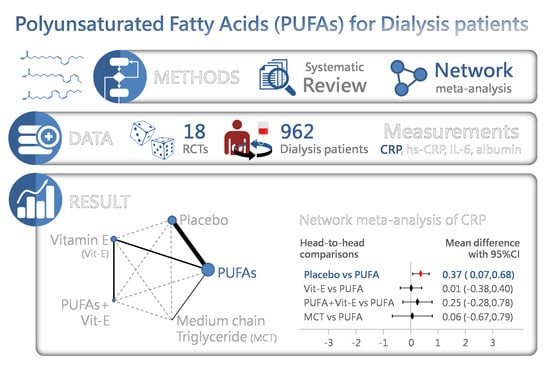
Efficacy of Polyunsaturated Fatty Acids on Inflammatory Markers in Patients Undergoing Dialysis: A Systematic Review with Network Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Results
2.1. Characteristics and Quality of Included Studies
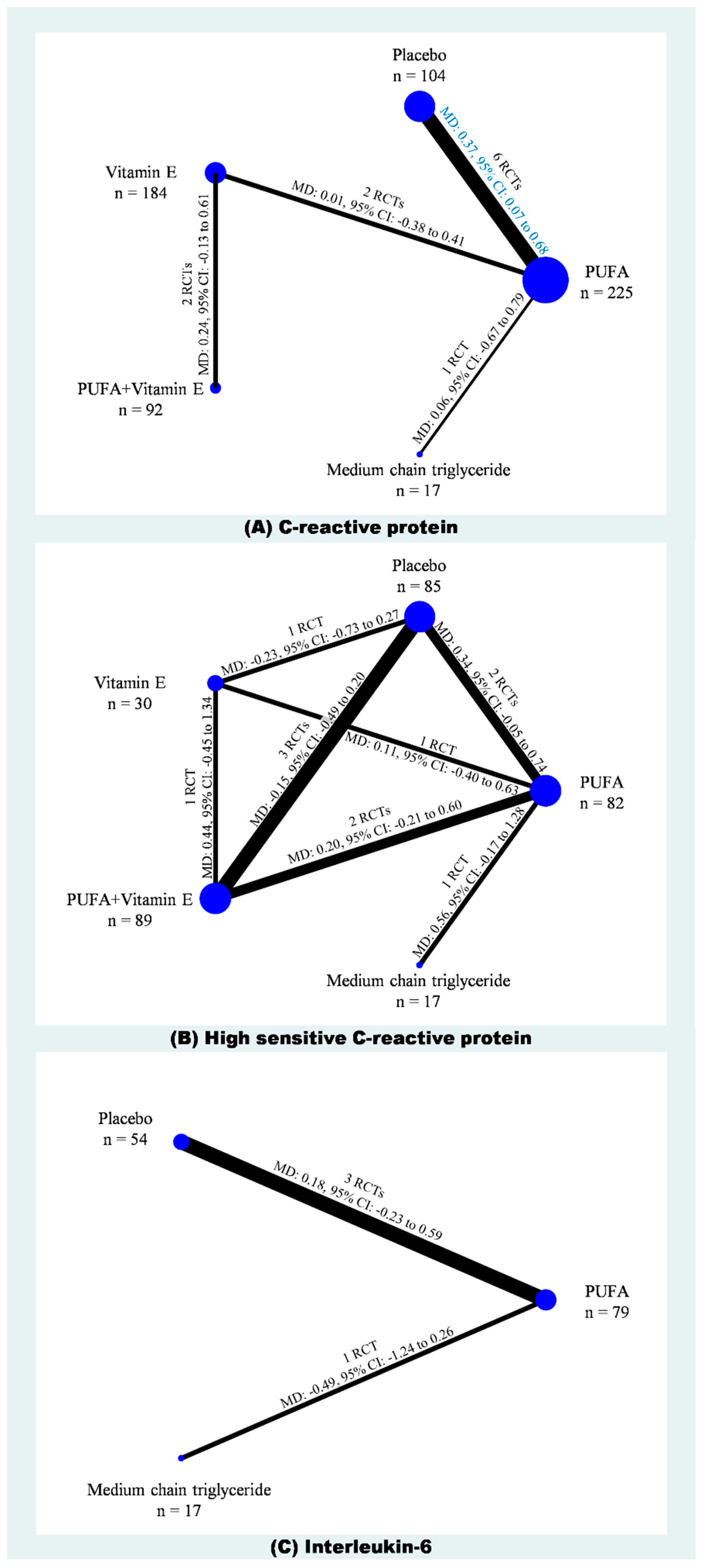
2.2. C-Reactive Protein
2.3. High-Sensitivity C-Reactive Protein
2.4. Interleukin-6
2.5. Further Analysis
3. Discussion
3.1. Comparing to the Previous Syntheses
3.2. Limitations
4. Materials and Methods
4.1. Study Selection Criteria
4.2. Search Strategy and Study Selection
4.3. Quality Assessment and Data Extraction
4.4. Evidence Synthesis and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| CRP | C-reactive protein |
| hs-CRP | high-sensitivity C-reactive protein |
| IQR | interquartile range |
| IL-6 | interleukin-6 |
| MCT | medium chain triglyceride |
| NF-κB | nuclear factor kappa B |
| PUFA | polyunsaturated fatty acids |
| RCT | randomized clinical trial |
| SD | standard deviation. |
| SE | standard error |
| SUCRA | surface under the cumulative ranking curve |
| WMD | weighted mean difference |
References
- Sachdeva, M.; Hung, A.; Kovalchuk, O.; Bitzer, M.; Mokrzycki, M.H. The initial vascular access type contributes to inflammation in incident hemodialysis patients. Int. J. Nephrol. 2012, 2012, 917465. [Google Scholar] [CrossRef] [PubMed]
- Caglar, K.; Peng, Y.; Pupim, L.B.; Flakoll, P.J.; Levenhagen, D.; Hakim, R.M.; Ikizler, T.A. Inflammatory signals associated with hemodialysis. Kidney Int. 2002, 62, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P. Inflammation in end-stage renal disease—What have we learned in 10 years? Semin. Dial. 2010, 23, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Bazeley, J.; Bieber, B.; Li, Y.; Morgenstern, H.; de Sequera, P.; Combe, C.; Yamamoto, H.; Gallagher, M.; Port, F.K.; Robinson, B.M. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 2452–2461. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Maggiore, U.; Taccola, D.; Migliori, M.; Rizza, G.M.; Consani, C.; Bertini, A.; Sposini, S.; Perez-Garcia, R.; Rindi, P.; et al. Interleukin-6 is a stronger predictor of total and cardiovascular mortality than c-reactive protein in haemodialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant. Assoc. Eur. Ren. Assoc. 2004, 19, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P. Inflammation in end-stage renal failure: Could it be treated? Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant. Assoc. Eur. Ren. Assoc. 2002, 17 (Suppl. 8), 33–38. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Alvestrand, A. Inflammation in end-stage renal disease: Sources, consequences, and therapy. Semin. Dial. 2002, 15, 329–337. [Google Scholar] [CrossRef]
- Machowska, A.; Carrero, J.J.; Lindholm, B.; Stenvinkel, P. Therapeutics targeting persistent inflammation in chronic kidney disease. Transl. Res. J. Lab. Clin. Med. 2016, 167, 204–213. [Google Scholar] [CrossRef]
- Barutcu, I.; Sezgin, A.T.; Sezgin, N.; Gullu, H.; Esen, A.M.; Topal, E.; Ozdemir, R.; Kosar, F.; Cehreli, S. Increased high sensitive crp level and its significance in pathogenesis of slow coronary flow. Angiology 2007, 58, 401–407. [Google Scholar] [CrossRef]
