Biofilms: The Microbial “Protective Clothing” in Extreme Environments
Abstract
1. Introduction
2. Microbial Biofilms
3. Biofilms in Extreme Environments
3.1. Biofilm in UV Radiation
3.2. Biofilm in Extreme Temperature
3.3. Biofilm in Extreme pH Environments
3.4. Biofilm in High-Salinity Environments
3.5. Biofilm in High-Pressure Environments
3.6. Biofilm in Oligotrophic Conditions
3.7. Tolerance and Resistance to Antibiotics in Biofilms
4. Regulation of Biofilms in Extreme Environments
4.1. Quorum Sensing-Based Signaling
4.2. Nucleotide Second Messenger-Based Signaling
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Capalash, N.; Sharma, P. Communication mechanisms in extremophiles: Exploring their existence and industrial applications. Microbiol. Res. 2019, 221, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Blanco, Y.; Rivas, L.A.; Gonzalez-Toril, E.; Ruiz-Bermejo, M.; Moreno-Paz, M.; Parro, V.; Palacin, A.; Aguilera, A.; Puente-Sanchez, F. Environmental parameters, and not phylogeny, determine the composition of extracellular polymeric substances in microbial mats from extreme environments. Sci. Total Environ. 2019, 650, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Gabani, P.; Singh, O.V. Radiation-resistant extremophiles and their potential in biotechnology and therapeutics. Appl. Microbiol. Biotechnol. 2013, 97, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Wakai, S. Biochemical and thermodynamic analyses of energy conversion in extremophiles. Biosci. Biotechnol. Biochem. 2019, 83, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Frols, S. Archaeal biofilms: Widespread and complex. Biochem. Soc. Trans. 2013, 41, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Huang, H.; Peng, C.; Peng, P.; Lin, Y.; Zhang, X.; Ren, H. Towards the biofilm characterization and regulation in biological wastewater treatment. Appl. Microbiol. Biotechnol. 2018, 103, 1115–1129. [Google Scholar] [CrossRef]
- Hoiby, N. A personal history of research on microbial biofilms and biofilm infections. Pathog. Dis. 2014, 70, 205–211. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Watnick, P.; Kolter, R. Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B. Filamentous microfossils in a 3235-million-year-old volcanogenic massive sulphide deposit. Nature 2000, 405, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Westall, F.; de Wit, M.J.; Dann, J.; van der Gaast, S.; de Ronde, C.E.J.; Gerneke, D. Early Archean fossil bacteria and biofilms in hydrothermally-influenced sediments from the Barberton greenstone belt, South Africa. Precambrian Res. 2001, 106, 93–116. [Google Scholar] [CrossRef]
- Taylor, C.D.; Wirsen, C.O.; Gaill, F. Rapid microbial production of filamentous sulfur mats at hydrothermal vents. Appl. Environ. Microbiol. 1999, 65, 2253–2255. [Google Scholar] [PubMed]
- Reysenbach, A.L.; Cady, S.L. Microbiology of ancient and modern hydrothermal systems. Trends Microbiol. 2001, 9, 79–86. [Google Scholar] [CrossRef]
- Koerdt, A.; Godeke, J.; Berger, J.; Thormann, K.M.; Albers, S.V. Crenarchaeal biofilm formation under extreme conditions. PLoS ONE 2010, 5, e14104. [Google Scholar] [CrossRef]
- Rinaudi, L.V.; Giordano, W. An integrated view of biofilm formation in rhizobia. FEMS Microbiol. Lett. 2010, 304, 1–11. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R. Biofilms: Microbial strategies for surviving UV exposure. Adv. Exp. Med. Biol. 2017, 996, 233–239. [Google Scholar]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef]
