The Cytoskeleton of the Retinal Pigment Epithelium: from Normal Aging to Age-Related Macular Degeneration
Abstract
1. Introduction
The Retinal Pigment Epithelium
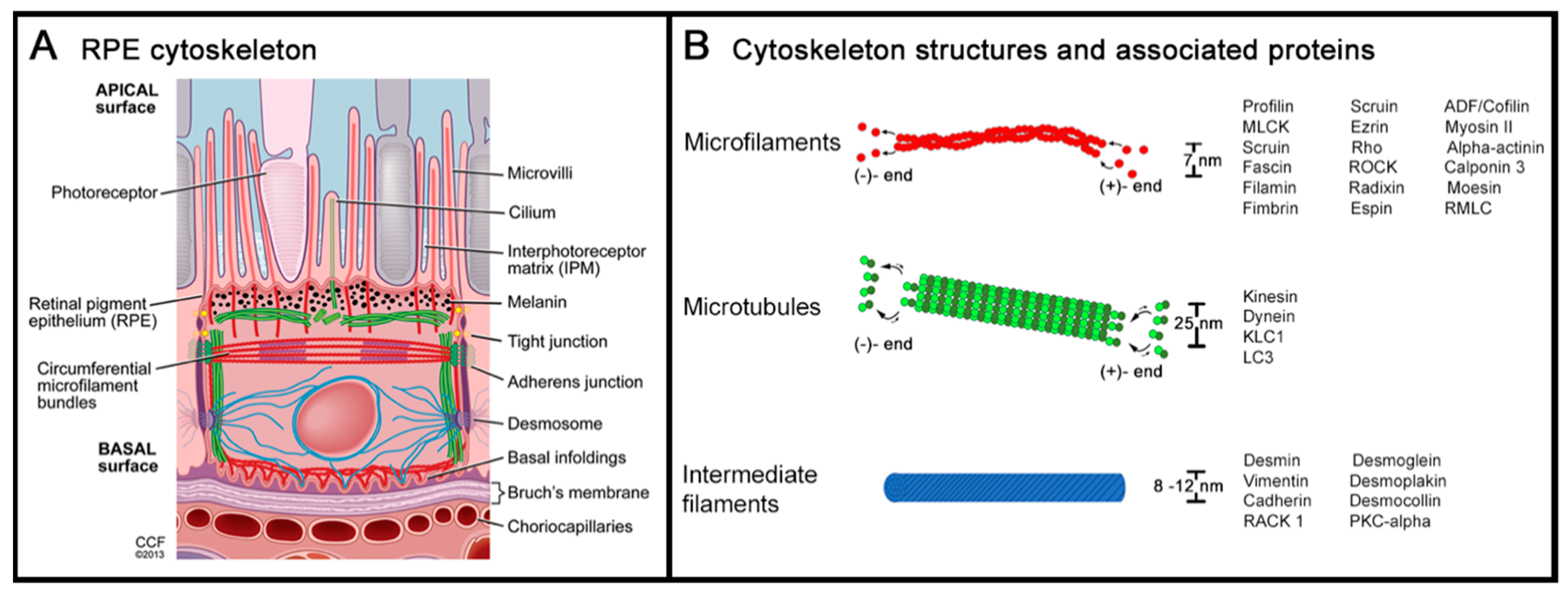
2. Cytoskeleton of the Retinal Pigment Epithelium
2.1. Actin and Non-Muscle Myosin
2.2. Microtubules and Intermediate Filaments
2.3. Cytoskeleton at the Apical RPE
2.4. Cytoskeleton at the Basolateral RPE
2.5. Extracellular Matrix (ECM)
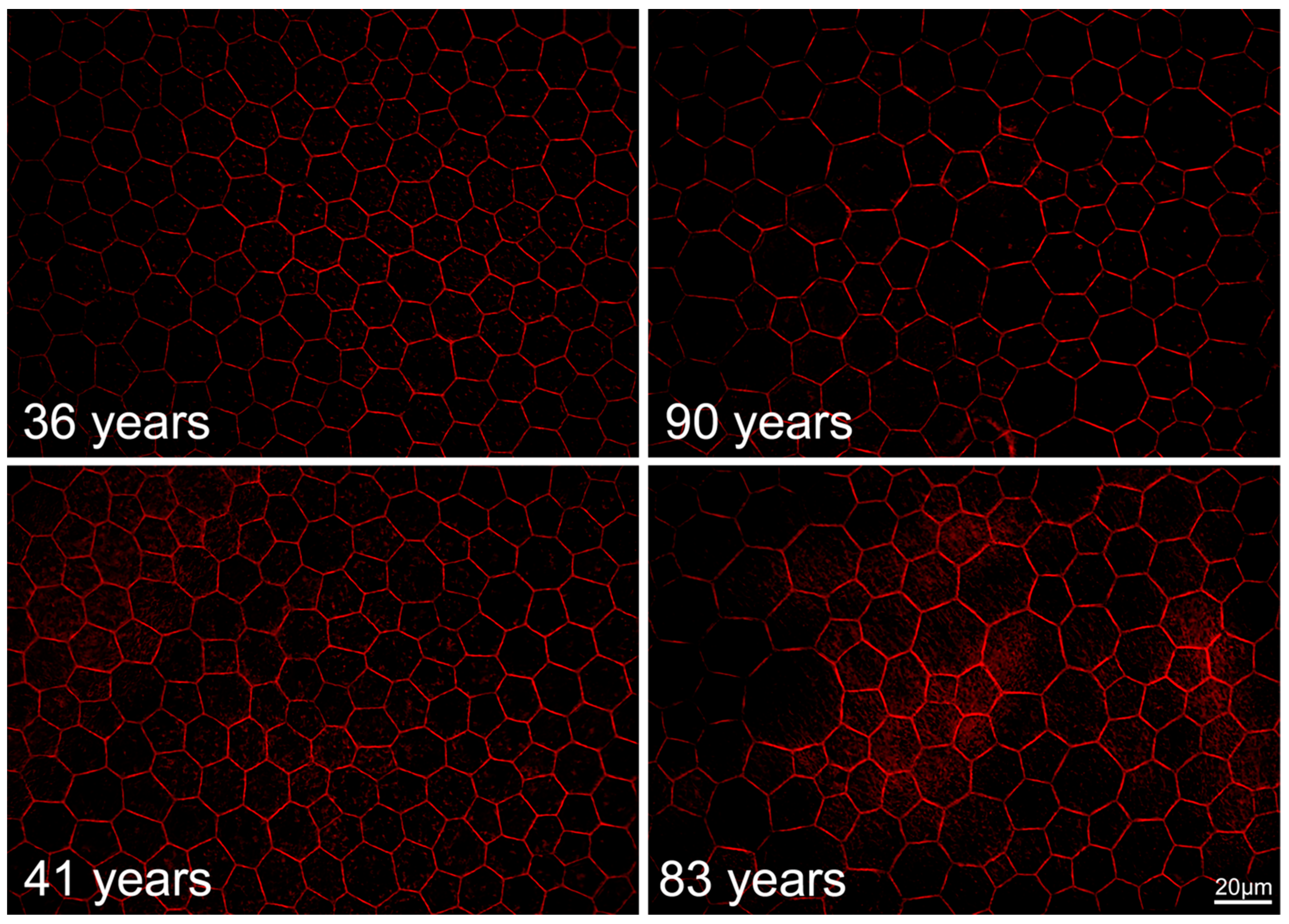
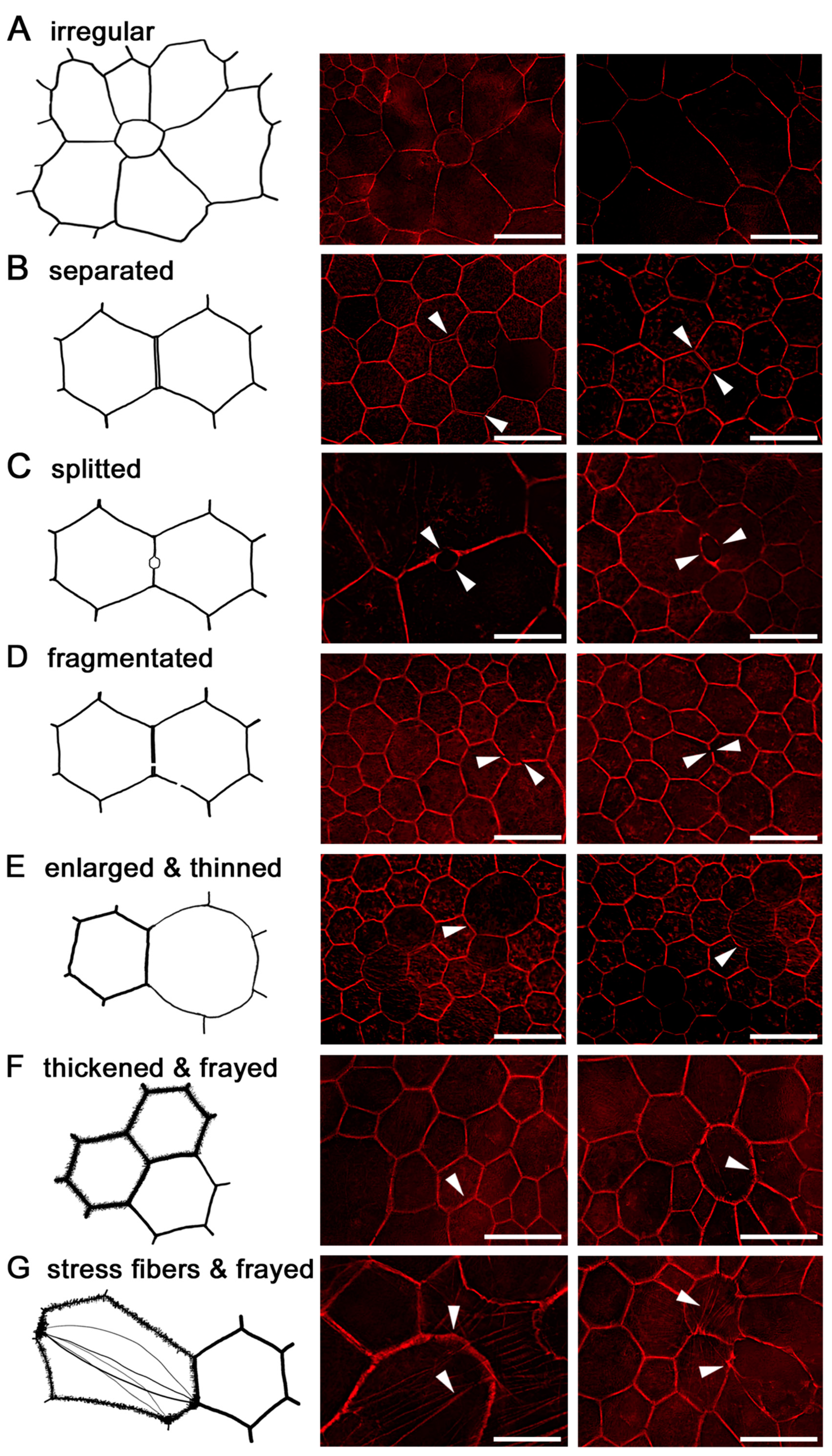
2.6. RPE Geometry in Normal Aging
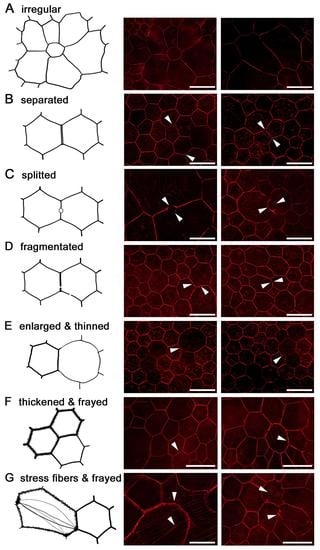
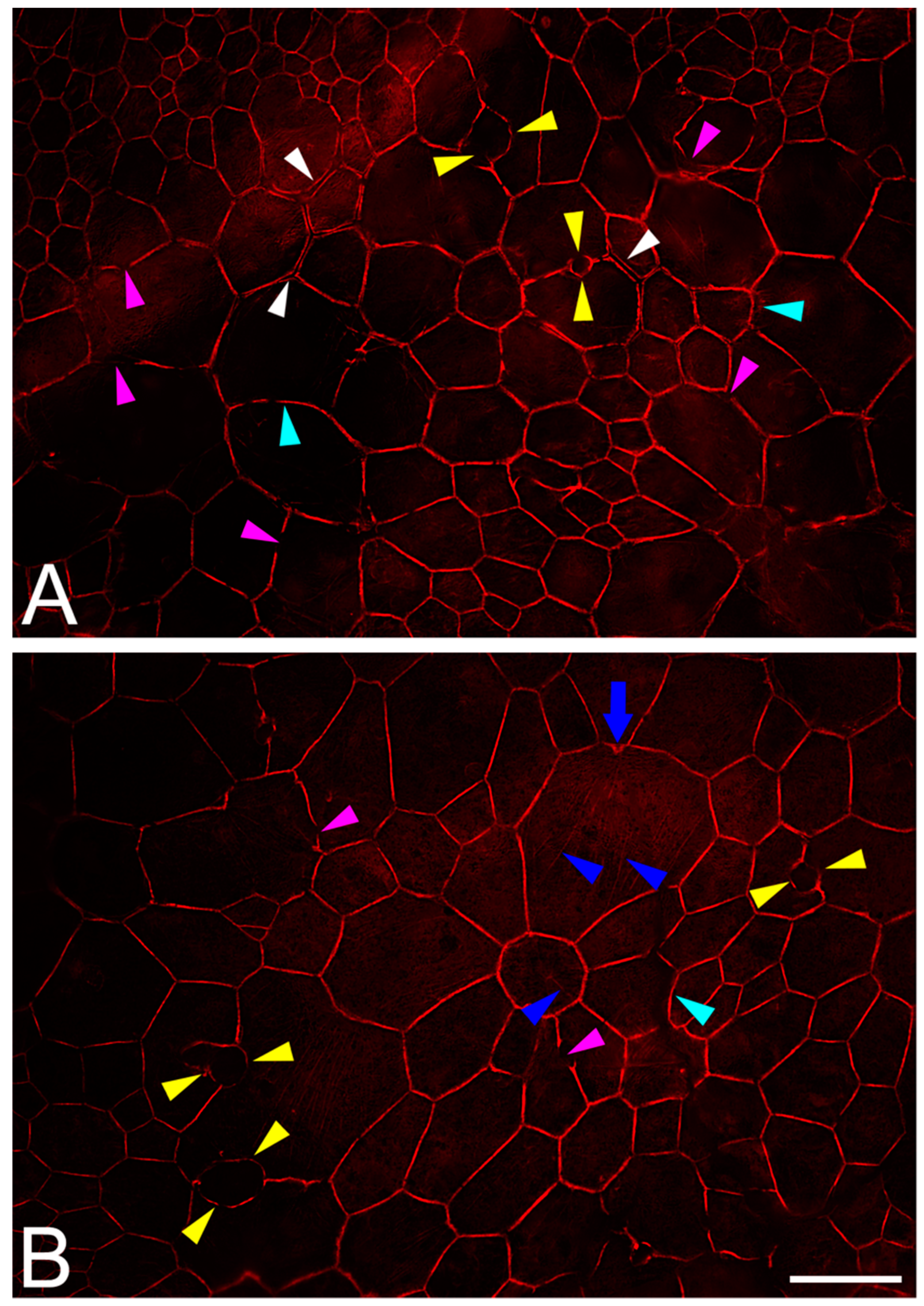
2.7. RPE Cytoskeleton in AMD
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Grierson, I.; Hiscott, P.; Hogg, P.; Robey, H.; Mazure, A.; Larkin, G. Development, repair and regeneration of the retinal pigment epithelium. Eye 1994, 8 Pt 2, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.L.; Benedicto, I.; Philp, N.J.; Rodriguez-Boulan, E. Plasma membrane protein polarity and trafficking in RPE cells: Past, present and future. Exp. Eye Res. 2014, 126, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Palczewski, K. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci. 2016, 2, 197–234. [Google Scholar] [CrossRef] [PubMed]
- Kevany, B.M.; Palczewski, K. Phagocytosis of Retinal Rod and Cone Photoreceptors. Physiology 2010, 25, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Fisher, S.K.; Steinberg, R.H. Mammalian cones: Disc shedding, phagocytosis, and renewal. Investig. Ophthalmol. Vis. Sci. 1978, 17, 117–133. [Google Scholar]
- Marmorstein, A.D. The Polarity of the Retinal Pigment Epithelium. Traffic 2001, 2, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo, L.J. Barrier properties of cultured retinal pigment epithelium. Exp. Eye Res. 2014, 126, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Blaauwgeers, H.G.; Holtkamp, G.M.; Rutten, H.; Witmer, A.N.; Koolwijk, P.; Partanen, T.A.; Alitalo, K.; Kroon, M.E.; Kijlstra, A.; van Hinsbergh, V.W. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris: Evidence for a trophic paracrine relation. Am. J. Pathol. 1999, 155, 421–428. [Google Scholar] [CrossRef]
- Adamis, A.; Shima, D.; Yeo, K.; Yeo, T.; Brown, L.; Berse, B.; Damore, P.; Folkman, J. Synthesis and Secretion of Vascular Permeability Factor/Vascular Endothelial Growth Factor by Human Retinal Pigment Epithelial Cells. Biochem. Biophys. Res. Commun. 1993, 193, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, G.; Kijlstra, A.; Peek, R.; De Vos, A. Retinal Pigment Epithelium-immune System Interactions: Cytokine Production and Cytokine-induced Changes. Prog. Retin. Eye Res. 2001, 20, 29–48. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and Exosomes in the Aged Retinal Pigment Epithelium: Possible Relevance to Drusen Formation and Age-Related Macular Degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef]
- Holtkamp, G.M.; Van Rossem, M.; De Vos, A.F.; Willekens, B.; Peek, R.; Kijlstra, A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin. Exp. Immunol. 1998, 112, 34–43. [Google Scholar] [CrossRef]
- Ablonczy, Z.; Dahrouj, M.; Tang, P.H.; Liu, Y.; Sambamurti, K.; Marmorstein, A.D.; Crosson, C.E. Human Retinal Pigment Epithelium Cells as Functional Models for the RPE In Vivo. Investig. Opthalmology Vis. Sci. 2011, 52, 8614–8620. [Google Scholar] [CrossRef] [PubMed]
- Feeney, L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Investig. Ophthalmol. Vis. Sci. 1978, 17, 583–600. [Google Scholar]
- Pollreisz, A.; Messinger, J.D.; Sloan, K.R.; Mittermueller, T.J.; Weinhandl, A.S.; Benson, E.K.; Kidd, G.J.; Schmidt-Erfurth, U.; Curcio, C.A. Visualizing melanosomes, lipofuscin, and melanolipofuscin in human retinal pigment epithelium using serial block face scanning electron microscopy. Exp. Eye Res. 2018, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Delori, F.C.; Dorey, C.K.; Staurenghi, G.; Arend, O.; Goger, D.G.; Weiter, J.J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investig. Ophthalmol. Vis. Sci. 1995, 36, 718–729. [Google Scholar]
- Schmitz-Valckenberg, S.; Holz, F.G.; Bird, A.C.; Spaide, R.F. Fundus autofluorescence imaging: Review and perspectives. Retina 2008, 28, 385–409. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Curcio, C.A. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: Literature review and model. Retina 2011, 31, 1609. [Google Scholar] [CrossRef]