- Asemi, Z.; Soleimani, A.; Shakeri, H.; Mazroii, N.; Esmaillzadeh, A. Effects of omega-3 fatty acid plus alpha-tocopherol supplementation on malnutrition-inflammation score, biomarkers of inflammation and oxidative stress in chronic hemodialysis patients. Int. Urol. Nephrol. 2016, 48, 1887–1895. [Google Scholar] [CrossRef]
- Bowden, R.G.; Wilson, R.L.; Deike, E.; Gentile, M. Fish oil supplementation lowers c-reactive protein levels independent of triglyceride reduction in patients with end-stage renal disease. Nutr. Clin. Pract. 2009, 24, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Daud, Z.A.; Tubie, B.; Adams, J.; Quainton, T.; Osia, R.; Tubie, S.; Kaur, D.; Khosla, P.; Sheyman, M. Effects of protein and omega-3 supplementation, provided during regular dialysis sessions, on nutritional and inflammatory indices in hemodialysis patients. Vasc. Health Risk Manag. 2012, 8, 187–195. [Google Scholar] [PubMed]
- Ewers, B.; Riserus, U.; Marckmann, P. Effects of unsaturated fat dietary supplements on blood lipids, and on markers of malnutrition and inflammation in hemodialysis patients. J. Ren. Nutr. 2009, 19, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, A.; Dashti-Khavidaki, S.; Lessan-Pezeshki, M.; Khatami, M.R. Potential effects of omega-3 fatty acids on insulin resistance and lipid profile in maintenance hemodialysis patients a randomized placebo-controlled trial. Iran. J. Kidney Dis. 2016, 10, 310–318. [Google Scholar] [PubMed]
- Gharekhani, A.; Khatami, M.R.; Dashti-Khavidaki, S.; Razeghi, E.; Abdollahi, A.; Hashemi-Nazari, S.S.; Mansournia, M.A. Effects of oral supplementation with omega-3 fatty acids on nutritional state and inflammatory markers in maintenance hemodialysis patients. J. Ren. Nutr. 2014, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, A.; Khatami, M.R.; Dashti-Khavidaki, S.; Razeghi, E.; Abdollahi, A.; Hashemi-Nazari, S.S.; Mansournia, M.A. Potential effects of omega-3 fatty acids on anemia and inflammatory markers in maintenance hemodialysis patients. DARU 2014, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, A.; Khatami, M.R.; Dashti-Khavidaki, S.; Razeghi, E.; Noorbala, A.A.; Hashemi-Nazari, S.S.; Mansournia, M.A. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: A randomized, placebo-controlled clinical trial. Eur. J. Clin. Pharmacol. 2014, 70, 655–665. [Google Scholar] [CrossRef]
- Harving, F.; Svensson, M.; Flyvbjerg, A.; Schmidt, E.B.; Jorgensen, K.A.; Eriksen, H.H.; Christensen, J.H. N-3 polyunsaturated fatty acids and adiponectin in patients with end-stage renal disease. Clin. Nephrol. 2015, 83, 279–285. [Google Scholar] [CrossRef]
- Himmelifarb, J.; Phinney, S.; Ikizler, T.A.; Kane, J.; McMonagle, E.; Miller, G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J. Ren. Nutr. 2007, 17, 296–304. [Google Scholar] [CrossRef]
- Hung, A.M.; Booker, C.; Ellis, C.D.; Siew, E.D.; Graves, A.J.; Shintani, A.; Abumrad, N.N.; Himmelfarb, J.; Ikizler, T.A. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol. Dial. Transplant. 2015, 30, 266–274. [Google Scholar] [CrossRef]
