Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. In Vitro Experiments
2.1.1. Resveratrol Effect on Cell Viability and Apoptosis
2.1.2. Resveratrol Effect on ROS Production/Oxidative Stress
2.1.3. Resveratrol Effect on Inflammatory Markers
2.1.4. Resveratrol Effect on VEGF
2.1.5. Other Cellular Effects of Resveratrol
2.2. In Vivo Experiments
2.2.1. Resveratrol Effect on Retinal Cell Apoptosis
2.2.2. Resveratrol Effect on Inflammatory Markers
2.2.3. Resveratrol Effect on Oxidative Stress
2.2.4. Resveratrol Effect on ERG Parameters
2.2.5. Resveratrol Effect on Vasculature
2.2.6. Other Effects or Mechanisms
3. Discussion
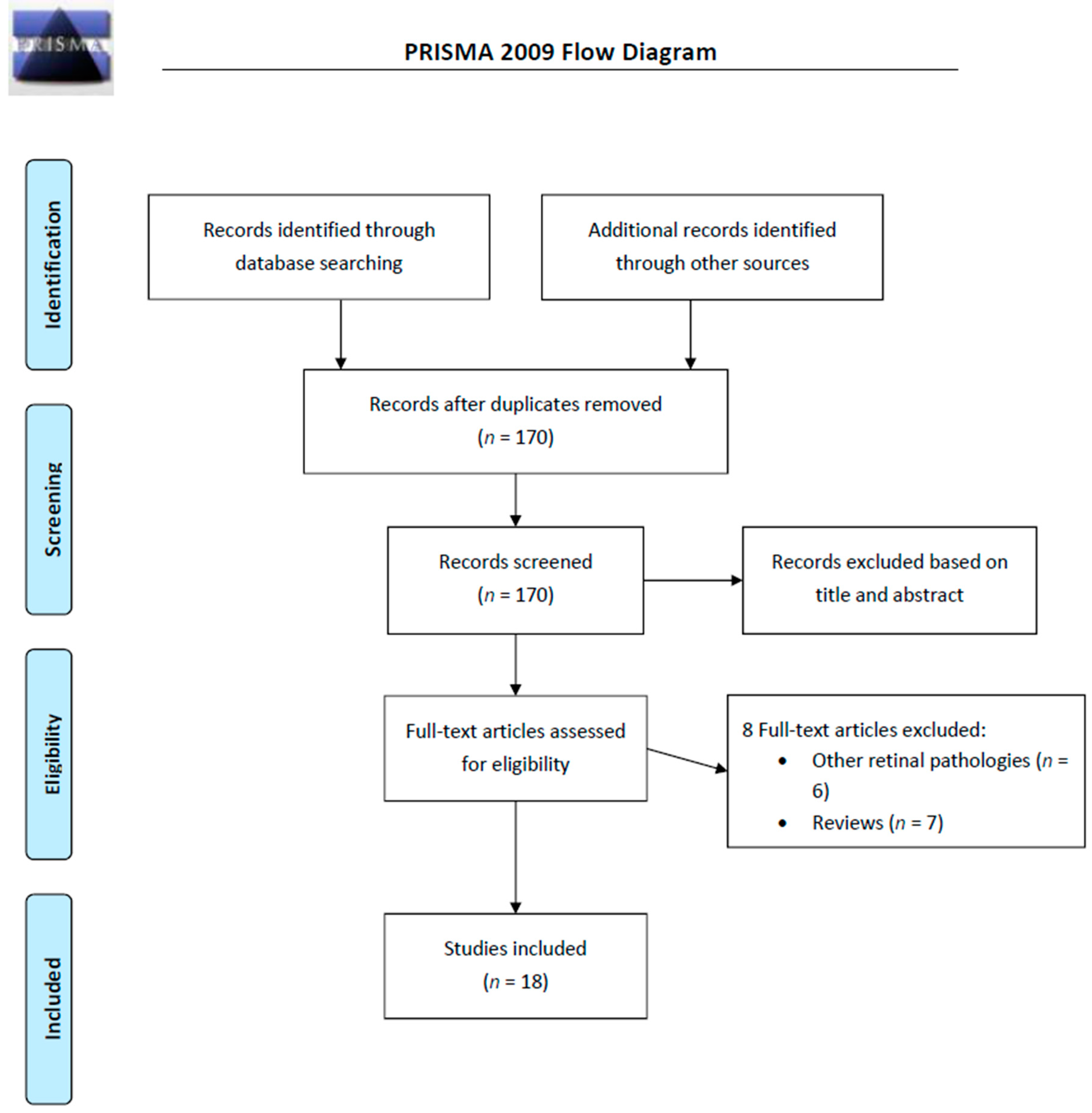
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of resveratrol. Comp. Rev. Food Sci. Food Saf. 2006, 5, 65–70. [Google Scholar] [CrossRef]
- Lyons, M.; Yu, C.; Toma, R.B.; Cho, S.Y.; Reiboldt, W.; Lee, J.; Van Breemen, R.B. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Catane, F.; Yang, Y.; Roderick, R.; Van Breemen, R.B. An LC-MS method for analysing total resveratrol in grape juice, cranberry juice and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cao, R.; Bråkenhielm, E. Antiangiogenic mechanisms of diet-derived polyphenols. J. Nutr. Biochem. 2002, 13, 380–390. [Google Scholar] [CrossRef]
- Bola, C.; Bartlett, H.; Eperjesi, F. Resveratrol and the eye: Activity and molecular mechanisms. Graefes. Arch. Clin. Exp. Ophthalmol. 2014, 252, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Bessis, R.; Maume, B.F.; Meunier, P.; Peyron, D.; Trollat, P. Effect of enological practices on the resveratrol isomer content of wine. J. Agric. Food Chem. 1995, 43, 316–319. [Google Scholar] [CrossRef]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 1–41. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. (Phila.) 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H. Retinal ganglion cell apoptotic pathway in glaucoma: Initiating and downstream mechanisms. Prog. Brain Res. 2015, 220, 37–57. [Google Scholar] [PubMed]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Dib, B.; Lin, H.; Maidana, D.E.; Tian, B.; Miller, J.B.; Bouzika, P.; Miller, J.W.; Vavvas, D.G. Mitochondrial DNA has a pro-inflammatory role in AMD. Biochim. Biophys. Acta 2015, 1853, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Rhone, M.; Basu, A. Phytochemicals and age-related eye diseases. Nutr. Rev. 2008, 66, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and Ophthalmic Diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of Resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic effects of resveratrol. Ann. N. Y. Acad Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, Y.U.; Liu, X. Protective effects of SIRT1 in patients with proliferative diabetic retinopathy via the inhibition of IL-17 expression. Exp. Ther. Med. 2016, 11, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wang, Y.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; et al. Resveratrol Inhibits Diabetic-Induced Muller Cells Apoptosis through MicroRNA-29b/Specificity Protein 1 Pathway. Mol. Neurobiol. 2017, 54, 4000–4014. [Google Scholar] [CrossRef]