- Norwood, D.E.; Gilmour, A. The differential adherence capabilities of two Listeria monocytogenes strains in monoculture and multispecies biofilms as a function of temperature. Lett. Appl. Microbiol. 2001, 33, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Hostacká, A.; Ciznár, I.; Stefkovicová, M. Temperature and pH affect the production of bacterial biofilm. Folia Microbiol. 2010, 55, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.H.; Chong, T.H. Physiological responses of salinity-stressed Vibrio sp. and the effect on the biofilm formation on a nanofiltration membrane. Environ. Sci. Technol. 2017, 51, 1249–1258. [Google Scholar] [CrossRef]
- Hou, J.; Veeregowda, D.H.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C. Extracellular polymeric matrix production and relaxation under fluid shear and mechanical pressure in Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2018, 84, e01516-17. [Google Scholar] [CrossRef] [PubMed]
- Marsden, A.E.; Grudzinski, K.; Ondrey, J.M.; DeLoney-Marino, C.R.; Visick, K.L. Impact of salt and nutrient content on biofilm formation by Vibrio fischeri. PLoS ONE 2017, 12, e0169521. [Google Scholar] [CrossRef]
- Hathroubi, S.; Mekni, M.A.; Domenico, P.; Nguyen, D.; Jacques, M. Biofilms: Microbial shelters against antibiotics. Microb. Drug Resist. 2017, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J.H. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef]
- Van Houdt, R.; Michiels, C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.C.; Pritchard, M.F.; Ferguson, E.L.; Powell, K.A.; Patel, S.U.; Rye, P.D.; Sakellakou, S.M.; Buurma, N.J.; Brilliant, C.D.; Copping, J.M.; et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbi. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.D. Current and recent advanced strategies for combating biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; EI Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. Biomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formation. Pathogens 2014, 3, 596–632. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 1–19. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Dufour, D.; Leung, V.; Lévesque, C.M. Bacterial biofilm: Structure, function, and antimicrobial resistance. Endod. Top. 2012, 22, 2–16. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Greinert, R.; Volkmer, B.; Henning, S.; Breitbart, E.W.; Greulich, K.O.; Cardoso, M.C.; Rapp, A. UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res. 2012, 40, 10263–10273. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.S.; Graindorge, D.; Martineau, S.; Machon, C.; Arnoux, P.; Guitton, J.; Francesconi, S.; Frochot, C.; Sage, E.; Girard, P.M. Singlet oxygen-mediated oxidation during UVA radiation alters the dynamic of genomic DNA replication. PLoS ONE 2015, 10, e0140645. [Google Scholar]
- Sorg, O.; Tran, C.; Carraux, P.; Grand, D.; Hugin, A.; Didierjean, L.; Saurat, J.H. Spectral properties of topical retinoids prevent DNA damage and apoptosis after acute UV-B exposure in hairless mice. Photochem. Photobiol. 2005, 81, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J. Effects of ultraviolet radiation on DNA. In Chromosomal Alterations; Obe, G., Natarajan, A.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 39–53. [Google Scholar]
- Elasri, M.O.; Miller, R.V. A Pseudomonas aeruginosa biosensor responds to exposure to ultraviolet radiation. Appl. Microbiol. Biotechnol. 1998, 50, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Elasri, M.O.; Miller, R.V. Study of the response of a biofilm bacterial community to UV radiation. Appl. Environ. Microb. 1999, 65, 2025–2031. [Google Scholar]
- Bernbom, N.; Vogel, B.F.; Gram, L. Listeria monocytogenes survival of UV-C radiation is enhanced by presence of sodium chloride, organic food material and by bacterial biofilm formation. Int. J. Food Microbiol. 2011, 147, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, N.T.; Anda, D.; Borsodi, A.K.; Szabó, A.; Pál, S.E.; Óvári, M.; Márialigeti, K.; Kovács-Bodor, P.; Mádl-Szőnyi, J.; Makk, J. Radioactive environment adapted bacterial communities constituting the biofilms of hydrothermal spring caves (Budapest, Hungary). J. Environ. Radioact. 2019, 203, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Omelchenko, M.V.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Lapidus, A.; Copeland, A.; Kim, E.; Land, M.; et al. Deinococcus geothermalis: The pool of extreme radiation resistance genes shrinks. PLoS ONE 2007, 2, e955. [Google Scholar] [CrossRef] [PubMed]