- Khalatbari Soltani, S.; Jamaluddin, R.; Tabibi, H.; Mohd Yusof, B.N.; Atabak, S.; Loh, S.P.; Rahmani, L. Effects of flaxseed consumption on systemic inflammation and serum lipid profile in hemodialysis patients with lipid abnormalities. Hemodial. Int. 2013, 17, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kooshki, A.; Taleban, F.A.; Tabibi, H.; Hedayati, M. Effects of marine omega-3 fatty acids on serum systemic and vascular inflammation markers and oxidative stress in hemodialysis patients. Ann. Nutr. Metab. 2011, 58, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Son, Y.K.; Kim, S.E.; An, W.S. The effects of omega-3 fatty acid on vitamin d activation in hemodialysis patients: A pilot study. Mar. Drugs 2015, 13, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.R.N.; de Alencastro, M.G.; Konrath, A.V.; Cargnin, M.; Manfro, R.C. Flaxseed oil supplementation decreases c-reactive protein levels in chronic hemodialysis patients. Nutr. Res. 2012, 32, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Mirfatahi, M.; Tabibi, H.; Nasrollahi, A.; Hedayati, M.; Taghizadeh, M. Effect of flaxseed oil on serum systemic and vascular inflammation markers and oxidative stress in hemodialysis patients: A randomized controlled trial. Int. Urol. Nephrol. 2016, 48, 1335–1341. [Google Scholar] [CrossRef]
- Naini, A.E.; Asiabi, R.E.; Keivandarian, N.; Moeinzadeh, F. Effect of omega-3 supplementation on inflammatory parameters in patients on chronic ambulatory peritoneal dialysis. Adv. Biomed. Res. 2015, 4, 167. [Google Scholar] [PubMed]
- Poulia, K.A.; Panagiotakos, D.B.; Tourlede, E.; Rezou, A.; Stamatiadis, D.; Boletis, J.; Zampelas, A. Omega-3 fatty acids supplementation does not affect serum lipids in chronic hemodialysis patients. J. Ren. Nutr. 2011, 21, 479–484. [Google Scholar] [CrossRef]
- Rodhe, Y.; Woodhill, T.; Thorman, R.; Moller, L.; Hylander, B. The effect of sea buckthorn supplement on oral health, inflammation, and DNA damage in hemodialysis patients: A double-blinded, randomized crossover study. J. Ren. Nutr. 2013, 23, 172–179. [Google Scholar] [CrossRef]
- Saifullah, A.; Watkins, B.A.; Saha, C.; Li, Y.; Moe, S.M.; Friedman, A.N. Oral fish oil supplementation raises blood omega-3 levels and lowers c-reactive protein in haemodialysis patients—A pilot study. Nephrol. Dial. Transplant. 2007, 22, 3561–3567. [Google Scholar] [CrossRef]
- Zakaria, H.; Mostafa, T.M.; El-Azab, G.A.; Abd El Wahab, A.M.; Elshahawy, H.; Sayed-Ahmed, N.A.H. The impact of fish oil and wheat germ oil combination on mineral-bone and inflammatory markers in maintenance hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Int. Urol. Nephrol. 2017, 49, 1851–1858. [Google Scholar] [CrossRef]
- Khor, B.H.; Narayanan, S.S.; Sahathevan, S.; Gafor, A.H.A.; Daud, Z.A.M.; Khosla, P.; Sabatino, A.; Fiaccadori, E.; Chinna, K.; Karupaiah, T. Efficacy of nutritional interventions on inflammatory markers in haemodialysis patients: A systematic review and limited meta-analysis. Nutrients 2018, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Bersch-Ferreira, A.C.; Sampaio, G.R.; Gehringer, M.O.; Ross-Fernandes, M.B.; Kovacs, C.; Alves, R.; Pereira, J.L.; Magnoni, C.D.; Weber, B.; Rogero, M.M. Association between polyunsaturated fatty acids and inflammatory markers in patients in secondary prevention of cardiovascular disease. Nutr. 2017, 37, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505s–1519s. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Laye, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-inflammatory effects of omega-3 fatty acids in the brain: Physiological mechanisms and relevance to pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The imbalance between n-6/n-3 polyunsaturated fatty acids and inflammatory bowel disease: A comprehensive review and future therapeutic perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 fatty acids in early prevention of inflammatory neurodegenerative disease: A focus on alzheimer’s disease. Biomed. Res. Int 2015, 2015, 172801. [Google Scholar] [CrossRef]
- Tortosa-Caparros, E.; Navas-Carrillo, D.; Marin, F.; Orenes-Pinero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592s–599s. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol. Asp. Med. 2007, 28, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liu, R.J.; Chang, M.; Huang, J.H.; Jin, Q.Z.; Wang, X.G. Effect of dietary alpha-linolenic acid on blood inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2018, 57, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto-Furuie, K.; Yoshimoto, K.; Tanaka, T.; Saima, S.; Kikuchi, Y.; Shay, J.; Horrobin, D.F.; Echizen, H. Effects of oral supplementation with evening primrose oil for six weeks on plasma essential fatty acids and uremic skin symptoms in hemodialysis patients. Nephron 1999, 81, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Taziki, O.; Lessan-Pezeshki, M.; Akha, O.; Vasheghani, F. The effect of low dose omega-3 on plasma lipids in hemodialysis patients. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ. Transplant. Saudi Arab. 2007, 18, 571–576. [Google Scholar]
- Tabibi, H.; Mirfatahi, M.; Hedayati, M.; Nasrollahi, A. Effects of flaxseed oil on blood hepcidin and hematologic factors in hemodialysis patients. Hemodial. Int. 2017, 21, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Schrnidt, E.B.; Jorgense, K.A.; Christensen, J.H. The effect of n-3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: A randomized placebo-controlled intervention study. Nephrol. Dial. Transplant. 2008, 23, 2918–2924. [Google Scholar] [CrossRef]
- Svensson, M.; Schmidt, E.B.; Jorgensen, K.A.; Christensen, J.H. The effect of n-3 fatty acids on heart rate variability in patients treated with chronic hemodialysis. J. Ren. Nutr. 2007, 17, 243–249. [Google Scholar] [CrossRef]
- Sorensen, G.V.B.; Svensson, M.; Strandhave, C.; Schmidt, E.B.; Jorgensen, K.A.; Christensen, J.H. The effect of n-3 fatty acids on small dense low-density lipoproteins in patients with end-stage renal disease: A randomized placebo-controlled intervention study. J. Ren. Nutr. 2015, 25, 376–380. [Google Scholar] [CrossRef]
- Schmitz, P.G.; McCloud, L.K.; Reikes, S.T.; Leonard, C.L.; Gellens, M.E. Prophylaxis of hemodialysis graft thrombosis with fish oil: Double-blind, randomized, prospective trial. J. Am. Soc. Nephrol. 2002, 13, 184–190. [Google Scholar]
- Rantanen, J.M.; Riahi, S.; Johansen, M.B.; Schmidt, E.B.; Christensen, J.H. Effects of marine n-3 polyunsaturated fatty acids on heart rate variability and heart rate in patients on chronic dialysis: A randomized controlled trial. Nutrients 2018, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Omrani, H.R.; Pasdar, Y.; Raisi, D.; Najafi, F.; Esfandiari, A. The effect of omega-3 on serum lipid profile in hemodialysis patients. J. Ren. Inj. Prev. 2015, 4, 68–72. [Google Scholar] [PubMed]