- Zeng, K.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; Yang, Y. Resveratrol prevents retinal dysfunction by regulating glutamate transporters, glutamine synthetase expression and activity in diabetic retina. Neurochem. Res. 2016, 41, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, C.W.; Hsieh, M.C.; Wu, H.J.; Wu, W.S.; Wu, W.C.; Kao, Y.H. High mobility group B1 up-regulates angiogenic and fibrogenic factors in human retinal pigment epithelial ARPE-19 cells. Cell. Signal. 2017, 40, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Soufi, F.G.; Mohammad-Nejad, D.; Ahmadieh, H. Resveratrol improves diabetic retinopathy possibly through oxidative stress-nuclear factor kappaB-apoptosis pathway. Pharmacol. Rep. PR 2012, 64, 1505–1514. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Ying, J.; Shi, T.; Wang, P. Resveratrol prevents ROS-induced apoptosis in high glucose-treated retinal capillary endothelial cells via the activation of AMPK/Sirt1/PGC-1alpha pathway. Oxid. Med. Cell. Longev. 2017, 7584691. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Truax, R.E.; Richard, G. Trans-resveratrol inhibits hyperglycemia-induced inflammation and connexin downregulation in retinal pigment epithelial cells. J. Agric. Food Chem. 2010, 58, 8246–8252. [Google Scholar] [CrossRef]
- Subramani, M.; Ponnalagu, M.; Krishna, L.; Jeyabalan, N.; Chevour, P.; Sharma, A.; Jayadev, C.; Shetty, R.; Begum, N.; Archunan, G.; et al. Resveratrol reverses the adverse effects of bevacizumab on cultured ARPE-19 cells. Sci. Rep. 2017, 7, 12242. [Google Scholar] [CrossRef]
- Chan, C.M.; Chang, H.H.; Wang, V.C.; Huang, C.L.; Hung, C.F. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRbeta, PI3K/Akt and MAPK pathways. PLoS ONE 2013, 8, e56819. [Google Scholar]
- Kowluru, R.A.; Santos, J.M.; Zhong, Q. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2014, 55, 5653–5660. [Google Scholar] [CrossRef]
- Michan, S.; Juan, A.M.; Hurst, C.G.; Cui, Z.; Evans, L.P.; Hatton, C.J.; Pei, D.T.; Ju, M.; Sinclair, D.A.; Smith, L.E.; et al. Sirtuin1 over-expression does not impact retinal vascular and neuronal degeneration in a mouse model of oxygen-induced retinopathy. PLoS ONE 2014, 9, e85031. [Google Scholar] [CrossRef]
- Al-Hussaini, H.; Kilarkaje, N. Effects of trans-resveratrol on type 1 diabetes-induced inhibition of retinoic acid metabolism pathway in retinal pigment epithelium of Dark Agouti rats. Eur. J. Pharmacol. 2018, 834, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Yar, A.S.; Menevse, S.; Dogan, I.; Alp, E.; Ergin, V.; Cumaoglu, A.; Aricioglu, A.; Ekmekci, A.; Menevse, A. Investigation of ocular neovascularization-related genes and oxidative stress in diabetic rat eye tissues after resveratrol treatment. J. Med. Food 2012, 15, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Soufi, F.G.; Vardyani, M.; Sheervalilou, R.; Mohammadi, M.; Somi, M.H. Long-term treatment with resveratrol attenuates oxidative stress pro-inflammatory mediators and apoptosis in streptozotocin-nicotinamide-induced diabetic rats. Gener. Physiol. Biophys. 2012, 31, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Ozawa, Y.; Kurihara, T.; Sasaki, M.; Yuki, K.; Miyake, S.; Noda, K.; Ishida, S.; Tsubota, K. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest. Ophthalmol. Vis. Sci. 2011, 52, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Soufi, F.G.; Arbabi-Aval, E.; Rezaei Kanavi, M.; Ahmadieh, H. Anti-inflammatory properties of resveratrol in the retinas of type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, Y.S.; Roh, G.S.; Choi, W.S.; Cho, G.J. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012, 90, e31–e37. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; Bogdan, C.; Pintea, A.; Rugină, D.; Ionescu, C. Antiangiogenic cytokines as potential new therapeutic targets for resveratrol in diabetic retinopathy. Drug Des. Devel. Ther. 2018, 12, 1985–1996. [Google Scholar] [CrossRef]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markò, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Zare Javid, A.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-Zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ruiz Ros, J.A.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Conti, V.; Scapagnini, G.; Filippelli, A.; Ferrara, N. Role of sirtuins, calorie restriction and physical activity in aging. Front. Biosci. 2012, 4, 768–778. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.L.; Cole, K.D.; Plant, A.L. Standards for Cell Line Authentication and Beyond. PLoS Biol. 2016, 14, e1002476. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.J.; Capes-Davis, A.; Davis, J.M.; Downward, J.; Freshney, R.I.; Knezevic, I.; Lovell-Badge, R.; Masters, J.R.; Meredith, J.; Stacey, G.N.; et al. Guidelines for the use of cell lines in biomedical research. Br. J. Cancer 2014, 111, 1021–1046. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Takekoshi, L.; Ramos, A.; Schaler, A.; Weinreb, S.; Blazeski, R.; Mason, C. Retinal pigment epithelial integrity is compromised in the developing albino mouse retina. J. Comp. Neurol. 2016, 524, 3696–3716. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Salinas-Navarro, M.; Jiménez-López, M.; Sobrado-Calvo, P.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Displaced retinal ganglion cells in albino and pigmented rats. Front. Neuroanat. 2014, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, S. Mind the gap-towards complete and transparent reporting of animal research. Med. Writ. 2018, 26, 24–27. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
| Author, Year (Country) | Cells | Origin | Identification of Cells/Authentication of Cell Line | Insult | Resveratrol Concentration (Incubation Time) | Laboratory Techniques | Major Findings |
|---|---|---|---|---|---|---|---|
| Chan Chi-Ming, 2013 (Taiwan, USA) [26] | ARPE-19 | Human cell line (retinal pigment epithelial cells) obtained from Food Industry Research and Development Institute (Hsinchu, Taiwan) | No data | PDGF-BB (20 ng/mL) at 37 °C for 30 minutes | 1, 3 or 10 µM | ECIS migration assay, MTT assay, dot binding assay, WB, in vitro scratch wound healing assay | Resveratrol inhibited PDGF- BB-induced migration and signaling in ARPE19 cells possibly through via PDGFRb, PI3K/Akt and MAPK pathways. Resveratrol had no effect on the RPE cell adhesion to fibronectin. |