- Frosler, J.; Panitz, C.; Wingender, J.; Flemming, H.C.; Rettberg, P. Survival of Deinococcus geothermalis in biofilms under desiccation and simulated space and martian conditions. Astrobiology 2017, 17, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Cihan, A.C.; Karaca, B.; Ozel, B.P.; Kilic, T. Determination of the bioflm production capacities and characteristics of members belonging to Bacillaceae family. World J. Microbiol. Biotechnol. 2017, 33, 118. [Google Scholar] [CrossRef] [PubMed]
- Inskeep, W.P.; Macur, R.E.; Harrison, G.; Bostick, B.C.; Fendorf, S. Biomineralization of as (V)-hydrous ferric oxyhydroxide in microbial mats of an acid-sulfate-chloride geothermal spring, Yellowstone National Park. Geochim. Cosmochim. Acta 2004, 68, 3141–3155. [Google Scholar] [CrossRef]
- Macur, R.E.; Langner, H.W.; Kocar, B.D.; Inskeep, W.P. Linking geochemical processes with microbial community analysis: Successional dynamics in an arsenic-rich, acid-sulphate-chloride geothermal spring. Geobiology 2004, 2, 163–177. [Google Scholar] [CrossRef]
- Kelley, J.I.; Turng, B.F.; Williams, H.N.; Baer, M.L. Effects of temperature, salinity, and substrate on the colonization of surfaces in situ by aquatic bdellovibrios. Appl. Environ. Microbiol. 1997, 63, 84–90. [Google Scholar] [PubMed]
- Williams, H.N.; Turng, B.F.; Kelley, J.I. Survival response of Bacteriovorax in surface biofilm versus suspension when stressed by extremes in environmental conditions. Microb. Ecol. 2009, 58, 474–484. [Google Scholar] [CrossRef]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Donato, P.D.; Finore, I.; Nicolaus, B.; Marco, G.D.; Michaud, L.; Giudice, A.L. Production and biotechnological potential of extracellular polymeric substances from sponge-associated antarctic bacteria. Appl. Environ. Microb. 2018, 84, e01624-17. [Google Scholar] [CrossRef]
- Hujslova, M.; Bystriansky, L.; Benada, O.; Gryndler, M. Fungi, a neglected component of acidophilic biofilms: Do they have a potential for biotechnology? Extremophiles 2019, 23, 267–275. [Google Scholar] [CrossRef]
- Li, T.; Sharp, C.E.; Ataeian, M.; Strous, M.; de Beer, D. Role of extracellular carbonic anhydrase in dissolved inorganic carbon uptake in alkaliphilic phototrophic biofilm. Front. Microbiol. 2018, 9, 2490. [Google Scholar] [CrossRef]
- Li, X.; Kappler, U.; Jiang, G.; Bond, P.L. The ecology of acidophilic microorganisms in the corroding concrete sewer environment. Front. Microbiol. 2017, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Bellenberg, S.; Huynh, D.; Poetsch, A.; Sand, W.; Vera, M. Proteomics reveal enhanced oxidative stress responses and metabolic adaptation in Acidithiobacillus ferrooxidans biofilm cells on pyrite. Front. Microbiol. 2019, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Souza-Egipsy, V.; Martín-Uriz, P.S.; Amils, R. Extracellular matrix assembly in extreme acidic eukaryotic biofilms and their possible implications in heavy metal adsorption. Aquat. Toxicol. 2008, 88, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.T.; Poyner, D.R.; Jackson, T.R.; Letcher, A.J.; Lander, D.A.; Irvine, R.F. Inhibition of iron-catalysed hydroxyl radical formation by inositol polyphosphates: A possible physiological function for myo-inositol hexakisphosphate. Biochem. J. 1993, 294, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Ray, A.; Garg, N.; Madamwar, D. Characterization of the extracellular polysaccharide produced by a marine cyanobacterium, Cyanothece sp. ATCC 51142, and its exploitation toward metal removal from solutions. Curr. Microbiol. 