Arsenic Neurotoxicity in Humans
Abstract
1. Introduction
2. Arsenic in the Environment
3. Arsenic Metabolic Pathway and Toxicity
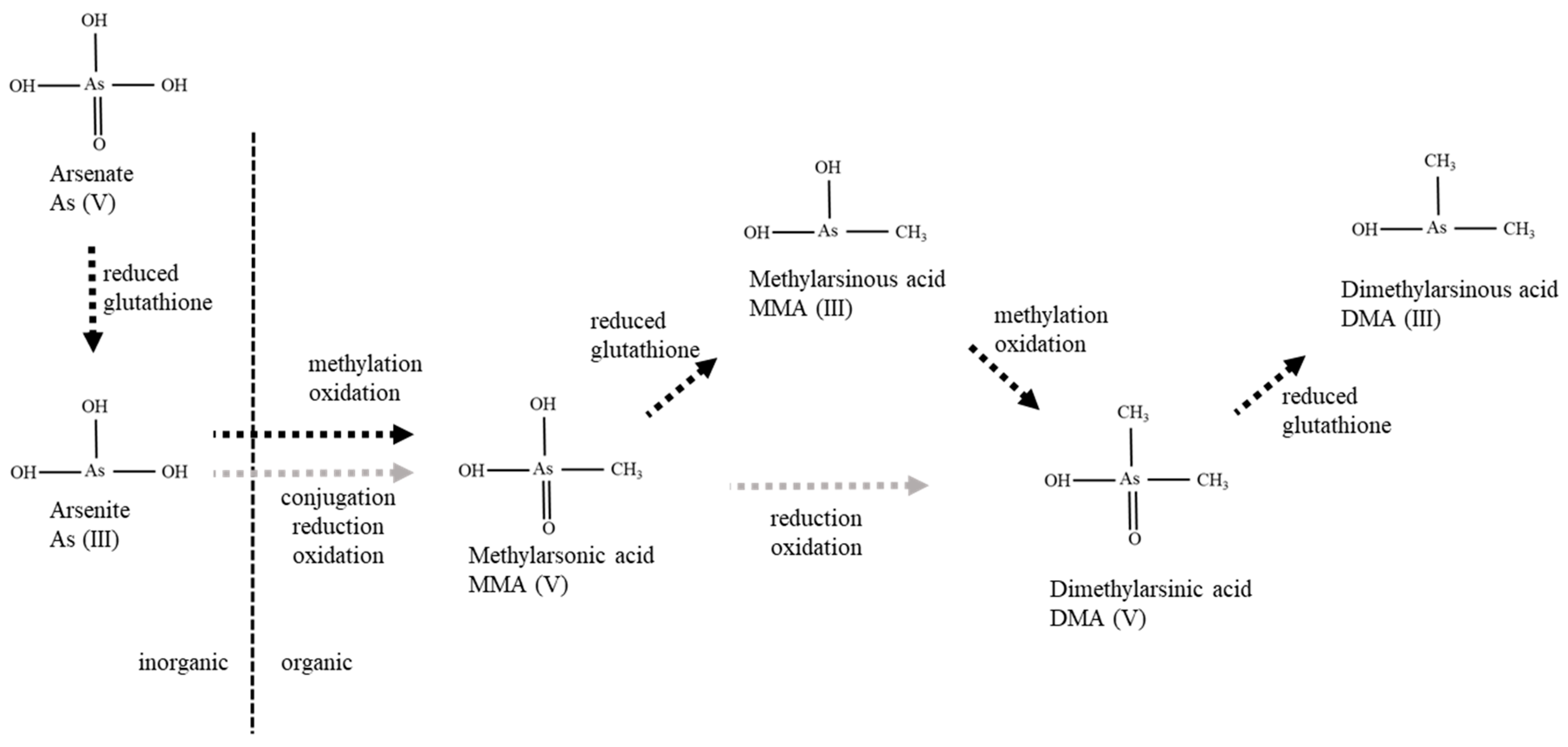
3.1. Metabolic Pathway
3.2. Toxic LD50 Concentrations
4. Toxic Mechanisms
4.1. Mitochondrial Dysfunction
4.2. Lipid Peroxidation
4.3. Apoptosis
4.4. Increased Calpain
4.5. Thiamine Deficiency
4.6. Decreased Acetylcholinesterase Activity
5. Clinical Neurological Symptoms
5.1. Acute As Poisoning
5.2. Toroku As Pollution
5.3. Arsenic Poisoning in Morinaga Dry Milk
5.4. Arsenic Contamination in Groundwater
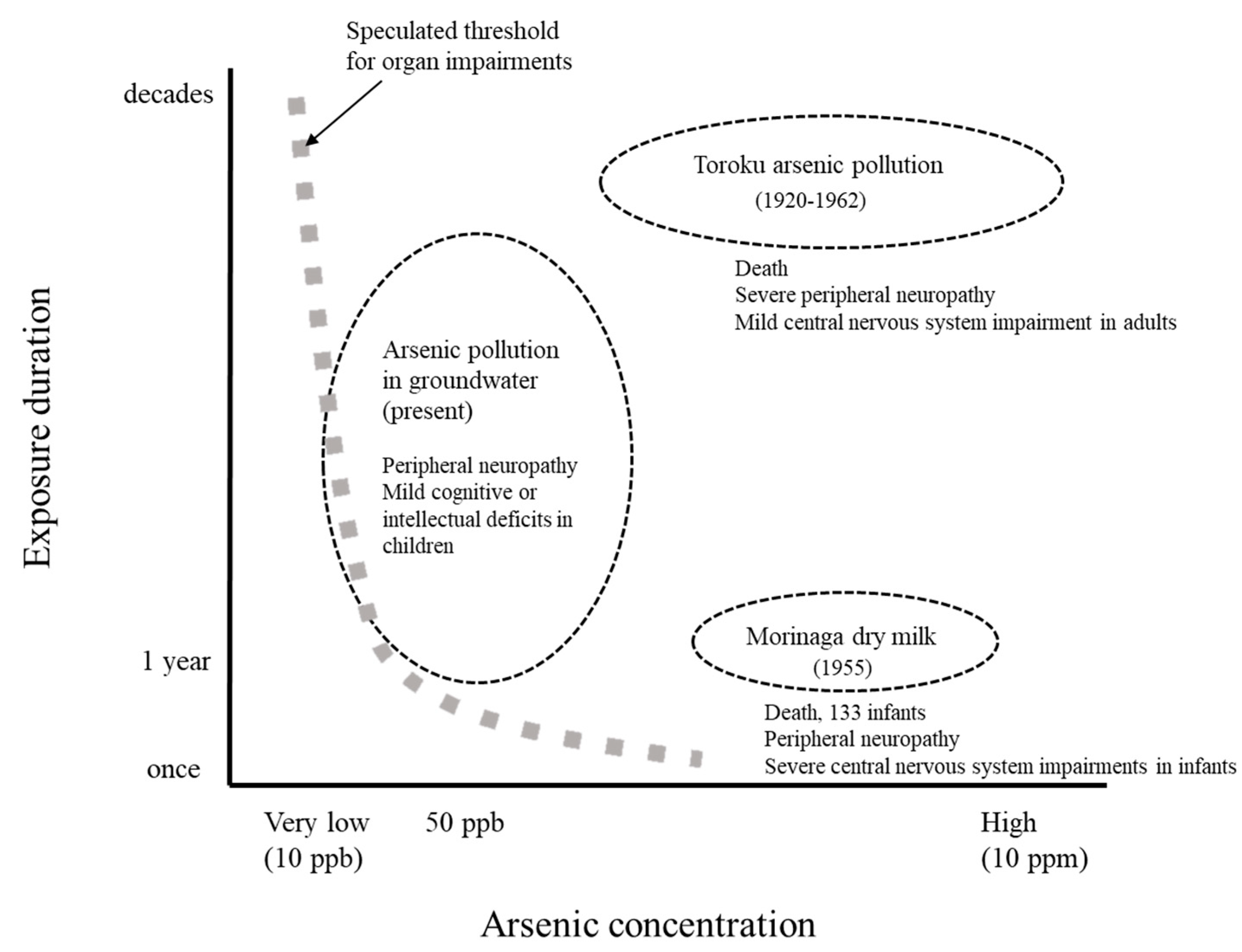
6. Exposure Dose–Response Relationship in Various Organs
7. Effect on Children
8. Factors to Consider
9. Conclusions
Funding
Conflicts of Interest
References
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution—A Global Synthesis; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Guha Mazumder, D.; Dasgupta, U.B. Chronic arsenic toxicity: Studies in West Bengal, India. Kaohsiung J. Med. Sci. 2011, 27, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Mochizuki, H.; Ebihara, Y.; Shiomi, K.; Nakazato, M. Clinical Symptoms, Neurological Signs, and Electrophysiological Findings in Surviving Residents with Probable Arsenic Exposure in Toroku, Japan. Arch. Environ. Contam. Toxicol. 