Abstract
Minimizing exposure of the fetus to medication and reducing adverse off-target effects in the mother are the primary challenges in developing novel drugs to treat pregnancy complications. Nanomedicine has introduced opportunities for the development of novel platforms enabling targeted delivery of drugs in pregnancy. This review sets out to discuss the advances and potential of surface-functionalized nanoparticles in the targeted therapy of pregnancy complications. We first describe the human placental anatomy, which is fundamental for developing placenta-targeted therapy, and then we review current knowledge of nanoparticle transplacental transport mechanisms. Meanwhile, recent surface-functionalized nanoparticles for targeting the uterus and placenta are examined. Indeed, surface-functionalized nanoparticles could help prevent transplacental passage and promote placental-specific drug delivery, thereby enhancing efficacy and improving safety. We have achieved promising results in targeting the placenta via placental chondroitin sulfate A (plCSA), which is exclusively expressed in the placenta, using plCSA binding peptide (plCSA-BP)-decorated nanoparticles. Others have also focused on using placenta- and uterus-enriched molecules as targets to deliver therapeutics via surface-functionalized nanoparticles. Additionally, we propose that placenta-specific exosomes and surface-modified exosomes might be potential tools in the targeted therapy of pregnancy complications. Altogether, surface-functionalized nanoparticles have great potential value as clinical tools in the targeted therapy of pregnancy complications.
1. Introduction
It is estimated that more than 131 million births occur globally each year [1], of which over 10% of mothers and fetuses are affected by one or more pregnancy complications. These complications have two adverse aspects: One is fetal complications, such as intrauterine growth restriction (IUGR), pregnancy loss, and preterm birth and the other is maternal complications, such as preeclampsia and gestational diabetes mellitus (GDM) [2,3,4]. Although these pregnancy disorders are distressing and lead to substantial maternal and fetal or neonatal morbidity and mortality, highly effective strategies or medications are not available for pregnant women. Indeed, the number of clinical drugs in obstetrics is extremely limited, and only three new medications have been licensed over the past two decades [5,6]. In addition, most common medications are small molecules, which readily cross the placenta via passive diffusion and reach the fetus [7], leading to serious fetotoxicity, such as hemorrhage [8], teratogenicity [9], and adenocarcinoma [10]. Minimizing the exposure of the fetus to medication and reducing the adverse off-target effects in the mother are the primary challenges in developing modern drugs to treat pregnancy complications.
Nanomedicine is capable of resolving the aforementioned problems, because it employs nanotechnology to increase the safety and efficacy of medications by selectively delivering them to the intended site [11]. A poorly functioning placenta is the main cause of pregnancy complications [3,12]. Therefore, nanoparticles can be exploited to precisely control therapeutic drug delivery to the placenta with minimal risk of side effects in the fetus and mother. This offers a new opportunity for targeted treatment of pregnancy complications.
Although nanoparticles have been actively studied and clinically used for more than two decades in the field of oncology [13,14], their application to treat pregnancy complications is still in its infancy. The development of novel nanoparticle delivery systems for use in pregnancy requires an in-depth understanding of the structure of the placenta and the mechanism of placental nanoparticle transfer. Therefore, in this review, we first describe the human placental anatomy and then summarize currently known mechanisms of nanoparticle transfer across the placenta. At the same time, we will review nanoparticle-based therapeutics, focusing especially on improved surface-functionalized nanoparticle formulations for treating pregnancy complications.
2. Human Placental Anatomy
The placenta attaches to the uterine wall and is an important organ for maternal–fetal physiologic exchange and determines the extent of fetal exposure to maternally delivered nanoparticles. Placental shape and structure vary enormously between different species [15]. The human placenta is discoidal and hemochorial in structure and is filled with both fetal and maternal blood (Figure 1A,B). It is formed from both fetal (chorionic plate and chorionic villi) and maternal tissue (decidua basalis of the uterus). Decidua septa arising from the decidua basalis divide the placenta into separate functional units know as cotyledons [16,17]. Each cotyledon contains a villus tree in which the maternal and fetal tissues are separated by a placental barrier (Figure 1C). This barrier consists of fetal capillaries, endothelial cells, and an outer trophoblast, containing the villus stroma, the multinucleated syncytiotrophoblast, and the cytotrophoblasts [17]. In addition, the unique syncytiotrophoblast, which expresses a substantial number of specific transporters, is the rate-limiting layer for substances crossing the placenta [18,19,20]. Prior to the tenth week of pregnancy, maternal blood does not perfuse the placenta, and the placental barrier is not yet established; compounds that are present in the maternal blood may be delivered to the fetus via diffusion through extracellular fluid. As the placental barrier develops (from the 10th to the 12th week of pregnancy), drugs and other chemicals that have small molecular weights, are lipophilic, exhibit low protein binding, and have a low degree of ionization readily cross the placenta barrier [21].
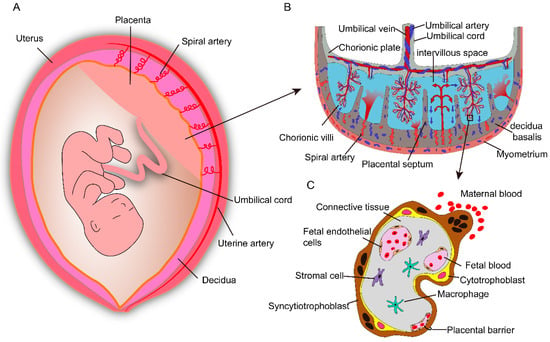
Figure 1.
Schematic depiction of anatomical structures of the human placenta. (A) The placenta is an important organ for maternal–fetal physiologic exchange and attaches to the uterine wall. (B) Anatomical structure and composition of the human placenta. The umbilical vein transport oxygen- and nutrient-rich blood from the placenta to fetus, while two umbilical arteries carry waste products from the fetus to the placenta. The intervillous space is filled with maternal blood, which enters through remodeled spiral arteries. (C) The major cell types and the placental barrier, it consists of syncytiotrophoblasts, cytotrophoblasts, and fetal endothelial cells.
3. Nanoparticle Transplacental Transport Mechanisms
The purpose of specifically controlling nanoparticles that reach the placenta is clear, yet our knowledge regarding nanoparticle transfer across the placental barrier is extremely limited, and several recent reviews have highlighted this as a new area in need of more research [22,23,24]. For nanoparticles to cross the placental barrier, they have to pass across two layers: (a) The syncytiotrophoblast and cytotrophoblasts layer and (b) the endothelial cell lining of fetal capillaries inside the villi. The possible transport routes for nanoparticles across the placenta are described below and summarized in Figure 2.
