and Endogenous Retroviruses. Uirusu 2016, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bastida-Ruiz, D.; Van Hoesen, K.; Cohen, M. The Dark Side of Cell Fusion. Int. J. Mol. Sci. 2016, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod. Immunol. 2014, 72, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Chuong, E.B. The placenta goes viral: Retroviruses control gene expression in pregnancy. PLoS Biol. 2018, 16, e3000028. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Liu, J.; Rycaj, K.; Huang, M.; Tsai, K.; Rosen, D.G.; Chen, D.T.; Lu, D.W.; Barnhart, K.F.; Johanning, G.L. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int. J. Cancer 2007, 120, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, J.Q.; Zheng, S. Expressional activation and functional roles of human endogenous retroviruses in cancers. Rev. Med. Virol. 2019, 29, e2025. [Google Scholar] [CrossRef]
- Paces, J.; Huang, Y.T.; Paces, V.; Ridl, J.; Chang, C.M. New insight into transcription of human endogenous retroviral elements. New Biotechnol. 2013, 30, 314–318. [Google Scholar] [CrossRef]
- Gimenez, J.; Montgiraud, C.; Pichon, J.P.; Bonnaud, B.; Arsac, M.; Ruel, K.; Bouton, O.; Mallet, F. Custom human endogenous retroviruses dedicated microarray identifies self-induced HERV-W family elements reactivated in testicular cancer upon methylation control. Nucleic Acids Res. 2010, 38, 2229–2246. [Google Scholar] [CrossRef]
- Grandi, N.; Tramontano, E. HERV Envelope Proteins: Physiological Role and Pathogenic Potential in Cancer and Autoimmunity. Front. Microbiol. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses. Front. Immunol. 2018, 9, 2039. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.S.; Linnebacher, M. Human endogenous retroviruses and cancer: Causality and therapeutic possibilities. World J. Gastroenterol. 2012, 18, 6027–6035. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L.; Kitova, M.; Maldener, E.; Meese, E.; Mayer, J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2). J. Virol. 2005, 79, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Mercorio, R.; Bonzini, M.; Angelici, L.; Iodice, S.; Delbue, S.; Mariani, J.; Apostoli, P.; Pesatori, A.C.; Bollati, V. Effects of metal-rich particulate matter exposure on exogenous and endogenous viral sequence methylation in healthy steel-workers. Environ. Res. 2017, 159, 452–457. [Google Scholar] [CrossRef]
- Zare, M.; Mostafaei, S.; Ahmadi, A.; Azimzadeh Jamalkandi, S.; Abedini, A.; Esfahani-Monfared, Z.; Dorostkar, R.; Saadati, M. Human endogenous retrovirus env genes: Potential blood biomarkers in lung cancer. Microb. Pathog. 2018, 115, 189–193. [Google Scholar] [CrossRef]
- Rhyu, D.W.; Kang, Y.J.; Ock, M.S.; Eo, J.W.; Choi, Y.H.; Kim, W.J.; Leem, S.H.; Yi, J.M.; Kim, H.S.; Cha, H.J. Expression of human endogenous retrovirus env genes in the blood of breast cancer patients. Int. J. Mol. Sci. 2014, 15, 9173–9183. [Google Scholar] [CrossRef]
- Perot, P.; Mullins, C.S.; Naville, M.; Bressan, C.; Huhns, M.; Gock, M.; Kuhn, F.; Volff, J.N.; Trillet-Lenoir, V.; Linnebacher, M.; et al. Expression of young HERV-H loci in the course of colorectal carcinoma and correlation with molecular subtypes. Oncotarget 2015, 6, 40095–40111. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, Y.J.; Jo, J.O.; Ock, M.S.; Baek, K.W.; Eo, J.; Lee, W.J.; Choi, Y.H.; Kim, W.J.; Leem, S.H.; et al. Elevation of human ERV3-1 env protein expression in colorectal cancer. J. Clin. Pathol. 2014, 67, 840–844. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, Z.; Xu, R.; Wu, L.; Zheng, S. Expression patterns of non-coding spliced transcripts from human endogenous retrovirus HERV-H elements in colon cancer. PLoS ONE 2012, 7, e29950. [Google Scholar] [CrossRef]