2000, 40, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.J.; Rout, S.P.; Garratt, E.J.; Patel, K.; Laws, A.P.; Humphreys, P.N. The enrichment of an alkaliphilic biofilm consortia capable of the anaerobic degradation of isosaccharinic acid from cellulosic materials incubated within an anthropogenic, hyperalkaline environment. FEMS Microbiol. Ecol. 2015, 91, fiv085. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.P.; Payne, L.; Walker, S.; Scott, T.; Heard, P.; Eccles, H.; Bond, G.; Shah, P.; Bills, P.; Jackson, B.R.; et al. The impact of alkaliphilic biofilm formation on the release and retention of carbon isotopes from nuclear reactor graphite. Sci. Rep. 2018, 8, 4455. [Google Scholar] [CrossRef]
- Charles, C.J.; Rout, S.P.; Patel, K.A.; Akbar, S.; Laws, A.P.; Jackson, B.R.; Boxall, S.A.; Humphreys, P.N. Floc formation reduces the pH stress experienced by microorganisms living in alkaline environments. Appl. Environ. Microbiol. 2017, 83, e02985-16. [Google Scholar] [CrossRef]
- Chávez de Paz, L.E.; Bergenholtz, G.; Dahlén, G.; Svensäter, G. Response to alkaline stress by root canal bacteria in biofilms. Int. Endod. J. 2007, 40, 344–355. [Google Scholar]
- Van der Waal, S.V.; van der Sluis, L.W.; Özok, A.R.; Exterkate, R.A.; van Marle, J.; Wesselink, P.R.; de Soet, J.J. The effects of hyperosmosis or high pH on a dual-species biofilm of Enterococcus faecalis and Pseudomonas aeruginosa: An in vitro study. Int. Endod. J. 2011, 44, 1110–1117. [Google Scholar] [CrossRef]
- Vyrides, I.; Stuckey, D.C. Adaptation of anaerobic biomass to saline conditions: Role of compatible solutes and extracellular polysaccharides. Enzyme Microb. Technol. 2009, 44, 46–51. [Google Scholar] [CrossRef]
- Kimata-Kino, N.; Ikeda, S.; Kurosawa, N.; Toda, T. Saline adaptation of granules in mesophilic UASB reactors. Int. Biodeter. Biodegr. 2011, 65, 65–72. [Google Scholar] [CrossRef]
- Gagliano, M.C.; Ismail, S.B.; Stams, A.J.M.; Plugge, C.M.; Temmink, H.; Van Lier, J.B. Biofilm formation and granule properties in anaerobic digestion at high salinity. Water Res. 2017, 121, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, J.; Otlewska, A.; Gutarowska, B. Halophilic microbial communities in deteriorated buildings. World J. Microb. Biot. 2015, 31, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Amjres, H.; Bejar, V.; Quesada, E.; Carranza, D.; Abrini, J.; Sinquin, C.; Ratiskol, J.; Colliec-Jouault, S.; Llamas, I. Characterization of haloglycan, an exopolysaccharide produced by Halomonas stenophila HK30. Int. J. Biol. Macromol. 2015, 72, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610–611, 1239–1250. [Google Scholar] [CrossRef]
- Zhao, L.; She, Z.; Jin, C.; Yang, S.; Guo, L.; Zhao, Y.; Gao, M. Characteristics of extracellular polymeric substances from sludge and biofilm in a simultaneous nitrification and denitrification system under high salinity stress. Bioprocess Biosyst. Eng. 2016, 39, 1375–1389. [Google Scholar] [CrossRef]
- You, G.; Hou, J.; Xu, Y.; Wang, C.; Wang, P.; Miao, L.; Ao, Y.; Li, Y.; Lv, B. Effects of CeO2 nanoparticles on production and physicochemical characteristics of extracellular polymeric substances in biofilms in sequencing batch biofilm reactor. Bioresour. Technol. 2015, 194, 91–98. [Google Scholar] [CrossRef]
- Kato, C.; Qureshi, M.H. Pressure response in deep-sea piezophilic bacteria. J. Molec. Microbiol. Biotechnol. 1999, 1, 87–92. [Google Scholar]
- Simonato, F.; Campanaro, S.; Lauro, F.M.; Vezzi, A.; D’Angelo, M.; Vitulo, N.; Valle, G.; Bartlett, D.H. Piezophilic adaptation: A genomic point of view. J. Biotechnol. 2006, 126, 11–25. [Google Scholar] [CrossRef]