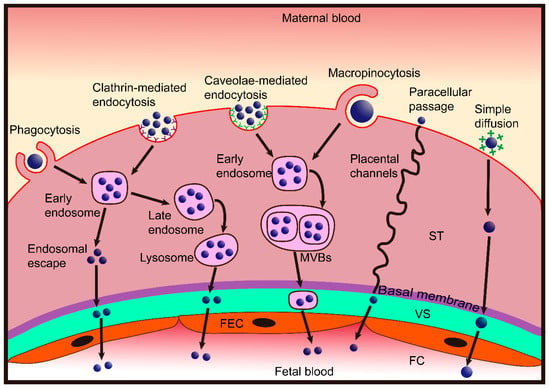
Figure 2.
Scheme showing the nanoparticle transplacental transport mechanisms. Nanoparticles cross the placental barrier by paracellular passage and transcellular passage. Very small nanoparticles can penetrate syncytiotrophoblasts (ST) via placental channels and thereby enter the villous stroma (VS). Diffusion may then occur through the fetal endothelial cells (FEC) into the lumen of the fetal capillaries (FC). The transcellular passage consists of endocytosis and exocytosis. Nanoparticles may be taken up by syncytiotrophoblasts via phagocytosis, clathrin-mediated endocytosis, caveolae-mediated endocytosis and macropinocytosis, then they may be exocytosed through the endosomal escape pathway, lysosomal secretion, and multivesicular bodies (MVBs)-related secretion. After entering the VS, nanoparticles may cross the FEC by the same pathway and eventually enter into the fetal blood. Some cationic nanoparticles may be able to directly fuse the negatively charged trophoblast cell membrane and move toward the basal membrane by simple diffusion, thereby, diffusing through the FEC into fetal blood.
3.1. Paracellular Passage
From the results of in vitro drug-transport experiments, researchers have suggested that the placenta is a lipid-pore membrane [25]. Subsequently, through a solid body of evidence based on electron microscopy analysis, Kertschanska et al. demonstrated placental pores or channels that extend from the basal trophoblastic surface into the syncytiotrophoblast and range from 15 to 25 nm in diameter under normal intravascular pressure [26]. Furthermore, some studies have shown that the flexural channels in the placenta are continuous and pass from the fetal to the maternal side [27,28,29,30]. Therefore, placental channels would allow nanoparticles with a size under 25 nm to cross the placenta via passive diffusion transport (also known as paracellular passage).
Paracellular passage is supported by an in vivo study showing that small quantum dots (QDs, < 25 nm) injected into pregnant mice cross the placenta more easily than larger QDs and the number of QDs transferred increases with increasing dosage [31]. However, data obtained from rodent experiments cannot be extrapolated to humans because the placenta is the most species-specific mammalian organ [32]. Ex vivo human placental perfusion provides a controlled and ethically accepted model that closely mimics the in vivo situation and can be used for studying transplacental transport of xenobiotics and particles. Using this model, studies have demonstrated that 25 and 50 nm silica particles [33] and 50 nm polystyrene nanoparticles can very quickly cross the placental barrier via simple passive diffusion [34]. Additionally, small dendrimer nanoparticles (5.6 nm) have been suggested to traverse the placenta through placental channels [35]. In contrast, bidirectional ex vivo perfusion studies have indicated that the transport of similar (50 nm) polystyrene nanoparticles in the fetal-to-maternal direction was significantly higher than transport in the reverse direction. The main mechanism of this translocation involves an active, energy-dependent pathway rather than passive diffusion [36]. Actually, the purported function of placental channels is to accommodate maternofetal blood flow. Under perfusion conditions, namely, hydrostatic and colloid osmotic pressure, the diameter of these channels can dilate to many times the normal size [37,38]. Enhanced uptake and passage of small gold nanoparticles was observed when placental structure and function were damaged by inflammation [39]. Hence, it is assumed that the conflicting findings may be ascribed to differences in perfusion pressure and placental conditions.
3.2. Transcellular Passage
Transcellular passage is an active process that includes both endocytosis and exocytosis, which involve complex vesicular systems and are strictly dependent on each other [40]. Endocytosis may be divided into phagocytosis (‘cellular eating’) and pinocytosis (‘cellular drinking’) [41,42]. Nanoparticle transport across the plasma membrane via transcellular passage may have multiple stages [40,43,44]. First, the nanoparticle is engulfed in membrane invaginations that form intracellular vesicles, also known as endosomes. Second, the vesicles deliver the nanoparticles to various specialized intracellular organelles, which enables sorting of vesicles toward different destinations. Finally, the nanoparticles loaded in the vesicles undergo intracellular trafficking to the opposite polar membrane and are excreted from the cells. The transport processes mentioned above are likely to be employed in mediating nanoparticle transport across the placenta.
3.2.1. Endocytosis
Phagocytosis is the preferred route for uptake of nanoparticles larger than 500 nm and primarily occurs in a few types of professional phagocytes, including macrophages, monocytes, and dendritic cells, which are responsible for host defense and uptake of pathogens, dead cells, and debris [45,46]. In general, large nanoparticles are more efficiently taken up via phagocytosis. For example, for polystyrene nanoparticles in a size range of 1.0–2.0 μm, larger particles exhibit maximal uptake by mouse peritoneal macrophages [47]. Placental trophoblasts have also been suggested to display phagocytic activity, but to a much lesser degree [48,49,50]. While interesting, whether placental trophoblasts can take up nanoparticles via phagocytosis has not yet been demonstrated.
Pinocytosis is the primary method for uptake of small nanoparticles and is classified as either clathrin-mediated endocytosis (CME) or clathrin-independent endocytosis [51]. Two major clathrin-independent endocytosis pathways are caveolae-mediated endocytosis and macropinocytosis. The placental syncytiotrophoblast displays many clathrin-coated regions between the microvilli, and coated vesicles, which are important participants in macropinocytosis, are abundant in the cytoplasm [52]. Hence, nanoparticles could be taken up by trophoblasts via pinocytosis. Evidence of this possibility came from studies showing that positively charged polymeric nanoparticles and gold nanoparticles were internalized by syncytiotrophoblasts via CME and caveolae-mediated endocytosis [53,54,55]. More recently, BeWo b30 placental barrier cells preincubated with a variety of different placental-transporter inhibitors also demonstrated that cells primarily internalized pullulan acetate nanoparticles through caveolae-mediated endocytosis and pinocytosis pathways [56]. Furthermore, macropinocytosis has been proposed for the uptake of small liposomes and nanoparticles (<60 nm) into placental trophoblasts and capillary endothelium [52,57].