- Madsen, T.; Hagstrup Christensen, J.; Toft, E.; Aardestrup, I.; Lundbye-Christensen, S.; Schmidt, E.B. Effect of intravenous ω-3 fatty acid infusion and hemodialysis on fatty acid composition of free fatty acids and phospholipids in patients with end-stage renal disease. J. Parenter. Enter. Nutr. 2011, 35, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.E.; Moist, L.; Hemmelgarn, B.R.; Tonelli, M.; Vazquez, M.A.; Dorval, M.; Oliver, M.; Donnelly, S.; Allon, M.; Stanley, K. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts a randomized controlled trial. JAMA J. Am. Med. Assoc. 2012, 307, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, H.T.; Dehgan, R.; Asl, B.H.; Safaian, A.; Panahi, F.; Estakhri, R.; Purasgar, B. Effect of omega-3 supplementation on serum level of homocysteine in hemodialysis patients. Iran. J. Kidney Dis. 2013, 7, 479–484. [Google Scholar]
- Khajehdehi, P. Lipid-lowering effect of polyunsaturated fatty acids in hemodialysis patients. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2000, 10, 191–195. [Google Scholar] [CrossRef]
- Kajbaf, M.H.; Khorvash, F.; Mortazavi, M.; Shahidi, S.; Moeinzadeh, F.; Farajzadegan, Z.; Tirani, S.A. Does omega-3 supplementation decrease carotid intima-media thickening in hemodialysis patients? J. Res. Pharm. Pract. 2016, 5, 252–256. [Google Scholar]
- Jabbari, M.; Khoshnevis, T.; Jenabi, A.; Yousefi, F. The effect of omega-3 supplement on serum lipid profile in patients undergoing hemodialysis: A randomized clinical trial. Rom. J. Intern. Med. Rev. Roum. Med. Interne 2016, 54, 222–227. [Google Scholar] [CrossRef][Green Version]
- Irish, A.B.; Viecelli, A.K.; Hawley, C.M.; Hooi, L.S.; Pascoe, E.M.; Paul-Brent, P.A.; Badve, S.V.; Mori, T.A.; Cass, A.; Kerr, P.G.; et al. Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis a randomized clinical trial. JAMA Intern. Med. 2017, 177, 184–193. [Google Scholar] [CrossRef]
- Ghanei, E.; Zeinali, J.; Borghei, M.; Homayouni, M. Efficacy of omega-3 fatty acids supplementation in treatment of uremic pruritus in hemodialysis patients: A double-blind randomized controlled trial. Iran. Red Crescent Med. J. 2012, 14, 515–522. [Google Scholar]
- Deger, S.M.; Hung, A.M.; Ellis, C.D.; Booker, C.; Bian, A.H.; Chen, G.H.; Abumrad, N.N.; Ikizler, T.A. High dose omega-3 fatty acid administration and skeletal muscle protein turnover in maintenance hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2016, 11, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- De Mattos, A.M.; da Costa, J.A.C.; Jordao, A.A.; Chiarello, P.G. Omega-3 fatty acid supplementation is associated with oxidative stress and dyslipidemia, but does not contribute to better lipid and oxidative status on hemodialysis patients. J. Ren. Nutr. 2017, 27, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Dashti-Khavidaki, S.; Gharekhani, A.; Khatami, M.R.; Miri, E.S.; Khalili, H.; Razeghi, E.; Hashemi-Nazari, S.S.; Mansournia, M.A. Effects of omega-3 fatty acids on depression and quality of life in maintenance hemodialysis patients. Am. J. Ther. 2014, 21, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Belury, M.A.; Burgess, J.R.; Peck, L.W. Supplementation with n-3 and n-6 polyunsaturated fatty acids: Effects on lipoxygenase activity and clinical symptoms of pruritus in hemodialysis patients. J. Ren. Nutr. 2004, 14, 233–241. [Google Scholar] [CrossRef]