| Chang Yo-Chen, 2017 (Taiwan) [21] | ARPE-19 | Human cell line (retinal pigment epithelial cells) obtained from ATCC | No data | CoCl2 (100–1000 μM)—hypoxic mimetic treatment | 20 µM | IP, WB, gelatin zymography, ELISA, RT PCR | Resveratrol reduced hypoxia-induced secretion of HMGB1.Oxidative and hypoxic stresses reduction; angiogenetic and fibrotic changes and tissue remodeling |
| Chen Yuhua, 2019 (China) [20] | Rat retinal endothelial cell (RREC) culture | Primary culture of rat cells | No data | High glucose conditions (30 mM glucose for 7 days) | 10, 50, 100, 200 or 500 μM (24 h) | MTT assay, WB, RT PCR | Incubation with resveratrol did not affect cell viability up to 100 µM in normal glucose concentration conditions. Inflammation suppression and increased expression of PON1 as well as suppression of active caspase-3 upregulation driven by culturing in exposure to elevated glucose levels. |
| Kowluru Renu A., 2014 (USA) [27] | Bovine retinal capillary endothelial cells (BRECs) | Primary culture of bovine cells | No data | H2O2 exposure (250 µM for 1 h) and high glucose conditions (20 mM for 4 days) | 25 µM | IP, RT PCR, WB, enzyme activity assay, ROS assay | Resveratrol ameliorated high glucose-induced inhibition of Sirt1 activity and prevented increase in the acetylation of p65, binding of p65 with MMP-9 promoter and activation of MMP-9. |
| Li Jun, 2017 (China) [23] | Bovine retinal capillary endothelial cells (BRECs) | Primary culture of bovine cells | Expression of Von Willebrand factor (IHC) | High glucose conditions (30mM glucose) | 1, 5, 10 or 20μM (48 h) | Flow cytometry, RT PCR, WB | Reduction of high glucose-induced intracellular ROS elevation through the activation of AMPK/Sirt1/PGC-1α pathway and apoptosis suppression. |
| Liu Shulin, 2016 (China) [17] | Peripheral blood mononuclear cells (PBMCs) | 19 patients with proliferative diabetic retinopathy and 20 controls | No data | No insult | 10 µM (72 h) | ELISA, WB, RT PCR | IL-17 expression was upregulated and SIRT1 expression levels were decreased in the PBMCs of patients with proliferative diabetic retinopathy |
| Losso Jack, 2010 (USA) [24] | ARPE-19 | Human cell line (retinal pigment epithelial cells) obtained from ATCC | No data | High glucose conditions (33 mM glucose) | 1.25, 2.5, 5, 10 µM (9 days) | crystal violet cell viability assay, ELISA, WB, scrape-Loading/dye transfer assay | Inhibitory effect on hyperglycemia-induced inflammation in retinal pigment epithelial cells: ameliorated decreased GJIC, secretion of cytokines IL-6 and IL-8, downregulation of Cx43, activation of TGF-β,PKCβ, and COX-2. |
| Shen Hongjie, 2015 (China) [22] | hRECs (Human Retinal Endothelial Cells) | Human cell line (retinal pigment epithelial cells) obtained from Angio-Proteomie (USA) | No data | High glucose conditions (33 mM glucose for 72 h) | homologous derivative of resveratrol, pterostilbene 1 mM (72 hours) | MTT assay, ELISA, enzyme activity assay, ROS assay | Regulation of oxidation balance by decreasing inflammation, and further regulation retinal cells over proliferation to delay diabetic retinopathy progress. |