3.2.2. Exocytosis
Once nanoparticles are taken up by cells, there can be multiple possible routes, which can be used by the cells for nanoparticle removal [40,58,59]. While nanoparticle transport across the placental barrier may be also through the following exocytosis mechanisms: (a) Following endocytosis, nanoparticles can be internalized into early endosomes. An early endosome can mature into multivesicular bodies (MVBs), which will eventually fuse with the plasma membrane and release the nanoparticles outside the trophoblasts, and eventually, the nanoparticles will enter fetal circulation. (b) Some of the nanoparticles can exit the vesicles during endosomal maturation. Diffusion out of the trophoblasts can be the only way for such nanoparticles to exit the cells. (c) Early endosomes deliver nanoparticles to lysosomes, which can also undergo exocytosis and release their content into the villous stroma, followed by release into fetal capillaries. (d) Some cationic nanoparticles may be able to directly fuse with the negatively charged membrane of trophoblasts and move toward the basal membrane through simple diffusion, eventually diffusing into the fetal capillaries.
Among the macromolecules involved in the placental transcytosis mechanism, the most commonly studied is immunoglobulin G (IgG). Fc receptors (FcRs) on the apical membrane of the syncytiotrophoblast mediate IgG transport from maternal to fetal circulation [60,61]. IgG binding to FcR is pH dependent; the IgG–FcR complex has high affinity at pH 6.0 and is released at pH 7.4 [62,63]. After uptake in early endosomes, IgG bound to FcR can be transported to the syncytiotrophoblast basolateral plasma membrane and be released into the villous interstitium. Then, FcγR receptor located on the endothelium of the villous vasculature delivers IgG from the placenta to the fetal circulation via a receptor-mediated diffusion mechanism [64,65].
4. Surface-Functionalized Nanoparticles for Targeting the Uterus to Treat Pregnancy Complications
In order to carry out targeted therapy of pregnancy complications, several surface-functionalized nanoparticles that target the uterus and the placenta have been exploited (Table 1).

Table 1.
Surface-functionalized nanoparticles as tools in targeted therapy of pregnancy complications.
Oxytocin receptors (OTRs) are abundantly expressed in placental decidual tissues, derived from the uterus and uterine myometrium [66,67] but, in other tissues, including the brain, pituitary, and mammary tissue, OTR expression levels are low [68]. This indicates that the OTR is a candidate for the development of a targeted drug delivery system for the treatment of pregnancy complications. Based on this principle, immunoliposomes with the ability to target the uterus for the treatment of preterm labor via surface decoration with an anti-OTR antibody have been fabricated [69,70,71]. In vitro experiments showed that immunoliposomes loaded with contraction-blocking agents, including nifedipine, salbutamol, and rolipram, consistently abolished both human and mouse uterine contractility [69]. The results of in vivo experiments with pregnant mice showed that immunoliposomes efficiently delivered indomethacin to the uterus and reduced the rates of preterm birth compared with untargeted liposomes. Additionally, no evidence of transplacental passage of the immunoliposomes to the fetus was observed [69].
Since the 1980s, immunoliposomes have been studied as a therapeutic tool for cancer and other diseases [88,89,90]. However, immunoliposomes have not yet entered clinical application. The highest risk associated with immunoliposomes is acute immune reactions, for example, anaphylaxis, anaphylactoid reactions, and serum sickness [91]. Pregnancy complications, such as preeclampsia and preterm birth, are proinflammatory in nature and characterized by immune cell activation [92]. Therefore, although fetal exposure to the drugs is minimized, administration of immunoliposomes may potentially aggravate maternal symptoms.
Similarly, liposomes conjugated with atosiban, a clinically available OTR antagonist [93], were also designed to target the uterus for preterm labor management in pregnant mice [72]. The atosiban-modified liposomes significantly increased the indomethacin concentration in the uterus, successfully prevented indomethacin passage to the fetus, and thus reduced the rate of preterm birth. At therapeutic concentrations, atosiban has been demonstrated to be safe [93], and the total injected dose of the OTR antagonist in this study was several times lower than the minimal therapeutic dose. Interestingly, in vitro studies comparing OTR antibody- and antagonist-decorated liposomes showed that these two drug delivery systems have similar OTR binding ability and cellular uptake through both clathrin- and caveolin-mediated mechanisms [71]. Altogether, OTR is a novel target for uterine drug delivery during pregnancy, and OTR-targeted nanoparticles may provide an effective tool for targeting the uterus to treat pregnancy complications.
5. Surface-Functionalized Nanoparticles for Targeting the Placenta to Treat Pregnancy Complications
5.1. EGFR Antibody
Several research groups across the globe are now devoted to developing placenta-targeted nanoparticles to treat pregnancy complications. Because epidermal growth factor receptor (EGFR) expression is highest in placental trophoblasts compared with all other nonmalignant normal human tissues, Kaitu’u-Lino et al. developed doxorubicin-loaded nanocells to treat ectopic pregnancy by coating their surface with EGFR-targeting bispecific antibodies [73]. The EGFR-targeted nanocells were readily taken up by human placental explants and significantly increased placental choriocarcinoma cell death in vitro. In a mouse subcutaneous tumor model, EGFR-targeted nanocells were found to successfully inhibit tumor cell growth and reduce tumor volume compared with nontargeted nanocells. This study was the first one to address the viability of surface-functionalized nanoparticle-mediated drug therapy in both in vitro and in vivo settings. However, toxicity and clearance data were not presented. It should be determined to what extent the EGFR-targeted nanocells will spare normal nonplacental tissues, such as the kidney and liver, from exposure to the drug in pregnant women.
Some investigators have observed that the use of full-length anti-EGFR antibodies for the treatment of diseases, especially cancer [94,95,96], has limitations due to the large size of the molecules and their long blood circulation half-life [80]. In addition, the specificity of EGFR-targeted nanoparticles may be affected by interaction of the whole antibody with Fc receptors on normal tissues [81]. To overcome these problems, single-chain antibody fragments against EGFR (ScFvEGFR) that consist of the heavy and light variable chains but lack the Fc region have been developed. ScFvEGFR-conjugated nanoparticles have exhibited the same affinity and specificity for the target antigen as the parent full-length antibodies [80,82]. While interesting, successful targeting to the EGFR-overexpressing placenta has not yet been demonstrated using ScFvEGFR, and its potential applicability remains to be determined.