- Litts, K.M.; Messinger, J.D.; Dellatorre, K.; Yannuzzi, L.A.; Freund, K.B.; Curcio, C.A. Clinicopathological Correlation of Outer Retinal Tubulation in Age-Related Macular Degeneration. JAMA Ophthalmol. 2015, 133, 609. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef] [PubMed]
- Ach, T.; Huisingh, C.; McGwin, G.; Messinger, J.D.; Zhang, T.; Bentley, M.J.; Gutierrez, D.B.; Ablonczy, Z.; Smith, R.T.; Sloan, K.R.; et al. Quantitative Autofluorescence and Cell Density Maps of the Human Retinal Pigment Epithelium. Investig. Opthalmol. Vis. Sci. 2014, 55, 4832–4841. [Google Scholar] [CrossRef] [PubMed]
- Starnes, A.C.; Huisingh, C.; McGwin, G.; Sloan, K.R.; Ablonczy, Z.; Smith, R.T.; Curcio, C.A.; Ach, T. Multi-nucleate retinal pigment epithelium cells of the human macula exhibit a characteristic and highly specific distribution. Vis. Neurosci. 2016, 33, E001. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo, L.J. Development and Role of Tight Junctions in the Retinal Pigment Epithelium. Adv. Appl. Microbiol. 2007, 258, 195–234. [Google Scholar]
- Hudspeth, A.J.; Yee, A.G. The intercellular junctional complexes of retinal pigment epithelia. Investig. Ophthalmol. Vis. Sci. 1973, 12, 354–365. [Google Scholar]
- Rizzolo, L.J.; Peng, S.; Luo, Y.; Xiao, W. Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium. Prog. Retin. Eye Res. 2011, 30, 296–323. [Google Scholar] [CrossRef]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-actin cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a Central Player in Cell Shape and Movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- McKechnie, N.M.; Boulton, M.; Robey, H.L.; Savage, F.J.; Grierson, I. The cytoskeletal elements of human retinal pigment epithelium: In vitro and in vivo. J. Cell Sci. 1988, 91, 303–312. [Google Scholar]
- Owaribe, K.; Franke, W.W.; Kartenbeck, J.; Rungger-Brändle, E. Cytoskeletons of retinal pigment epithelial cells: Interspecies differences of expression patterns indicate independence of cell function from the specific complement of cytoskeletal proteins. Cell Tissue Res. 1988, 254, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, V.L. Retinal pigment epithelium (RPE) cytoskeleton in vivo and in vitro. Exp. Eye Res. 2014, 126, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Dehghani, F. The Cytoskeleton—A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Rotty, J.D.; Bear, J.E. Competition and collaboration between different actin assembly pathways allows for homeostatic control of the actin cytoskeleton. Bioarchitecture 2014, 5, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Achard, V.; Martiel, J.-L.; Michelot, A.; Guérin, C.; Reymann, A.-C.; Blanchoin, L.; Boujemaa-Paterski, R. A “Primer”-Based Mechanism Underlies Branched Actin Filament Network Formation and Motility. Curr. Boil. 2010, 20, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Kawska, A.; Carvalho, K.; Manzi, J.; Boujemaa-Paterski, R.; Blanchoin, L.; Martiel, J.-L.; Sykes, C. How actin network dynamics control the onset of actin-based motility. Proc. Natl. Acad. Sci. USA 2012, 109, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Sept, D.; Xu, J.; Pollard, T.D.; McCammon, J.A. Annealing Accounts for the Length of Actin Filaments Formed by Spontaneous Polymerization. Biophys. J. 1999, 77, 2911–2919. [Google Scholar] [CrossRef]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harbor Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef]
- Bretscher, A.; Weber, K. Villin is a major protein of the microvillus cystoskeleton which binds both G and F actin in a calcium-dependent manner. Cell 1980, 20, 839–847. [Google Scholar] [CrossRef]
- Vasioukhin, V.; Bauer, C.; Yin, M.; Fuchs, E. Directed Actin Polymerization Is the Driving Force for Epithelial Cell–Cell Adhesion. Cell 2000, 100, 209–219. [Google Scholar] [CrossRef]
- Mooseker, M.S.; Kramer, M.F.; Geuze, J.J. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J. Cell Boil. 1975, 67, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Kovar, D.R.; Harris, E.S.; Mahaffy, R.; Higgs, H.N.; Pollard, T.D. Control of the Assembly of ATP- and ADP-Actin by Formins and Profilin. Cell 2006, 124, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Andrianantoandro, E.; Pollard, T.D. Mechanism of Actin Filament Turnover by Severing and Nucleation at Different Concentrations of ADF/Cofilin. Mol. Cell 2006, 24, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Purich, D.L.; Southwick, F.S. Profilin Promotes Barbed-end Actin Filament Assembly without Lowering the Critical Concentration. J. Boil. Chem. 1999, 274, 36963–36972. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, R.S.; Eisenberg, E. Regulation and Kinetics of the Actin-Myosin-ATP Interaction. Annu. Rev. Biochem. 1980, 49, 921–956. [Google Scholar] [CrossRef] [PubMed]