2018, 75, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Phyu, K.P.; Aung, M.N.; Zin, P.W.; Yano, Y.; Myint, M.Z.; Thit, W.M.; Yamamoto, Y.; Hishikawa, Y.; Thant, K.Z.; et al. Peripheral neuropathy induced by drinking water contaminated with low-dose arsenic in Myanmar. Environ. Health Prev. Med. 2019, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H. Arsenic. In Handbook of Geochemistry; Wedepohl, K.H., Ed.; Springer: New York, NY, USA, 1969; Volume II. [Google Scholar]
- Lunde, G. Occurrence and transformation of arsenic in the marine environment. Environ. Health Perspect. 1977, 19, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, C.; Raab, A.; Williams, P.N.; Deacon, C.; Haris, P.I.; Meharg, A.A.; Feldmann, J. Accumulation or production of arsenobetaine in humans? J. Environ. Monit. 2010, 12, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Aposhian, H.V.; Gurzau, E.S.; Le, X.C.; Gurzau, A.; Healy, S.M.; Lu, X.; Ma, M.; Yip, L.; Zakharyan, R.A.; Maiorino, R.M.; et al. Occurrence of monomethylarsonous acid in urine of humans exposed to inorganic arsenic. Chem. Res. Toxicol. 2000, 13, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905–23926. [Google Scholar] [CrossRef]