5.2. Tumor Homing Peptides
The tumor homing peptides CGKRK and iRGD, which selectively target integrins on the placenta and uterine spiral arteries, have also been used for placenta-specific drug delivery [74]. Lynda Harris and team demonstrated that liposomes decorated with the tumor homing peptides were able to successfully target the placental trophoblasts and the endothelium of established uterine spiral arteries. When the targeted liposomes were loaded with insulin-like growth factor 2 and administered to a fetal growth restriction mouse model, significantly enhanced fetal and placental growth was observed, and there was growth factor transfer to the fetus. Additionally, this group developed homing-peptide miRNA inhibitor conjugates [97]. In vivo experiments showed that the conjugates significantly increased fetal and placental weights compared with controls. This team also identified other novel uteroplacental-targeted peptides using a phage screening method [75]. Similarly, targeted peptide-decorated liposomes specifically bound to the spiral arteries and placental labyrinth in pregnant mice. The targeted liposomes efficiently delivered a NO donor (SE175) to the placenta and significantly increased fetal weight and mean spiral artery diameter in pregnant endothelial nitric oxide synthase (eNOS) knockout mice. This group has developed a number of placental homing peptide-decorated liposomes that show great promise as potential therapeutics for fetal growth restriction.
5.3. plCSA-BP
Plasmodium falciparum has evolved a protein, VAR2CSA, that mediates the binding of infected erythrocytes to placental chondroitin sulfate A (plCSA) on the surface of syncytiotrophoblasts, leading to accumulation in the placenta but not in other tissues [98,99,100]. Meanwhile, a peptide from the minimal plCSA-binding region of VAR2CSA was identified through a phage screening approach [101], and we named the synthetic peptide placental CSA binding peptide (plCSA-BP). Through histological analysis, we demonstrated that plCSA-BP could specifically bind to a distinct placental-type CSA expressed on trophoblasts in human and mouse placentas. Notably, plCSA-BP has the ability to specifically bind to trophoblasts throughout gestation in the mouse [76]. We also synthesized plCSA-BP-conjugated nanoparticles (plCSA-NPs) [77] and demonstrated successful delivery of methotrexate specifically to placental trophoblasts via plCSA-NPs [76]. Importantly, plCSA-NPs had no adverse effects on maternal tissues and did not detectably cross the placenta. In addition, nanoparticles decorated with plCSA-BP were efficiently taken up into lysosomes by choriocarcinoma JEG3 cells, and in vivo experiments showed that targeted nanoparticles loaded with doxorubicin significantly inhibited tumor growth and metastasis relative to controls [78]. Meanwhile, we developed the first three-complementary method that utilizes in vivo imaging, high-frequency ultrasound, and high-performance liquid chromatography (HPLC) as a novel tool to evaluate the effectiveness and safety of placenta-targeted drug delivery systems [79]. Altogether, plCSA is a specific molecule for targeting the placenta, and plCSA-BP-decorated nanoparticles could be an effective tool for delivering large amounts of therapeutic drugs to treat pregnancy complications.
6. Untapped Potential of Placenta-Targeted Tools for Treatment of Pregnancy Complications
In multicellular organisms, cells have the ability to communicate with each other in order to proliferate and maintain homeostasis. In recent years, an extensive number of studies have demonstrated that exosomes serve important roles in cell communication with neighboring and distant cells [102,103]. Exosomes are lipid bilayer nanovesicles with a distinct size (between 30 and 150 nm) and a buoyant density of 1.12–1.19 g/mL. They are formed when MVBs undergo reverse budding and are released when MVBs fuse with the cytoplasmic membrane [104].
Exosomes are continuously shed from trophoblast cells into maternal circulation throughout gestation. Recently, it was demonstrated that chorionic villous trophoblasts (CVTs) are capable of modulating the activity of extravillous trophoblasts (EVTs) via exosomal miRNA [105,106,107]. Moreover, the number of exosomes produced during gestation is higher when pregnancy complications occur, such as preeclampsia and gestational diabetes mellitus (GDM), than in normal pregnancies [83]. Specifically, the number of placenta-derived exosomes is massive, and they can be taken up by trophoblasts. The level and content of placenta-specific exosomes may be a useful tool for the delivery of payloads to the placenta. Exosomes loaded with doxorubicin and siRNA have been reported as a feasible approach with great potential value for clinical applications [84,85]. Exosomes as drug delivery vehicles have multiple advantages. First, exosomes can be derived from a patient’s own cells, and thus, they may be less immunogenic and toxic than artificial nanoparticles [108]. Second, exosomes have lipid bilayers, which may directly fuse with target cell membranes, thereby increasing cellular internalization of loaded drugs [109]. Third, the naturally small size of exosomes allows them to avoid phagocytosis by the mononuclear phagocyte system and facilitates their uptake in target tissues [85]. In addition, exosomes have also been proposed to specifically recognize their target cells, a feature that would reduce off-target effects [110,111]. Hence, placenta-specific exosomes may deliver payloads to the placenta by directly targeting trophoblasts. If not, placenta-specific exosome surfaces can be modified with targeting peptides or antibodies by manipulating trophoblasts, either through genetic or metabolic engineering or by introducing exogenous material that is subsequently incorporated into secreted exosomes (Figure 3). For example, the targeting peptides RVG (rabies viral glycoprotein peptide) and iRGD were successfully inserted into exosomes from immature dendritic cells using a genetic method [86,87]. Overall, re-engineering placenta-specific exosomes is a very promising approach for the development of bio-inspired, targeted drug delivery systems to treat pregnancy complications.
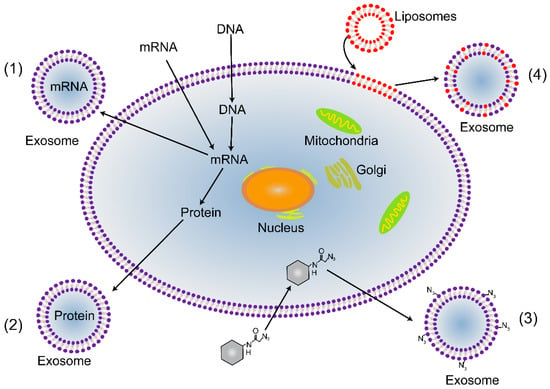
Figure 3.
Strategies for re-engineering placenta-targeted exosomes. (1) Genetic engineering can be used to introduce mRNA and DNA into trophoblasts. It can be incorporated into exosomes to promote gene expression or regulate transcription in recipient trophoblasts. (2) Transgenic proteins can be packaged into exosomes, for example, as targeting moieties and fluorescent reporters. (3) Metabolic engineering, in which metabolite analogs are packaged into trophoblast biosynthesis. This method can be used to introduce functional groups; for instance, azides can be attached to the surface of exosomes, which allows subsequent bio-orthogonal reactions to be performed. (4) Material can be incorporated into exosomes via liposomes that fuse with cytoplasmic membranes.
The untapped potential of certain placenta-enriched molecules, including placental growth factor (PlGF), pregnancy-associated plasma protein-A (PAPP-A), and soluble FMS-like tyrosine kinase 1 (sFlt-1), have been highlighted previously [112]. Targeting these circulating placental proteins might be another method to treat pregnancy complications. Recently, direct targeting of sFlt-1 has been reported via siRNA therapies administered in a baboon preeclampsia model [113]. Meanwhile, a better understanding of placenta biology and the pathogenesis of pregnancy complications may yield more placenta-targeted tools.