- Beavers, K.M.; Beavers, D.P.; Bowden, R.G.; Wilson, R.L.; Gentile, M. Effect of over-the-counter fish-oil administration on plasma lp(a) levels in an end-stage renal disease population. J. Ren. Nutr. 2009, 19, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Beavers, D.P.; Bowden, R.G.; Wilson, R.L.; Gentile, M. Omega-3 fatty acid supplementation and total homocysteine levels in end-stage renal disease patients. Nephrology 2008, 13, 284–288. [Google Scholar] [CrossRef]
- Ateya, A.M.; Sabri, N.A.; El Hakim, I.; Shaheen, S.M. Effect of omega-3 fatty acids on serum lipid profile and oxidative stress in pediatric patients on regular hemodialysis: A randomized placebo-controlled study. J. Ren. Nutr. 2017, 27, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Allawi, A.A.D.; Wahab Alwardi, M.A.; Altemimi, H.M. Effects of omega-3 on vitamin d activation in iraqi patients with chronic kidney disease treated by maintenance hemodialysis. J. Pharm. Sci. Res. 2017, 9, 1812–1816. [Google Scholar]
- Xu, T.H.; Sun, Y.T.; Sun, W.; Yao, L.; Sun, L.; Liu, L.L.; Ma, J.F.; Wang, L.N. Effect of omega-3 fatty acid supplementation on serum lipids and vascular inflammation in patients with end-stage renal disease: A meta-analysis. Sci Rep. 2016, 6, 39346. [Google Scholar] [CrossRef]
- Khan, A.Q.; Khan, R.; Rehman, M.U.; Lateef, A.; Tahir, M.; Ali, F.; Sultana, S. Soy isoflavones (daidzein & genistein) inhibit 12-o-tetradecanoylphorbol-13-acetate (tpa)-induced cutaneous inflammation via modulation of cox-2 and nf-kappab in swiss albino mice. Toxicology 2012, 302, 266–274. [Google Scholar]
- Mori, T.A.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: A hypothesis proposal. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4 (Suppl. 1), S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Stenvinkel, P.; Qureshi, A.R.; Riserus, U.; Cederholm, T.; Barany, P.; Heimburger, O.; Lindholm, B.; Carrero, J.J. Essential polyunsaturated fatty acids, inflammation and mortality in dialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transplant. Assoc. Eur. Ren. Assoc. 2012, 27, 3615–3620. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1998, 32, S112–S119. [Google Scholar] [CrossRef]
- Mills, E.J.; Thorlund, K.; Ioannidis, J.P. Demystifying trial networks and network meta-analysis. BMJ (Clin. Res. Ed.) 2013, 346, f2914. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The prisma extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.C.; Tuan, H.I.; Kang, Y.N. Effects of polyunsaturated fatty acids on nonspecific typical dry eye disease: A systematic review and meta-analysis of randomized clinical trials. Nutrients 2019, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.N.; Chi, S.C.; Wu, M.H.; Chiu, H.H. The effects of losartan versus beta-blockers on cardiovascular protection in marfan syndrome: A systematic review and meta-analysis. J. Formos. Med Assoc. Taiwan Yi Zhi 2019, in press. [Google Scholar] [CrossRef]