- Challenger, F. Biological methylation. Sci. Prog. 1947, 35, 396–416. [Google Scholar]
- Hayakawa, T.; Kobayashi, Y.; Cui, X.; Hirano, S. A new metabolic pathway of arsenite: Arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch. Toxicol. 2005, 79, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Pinyayev, T.S.; Kohan, M.J.; Herbin-Davis, K.; Creed, J.T.; Thomas, D.J. Preabsorptive metabolism of sodium arsenate by anaerobic microbiota of mouse cecum forms a variety of methylated and thiolated arsenicals. Chem. Res. Toxicol. 2011, 24, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Kumana, C.R.; Au, W.Y.; Lee, N.S.; Kou, M.; Mak, R.W.; Lam, C.W.; Kwong, Y.L. Systemic availability of arsenic from oral arsenic-trioxide used to treat patients with hematological malignancies. Eur. J. Clin. Pharmacol. 2002, 58, 521–526. [Google Scholar] [PubMed]
- Buchet, J.P.; Lauwerys, R.; Roels, H. Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int. Arch. Occup. Environ. Health 1981, 48, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Xie, S.; Hafeez, M.A.; Wang, X.; Hussain, H.I.; Iqbal, Z.; Pan, Y.; Iqbal, M.; Shabbir, M.A.; Yuan, Z. Metabolism and toxicity of arsenicals in mammals. Environ. Toxicol. Pharmacol. 2016, 48, 214–224. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007.