7. Conclusions and Perspectives
Nanomedicines for the treatment of a variety of diseases are already on the market, and the number of nanomedicine products is growing rapidly with clinical development [114]. In line with that, nanomedicine for the targeted therapy of pregnancy complications is showing great promise; however, more studies are needed. A deep understanding of the translocation and effects of nanoparticles in the human placenta is necessary for the development of novel nanoparticle platforms to treat pregnancy complications while reducing fetal exposure. A key consideration when studying nanoparticle transfer across the placenta is that it may involve multiple mechanisms. To give one example, polystyrene nanoparticles with a size of approximately 50 nm may cross the placenta via diffusion and the transtrophoblastic channel system, but those with sizes up to 120 nm may cross the placenta through caveolin-coated vesicles [34]. Hence, nanoparticle transfer across the placental barrier is dependent on particle characteristics and functionalization [115]. Additionally, restricting nanoparticle retention in the placenta by inhibiting the placenta transport system is difficult. Surface-functionalized nanoparticles are a good choice for promoting nanoparticle uptake by the placenta. Meanwhile, one of the key problems is finding a target for binding that is exclusively placental. In our study, plCSA-BP achieved that goal and it is an efficient tool that can selectively bind placental trophoblasts. The clinical need for new methods to deliver drugs in pregnancy is substantial, and therefore, exploring more tools is a timely endeavor. Placenta-specific exosomes may have placenta homing characteristics, and we propose that re-engineered placenta-specific exosomes can be used directly as a tool for the targeted delivery of therapeutics to the placenta. If not, surface-functionalized exosomes constitute a very promising approach for development of bio-inspired, targeted drug delivery systems to treat pregnancy complications. In summary, the use of nanomedicine in pregnancy is a new frontier in perinatal therapeutics, and surface-functionalized nanoparticles could be efficient tools in the targeted therapy of pregnancy complications.
Author Contributions
B.Z. and R.L. drafted the manuscript and M.Z., L.C., and X.F. made critical revisions.
Funding
This research was funded by the National Key Research and Development Program of China (2018YFC1004103), National Natural Sciences Foundation of China (81830041 and 81771617), the Shenzhen Peacock Program (KQJSCX2017033116131058), and the Basic Research Program Fund of Shenzhen (JCY J20170413165233512) to X.F
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CME | Clathrin-mediated endocytosis |
| CVT | Chorionic villous trophoblasts |
| EGFR | Epidermal growth factor receptor |
| eNOS | Endothelial nitric oxide synthase |
| EVT | Extravillous trophoblast |
| FcR | Fc receptors |
| GDM | Gestational diabetes mellitus |
| IgG | Immunoglobulin G |
| IUGR | Intrauterine growth restriction |
| MVB | Multivesicular bodies |
| OTR | Oxytocin receptors |
| PlGF | Placental growth factor |
| PAPP-A | Pregnancy-associated plasma protein-A |
| plCSA | Placental chondroitin sulfate A |
| plCSA-BP | Placental CSA binding peptide |
| sFlt-1 | Soluble FMS-like tyrosine kinase 1 |
References
- Wang, H.; Liddell, C.A.; Coates, M.M.; Mooney, M.D.; Levitz, C.E.; Schumacher, A.E.; Apfel, H.; Iannarone, M.; Phillips, B.; Lofgren, K.T.; et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 957–979. [Google Scholar] [CrossRef]
- Rodger, M.A.; Betancourt, M.T.; Clark, P.; Lindqvist, P.G.; Dizon-Townson, D.; Said, J.; Seligsohn, U.; Carrier, M.; Salomon, O.; Greer, I.A. The association of factor V leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: A systematic review and meta-analysis of prospective cohort studies. PLoS Med. 2010, 7, e1000292. [Google Scholar] [CrossRef] [PubMed]
- Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA 2011, 306, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F.A. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Fisk, N.M.; Atun, R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008, 5, e22. [Google Scholar] [CrossRef] [PubMed]
- Fisk, N.M.; McKee, M.; Atun, R. Relative and absolute addressability of global disease burden in maternal and perinatal health by investment in R&D. Trop. Med. Int. Health 2011, 16, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Syme, M.R.; Paxton, J.W.; Keelan, J.A. Drug transfer and metabolism by the human placenta. Clin Pharm. 2004, 43, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.D.; Grobman, W.A.; Parilla, B.V. Indomethacin tocolysis and intraventricular hemorrhage. Obstet. Gynecol. 2001, 97, 921–925. [Google Scholar]
- Kulaga, S.; Sheehy, O.; Zargarzadeh, A.H.; Moussally, K.; Berard, A. Antiepileptic drug use during pregnancy: Perinatal outcomes. Seizure 2011, 20, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Venn, A.; Bruinsma, F.; Werther, G.; Pyett, P.; Baird, D.; Jones, P.; Rayner, J.; Lumley, J. Oestrogen treatment to reduce the adult height of tall girls: Long-term effects on fertility. Lancet 2004, 364, 1513–1518. [Google Scholar] [CrossRef]
- Teli, M.K.; Mutalik, S.; Rajanikant, G.K. Nanotechnology and nanomedicine: Going small means aiming big. Curr. Pharm. Des. 2010, 16, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.P.; Turner, M.A.; Cetin, I.; Ayuk, P.; Boyd, C.A.; D’Souza, S.W.; Glazier, J.D.; Greenwood, S.L.; Jansson, T.; Powell, T. Placental phenotypes of intrauterine growth. Pediatr. Res. 2005, 58, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control. Release Soc. 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Van der Aa, E.M.; Copius Peereboom-Stegeman, J.H.J.; Noordhoek, J.; Gribnau, F.W.J.; Russel, F.G.M. Mechanisms of drug transfer across the human placenta. Pharm. World Sci. 1998, 20, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Neesen, J.; Straka, E.; Ellinger, I.; Dolznig, H.; Hengstschläger, M. Genetics of the human placenta: implications for toxicokinetics. Arch. Toxicol. 2016, 90, 2563–2581. [Google Scholar] [CrossRef] [PubMed]