- Finer, J.T.; Simmons, R.M.; Spudich, J.A. Single myosin molecule mechanics: Piconewton forces and nanometre steps. Nature 1994, 368, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.K.; Pardee, J.D. Assembly mechanism of Dictyostelium myosin II: Regulation by K+, Mg2+, and actin filaments. Biochemistry 1996, 35, 15504–15514. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Chauhan, V.P.S.; Elzinga, M. Two Nonmuscle Myosin II Heavy Chain Isoforms Expressed in Rabbit Brains: Filament Forming Properties, the Effects of Phosphorylation by Protein Kinase C and Casein Kinase II, and Location of the Phosphorylation Sites. Biochemistry 1998, 37, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Yumura, S.; Yoshida, M.; Betapudi, V.; Licate, L.S.; Iwadate, Y.; Nagasaki, A.; Uyeda, T.Q.; Egelhoff, T.T.; McIntosh, J.R. Multiple Myosin II Heavy Chain Kinases: Roles in Filament Assembly Control and Proper Cytokinesis in Dictyostelium. Mol. Boil. Cell 2005, 16, 4256–4266. [Google Scholar] [CrossRef]
- Murakami, N.; Kotula, L.; Hwang, Y.-W. Two Distinct Mechanisms for Regulation of Nonmuscle Myosin Assembly via the Heavy Chain: Phosphorylation for MIIB and Mts 1 Binding for MIIA. Biochemistry 2000, 39, 11441–11451. [Google Scholar] [CrossRef]
- Rosenberg, M.; Ravid, S.; Ridley, A. Protein Kinase Cγ Regulates Myosin IIB Phosphorylation, Cellular Localization, and Filament Assembly. Mol. Boil. Cell 2006, 17, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Scholey, J.M.; Taylor, K.A.; Kendrick-Jones, J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature 1980, 287, 233–235. [Google Scholar] [CrossRef] [PubMed]
- E Kamm, K.; Stull, J.T. The Function of Myosin and Myosin Light Chain Kinase Phosphorylation in Smooth Muscle. Annu. Rev. Pharmacol. Toxicol. 1985, 25, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Boil. 2005, 15, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Bendix, P.M.; Koenderink, G.H.; Cuvelier, D.; Dogic, Z.; Koeleman, B.N.; Brieher, W.M.; Field, C.M.; Mahadevan, L.; Weitz, D.A. A Quantitative Analysis of Contractility in Active Cytoskeletal Protein Networks. Biophys. J. 2008, 94, 3126–3136. [Google Scholar] [CrossRef]
- Koenderink, G.H.; Dogic, Z.; Nakamura, F.; Bendix, P.M.; MacKintosh, F.C.; Hartwig, J.H.; Stossel, T.P.; Weitz, D.A. An active biopolymer network controlled by molecular motors. Proc. Natl. Acad. Sci. USA 2009, 106, 15192–15197. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Tharmann, R.; Haase, I.; Fischer, M.; Bausch, A.R. Cytoskeletal polymer networks: The molecular structure of cross-linkers determines macroscopic properties. Proc. Natl. Acad. Sci. USA 2006, 103, 13974–13978. [Google Scholar] [CrossRef]
- Kohler, S.; Schaller, V.; Bausch, A.R. Collective Dynamics of Active Cytoskeletal Networks. PLoS ONE 2011, 6, e23798. [Google Scholar] [CrossRef]
- Ingerman, E.; Hsiao, J.Y.; Mullins, R.D. Arp2/3 complex ATP hydrolysis promotes lamellipodial actin network disassembly but is dispensable for assembly. J. Cell Boil. 2013, 200, 619–633. [Google Scholar] [CrossRef]
- Reymann, A.-C.; Suarez, C.; Guérin, C.; Martiel, J.-L.; Staiger, C.J.; Blanchoin, L.; Boujemaa-Paterski, R. Turnover of branched actin filament networks by stochastic fragmentation with ADF/cofilin. Mol. Boil. Cell 2011, 22, 2541–2550. [Google Scholar] [CrossRef]
- Ngo, K.X.; Kodera, N.; Katayama, E.; Ando, T.; Uyeda, T.Q. Cofilin-induced unidirectional cooperative conformational changes in actin filaments revealed by high-speed atomic force microscopy. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Elam, W.A.; Kang, H.; De La Cruz, E.M. Biophysics of Actin Filament Severing by Cofilin. FEBS Lett. 2013, 587, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Talas, G.; Porter, R.A.; McGrouther, D.A.; Eastwood, M. Balanced mechanical forces and microtubule contribution to fibroblast contraction. J. Cell. Physiol. 1996, 169, 439–447. [Google Scholar] [CrossRef]