- Vahter, M.; Concha, G. Role of metabolism in arsenic toxicity. Pharmacol. Toxicol. 2001, 89, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Aposhian, H.V.; Zakharyan, R.A.; Avram, M.D.; Sampayo-Reyes, A.; Wollenberg, M.L. A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species. Toxicol. Appl. Pharmacol. 2004, 198, 327–335. [Google Scholar] [PubMed]
- Petrick, J.S.; Ayala-Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Himeno, S. Versatile health effects of arsenic in humans. Chikyu Kankyo 2017, 22, 81–90. [Google Scholar]
- Kligerman, A.D.; Doerr, C.L.; Tennant, A.H.; Harrington-Brock, K.; Allen, J.W.; Winkfield, E.; Poorman-Allen, P.; Kundu, B.; Funasaka, K.; Roop, B.C.; et al. Methylated trivalent arsenicals as candidate ultimate genotoxic forms of arsenic: Induction of chromosomal mutations but not gene mutations. Environ. Mol. Mutagen. 2003, 42, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Tamaoka, A.; Otsuka, F.; Iwasaki, N.; Shin, K.; Matsui, A.; Endo, G.; Kumagai, Y.; Ishii, T.; Shoji, S.; et al. Diphenylarsinic acid poisoning from chemical weapons in Kamisu, Japan. Ann. Neurol. 2004, 56, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Ministry of the Environment Japan. The Result of Cytotoxicity Test of Organic Arsenic Compounds (Japanese); Ministry of the Environment Japan: Tokyo, Japan, 2007.
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Flora, S.J. Concomitant exposure to arsenic and organophosphates on tissue oxidative stress in rats. Food Chem. Toxicol. 2011, 49, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Goel, R.K.; Kaur, T. Mechanisms pertaining to arsenic toxicity. Toxicol. Int. 2011, 18, 87–93. [Google Scholar] [PubMed]
- Chandravanshi, L.P.; Gupta, R.; Shukla, R.K. Developmental Neurotoxicity of Arsenic: Involvement of Oxidative Stress and Mitochondrial Functions. Biol. Trace Elem. Res. 2018, 186, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Kumar, V. Arsenic-induced mitochondrial oxidative damage is mediated by decreased PGC-1alpha expression and its downstream targets in rat brain. Chem. Biol. Interact. 2016, 256, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Chandravanshi, L.P.; Shukla, R.K.; Sultana, S.; Pant, A.B.; Khanna, V.K. Early life arsenic exposure and brain dopaminergic alterations in rats. Int. J. Dev. Neurosci. 2014, 38, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Yadav, R.S.; Chandravanshi, L.P.; Shukla, R.K.; Dhuriya, Y.K.; Chauhan, L.K.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats. Toxicol. Appl. Pharmacol. 2014, 279, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Cali, T.; Ottolini, D.; Brini, M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. BioFactors 2011, 37, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants: A personal view. Nutr. Rev. 1994, 52, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Felix, K.; Manna, S.K.; Wise, K.; Barr, J.; Ramesh, G.T. Low levels of arsenite activates nuclear factor-kappaB and activator protein-1 in immortalized mesencephalic cells. J. Biochem. Mol. Toxicol. 2005, 19, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Hwang, L.C.; Kao, J.S.; Yiin, S.J.; Lin, S.F.; Lin, C.H.; Lin, Y.C.; Aw, T.C. Lipid peroxidation in workers exposed to aluminium, gallium, indium, arsenic, and antimony in the optoelectronic industry. J. Occup. Environ. Med. 2006, 48, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Namgung, U.; Xia, Z. Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol. Appl. Pharmacol. 2001, 174, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Wurstle, M.L.; Laussmann, M.A.; Rehm, M. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp. Cell Res. 2012, 318, 1213–1220. [Google Scholar] [CrossRef]