- Mullins, C.S.; Linnebacher, M. Endogenous retrovirus sequences as a novel class of tumor-specific antigens: an example of HERV-H env encoding strong CTL epitopes. Cancer Immunol. Immunother. CII 2012, 61, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Coy, J.F.; Knaebel, H.P.; Linnebacher, M.; Wilz, B.; Gebert, J.; von Knebel Doeberitz, M. Expression of an endogenous retroviral sequence from the HERV-H group in gastrointestinal cancers. Int. J. Cancer 2007, 121, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology (Basel) 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, U.; Schulz, W.A.; Koch, A.; Niegisch, G.; Goering, W. HERV-K and LINE-1 DNA Methylation and Reexpression in Urothelial Carcinoma. Front. Oncol. 2013, 3, 255. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Laird, P.W. Interplay between the cancer genome and epigenome. Cell 2013, 153, 38–55. [Google Scholar] [CrossRef]
- Anwar, S.L.; Wulaningsih, W.; Lehmann, U. Transposable Elements in Human Cancer: Causes and Consequences of Deregulation. Int. J. Mol. Sci. 2017, 18, 974. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.S.; Huhns, M.; Krohn, M.; Peters, S.; Cheynet, V.; Oriol, G.; Guillotte, M.; Ducrot, S.; Mallet, F.; Linnebacher, M. Generation, Characterization and Application of Antibodies Directed against HERV-H Gag Protein in Colorectal Samples. PLoS ONE 2016, 11, e0153349. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.; Levy, N.; Stevenson, B.J.; Bouzourene, H.; Theiler, G.; Bricard, G.; Viatte, S.; Ayyoub, M.; Vuilleumier, H.; Givel, J.C.; et al. Identification of tumor-associated antigens by large-scale analysis of genes expressed in human colorectal cancer. Cancer Immun. 2008, 8, 11. [Google Scholar] [PubMed]
- Cegolon, L.; Salata, C.; Weiderpass, E.; Vineis, P.; Palu, G.; Mastrangelo, G. Human endogenous retroviruses and cancer prevention: evidence and prospects. BMC Cancer 2013, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Traver, D.; Herbomel, P.; Patton, E.E.; Murphey, R.D.; Yoder, J.A.; Litman, G.W.; Catic, A.; Amemiya, C.T.; Zon, L.I.; Trede, N.S. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 2003, 81, 253–330. [Google Scholar] [PubMed]
- Al-Samadi, A.; Awad, S.A.; Tuomainen, K.; Zhao, Y.; Salem, A.; Parikka, M.; Salo, T. Crosstalk between tongue carcinoma cells, extracellular vesicles, and immune cells in in vitro and in vivo models. Oncotarget 2017, 8, 60123–60134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kammerer, U.; Germeyer, A.; Stengel, S.; Kapp, M.; Denner, J. Human endogenous retrovirus K (HERV-K) is expressed in villous and extravillous cytotrophoblast cells of the human placenta. J. Reprod. Immunol. 2011, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kassiotis, G.; Stoye, J.P. Making a virtue of necessity: The pleiotropic role of human endogenous retroviruses in cancer. Philos. Trans. R. Soc. Lond. Ser. Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.A.; Dao Thi, V.L.; Denner, J. The transmembrane protein of the human endogenous retrovirus--K (HERV-K) modulates cytokine release and gene expression. PLoS ONE 2013, 8, e70399. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Pergoli, L.; Cantone, L.; Favero, C.; Angelici, L.; Iodice, S.; Pinatel, E.; Hoxha, M.; Dioni, L.; Letizia, M.; Albetti, B.; et al. Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation. Part. Fibre Toxicol. 2017, 14, 32. [Google Scholar] [CrossRef]
- Bollati, V.; Favero, C.; Albetti, B.; Tarantini, L.; Moroni, A.; Byun, H.M.; Motta, V.; Conti, D.M.; Tirelli, A.S.; Vigna, L.; et al. Nutrients intake is associated with DNA methylation of candidate inflammatory genes in a population of obese subjects. Nutrients 2014, 6, 4625–4639. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Moncini, S.; Bonati, M.T.; Morella, I.; Ferrari, L.; Brambilla, R.; Riva, P. Differential allelic expression of SOS1 and hyperexpression of the activating SOS1 c.755C variant in a Noonan syndrome family. Eur. J. Hum. Genet. 2015, 23, 1531–1537. [Google Scholar] [CrossRef]
- Casadei, R.; Pelleri, M.C.; Vitale, L.; Facchin, F.; Lenzi, L.; Canaider, S.; Strippoli, P.; Frabetti, F. Identification of housekeeping genes suitable for gene expression analysis in the zebrafish. Gene Expr. Patterns 2011, 11, 271–276. [Google Scholar] [CrossRef]