- Rudolph, R.; Woodward, M. Spatial orientation of microtubules in contractile fibroblasts in vivo. Anat. Rec. 1978, 191, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J. Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J. Cell Boil. 1984, 99, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Esteve-Rudd, J.; Lopes, V.S.; Diemer, T.; Lillo, C.; Rump, A.; Williams, D.S. Microtubule motors transport phagosomes in the RPE, and lack of KLC1 leads to AMD-like pathogenesis. J. Cell Boil. 2015, 210, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.; Bell, B.; Peachey, N.S.; Daniele, L.L.; Reyes-Reveles, J.; Sharp, R.C.; Jun, B.; Bazan, N.G.; Sparrow, J.R.; Kim, H.J. Microtubule-Associated Protein 1 light Chain 3B,(LC3B) is Necessary to Maintain Lipid-Mediated Homeostasis in the Retinal Pigment Epithelium. Front. Cell. Neurosci. 2018, 12, 351. [Google Scholar] [CrossRef]
- Lee, C.F.; Liu, C.Y.; Hsieh, R.H.; Wei, Y.H. Oxidative stress-induced depolymerization of microtubules and alteration of mitochondrial mass in human cells. Ann. N. Y. Acad. Sci. 2005, 1042, 246–254. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Cytoplasmic Intermediate Filaments in Cell Biology. Annu. Rev. Cell Dev. Boil. 2018, 34, 1–28. [Google Scholar] [CrossRef]
- Chang, L.; Goldman, R.D. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Boil. 2004, 5, 601–613. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Molecular Structure, Assembly Mechanism, and Integration Into Functionally Distinct Intracellular Scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Stamenović, D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am. J. Physiol. Physiol. 2000, 279, C188–C194. [Google Scholar] [CrossRef] [PubMed]
- Godsel, L.M.; Hobbs, R.P.; Green, K.J. Intermediate filament assembly: Dynamics to disease. Trends Cell Boil. 2008, 18, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate Filaments at the Junction of Mechanotransduction, Migration, and Development. Front. Cell Dev. Boil. 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Favre, B.; Schneider, Y.; Lingasamy, P.; Bouameur, J.-E.; Begré, N.; Gontier, Y.; Steiner-Champliaud, M.-F.; Frias, M.A.; Borradori, L.; Fontao, L. Plectin interacts with the rod domain of type III intermediate filament proteins desmin and vimentin. Eur. J. Cell Boil. 2011, 90, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Sripathi, S.R.; He, W.; Sylvester, O.; Neksumi, M.; Um, J.-Y.; Dluya, T.; Bernstein, P.S.; Jahng, W.J.; Sripathi, S.R. Altered Cytoskeleton as a Mitochondrial Decay Signature in the Retinal Pigment Epithelium. Protein J. 2016, 35, 179–192. [Google Scholar] [CrossRef]
- Matveeva, E.A.; Venkova, L.S.; Chernoivanenko, I.S.; Minin, A.A. Vimentin is involved in regulation of mitochondrial motility and membrane potential by Rac1. Boil. Open 2015, 4, 1290–1297. [Google Scholar] [CrossRef]
- Kröger, C.; Loschke, F.; Schwarz, N.; Windoffer, R.; Leube, R.E.; Magin, T.M. Keratins control intercellular adhesion involving PKC-α–mediated desmoplakin phosphorylation. J. Cell Boil. 2013, 201, 681–692. [Google Scholar] [CrossRef]
- Toivola, D.M.; Tao, G.-Z.; Habtezion, A.; Liao, J.; Omary, M.B. Cellular integrity plus: Organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Boil. 2005, 15, 608–617. [Google Scholar] [CrossRef]
- Geisler, F.; Leube, R.E. Epithelial Intermediate Filaments: Guardians against Microbial Infection? Cells 2016, 5, 29. [Google Scholar] [CrossRef]
- Pekny, M.; Lane, E.B. Intermediate filaments and stress. Exp. Cell Res. 2007, 313, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Ramms, L.; Fabris, G.; Windoffer, R.; Schwarz, N.; Springer, R.; Zhou, C.; Lazar, J.; Stiefel, S.; Hersch, N.; Schnakenberg, U. Keratins as the main component for the mechanical integrity of keratinocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 18513–18518. [Google Scholar] [CrossRef] [PubMed]
- Toivola, D.; Strnad, P.; Habtezion, A.; Omary, M. Intermediate filaments take the heat as stress proteins. Trends Cell Boil. 2010, 20, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, U.; Kivelä, T.; Tarkkanen, A. Cytoskeleton in normal and reactive human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3178–3186. [Google Scholar]
- Matsumoto, B.; Guérin, C.J.; Anderson, D.H. Cytoskeletal redifferentiation of feline, monkey, and human RPE cells in culture. Investig. Ophthalmol. Vis. Sci. 1990, 31, 879–889. [Google Scholar]