- Lin, T.M.; Chi, J.E.; Chang, C.C.; Kang, Y.N. Do etoricoxib and indometacin have similar effects and safety for gouty arthritis? A meta-analysis of randomized controlled trials. J. Pain Res. 2019, 12, 83–91. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.W.; Wu, M.S.; Chen, K.C.; Peng, C.C.; Kang, Y.N. Effects of calcium channel blockers comparing to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with hypertension and chronic kidney disease stage 3 to 5 and dialysis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0188975. [Google Scholar] [CrossRef]
- Kao, C.C.; Lin, Y.S.; Chu, H.C.; Fang, T.C.; Wu, M.S.; Kang, Y.N. Association of renal function and direct-acting antiviral agents for hcv: A network meta-analysis. J. Clin. Med. 2018, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Kuo, Y.K.; Kang, Y.N. Effects of three common lumbar interbody fusion procedures for degenerative disc disease: A network meta-analysis of prospective studies. Int. J. Surg. 2018, 60, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]


| Author | Location | Inclusion | Treatments | Patients | Mean Age | Sex (M/F) | Dialysis Period |
|---|---|---|---|---|---|---|---|
| Year | |||||||
| Asemi | Iran | 2014 | 1. ω-3 | 30 | 55.2 | 20/10 | 3.6 |
| [10] | 2. αT | 30 | 61.2 | 20/10 | 3.5 | ||
| 3. ω-3 + αT | 30 | 54.9 | 20/10 | 3.4 | |||
| 4. Placebo | 30 | 59.9 | 20/10 | 3.4 | |||
| Bowden | USA | NR | 1. ω-3 | 18 | 57.2 | 11/7 | 1.5 |
| [11] | 2. corn oil | 15 | 64.3 | 8/7 | 2.8 | ||
| Daud | USA | NR | 1. ω-3 | 28 | 59 | 20/11 | 3.6 |
| [12] | 2. placebo | 27 | 58 | 12/20 | 3.3 | ||
| Ewers | Denmark | 2007 | 1. ω-3 | 14 | 64.6 | 30/10 | NR |
| [13] | 2. No supplement | 14 | 64.6 | 30/10 | NR | ||
| Gharekhani | Iran | NR | 1. ω-3 | 25 | 56.8 | 12/13 | 5 |
| [14,15,16,17] | 2. paraffin (placebo) | 20 | 57.2 | 8/12 | 6 | ||
| Harving | Denmark | NR | 1. ω-3 | 83 | 65.5 | 55/28 | 4 |
| [18] | 2. Olive oil | 79 | 68 | 51/28 | 3.6 | ||
| Himmelifarb | USA | 2008 to | 1. ω-3 | 31 | 58 | 23/8 | 2.1 |
| [19] | 2011 | 2. placebo | 32 | 61.2 | 17/15 | 2.6 | |
| Hung | USA | 2008 to | 1. ω-3 | 17 | 50 | 14/3 | 4.2 |
| [20] | 2011 | 2. placebo | 17 | 53 | 13/4 | 3.6 | |
| Khalatbari | Iran | NR | 1. ground flaxseed | 15 | 54 | 10/5 | 2.6 |
| Soltani [21] | 2. Usual diet | 15 | 54.5 | 6/9 | 2.8 | ||
| Kooshki | Iran | NR | 1. ω-3 | 17 | 50 | 10/7 | 1.75 |
| [22] | 2. placebo | 17 | 50 | 11/6 | 2.3 | ||
| Lemos | Brazil | NR | 1. flaxseed oil + αT | 70 | 55.7 | 39/31 | 2.4 |
| [24] | 2. mineral oil + αT | 75 | 58.3 | 46/29 | 2.9 | ||
| Lee | Korea | 2012 | 1. ω-3 | 8 | 60 | 2/6 | NR |
| [23] | 2. Olive oil | 7 | 64 | 3/4 | NR | ||
| Mirfatahi | Iran | NR | 1. flaxseed oil | 17 | 68 | 12/5 | 4.4 |
| [25] | 2. medium-chain | 17 | 59 | 10/7 | 4.6 | ||
| triglycerides oil | |||||||
| Naini | Iran | NR | 1. ω-3 | 20 | 57.7 | 11/9 | NR |
| [26] | 2. placebo | 20 | 59.3 | 12/8 | NR | ||
| Poulia | Greece | NR | 1. ω-3 + αT | 22 | 51 | 16/9 | 9.4 |
| [27] | 2. αT | 23 | 51 | 16/9 | |||
| Rodhe | Sweden | NR | 1. sea buckthorn + vit-E | 24 | 62 | 29/16 | NR |
| [28] | 2. Coconut oil | 21 | 62 | 29/16 | NR | ||
| Saifullah | USA | NR | 1. ω-3 | 15 | 58 | 11/4 | NR |
| [29] | 2. placebo | 8 | 57 | 7/1 | NR | ||