- Masanta, W.O.; Hinz, R.; Zautner, A.E. Infectious causes of cholesteatoma and treatment of infected ossicles prior to reimplantation by hydrostatic high-pressure inactivation. BioMed Res. Int. 2015, 2015, 761259. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, M.; Hormann, S.; Vogel, R.F.; Ehrmann, M.A. Transcriptional response reveals translation machinery as target for high pressure in Lactobacillus sanfranciscensis. Arch. Microbiol. 2005, 184, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.R.; den Besten, H.M.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Combrouse, T.; Sadovskaya, I.; Faille, C.; Kol, O.; Guerardel, Y.; Midelet-Bourdin, G. Quantification of the extracellular matrix of the Listeria monocytogenes biofilms of different phylogenic lineages with optimization of culture conditions. J. Appl. Microbiol. 2013, 114, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, T.; Jacques, M.; Quessy, S.; Fravalo, P. Impact of nutrient restriction on the structure of Listeria monocytogenes biofilm grown in a microfluidic system. Front. Microbiol. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Maestre-Reyna, M.; Lee, G.; Gerard, H.; Wang, A.H.; Watnick, P.I. In situ proteolysis of the Vibrio cholerae matrix protein RbmA promotes biofilm recruitment. Proc. Natl. Acad. Sci. USA 2015, 112, 10491–10496. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.C.; Yildiz, F.H. Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J. Bacteriol. 2008, 190, 6646–6659. [Google Scholar] [CrossRef]
- Falkinham, J.O., 3rd. Surrounded by mycobacteria: Nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 2009, 107, 356–367. [Google Scholar] [CrossRef]
- Mittelman, M.W.; Jones, A.D.G. A pure life: The microbial ecology of high purity industrial waters. Microb. Ecol. 2018, 76, 9–18. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Dunne, W.M., Jr.; Mason, E.O., Jr.; Kplan, S.L. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 1993, 37, 2522–2526. [Google Scholar] [CrossRef] [PubMed]
- Nadell, C.D.; Drescher, K.; Wingreen, N.S.; Bassler, B.L. Extracellular matrix structure governs invasion resistance in bacterial biofilms. ISME J. 2015, 9, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Kumon, H.; Tomochika, K.; Matunaga, T.; Ogawa, M.; Ohmoril, H. A sandwich cup method for the penetration assay of antimicrobial agents through Pseudomonas exopolysaccharides. Microbiol. Immunol. 1994, 38, 615–619. [Google Scholar] [CrossRef]
- Giwercman, B.; Jensen, E.T.; Høiby, N.; Kharazmi, A.; Costerton, J.W. Induction of β-lactamase production in Pseudomonas aeruginosa biofilm. Antimicrob. Agents Chemother. 1991, 35, 1008–1010. [Google Scholar] [CrossRef]
- Stewart, P.S. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 1996, 40, 2517–2522. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.; Parsek, M.R. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Dogtrid, I.G.; Evans, E.; Brown, M.R.W.; Gilbert, P. Growth-rate-independent killing by ciprofloxacin of biofilm-derived Staphylococcus epidermidis evidence for cell-cycle dependency. J. Antimicrob. Chemother. 1992, 30, 791–802. [Google Scholar]
- Evans, D.J.; Allison, D.G.; Brown, M.R.W.; Gilbert, P. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: Effect of specific growth rate. J. Antimicrob. Chemother. 1991, 27, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Collier, P.J.; Brown, M.R.W. Influence of growth rate on susceptibility to antimicrobial agents: Biofilms, cell cycle, dormancy, and stringent response. Antimicrob. Agents Chemother. 1990, 34, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.; Strap, J.L.; Costerton, J.W. Establishment of aging biofilms: Possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob. Agents Chemother. 1992, 36, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Mcleod, G.I.; Spector, M.P. Starvation- and stationary-phase-induced resistance to the antimicrobial peptide polymyxin B in Salmonella typhimurium is RpoS (σS) independent and occurs through both phoP-dependent and -independent pathway. J. Bacteriol. 1996, 178, 3683–3688. [Google Scholar] [CrossRef]