- Burnside, B.; Laties, A.M. Actin filaments in apical projections of the primate pigmented epithelial cell. Investig. Ophthalmol. 1976, 15, 570–575. [Google Scholar]
- Bretscher, A.; Weber, K. Purification of microvilli and an analysis of the protein components of the microfilament core bundle. Exp. Cell Res. 1978, 116, 397–407. [Google Scholar] [CrossRef]
- Gungor-Ordueri, N.E.; Celik-Ozenci, C.; Cheng, C.Y. Ezrin: A regulator of actin microfilaments in cell junctions of the rat testis. Asian J. Androl. 2015, 17, 653–658. [Google Scholar]
- Law, A.L.; Parinot, C.; Chatagnon, J.; Gravez, B.; Sahel, J.A.; Bhattacharya, S.S.; Nandrot, E.F. Cleavage of Mer tyrosine kinase (MerTK) from the cell surface contributes to the regulation of retinal phagocytosis. J. Biol. Chem. 2015, 290, 4941–4952. [Google Scholar] [CrossRef]
- Stossel, T.P. Phagocytosis. N. Engl. J. Med. 1974, 290, 717–723. [Google Scholar] [CrossRef]
- Simson, J.V.; Spicer, S.S. Activities of specific cell constituents in phagocytosis (endocytosis). Int. Rev. Exp. Pathol. 1973, 12, 79–118. [Google Scholar] [PubMed]
- Malawista, S.E. Microtubules and the mobilization of lysosomes in phagocytizing human leukocytes. Ann. N. Y. Acad. Sci. 1975, 253, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, G.; Goldstein, I.; Hoffstein, S.; Tsung, P.-K. Reciprocal effects of camp and cgmp on microtubule-dependent release of lysosomal enzymes. Ann. N. Y. Acad. Sci. 1975, 253, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.; Kitamoto, J.; Williams, D.S. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc. Natl. Acad. Sci. USA 2003, 100, 6481–6486. [Google Scholar] [CrossRef] [PubMed]
- Barral, D.C.; Seabra, M.C. The Melanosome as a Model to Study Organelle Motility in Mammals. Pigment. Cell Res. 2004, 17, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, T.; O’Connor, M.N.; Seabra, M.C.; Cutler, D.F.; Futter, C.E. Regulation of melanosome number, shape and movement in the zebrafish retinal pigment epithelium by OA1 and PMEL. J. Cell Sci. 2015, 128, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.H.; Wood, I.; Hogan, M.J. Pigment Epithelial Ensheathment and Phagocytosis of Extrafoveal Cones in Human Retina. Philos. Trans. R. Soc. B Boil. Sci. 1977, 277, 459–471. [Google Scholar] [CrossRef]
- Gibbs, D.; Azarian, S.M.; Lillo, C.; Kitamoto, J.; Klomp, A.E.; Libby, R.T.; Steel, K.P.; Williams, D.S. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J. Cell Sci. 2004, 117, 6459–6471. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Rodriguez-Boulan, E. Polarity and developmental regulation of two PDZ proteins in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3274–3282. [Google Scholar]
- Kivelä, T.; Jääskeläine, J.; Vaheri, A.; Carpén, O. Ezrin, a membrane-organizing protein, as a polarization marker of the retinal pigment epithelium in vertebrates. Cell Tissue Res. 2000, 301, 217–223. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Finnemann, S.C.; Rodriguez-Boulan, E. Ezrin Promotes Morphogenesis of Apical Microvilli and Basal Infoldings in Retinal Pigment Epithelium. J. Cell Boil. 1999, 147, 1533–1548. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, M.; West, K.; Possin, D.E.; Crabb, J.W.; Huang, J.; Bretscher, A.; Saari, J.C. Cellular Retinaldehyde-Binding Protein Interacts with ERM-Binding Phosphoprotein 50 in Retinal Pigment Epithelium. Investig. Opthalmol. Vis. Sci. 2004, 45, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Noa, N. Retinoid-binding proteins: Mediators of retinoid action. Biochem. J. 2000, 348, 481–495. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, F.; Kittredge, K.L.; Rayborn, M.E.; Hollyfield, J.G.; Landers, R.A.; Saha, M.; Grainger, R.M. Interphotoreceptor retinoid-binding protein (IRBP), a major 124 kDa glycoprotein in the interphotoreceptor matrix of Xenopus laevis. Characterization, molecular cloning and biosynthesis. J. Cell Sci. 1993, 105, 7–21. [Google Scholar] [PubMed]
- Gonzalez-Fernandez, F.; Ghosh, D. Focus on Molecules: Interphotoreceptor retinoid-binding protein (IRBP). Exp. Eye Res. 2008, 86, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Carson, N.; Simpson, N. A physical map of 13 markers on chromosome-10 from dosage studies on abnormal-cell lines. In Cytogenetics and Cell Genetics; Karger: Basel, Switzerlands, 1989; pp. 974–975. [Google Scholar]