| Subramani Murali, 2017 (India) [25] | ARPE-19 | Human cell line (retinal pigment epithelial cells) obtained from Karolinska Institute, Sweden | No data | Bevacizumab (0.25 mg/ml for 2 h) | 100 µM (48 h) | Trypan blue assay, MTT assay, FLICA, RT PCR, BrdU assay, IHC, WB, scratch assay | Downregulation of VEGFR-2 and its activation, reduction by 50% of VEGF-A, decrease in the proliferation of cultured RPE cells, restoring the membrane integrity of blood-retinal barrier |
| Zeng Kaihong, 2016 (China) [19] | Rat Müller cells | Primary culture of rat cells | Müller cells were identified by expression of glutamine synthetase, vimentin and glutamate transporter (IHC) | High glucose conditions (25 mM for at least 3 days) | 10, 20 or 30 mM (for at least 3 days) | Glutamate uptake assay, enzymatic activity assay, IHC, RT PCR | Resveratrol prevented high glucose –induced decrease of glutamate transporters (GLAST) expression and decrease in glutamate uptake. |
| Zeng Kaihong, 2017 (China) [18] | Rat Müller cells | Primary culture of rat cells | Müller cells were identified by expression of glutamine synthetase, vimentin and glutamate transporter (IHC) | High glucose conditions (25 mM for at least 3 days) | 10, 20 or 30 mM (for at least 3 days) | RT PCR, enzymatic activity assay, IHC | Resveratrol prevented high glucose-induced retinal Müller cells apoptosis via microRNA-29b (miR-29b): decreased Bax and specificity protein 1 (SP1) expression and increased Bcl-2. miR-29b inhibitor reversed the anti-apoptotic effect of resveratrol. |
| Al-Hussaini et al. [29] | Chen et al. [20] | Kim et al. [34] | Kubota et al. [32] | Michan et al. [28] | Soufi et al. [22] | Soufi et al. [31] | Soufi et al. [33] | Yar et al. [30] | Zeng et al. [19] | Zeng et al. [18] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Random allocation | No data | No data | No data | No data | No data | Yes | Yes | Yes | Yes | Yes | Yes |
| Blinding of outcome assessment | No data | No data | No data | No data | Yes | No data | No data | No data | No data | No data | No data |
| Sample size calculation | No | No | No | No | No | No | No | No | No | No | No |
| Author, Year (Country) | Animals | Sample Size (n) | Animal Model | Resveratrol Dosing) | Follow Up | Laboratory Techniques | Tissues Studied | Major Findings |
|---|---|---|---|---|---|---|---|---|
| Al-Hussaini Heba 2018 (Kuwait) [29] | Male Dark Agouti rats (16 weeks old) | n = 5, n = 6 or n = 10 per group | Streptozotocin (60 mg/kg body weight; single, ip) | 5 mg/kg; i.p., daily (6 days a weeks) starting from day 2 after STZ till the end of experiment | 14 days or 30 days | RT PCR, microarrays, WB | RPE | Exageration of type 1 diabetes-induced gene inhibition (normalization of diabetes-induced decreases in expressions of Lpl, Rdh12, Aldh1a3, Cralbp1, Cralbp2 but not Lpl, Lrat, Rdh5, Rdh10, RPE65, Rlbp1, and Rbp1 genes). Long term (30 days) but not short term (14 days) supplementation upregulated transcription of key retinoic acid metabolism pathway enzymes |