| Element | Cell Type | Mean | Standard Deviation | Median | Interquartile Range | T-Test | Wilcoxon |
|---|---|---|---|---|---|---|---|
| HERV K | Caco-2 | 54.6 | 1.6 | 55.5 | 2.9 | 0.016 | 0.036 |
| SK-CO-1 | 45.5 | 5.3 | 43.3 | 0.8 | |||
| HERV W | Caco-2 | 92.9 | 1.1 | 93.2 | 1.6 | 0.014 | 0.095 |
| SK-CO-1 | 88.4 | 3.0 | 87.1 | 0.7 | |||
| HERV H | Caco-2 | 69.9 | 2.4 | 70.1 | 3.3 | <0.001 | 0.020 |
| SK-CO-1 | 16.5 | 2.6 | 17.3 | 3.7 | |||
| HERV P | Caco-2 | 57.0 | 0.8 | 93.2 | 1.6 | <0.001 | 0.019 |
| SK-CO-1 | 5.2 | 2.0 | 87.1 | 0.7 | |||
| LINE1 | Caco-2 | 56.9 | 2.3 | 56.6 | 1.0 | 0.002 | 0.012 |
| SK-CO-1 | 51.5 | 1.6 | 50.8 | 0.8 |
| Caco-2 Cells | |||||||
| Element | Treatment | Mean | Standard Deviation | Median | Interquartile Range | T-Test | Wilcoxon |
| HERV-K | Untreated | 54.6 | 1.6 | 55.5 | 2.9 | <0.001 | 0.012 |
| Treated | 30.0 | 1.3 | 30.5 | 0.9 | |||
| HERV-W | Untreated | 92.9 | 1.1 | 93.2 | 1.6 | <0.001 | 0.012 |
| Treated | 64.1 | 3.0 | 63.2 | 63.2 | |||
| HERV-H | Untreated | 69.9 | 2.4 | 70.1 | 3.3 | <0.001 | 0.012 |
| Treated | 42.9 | 1.5 | 43.5 | 2.1 | |||
| HERV-P | Untreated | 57.0 | 0.8 | 56.8 | 1.3 | <0.001 | 0.012 |
| Treated | 29.6 | 1.9 | 29.2 | 1.3 | |||
| SK-CO-1 Cells | |||||||
| Element | Treatment | Mean | Standard Deviation | Median | Interquartile Range | T-Test | Wilcoxon |
| HERV-K | Untreated | 45.5 | 5.3 | 43.3 | 0.8 | <0.001 | 0.012 |
| Treated | 30.8 | 2.8 | 29.9 | 0.4 | |||
| HERV-W | Untreated | 88.4 | 3.0 | 87.1 | 0.7 | <0.001 | 0.012 |
| Treated | 65.2 | 6.7 | 67.5 | 3.3 | |||
| HERV-H | Untreated | 16.5 | 2.6 | 17.3 | 3.7 | 0.210 | 0.312 |
| Treated | 14.3 | 2.0 | 13.9 | 3.0 | |||
| HERV-P | Untreated | 5.2 | 2.0 | 5.0 | 3.4 | 0.137 | 0.183 |
| Treated | 3.4 | 0.8 | 3.2 | 1.2 | |||
| Treatment | Caco-2 | SK-CO-1 | |||
|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | ||
| Untreated | HERV-K | 5.5 | 3.9 | 10.9 | 4.1 |
| HERV-W | 9 | 3.4 | 13.3 | 4.5 | |
| EpCAM | 145.5 | 132.1 | 119.7 | 105.1 | |
| CD63 | 131.7 | 173.2 | 34.7 | 25.1 | |
| Treated | HERV-K | 9.1 | 6 | 8.3 | 4.1 |
| HERV-W | 10.5 | 3.6 | 10.8 | 4.6 | |
| EpCAM | 204.6 | 184.2 | 81.4 | 58.5 | |
| CD63 | 133.4 | 190.4 | 58.2 | 59.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, L.; Cafora, M.; Rota, F.; Hoxha, M.; Iodice, S.; Tarantini, L.; Dolci, M.; Delbue, S.; Pistocchi, A.; Bollati, V. Extracellular Vesicles Released by Colorectal Cancer Cell Lines Modulate Innate Immune Response in Zebrafish Model: The Possible Role of Human Endogenous Retroviruses. Int. J. Mol. Sci. 2019, 20, 3669. https://doi.org/10.3390/ijms20153669
Ferrari L, Cafora M, Rota F, Hoxha M, Iodice S, Tarantini L, Dolci M, Delbue S, Pistocchi A, Bollati V. Extracellular Vesicles Released by Colorectal Cancer Cell Lines Modulate Innate Immune Response in Zebrafish Model: The Possible Role of Human Endogenous Retroviruses. International Journal of Molecular Sciences. 2019; 20(15):3669. https://doi.org/10.3390/ijms20153669
Chicago/Turabian StyleFerrari, Luca, Marco Cafora, Federica Rota, Mirjam Hoxha, Simona Iodice, Letizia Tarantini, Maria Dolci, Serena Delbue, Anna Pistocchi, and Valentina Bollati. 2019. "Extracellular Vesicles Released by Colorectal Cancer Cell Lines Modulate Innate Immune Response in Zebrafish Model: The Possible Role of Human Endogenous Retroviruses" International Journal of Molecular Sciences 20, no. 15: 3669. https://doi.org/10.3390/ijms20153669
APA StyleFerrari, L., Cafora, M., Rota, F., Hoxha, M., Iodice, S., Tarantini, L., Dolci, M., Delbue, S., Pistocchi, A., & Bollati, V. (2019). Extracellular Vesicles Released by Colorectal Cancer Cell Lines Modulate Innate Immune Response in Zebrafish Model: The Possible Role of Human Endogenous Retroviruses. International Journal of Molecular Sciences, 20(15), 3669. https://doi.org/10.3390/ijms20153669