- Williamson, K.S.; Richards, L.A.; Perez-Osorio, A.C.; Pitts, B.; McInnerney, K.; Stewart, P.S.; Franklin, M.J. Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J. Bacteriol. 2012, 194, 2062–2073. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells: Molecular mechanisms related to antibiotic tolerance. Handb. Exp. Pharmacol. 2012, 211, 121–133. [Google Scholar]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef]
- Bae, J.; Oh, E.; Jeon, B. Enhanced transmission of antibiotic resistance in Campylobacter jejuni biofilms by natural transformation. Antimicrob. Agents Chemother. 2014, 58, 7573–7575. [Google Scholar] [CrossRef]
- Limoli, D.H.; Rockel, A.B.; Host, K.M.; Jha, A.; Kopp, B.T.; Hollis, T.; Wozniak, D.J. Cationic antimicrobial peptides promote microbial mutagenesis and pathoadaptation in chronic infections. PLoS Pathog. 2014, 10, e1004083. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.C.; Dunny, G.M. Effects of biofilm growth on plasmid copy number and expression of antibiotic resistance genes in Enterococcus faecalis. Antimicrob. Agents Chemother. 2013, 57, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Multidrug resistance in Gram-negative bacteria. Curr. Opin. Microbiol. 2001, 4, 500–508. [Google Scholar] [CrossRef]
- Sun, J.; Deng, Z.; Yan, A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Fraud, S.; Poole, K. Oxidative stress induction of the MexXY multidrug efflux genes and promotion of aminoglycoside resistance development in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 1068–1074. [Google Scholar] [CrossRef]
- Zhang, L.; Mah, T.F. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, F.J.; Liu, Y.; Xiong, L.R.; Xie, L.L.; Xia, P.Y. Enhancement of biofilm formation by subinhibitory concentrations of macrolides in icaADBC-positive and -negative clinical isolates of Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2010, 54, 2707–2711. [Google Scholar] [CrossRef]
- Rachid, S.; Ohlsen, K.; Witte, W.; Hacker, J.R.; Ziebuhr, W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2000, 44, 3357–3363. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Gunawardana, M.; Maguire, K.; Guerrero-Given, D.; Schaudinn, C.; Wang, C.; Baum, M.M.; Webster, P. Beta-lactam antibiotics stimulate biofilm formation in non-typeable Haemophilus influenzae by up-regulating carbohydrate metabolism. PLoS ONE 2014, 9, e99204. [Google Scholar] [CrossRef]
- Burmolle, M.; Ren, D.; Bjarnsholt, T.; Sorensen, S.J. Interactions in multispecies biofilms: Do they actually matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef]
- Roder, H.L.; Sorensen, S.J.; Burmolle, M. Studying bacterial multispecies biofilms: Where to start? Trends Microbiol. 2016, 24, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Burmolle, M.; Webb, J.S.; Rao, D.; Hansen, L.H.; Sorensen, S.J.; Kjelleberg, S. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl. Environ. Microbiol. 2006, 72, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- De Brucker, K.; Tan, Y.; Vints, K.; De Cremer, K.; Braem, A.; Verstraeten, N.; Michiels, J.; Vleugels, J.; Cammue, B.P.; Thevissen, K. Fungal beta-1,3-glucan increases ofloxacin tolerance of Escherichia coli in a polymicrobial E. coli/Candida albicans biofilm. Antimicrob. Agents Chemother. 2015, 59, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef]
- Peters, B.M.; Jabra-Rizk, M.A.; Scheper, M.A.; Leid, J.G.; Costerton, J.W.; Shirtliff, M.E. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 493–503. [Google Scholar] [CrossRef]