| Chen Yuhua, 2019 (China) [20] | Male Sprague-Dawley rats (14 weeks old) | n = 5 or n = 4 per group | Streptozotocin (60 mg/kg body weight; single, iv) | intravitreal injection 2 wks after STZ (0.1 μg/mL or 1 μg/mL in one eye) or daily intravenous injections (5, 10 or 50 μg/kg/d) for 12 weeks. | 12 weeks | RT PCR, WB, enzymatic activity assay, ELISA | Whole retina | Inhibition of apoptosis (lower expression of active capsase 3), reduction of inflammation: reduced inflammatory factors, reduced ox-LDL. Inflamation suppression might be driven by increased expression and activity of PON1. |
| Kim Young Hee, 2012 (Korea) [34] | Male C57BL⁄ 6 mice (8 weeks old) | n = 4 per group | Streptozotocin (55 mg/kg body weight; for 5 consecutive days, ip) | Oral administration one month after the last injection of STZ, 20 mg/kg once daily for 4 weeks | 8 weeks | Fluorescein angiography, Evans blue BBB leakage assay, IHC, WB, ELISA | Whole retina | Blockage of diabetes-induced early vascular lesions and pericyte loss. Blockage of diabetes-induced increase of VEGF expression. |
| Kubota Shunsuke, 2011 (Japan) [32] | C57BL/6 mice (6 weeks old, sex not specified) | n = 7 or n = 8 per group | Streptozotocin (60 mg/kg body weight; for 5 days, ip) | Oral administration (by gastric intubation) seven weeks after the first injection of STZ, 50 mg/kg daily for 7 days | WB, ELISA, enzymatic activity assay, perfusion labeling | Whole retina | Anti-inflammatory effects, suppression of leukocyte adhesion to the retinal vasculature Normalization of diabetes-induced AMPK deactivation, recovery of SIRT1 activity. Suppression of diabetes-induced upregulation of NF- κB signaling by activating the AMPK pathway. Suppression of leukocyte adhesion to the retinal vasculature. | |
| Michan Shaday, 2014 (USA, Mexico, Australia) [28] | Nestin-Cre mice, Tie2-Cre mice, and C57Bl/6J mice (neonatal, both sexes?) | n = 6 or n = 8–20 per group | Oxygen induced retinopathy (neonatal mice exposed to 75% oxygen from P7 to P12) | Oral administration, micronized formulation of resveratrol, SRT501 (400 mg/kg body weight) given daily from P5 to P17. | 13 days | IHC, HE staining, RT PCR, WB | Whole retina | Increase of vaso-obliteration and no significant differences in pathologic neovascularization, (although there was a trend toward suppressing). Resveratrol did not show protective effects against the development of retinopathy. |
| Soufi Farhad Ghadiri, 2012 (Iran) [31] | Male Wistar rats (320–350g) | 48 | Streptozotocin (50 mg/kg body weight single, ip) | Oral administration 5/mg/kg/day | 4 months | TBARS assay, ELISA | Whole retina | Anti-hyperglycemic and antioxidant effects, reduction of inflammatory mediators (TNFα, IL-6 and NF-κB). Reversion of apoptosis. Prevention from disarrangement and reduction in thickness of retinal layers. |
| Soufi Farhad Ghadiri, 2012 (Iran) [22] | Male Wistar rats (320–350g) | 48 | Streptozotocin (50 mg/kg body weight; single, ip) | Oral administration 5/mg/kg/day for 4 months | 4 months | HE staining ELISA, enzymatic assay | Whole retina | Reduction of apoptosis, oxidative stress and anti-hyperglycemic effect with decrease of inflammation (prevents STZ-induced activation of NF-κB). |