- Maw, M.A.; Kennedy, B.; Knight, A.; Bridges, R.; Roth, K.E.; Mani, E.; Mukkadan, J.; Nancarrow, D.; Crabb, J.W.; Denton, M.J. Mutation of the gene encoding cellular retinaldehyde–binding protein in autosomal recessive retinitis pigmentosa. Nat. Genet. 1997, 17, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Loredo, A.Y.; Lopez-Colome, A.M. New Insights into the Regulation of Myosin Light Chain Phosphorylation in Retinal Pigment Epithelial Cells. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 293, pp. 85–121. [Google Scholar]
- Gunning, P.; O’Neill, G.; Hardeman, E. Tropomyosin-Based Regulation of the Actin Cytoskeleton in Time and Space. Physiol. Rev. 2008, 88, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ciuba, K.; Hawkes, W.; Tojkander, S.; Kogan, K.; Engel, U.; Iskratsch, T.; Lappalainen, P. Calponin-3 is critical for coordinated contractility of actin stress fibers. Sci. Rep. 2018, 8, 17670. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Boil. 2009, 10, 778–790. [Google Scholar] [CrossRef]
- Totsukawa, G.; Yamakita, Y.; Yamashiro, S.; Hartshorne, D.J.; Sasaki, Y.; Matsumura, F. Distinct Roles of Rock (Rho-Kinase) and Mlck in Spatial Regulation of Mlc Phosphorylation for Assembly of Stress Fibers and Focal Adhesions in 3t3 Fibroblasts. J. Cell Boil. 2000, 150, 797–806. [Google Scholar] [CrossRef]
- Maekawa, M. Signaling from Rho to the Actin Cytoskeleton Through Protein Kinases ROCK and LIM-kinase. Sci. 1999, 285, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers—Assembly, dynamics and biological roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Cramer, L.P. Identification of Novel Graded Polarity Actin Filament Bundles in Locomoting Heart Fibroblasts: Implications for the Generation of Motile Force. J. Cell Boil. 1997, 136, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Lazarides, E.; Burridge, K. α-Actinin: Immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell 1975, 6, 289–298. [Google Scholar] [CrossRef]
- Naumanen, P.; Lappalainen, P.; Hotulainen, P. Mechanisms of actin stress fibre assembly. J. Microsc. 2008, 231, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, B.; Salmazo, A.; Djinovic-Carugo, K. α-Actinin structure and regulation. Cell. Mol. Life Sci. 2008, 65, 2688–2701. [Google Scholar] [CrossRef]
- Koenderink, G.H.; Paluch, E.K. Architecture shapes contractility in actomyosin networks. Curr. Opin. Cell Boil. 2018, 50, 79–85. [Google Scholar] [CrossRef]
- Hotulainen, P.; Lappalainen, P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Boil. 2006, 173, 383–394. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Schevzov, G.; Hotulainen, P.; Naumanen, P.; Martin, C.; Gunning, P.W.; Lappalainen, P. A Molecular Pathway for Myosin II Recruitment to Stress Fibers. Curr. Boil. 2011, 21, 539–550. [Google Scholar] [CrossRef]
- Endlich, N.; Schordan, E.; Cohen, C.D.; Kretzler, M.; Lewko, B.; Welsch, T.; Kriz, W.; Otey, C.A.; Endlich, K. Palladin is a dynamic actin-associated protein in podocytes. Kidney Int. 2009, 75, 214–226. [Google Scholar] [CrossRef]
- Schmidt, K.; Nichols, B.J. Functional interdependence between septin and actin cytoskeleton. BMC Cell Boil. 2004, 5, 43. [Google Scholar]
- Burnette, D.T.; Manley, S.; Sengupta, P.; Sougrat, R.; Davidson, M.W.; Kachar, B.; Lippincott-Schwartz, J. A role for actin arcs in the leading-edge advance of migrating cells. Nat. Cell Biol. 2011, 13, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dasbiswas, K.; Guo, Z.; Tee, Y.-H.; Thiagarajan, V.; Hersen, P.; Chew, T.-L.; Safran, S.A.; Zaidel-Bar, R.; Bershadsky, A.D. Long-range self-organization of cytoskeletal myosin II filament stacks. Nat. Cell Biol. 2017, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tojkander, S.; Gateva, G.; Husain, A.; Krishnan, R.; Lappalainen, P. Generation of contractile actomyosin bundles depends on mechanosensitive actin filament assembly and disassembly. eLife 2015, 4, e06126. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Guilluy, C. Focal adhesions, stress fibers and mechanical tension. Exp. Cell Res. 2016, 343, 14–20. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Messinger, J.D.; Sloan, K.R.; Yannuzzi, L.A.; Freund, K.B.; Curcio, C.A. Histology and optical coherence tomographic correlates in drusenoid pigment epithelium detachment in age-related macular degeneration. Ophthalmology 2017, 124, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Elner, S.G.; Elner, V.M. The integrin superfamily and the eye. Investig. Ophthalmol. Vis. Sci. 1996, 37, 696–701. [Google Scholar]