| Zakaria | Egypt | NR | 1. ω-3 + vit-E | 20 | 50.2 | 12/8 | 4 |
| [30] | 2. Placebo | 20 | 46.4 | 11/9 | 4.5 |
| Study | Randomization | Concealment | Blinding | Follow-Up | Loss | Type of | Relevant | Quality |
|---|---|---|---|---|---|---|---|---|
| Duration | Follow-Up | Analysis | Outcomes | Judgement | ||||
| Asemi | Computer generated | Yes | Double-blind | 12 weeks | 0 | ITT | hs-CRP | Low risk |
| Bowden | 4-block permuted randomization | No | Double-blind | 26 weeks | 7 | PP | hs-CRP | High risk |
| Daud | NR | NR | Triple-blind | 26 weeks | 2 | ITT | CRP | Moderate |
| Ewers | Computer generated | NR | Single-blind | 6 weeks | 10 | PP | CRP | High risk |
| Gharekhani | Blocked randomization | NR | Single-blind | 16 weeks | 9 | PP | CRP, IL-6 | High risk |
| Harving | NR | NR | NR | 12 weeks | 44 | PP | hs-CRP | High risk |
| Himmelifarb | 4-block permuted randomization | NR | Double-blind | 8 weeks | 0 | ITT | CRP, IL-6 | Moderate |
| Hung | Randomized in 1:1 ratio | NR | Double-blind | 12 weeks | 4 | PP | hs-CRP, IL-6 | Moderate |
| KhalatbariSoltani | NR | NR | Unblind | 8 weeks | 8 | PP | CRP | High risk |
| Kooshki | Blocked randomization | Yes | Double-blind | 10 weeks | 0 | ITT | CRP, IL-6 | Low risk |
| Lee | Random number table | NR | Double-blind | 12 weeks | 0 | PP | CRP | Moderate |
| Lemos | NR | Yes | Double-blind | 7 weeks | 22 | ITT | CRP | High risk |
| Mirfatahi | Blocked randomization | NR | Double-blind | 8 weeks | 0 | PP | hs-CRP | Moderate |
| Naini | NR | Yes | Double-blind | 8 weeks | 0 | ITT | CRP, IL-6 | Moderate |
| Poulia | Flip coin | NR | Single-blind | 4 weeks | 8 | PP | CRP | High risk |
| Rodhe | NR | Yes | Double-blind | 8 weeks | 21 | PP | hs-CRP | High risk |
| Saifullah | Computer generated | Yes | Double-blind | 12 weeks | 3 | PP | CRP | Moderate |
| Zakaria | Flip coin | NR | Double-blind | 16 weeks | 0 | PP | hs-CRP | Moderate |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-K.; Yeh, S.-C.; Li, S.-J.; Kang, Y.-N. Efficacy of Polyunsaturated Fatty Acids on Inflammatory Markers in Patients Undergoing Dialysis: A Systematic Review with Network Meta-Analysis of Randomized Clinical Trials. Int. J. Mol. Sci. 2019, 20, 3645. https://doi.org/10.3390/ijms20153645
Wu P-K, Yeh S-C, Li S-J, Kang Y-N. Efficacy of Polyunsaturated Fatty Acids on Inflammatory Markers in Patients Undergoing Dialysis: A Systematic Review with Network Meta-Analysis of Randomized Clinical Trials. International Journal of Molecular Sciences. 2019; 20(15):3645. https://doi.org/10.3390/ijms20153645
Chicago/Turabian StyleWu, Po-Kuan, Shu-Ching Yeh, Shan-Jen Li, and Yi-No Kang. 2019. "Efficacy of Polyunsaturated Fatty Acids on Inflammatory Markers in Patients Undergoing Dialysis: A Systematic Review with Network Meta-Analysis of Randomized Clinical Trials" International Journal of Molecular Sciences 20, no. 15: 3645. https://doi.org/10.3390/ijms20153645
APA StyleWu, P.-K., Yeh, S.-C., Li, S.-J., & Kang, Y.-N. (2019). Efficacy of Polyunsaturated Fatty Acids on Inflammatory Markers in Patients Undergoing Dialysis: A Systematic Review with Network Meta-Analysis of Randomized Clinical Trials. International Journal of Molecular Sciences, 20(15), 3645. https://doi.org/10.3390/ijms20153645