- Ceckova-Novotna, M.; Pavek, P.; Staud, F. P-glycoprotein in the placenta: Expression, localization, regulation and function. Reprod. Toxicol. 2006, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.C.; Blankenship, T.N. Comparative placental structure. Adv. Drug Deliv. Rev. 1999, 38, 3–15. [Google Scholar] [CrossRef]
- Evseenko, D.; Paxton, J.W.; Keelan, J.A. Active transport across the human placenta: Impact on drug efficacy and toxicity. Expert Opin. Drug Metab. Toxicol. 2006, 2, 51–69. [Google Scholar] [CrossRef]
- Ganapathy, V.; Prasad, P.D.; Ganapathy, M.E.; Leibach, F.H. Placental transporters relevant to drug distribution across the maternal-fetal interface. J. Pharmacol. Exp. Ther. 2000, 294, 413–420. [Google Scholar]
- Audus, K.L. Controlling drug delivery across the placenta. Eur. J. Pharm. Sci. 1999, 8, 161–165. [Google Scholar] [CrossRef][Green Version]
- Keelan, J.A.; Leong, J.W.; Ho, D.; Iyer, K.S. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 2015, 10, 2229–2247. [Google Scholar] [CrossRef] [PubMed]
- Menezes, V.; Malek, A.; Keelan, J.A. Nanoparticulate drug delivery in pregnancy: Placental passage and fetal exposure. Curr. Pharm. Biotechnol. 2011, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M. Transplacental transport of nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Kurz, H.; Fasching, H. The permeation of drugs across the placental barrier. Naunyn-Schmiedebergs Arch. Fur Exp. Pathol. Und Pharmakol. 1968, 259, 214. [Google Scholar] [CrossRef]
- Kertschanska, S.; Schröder, H.; Kaufmann, P. The ultrastructure of the trophoblastic layer of the degu (Octodon degus) Placenta: A Re-evaluation of the ‘Channel Problem’. Placenta 1997, 18, 219–225. [Google Scholar] [CrossRef]
- Kertschanska, S.; Kosanke, G.; Kaufmann, P. Is there morphological evidence for the existence of transtrophoblastic channels in human placental villi? Placenta 1994, 15, 581–596. [Google Scholar] [CrossRef]
- Enders, A.C.; Blankenship, T.N.; Lantz, K.C.; Enders, S.S. Morphological variation in the interhemal areas of chorioallantoic placentae: A review. Placenta 1998, 19, 1–19. [Google Scholar] [CrossRef]
- Bosco, C.; Buffet, C.; Bello, M.A.; Rodrigo, R.; Gutierrez, M.; García, G. Placentation in the degu (Octodon degus): Analogies with extrasubplacental trophoblast and human extravillous trophoblast. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 475–485. [Google Scholar] [CrossRef]
- Kertschanska, S.; Stulcova, B.; Kaufmann, P.; Stulc, J. Distensible transtrophoblastic channels in the rat placenta. Placenta 2000, 21, 670–677. [Google Scholar] [CrossRef]
- Chu, M.; Wu, Q.; Yang, H.; Yuan, R.; Hou, S.; Yang, Y.; Zou, Y.; Xu, S.; Xu, K.; Ji, A.; et al. Transfer of quantum dots from pregnant mice to pups across the placental barrier. Small 2010, 6, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Guschanski, K.; Warnefors, M.; Kaessmann, H. The evolution of duplicate gene expression in mammalian organs. Genome Res. 2017, 27, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.S.; Mose, T.; Maroun, L.L.; Mathiesen, L.; Knudsen, L.E.; Rytting, E. Kinetics of silica nanoparticles in the human placenta. Nanotoxicology 2015, 9 (Suppl. 1), 79–86. [Google Scholar] [CrossRef]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.A.; Zisch, A.; Krug, H.F.; von Mandach, U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Menjoge, A.R.; Rinderknecht, A.L.; Navath, R.S.; Faridnia, M.; Kim, C.J.; Romero, R.; Miller, R.K.; Kannan, R.M. Transfer of PAMAM dendrimers across human placenta: Prospects of its use as drug carrier during pregnancy. J. Control. Release Off. J. Control. Release Soc. 2011, 150, 326–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grafmueller, S.; Manser, P.; Diener, L.; Diener, P.A.; Maeder-Althaus, X.; Maurizi, L.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; von Mandach, U.; et al. Bidirectional Transfer Study of Polystyrene Nanoparticles across the Placental Barrier in an ex Vivo Human Placental Perfusion Model. Env. Health Perspect. 2015, 123, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Kertschanska, S.; Kosanke, G.; Kaufmann, P. Pressure dependence of so-called transtrophoblastic channels during fetal perfusion of human placental villi. Microsc. Res. Tech. 1997, 38, 52–62. [Google Scholar] [CrossRef]
- Leach, L.; Firth, J.A. Structure and permeability of human placental microvasculature. Microsc. Res. Tech. 1997, 38, 137–144. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, M.; Du, L.; Wang, J.; Fan, Z.; Liu, J.; Zhao, Y.; Nie, G. Intrauterine inflammation increases materno-fetal transfer of gold nanoparticles in a size-dependent manner in murine pregnancy. Small 2013, 9, 2432–2439. [Google Scholar] [CrossRef]
- Sakhtianchi, R.; Minchin, R.F.; Lee, K.B.; Alkilany, A.M.; Serpooshan, V.; Mahmoudi, M. Exocytosis of nanoparticles from cells: Role in cellular retention and toxicity. Adv. Colloid Interface Sci. 2013, 201–202, 18–29. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, R.N.; Maxfield, F.R. Endocytosis. Physiol. Rev. 1997, 77, 759–803. [Google Scholar] [CrossRef] [PubMed]
- Tetro, N.; Moushaev, S.; Rubinchik-Stern, M.; Eyal, S. The Placental Barrier: The Gate and the Fate in Drug Distribution. Pharm. Res. 2018, 35, 71. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release Off. J. Control. Release Soc. 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Germain, R.N. An innately interesting decade of research in immunology. Nat. Med. 2004, 10, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ibricevic, A.; Cohen, J.A.; Cohen, J.L.; Gunsten, S.P.; Frechet, J.M.; Walter, M.J.; Welch, M.J.; Brody, S.L. Impact of hydrogel nanoparticle size and functionalization on in vivo behavior for lung imaging and therapeutics. Mol. Pharm. 2009, 6, 1891–1902. [Google Scholar] [CrossRef]
- Choy, M.Y.; Manyonda, I.T. The phagocytic activity of human first trimester extravillous trophoblast. Hum. Reprod. 1998, 13, 2941–2949. [Google Scholar] [CrossRef]