- Yang, X.; Chung, J.-Y.; Rai, U.; Esumi, N. Cadherins in the retinal pigment epithelium (RPE) revisited: P-cadherin is the highly dominant cadherin expressed in human and mouse RPE in vivo. PLoS ONE 2018, 13, e0191279. [Google Scholar] [CrossRef]
- Sorkio, A.; Hongisto, H.; Kaarniranta, K.; Uusitalo, H.; Juuti-Uusitalo, K.; Skottman, H. Structure and Barrier Properties of Human Embryonic Stem Cell–Derived Retinal Pigment Epithelial Cells Are Affected by Extracellular Matrix Protein Coating. Tissue Eng. Part A 2014, 20, 622–634. [Google Scholar] [CrossRef]
- Ooto, S.; Vongkulsiri, S.; Sato, T.; Suzuki, M.; Curcio, C.A.; Spaide, R.F. Outer Retinal Corrugations in Age-Related Macular Degeneration. JAMA Ophthalmol. 2014, 132, 806. [Google Scholar] [CrossRef]
- Tan, A.C.S.; Astroz, P.; Dansingani, K.K.; Slakter, J.S.; Yannuzzi, L.A.; Curcio, C.A.; Freund, K.B. The Evolution of the Plateau, an Optical Coherence Tomography Signature Seen in Geographic Atrophy. Investig. Opthalmol. Vis. Sci. 2017, 58, 2349. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.B.; Bressler, N.M.; Sarks, S.H.; Sarks, J.P. Age-related macular degeneration: Nonneovascular early AMD, intermediate AMD, and geographic atrophy. In Volume 2: Medical Retina; Elsevier Inc.: Amsterdam, The Netherlands, 2005; pp. 1041–1074. [Google Scholar]
- Li, M.; Huisingh, C.; Messinger, J.; Dolz-Marco, R.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Histology of geographic atrophy secondary to age-related macular degeneration: A multilayer approach. Retina 2018, 38, 1937. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, P.; Sheridan, C.; Magee, R.M.; Grierson, I. Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog. Retin. Eye Res. 1999, 18, 167–190. [Google Scholar] [CrossRef]
- Friedlander, M.; Theesfeld, C.L.; Sugita, M.; Fruttiger, M.; Thomas, M.A.; Chang, S.; Cheresh, D.A. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. USA 1996, 93, 9764–9769. [Google Scholar] [CrossRef] [PubMed]
- Ts’O, M.O.; Friedman, E. The retinal pigment epithelium. I. Comparative histology. Arch. Ophthalmol. 1967, 78, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Østerberg, C. Topography ofthe layer of rods and cones in the human retina. Acta Ophthalmol. 1935, 13, 1–102. [Google Scholar]
- Wing, G.L.; Blanchard, G.C.; Weiter, J.J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1978, 17, 601–607. [Google Scholar]
- Gao, H.; Hollyfield, J.G. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Del Priore, L.V.; Kuo, Y.-H.; Tezel, T.H. Age-related changes in human RPE cell density and apoptosis proportion in situ. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3312–3318. [Google Scholar]
- Ach, T.; Tolstik, E.; Messinger, J.D.; Zarubina, A.V.; Heintzmann, R.; Curcio, C.A. Lipofuscin Redistribution and Loss Accompanied by Cytoskeletal Stress in Retinal Pigment Epithelium of Eyes With Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2015, 56, 3242–3252. [Google Scholar] [CrossRef]
- Greferath, U.; Guymer, R.H.; Vessey, K.A.; Brassington, K.; Fletcher, E.L. Correlation of Histologic Features with In Vivo Imaging of Reticular Pseudodrusen. Ophthalmology 2016, 123, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Girouard, M.-P.; Pool, M.; Alchini, R.; Rambaldi, I.; Fournier, A.E. RhoA Proteolysis Regulates the Actin Cytoskeleton in Response to Oxidative Stress. PLoS ONE 2016, 11, 0168641. [Google Scholar] [CrossRef] [PubMed]
- Leri, A.; Claudio, P.P.; Li, Q.; Wang, X.; Reiss, K.; Wang, S.; Malhotra, A.; Kajstura, J.; Anversa, P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J. Clin. Investig. 1998, 101, 1326–1342. [Google Scholar] [CrossRef] [PubMed]
- Mazzitello, K.; Zhang, Q.; Chrenek, M.; Family, F.; Grossniklaus, H.; Nickerson, J.; Jiang, Y. Druse-Induced Morphology Evolution in Retinal Pigment Epithelium. arXiv 2016, arXiv:1609.04496 2016. [Google Scholar]
- Wu, S.; Lu, Q.; Wang, N.; Zhang, J.; Liu, Q.; Gao, M.; Chen, J.; Liu, W.; Xu, L. Cyclic stretch induced-retinal pigment epithelial cell apoptosis and cytokine changes. BMC Ophthalmol. 2017, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.D.; Overby, D.R.; Mannix, R.; Ingber, D.E. Cellular adaptation to mechanical stress: Role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J. Cell Sci. 2006, 119, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Butler, J.; Ingber, D. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef]