| Soufi Farhad Ghadiri, 2015 (Iran) [33] | Male Wistar rats (12-week old, 320-350 g) | n =6 or n=12 per group | Streptozotocin (50 mg/kg body weight; single, ip) | Oral administration 5 mg/kg/day for 4 months | 4 months | RT PCR, ELISA | Whole retina | Inhibition of STZ-induced enhancement of retinal apoptosis and upregulation of pro-inflammatory mediators (TNF-a, IL-6 and COX-2), reduction of STZ-induced retinal NF-κB activity and mRNA expression. |
| Yar Seda Atiye, 2012 (Turkey) [30] | Male Wistar rats (3-month old, 250-300g) | n = 24 (n = 6 per group) | Streptozotocin (55 mg/kg body weight; single, ip) | Oral administration 10 mg/kg/day for 4 weeks starting 4 weeks after STZ | 8 weeks | RT PCR, biochemical measurements | Eye tissues | Suppression the expression of eNOS, but mRNA levels of VEGF, MMP-9, and ACE genes associated with vascular remodeling did not change significantly. |
| Zeng Kaihong, 2016 (China) [19] | Sprague–Dawley rats (14-week old, sex not specified) | n = 408 (n = 68 per group) | Streptozotocin (60 mg/kg body weight; single, ip) | Oral administration 5 or 10 mg/kg/day for 1, 3 5 or 7 months | 1, 3 5 or 7 months | ERG, RT PCR, WT | Whole retina | Attenuation diabetes-induced decreases in amplitude of a-wave in rod response, a- and b-wave in cone and rod response or OP2 in oscillatory potentials, significantly repressed diabetes-induced delay in OP2 implicit times in scotopic 3.0 OPS test. Upregulation of glutamate transporters (GLAST) and glutamine synthetase (GS). |
| Zeng Kaihong, 2017 (China) [18] | Male Sprague–Dawley rats (14-week old, sex not specified) | n = 408 (n = 68 per group) | Streptozotocin (60 mg/kg body weight; single, ip) | Oral administration 5 or 10 mg/kg/day starting 3 days after STZ | 1, 3 5 or 7 months | TUNEL staining, IHC, RT PCR, WB, caspase-3 assay | Whole retina | Suppression of the elevated levels of plasma glucose and fructosamine in STZ-treated rats. Suppresion of STZ-induced retinal cells apoptosis. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, M.D.; Nowomiejska, K.; Avitabile, T.; Rejdak, R.; Tripodi, S.; Porta, A.; Reibaldi, M.; Figus, M.; Posarelli, C.; Fiedorowicz, M. Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 3503. https://doi.org/10.3390/ijms20143503
Toro MD, Nowomiejska K, Avitabile T, Rejdak R, Tripodi S, Porta A, Reibaldi M, Figus M, Posarelli C, Fiedorowicz M. Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review. International Journal of Molecular Sciences. 2019; 20(14):3503. https://doi.org/10.3390/ijms20143503
Chicago/Turabian StyleToro, Mario D., Katarzyna Nowomiejska, Teresio Avitabile, Robert Rejdak, Sarah Tripodi, Alessandro Porta, Michele Reibaldi, Michele Figus, Chiara Posarelli, and Michal Fiedorowicz. 2019. "Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review" International Journal of Molecular Sciences 20, no. 14: 3503. https://doi.org/10.3390/ijms20143503
APA StyleToro, M. D., Nowomiejska, K., Avitabile, T., Rejdak, R., Tripodi, S., Porta, A., Reibaldi, M., Figus, M., Posarelli, C., & Fiedorowicz, M. (2019). Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review. International Journal of Molecular Sciences, 20(14), 3503. https://doi.org/10.3390/ijms20143503