- Gambino, M.; Cappitelli, F. Mini-review: Biofilm responses to oxidative stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef]
- Matin, A.; Lynch, S.V. Investigating the threat of bacteria grown in space. ASM News 2005, 71, 235–240. [Google Scholar]
- Montgomery, K.; Charlesworth, J.C.; LeBard, R.; Visscher, P.T.; Burns, B.P. Quorum sensing in extreme environments. Life 2013, 3, 131–148. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.C.; Huang, C.T. Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J. Antimicrob. Chemother. 2002, 49, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Jensen, P.O.; Burmolle, M.; Hentzer, M.; Haagensen, J.A.; Hougen, H.P.; Calum, H.; Madsen, K.G.; Moser, C.; Molin, S.; et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiol. -SGM 2005, 151, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.O.; Wang, H.; Hoiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mekalanos, J.J. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 2003, 5, 647–656. [Google Scholar] [CrossRef]
- March, J.C.; Bentley, W.E. Quorum sensing and bacterial cross-talk in biotechnology. Curr. Opin. Biotechnol. 2004, 15, 495–502. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharma, P.; Capalash, N. N-acyl homoserine lactone mediated interspecies interactions between A. baumannii and P. aeruginosa. Biofouling 2012, 28, 813–822. [Google Scholar] [CrossRef]
- Sun, Z.; He, X.; Brancaccio, V.F.; Yuan, J.; Riedel, C.U. Bifidobacteria exhibit LuxS-dependent autoinducer 2 activity and biofilm formation. PLoS ONE 2014, 9, e88260. [Google Scholar] [CrossRef]
- Díaz, M.; Castro, M.; Copaja, S.; Guiliani, N. Biofilm formation by the acidophile bacterium Acidithiobacillus thiooxidans involves c-di-GMP pathway and Pel exopolysaccharide. Genes 2018, 9, 113. [Google Scholar] [CrossRef]
- Huynh, T.T.; McDougald, D.; Klebensberger, J.; Al Qarni, B.; Barraud, N.; Rice, S.A.; Kjelleberg, S.; Schleheck, D. Glucose starvation-induced dispersal of Pseudomonas aeruginosa biofilms is cAMP and energy dependent. PLoS ONE 2012, 7, e42874. [Google Scholar] [CrossRef]
- Galloway, W.R.J.D.; Hodgkinson, J.T.; Bowden, S.D.; Welch, M.; Spring, D.R. Quorum sensing in Gram-negative bacteria: Small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 2011, 111, 28–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dai, S.; Tian, B.; Li, T.; Yu, J.; Liu, C.; Wang, L.; Xu, H.; Zhao, Y.; Hua, Y. DqsIR quorum sensing-mediated gene regulation of the extremophilic bacterium Deinococcus radiodurans in response to oxidative stress. Mol. Microbiol. 2016, 100, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Mamani, S.; Moinier, D.; Denis, Y.; Soulere, L.; Queneau, Y.; Talla, E.; Bonnefoy, V.; Guiliani, N. Insights into the quorum sensing regulon of the acidophilic Acidithiobacillus ferrooxidans revealed by transcriptomic in the presence of an Acyl homoserine lactone superagonist analog. Front. Microbiol. 2016, 7, 1365. [Google Scholar] [CrossRef] [PubMed]
- Moya-Beltran, A.; Rojas-Villalobos, C.; Diaz, M.; Guiliani, N.; Quatrini, R.; Castro, M. Nucleotide second messenger-based signaling in extreme acidophiles of the Acidithiobacillus species complex: Partition between the core and variable gene complements. Front. Microbiol. 2019, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Coggan, K.A.; Wolfgang, M.C. Global regulatory pathways and cross-talk control Pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr. Issues Mol. Biol. 2012, 14, 47–70. [Google Scholar] [PubMed]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Amikam, D. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 2006, 9, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Borlee, B.R.; O’Connor, J.R.; Hill, P.J.; Harwood, C.S.; Wozniak, D.J.; Parsek, M.R. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2012, 109, 20632–20636. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Behrens, A.J.; Kaever, V.; Kazmierczak, B.I. Type IV pilus assembly in Pseudomonas aeruginosa over a broad range of cyclic di-GMP concentrations. J. Bacteriol. 2012, 194, 4285–4294. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Wood, T.K. Tyrosine phosphatase TpbA of Pseudomonas aeruginosa controls extracellular DNA via cyclic diguanylic acid concentrations. Environ. Microbiol. Rep. 2010, 2, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Tischler, A.D.; Camilli, A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 2004, 53, 857–869. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.B.T.; Whitchurch, C.B.; Mattick, J.S. A re-examination of twitching motility in Pseudomonas aeruginosa. Microbiol. -SGM 1999, 145, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Paytubi, S.; Cansado, C.; Madrid, C.; Balsalobre, C. Nutrient composition promotes switching between pellicle and bottom biofilm in Salmonella. Front. Microbiol. 2017, 8, 2160. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef] [PubMed]
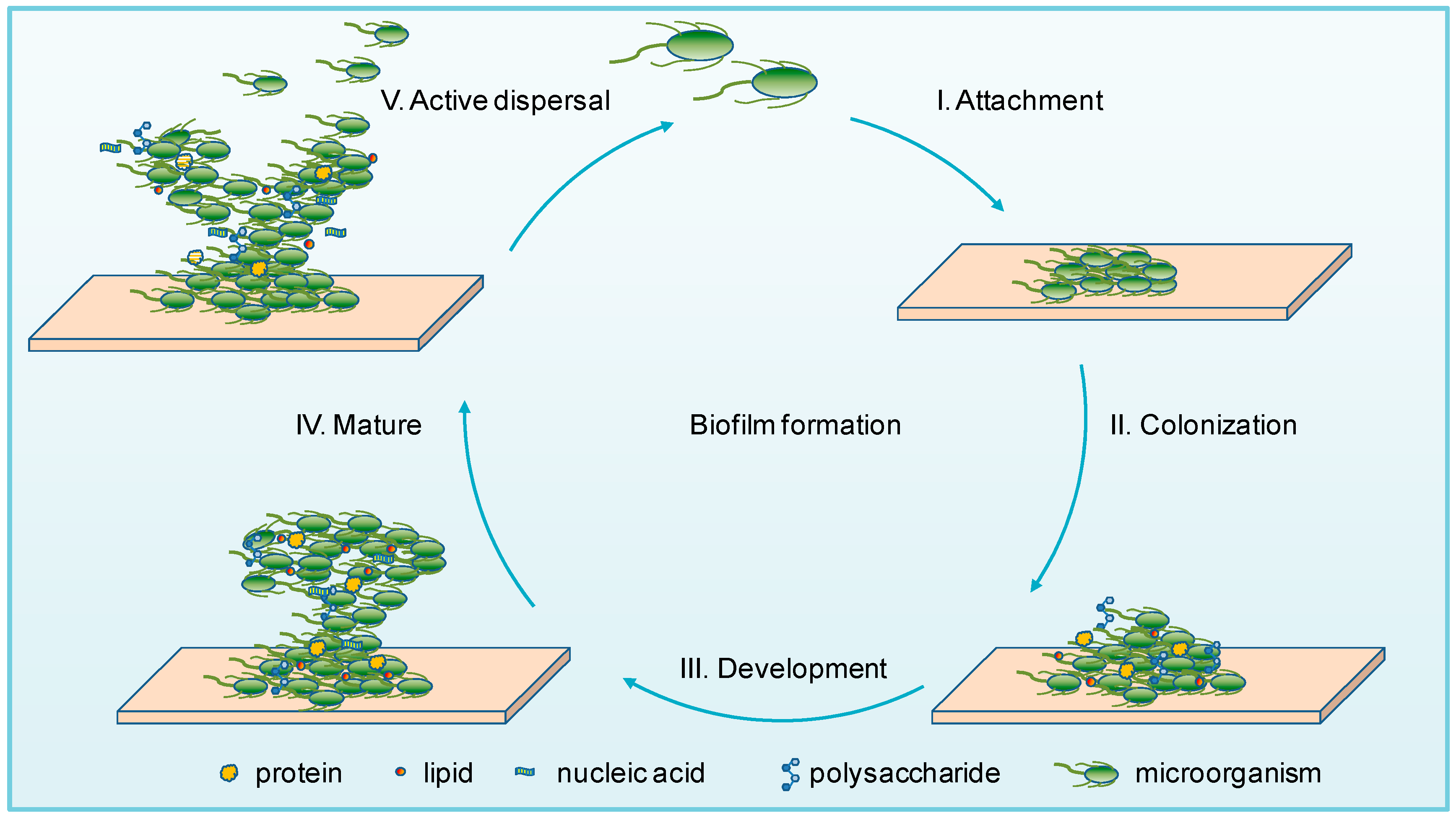
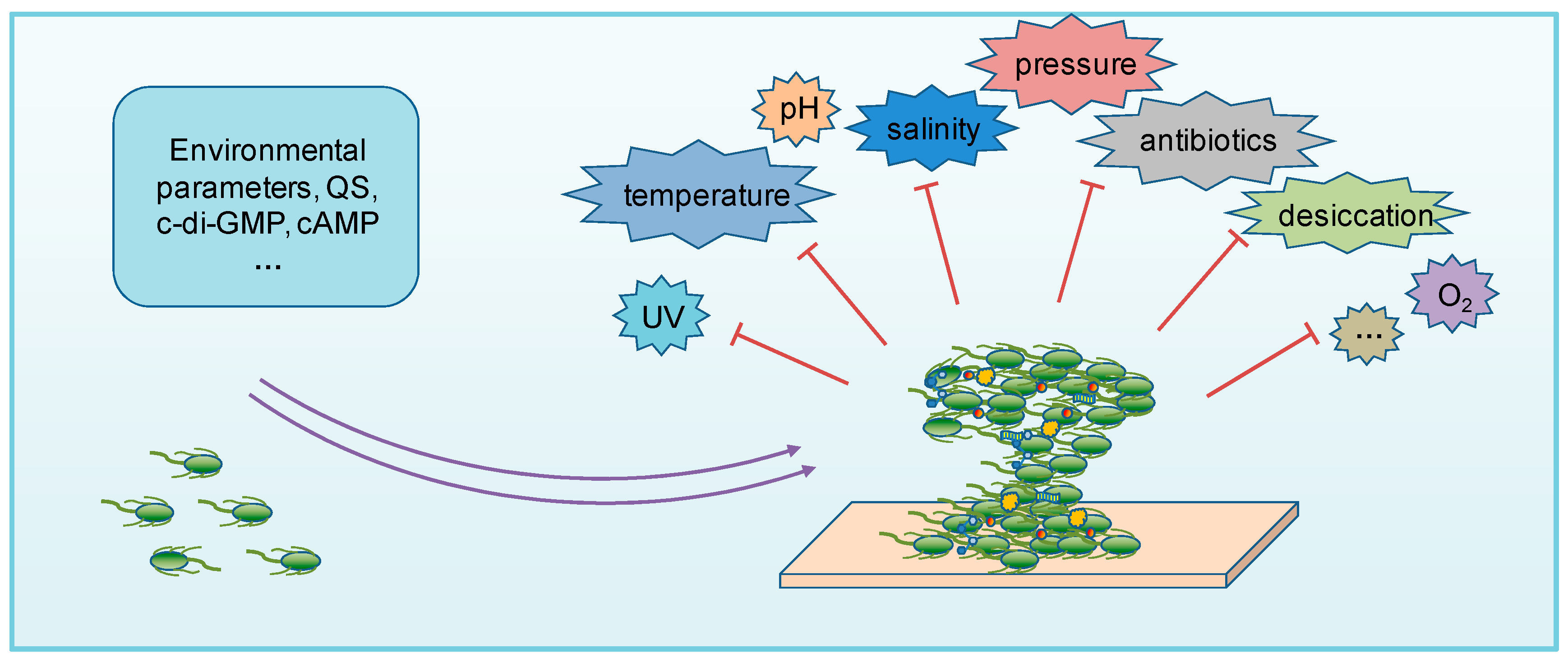
| Factor | Brief Description | Strain | Reference | |
|---|---|---|---|---|
| Environmental parameter | Ultraviolet (UV) | Matrix of extracellular polymeric substances (EPS) shows a protective property by physical shielding against UV radiation | Listeria monocytogenes, Deinococcus geothermalis | [51,53] |
| Temperature | Biofilm formation increases at high temperature, and the composition of biofilms change at low temperature. They both enhance microbial resistance | Thermolongibacillus, Sulfolobus, etc. | [17,54] | |
| Extreme pH | At extreme pH, biofilms increase bacterial resistance by altering EPS content | Enterococcus faecalis, Alishewanella, etc. | [69,70] | |
| Salinity | Bacteria can withstand high salt damage by aggregating into granular biofilm forms | Halomonas stenophila, Kocuria flava AB402, etc. | [76,77] | |
| High pressure | High pressure yield an immediate increase in the polysaccharide band area of bacterial biofilms | Staphylococcus aureus | [24] | |
| Poor nutrient | Under poor nutrient conditions, the biofilm formation is enhanced | Listeria monocytogenes, non-tuberculous mycobacteria | [86,89] | |
| Quorum sensing (QS) | Autoinducer-1 (AI-1) | AI-1 system is related to biofilm formation, and can adjust its amount in extreme environments | Acinetobacter baumannii, Pseudomonas aeruginosa | [138] |
| Autoinducer-2 (AI-2) | AI-2 regulates biofilm formation against environmental stresses | Bifidobacterium longum | [139] | |
| Messenger molecule | cyclic dimeric guanosine 3’,5’-monophosphate (c-di-GMP) | c-di-GMP can control biofilm formation in response to different environments | Acidithiobacillus thioooxidans | [140] |
| cyclic adenosine 3’,5’-monophosphate (cAMP) | cAMP is crucial for the formation of pellicle biofilm | Pseudomonas aeruginosa,Vibrio cholerae | [88,141] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. https://doi.org/10.3390/ijms20143423
Yin W, Wang Y, Liu L, He J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. International Journal of Molecular Sciences. 2019; 20(14):3423. https://doi.org/10.3390/ijms20143423
Chicago/Turabian StyleYin, Wen, Yiting Wang, Lu Liu, and Jin He. 2019. "Biofilms: The Microbial “Protective Clothing” in Extreme Environments" International Journal of Molecular Sciences 20, no. 14: 3423. https://doi.org/10.3390/ijms20143423
APA StyleYin, W., Wang, Y., Liu, L., & He, J. (2019). Biofilms: The Microbial “Protective Clothing” in Extreme Environments. International Journal of Molecular Sciences, 20(14), 3423. https://doi.org/10.3390/ijms20143423