- Bevilacqua, E.; Hoshida, M.S.; Amarante-Paffaro, A.; Albieri-Borges, A.; Zago Gomes, S. Trophoblast phagocytic program: Roles in different placental systems. Int. J. Dev. Biol. 2010, 54, 495–505. [Google Scholar] [CrossRef]
- Matsubara, S.; Takizawa, T.; Yamada, T.; Minakami, H.; Sato, I. Phagocytosis of chorion laeve trophoblasts in patients with chorioamnionitis-associated preterm delivery: Ultrastructural and enzyme-histochemical observations. Placenta 2000, 21, 273–279. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ockleford, C.D.; Whyte, A. Differeniated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: Coated vesicles. J. Cell Sci. 1977, 25, 293–312. [Google Scholar] [PubMed]
- Kaul, G.; Clemons, T.D.; Iyer, K.S.; Pugazhenthi, K.; Keelan, J.A. Mechanism of uptake of cationic nanoparticles by human placental syncytiotrophoblast cells. Reprod. Sci. 2013, 20, A113. [Google Scholar]
- Rattanapinyopituk, K.; Shimada, A.; Morita, T.; Sakurai, M.; Asano, A.; Hasegawa, T.; Inoue, K.; Takano, H. Demonstration of the clathrin- and caveolin-mediated endocytosis at the maternal-fetal barrier in mouse placenta after intravenous administration of gold nanoparticles. J. Vet. Med. Sci. 2014, 76, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Myllynen, P.K.; Loughran, M.J.; Howard, C.V.; Sormunen, R.; Walsh, A.A.; Vahakangas, K.H. Kinetics of gold nanoparticles in the human placenta. Reprod. Toxicol. 2008, 26, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Jiang, Z.; He, H.; Li, X.; Hu, H.; Zhang, N.; Dai, Y.; Zhou, Z. Uptake and transport of pullulan acetate nanoparticles in the BeWo b30 placental barrier cell model. Int. J. Nanomed. 2018, 13, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Ockleford, C.D.; Menon, G. Differentiated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: A new organelle and the binding of iron. J. Cell Sci. 1977, 25, 279–291. [Google Scholar]
- Dahiya, U.R.; Ganguli, M. Exocytosis—A putative road-block in nanoparticle and nanocomplex mediated gene delivery. J. Control. Release Off. J. Control. Release Soc. 2019. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. 1), 51–63. [Google Scholar] [CrossRef]
- Gu, J.; Lei, Y.; Huang, Y.; Zhao, Y.; Li, J.; Huang, T.; Zhang, J.; Wang, J.; Deng, X.; Chen, Z.; et al. Fab fragment glycosylated IgG may play a central role in placental immune evasion. Hum. Reprod. 2015, 30, 380–391. [Google Scholar] [CrossRef]
- Schneider, H.; Miller, R.K. Receptor-mediated uptake and transport of macromolecules in the human placenta. Int. J. Dev. Biol. 2010, 54, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.L.; West, A.P.; Gan, L.; Bjorkman, P.J. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Mol. Cell 2001, 7, 867–877. [Google Scholar] [CrossRef]
- Stapleton, N.M.; Armstrong-Fisher, S.S.; Andersen, J.T.; van der Schoot, C.E.; Porter, C.; Page, K.R.; Falconer, D.; de Haas, M.; Williamson, L.M.; Clark, M.R.; et al. Human IgG lacking effector functions demonstrate lower FcRn-binding and reduced transplacental transport. Mol. Immunol. 2018, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Anderson, C.L.; Robinson, J.M. A novel Fc gamma R-defined, IgG-containing organelle in placental endothelium. J. Immunol. 2005, 175, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.-R.; Fuchs, F.; Husslein, P.; Soloff, M.S. Oxytocin receptors in the human uterus during pregnancy and parturition. Am. J. Obstet. Gynecol. 1984, 150, 734–741. [Google Scholar] [CrossRef]
- Wathes, D.C.; Borwick, S.C.; Timmons, P.M.; Leung, S.T.; Thornton, S. Oxytocin receptor expression in human term and preterm gestational tissues prior to and following the onset of labour. J. Endocrinol. 1999, 161, 143–151. [Google Scholar] [CrossRef]
- Adan, R.A.; Van Leeuwen, F.W.; Sonnemans, M.A.; Brouns, M.; Hoffman, G.; Verbalis, J.G.; Burbach, J.P. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: Partial sequence and immunocytochemical localization. Endocrinology 1995, 136, 4022–4028. [Google Scholar] [CrossRef]
- Paul, J.W.; Hua, S.; Ilicic, M.; Tolosa, J.M.; Butler, T.; Robertson, S.; Smith, R. Drug delivery to the human and mouse uterus using immunoliposomes targeted to the oxytocin receptor. Am. J. Obs. Gynecol 2017, 216, 283.e1–283.e14. [Google Scholar] [CrossRef]
- Hua, S. Synthesis and in vitro characterization of oxytocin receptor targeted PEGylated immunoliposomes for drug delivery to the uterus. J. Liposome Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Hua, S.; Vaughan, B. In vitro comparison of liposomal drug delivery systems targeting the oxytocin receptor: A potential novel treatment for obstetric complications. Int. J. Nanomed. 2019, 14, 2191–2206. [Google Scholar] [CrossRef] [PubMed]
- Refuerzo, J.S.; Leonard, F.; Bulayeva, N.; Gorenstein, D.; Chiossi, G.; Ontiveros, A.; Longo, M.; Godin, B. Uterus-targeted liposomes for preterm labor management: Studies in pregnant mice. Sci. Rep. 2016, 6, 34710. [Google Scholar] [CrossRef] [PubMed]
- Kaitu’u-Lino, T.J.; Pattison, S.; Ye, L.; Tuohey, L.; Sluka, P.; MacDiarmid, J.; Brahmbhatt, H.; Johns, T.; Horne, A.W.; Brown, J.; et al. Targeted nanoparticle delivery of doxorubicin into placental tissues to treat ectopic pregnancies. Endocrinology 2013, 154, 911–919. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef] [PubMed]
- Cureton, N.; Korotkova, I.; Baker, B.; Greenwood, S.; Wareing, M.; Kotamraju, V.R.; Teesalu, T.; Cellesi, F.; Tirelli, N.; Ruoslahti, E.; et al. Selective Targeting of a Novel Vasodilator to the Uterine Vasculature to Treat Impaired Uteroplacental Perfusion in Pregnancy. Theranostics 2017, 7, 3715–3731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tan, L.; Yu, Y.; Wang, B.; Chen, Z.; Han, J.; Li, M.; Chen, J.; Xiao, T.; Ambati, B.K.; et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics 2018, 8, 2765–2781. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, M.; Cai, L.; Fan, X. Synthesis and Characterization of Placental Chondroitin Sulfate A (plCSA)-Targeting Lipid-Polymer Nanoparticles. J. Vis. Exp. Jove 2018. [Google Scholar] [CrossRef]