- Galbraith, C.; Skalak, R.; Chien, S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil. Cytoskelet. 1998, 40, 317–330. [Google Scholar] [CrossRef]
- Davies, P.F.; Tripathi, S.C. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ. Res. 1993, 72, 239–245. [Google Scholar] [CrossRef]
- Zanzottera, E.C.; Ach, T.; Huisingh, C.; Messinger, J.D.; Spaide, R.F.; Curcio, C.A. Visualizing retinal pigment epithelium phenotypes in the transition to geographic atrophy in age-related macular degeneration. Retina 2016, 36, S12. [Google Scholar] [CrossRef]
- Zanzottera, E.C.; Messinger, J.D.; Ach, T.; Smith, R.T.; Freund, K.B.; Curcio, C.A.; Smith, T. The Project MACULA Retinal Pigment Epithelium Grading System for Histology and Optical Coherence Tomography in Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2015, 56, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Zanzottera, E.C.; Ach, T.; Balaratnasingam, C.; Freund, K.B. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 2017, 58, BIO211–BIO226. [Google Scholar]
- Zanzottera, E.C.; Messinger, J.D.; Ach, T.; Smith, R.T.; Curcio, C.A. Subducted and Melanotic Cells in Advanced Age-Related Macular Degeneration Are Derived From Retinal Pigment Epithelium. Investig. Opthalmol. Vis. Sci. 2015, 56, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.K.; Kholodenko, B.N.; Von Kriegsheim, A. Rac1 and RhoA: Networks, loops and bistability. Small GTPases 2018, 9, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Exp. Med. 2010, 207, 11–19. [Google Scholar] [CrossRef]
- Grisanti, S.; Guidry, C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Investig. Ophthalmol. Vis. Sci. 1995, 36, 391–405. [Google Scholar]
- Ding, J.-D.; Johnson, L.V.; Herrmann, R.; Farsiu, S.; Smith, S.G.; Groelle, M.; Mace, B.E.; Sullivan, P.; Jamison, J.A.; Kelly, U. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, E279–E287. [Google Scholar] [CrossRef]
- Bruban, J.; Glotin, A.-L.; Dinet, V.; Chalour, N.; Sennlaub, F.; Jonet, L.; An, N.; Faussat, A.M.; Mascarelli, F. Amyloid-β(1-42) alters structure and function of retinal pigmented epithelial cells. Aging Cell 2009, 8, 162–177. [Google Scholar] [CrossRef]
- Müller, C.; Charniga, C.; Temple, S.; Finnemann, S.C. Quantified F-Actin Morphology Is Predictive of Phagocytic Capacity of Stem Cell-Derived Retinal Pigment Epithelium. Stem Cell Rep. 2018, 10, 1075–1087. [Google Scholar] [CrossRef]
- Gambril, J.A.; Sloan, K.R.; Swain, T.A.; Huisingh, C.; Zarubina, A.V.; Messinger, J.D.; Ach, T.; Curcio, C.A. Quantifying Retinal Pigment Epithelium Dysmorphia and Loss of Histologic Autofluorescence in Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2019, 60, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.E.; Yang, Q.; Song, H.; Saito, K.; Nozato, K.; Latchney, L.R.; Leonard, B.T.; Chung, M.M.; Williams, D.R.; Rossi, E.A. Human Retinal Pigment Epithelium: In Vivo Cell Morphometry, Multispectral Autofluorescence, and Relationship to Cone Mosaic. Investig. Opthalmol. Vis. Sci. 2018, 59, 5705–5716. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jung, H.; Liu, J.; Droettboom, M.; Tam, J. Noninvasive near infrared autofluorescence imaging of retinal pigment epithelial cells in the human retina using adaptive optics. Biomed. Opt. Express 2017, 8, 4348–4360. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarau, I.-S.; Berlin, A.; Curcio, C.A.; Ach, T. The Cytoskeleton of the Retinal Pigment Epithelium: from Normal Aging to Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3578. https://doi.org/10.3390/ijms20143578
Tarau I-S, Berlin A, Curcio CA, Ach T. The Cytoskeleton of the Retinal Pigment Epithelium: from Normal Aging to Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2019; 20(14):3578. https://doi.org/10.3390/ijms20143578
Chicago/Turabian StyleTarau, Ioana-Sandra, Andreas Berlin, Christine A. Curcio, and Thomas Ach. 2019. "The Cytoskeleton of the Retinal Pigment Epithelium: from Normal Aging to Age-Related Macular Degeneration" International Journal of Molecular Sciences 20, no. 14: 3578. https://doi.org/10.3390/ijms20143578
APA StyleTarau, I.-S., Berlin, A., Curcio, C. A., & Ach, T. (2019). The Cytoskeleton of the Retinal Pigment Epithelium: from Normal Aging to Age-Related Macular Degeneration. International Journal of Molecular Sciences, 20(14), 3578. https://doi.org/10.3390/ijms20143578