- Zhang, B.; Cheng, G.; Zheng, M.; Han, J.; Wang, B.; Li, M.; Chen, J.; Xiao, T.; Zhang, J.; Cai, L.; et al. Targeted delivery of doxorubicin by CSA-binding nanoparticles for choriocarcinoma treatment. Drug Deliv. 2018, 25, 461–471. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Z.; Han, J.; Li, M.; Nayak, N.R.; Fan, X. Comprehensive Evaluation of the Effectiveness and Safety of Placenta-Targeted Drug Delivery Using Three Complementary Methods. J. Vis. Exp. Jove 2018. [Google Scholar] [CrossRef]
- Chopra, A. Single-Chain Anti-Epidermal Growth Factor Receptor Antibody Fragment Conjugated to Magnetic Iron Oxide Nanoparticles. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Peng, X.H.; Wang, Y.; Huang, D.; Wang, Y.; Shin, H.J.; Chen, Z.; Spewak, M.B.; Mao, H.; Wang, X.; Wang, Y.L.; et al. Targeted delivery of cisplatin to lung cancer using ScFvEGFR-heparin-cisplatin nanoparticles. ACS Nano 2011, 5, 9480–9493. [Google Scholar] [CrossRef]
- Honegger, A. Engineering antibodies for stability and efficient folding. Handb. Exp. Pharmacol. 2008, 47–68. [Google Scholar] [CrossRef]
- Salomon, C.; Rice, G.E. Role of Exosomes in Placental Homeostasis and Pregnancy Disorders. Prog. Mol. Biol. Transl. Sci. 2017, 145, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Arrighetti, N.; Corbo, C.; Evangelopoulos, M.; Pasto, A.; Zuco, V.; Tasciotti, E. Exosome-like nanovectors for drug delivery in cancer. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Kennel, S.J.; Huang, L. Interactions of immunoliposomes with target cells. J. Biol. Chem. 1983, 258, 14034–14040. [Google Scholar] [PubMed]
- Eloy, J.O.; Petrilli, R.; Trevizan, L.N.F.; Chorilli, M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, Y.; Liu, Y.; Meng, F.; Lee, R.J. Clinical translation of immunoliposomes for cancer therapy: Recent perspectives. Expert. Opin. Drug Deliv. 2018, 15, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325. [Google Scholar] [CrossRef]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F.; Petraglia, F. Inflammation and Pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Flenady, V.; Reinebrant, H.E.; Liley, H.G.; Tambimuttu, E.G.; Papatsonis, D.N. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst. Rev. 2014, Cd004452. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Jeong, H.Y.; Kim, M.W.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Park, Y.S. Anti-EGFR lipid micellar nanoparticles co-encapsulating quantum dots and paclitaxel for tumor-targeted theranosis. Nanoscale 2018, 10, 19338–19350. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, W.; Zhang, Z.; Lin, X.; Lin, H.; Peng, L.; Chen, T. Highly Uniform Synthesis of Selenium Nanoparticles with EGFR Targeting and Tumor Microenvironment-Responsive Ability for Simultaneous Diagnosis and Therapy of Nasopharyngeal Carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 11177–11193. [Google Scholar] [CrossRef] [PubMed]
- Groysbeck, N.; Stoessel, A.; Donzeau, M.; da Silva, E.C.; Lehmann, M.; Strub, J.M.; Cianferani, S.; Dembele, K.; Zuber, G. Synthesis and biological evaluation of 2.4 nm thiolate-protected gold nanoparticles conjugated to Cetuximab for targeting glioblastoma cancer cells via the EGFR. Nanotechnology 2019, 30, 184005. [Google Scholar] [CrossRef] [PubMed]
- Beards, F.; Jones, L.E.; Charnock, J.; Forbes, K.; Harris, L.K. Placental Homing Peptide-microRNA Inhibitor Conjugates for Targeted Enhancement of Intrinsic Placental Growth Signaling. Theranostics 2017, 7, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Salanti, A.; Dahlback, M.; Turner, L.; Nielsen, M.A.; Barfod, L.; Magistrado, P.; Jensen, A.T.; Lavstsen, T.; Ofori, M.F.; Marsh, K.; et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2004, 200, 1197–1203. [Google Scholar] [CrossRef]
- Salanti, A.; Clausen, T.M.; Agerbaek, M.O.; Al Nakouzi, N.; Dahlback, M.; Oo, H.Z.; Lee, S.; Gustavsson, T.; Rich, J.R.; Hedberg, B.J.; et al. Targeting Human Cancer by a Glycosaminoglycan Binding Malaria Protein. Cancer Cell 2015, 28, 500–514. [Google Scholar] [CrossRef]
- Salanti, A.; Staalsoe, T.; Lavstsen, T.; Jensen, A.T.; Sowa, M.P.; Arnot, D.E.; Hviid, L.; Theander, T.G. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003, 49, 179–191. [Google Scholar] [CrossRef]
- Resende, M.; Nielsen, M.A.; Dahlback, M.; Ditlev, S.B.; Andersen, P.; Sander, A.F.; Ndam, N.T.; Theander, T.G.; Salanti, A. Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA. Malar. J. 2008, 7, 104. [Google Scholar] [CrossRef]
- Bastos, N.; Ruivo, C.F.; da Silva, S.; Melo, S.A. Exosomes in cancer: Use them or target them? Semin. Cell Dev. Biol. 2018, 78, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 2015, 219, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ohkuchi, A.; Kuwata, T.; Usui, R.; Baba, Y.; Suzuki, H.; Chaw Kyi, T.T.; Matsubara, S.; Saito, S.; Takizawa, T. Endogenous and exogenous miR-520c-3p modulates CD44-mediated extravillous trophoblast invasion. Placenta 2017, 50, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Biro, O.; Fothi, A.; Alasztics, B.; Nagy, B.; Orban, T.I.; Rigo, J., Jr. Circulating exosomal and Argonaute-bound microRNAs in preeclampsia. Gene 2019, 692, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Prieto, S.; Chaiwangyen, W.; Herrmann, J.; Groten, T.; Schleussner, E.; Markert, U.R.; Morales-Prieto, D.M. MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl. Res. J. Lab. Clin. Med. 2016, 172, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Herrmann, I.K.; Stevens, M.M. Cell-derived vesicles for drug therapy and diagnostics: Opportunities and challenges. Nano Today 2015, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Whigham, C.A.; MacDonald, T.M.; Walker, S.P.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta 2019. [Google Scholar] [CrossRef] [PubMed]
- Turanov, A.A.; Lo, A.; Hassler, M.R.; Makris, A.; Ashar-Patel, A.; Alterman, J.F.; Coles, A.H.; Haraszti, R.A.; Roux, L.; Godinho, B.M.D.C.; et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat. Biotechnol. 2018, 36, 1164. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Muoth, C.; Aengenheister, L.; Kucki, M.; Wick, P.; Buerki-Thurnherr, T. Nanoparticle transport across the placental barrier: Pushing the field forward! Nanomedicine 2016, 11, 941–957. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).