- Vahidnia, A.; Romijn, F.; Tiller, M.; van der Voet, G.B.; de Wolff, F.A. Arsenic-induced toxicity: Effect on protein composition in sciatic nerve. Human Exp. Toxicol. 2006, 25, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Vahidnia, A.; van der Straaten, R.J.; Romijn, F.; van Pelt, J.; van der Voet, G.B.; de Wolff, F.A. Mechanism of arsenic-induced neurotoxicity may be explained through cleavage of p35 to p25 by calpain. Toxicol. In Vitro 2008, 22, 682–687. [Google Scholar] [CrossRef]
- Vahidnia, A.; Romijn, F.; van der Voet, G.B.; de Wolff, F.A. Arsenic-induced neurotoxicity in relation to toxicokinetics: Effects on sciatic nerve proteins. Chem. Biol. Interact. 2008, 176, 188–195. [Google Scholar] [CrossRef]
- Gopalkrishnan, A.; Rao, M.V. Amelioration by vitamin A upon arsenic induced metabolic and neurotoxic effects. J. Health Sci. 2006, 52, 568–577. [Google Scholar] [CrossRef]
- Samikkannu, T.; Chen, C.H.; Yih, L.H.; Wang, A.S.; Lin, S.Y.; Chen, T.C.; Jan, K.Y. Reactive oxygen species are involved in arsenic trioxide inhibition of pyruvate dehydrogenase activity. Chem. Res. Toxicol. 2003, 16, 409–414. [Google Scholar] [CrossRef]
- Szinicz, L.; Forth, W. Effect of As2O3 on gluconeogenesis. Arch. Toxicol. 1988, 61, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, A.K.; Tchounwou, P.B. Serum acetyl cholinesterase as a biomarker of arsenic induced neurotoxicity in sprague-dawley rats. Int. J. Environ. Res. Public Health 2005, 2, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Yazawa, S.; Ohnishi, A.; Ohi, T. Chronic and predominantly sensory polyneuropathy in Toroku Valley where a mining company produced arsenic. Clin. Neurol. 2002, 42, 504–511. [Google Scholar]
- Le Quesne, P.M.; McLeod, J.G. Peripheral neuropathy following a single exposure to arsenic. Clincal course in four patients with electrophysiological and histological studies. J. Neurol. Sci. 1977, 32, 437–451. [Google Scholar] [CrossRef]
- Vibol, S.; Hashim, J.H.; Sarmani, S. Neurobehavioral effects of arsenic exposure among secondary school children in the Kandal Province, Cambodia. Environ. Res. 2015, 137, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Yagi, K.; Tsuruta, K.; Taniguchi, A.; Ishii, N.; Shiomi, K.; Nakazato, M. Prolonged central sensory conduction time in patients with chronic arsenic exposure. J. Neurol. Sci. 2016, 361, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Susan, A.; Rajendran, K.; Sathyasivam, K.; Krishnan, U.M. An overview of plant-based interventions to ameliorate arsenic toxicity. Biomed. Pharmacother. 2019, 109, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Beckett, W.S.; Moore, J.L.; Keogh, J.P.; Bleecker, M.L. Acute encephalopathy due to occupational exposure to arsenic. Br. J. Ind. Med. 1986, 43, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, P.D.; Wilbourn, A.J.; Albers, J.W.; Rogers, L.; Salanga, V.; Greenberg, H.S. Acute arsenic intoxication presenting as Guillain-Barre-like syndrome. Muscle Nerve 1987, 10, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki Prefecture. Toroku Chiku no Kougai ni Kakawaru Shakaiigakutekityousaseiseki (Japanese); Miyazaki Prefecture: Miyazaki, Japan, 1972. [Google Scholar]
- Ishii, N.; Mochizuki, H.; Yamashita, M.; Yagi, K.; Shiomi, K.; Tsuruta, K.; Nakazato, M. Auditory brainstem response analysis for long-term central auditory function sequelae in patients with chronic arsenic intoxication: A cross-sectional study. J. Neurol. Sci. 2019, 398, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Dakeishi, M.; Murata, K.; Grandjean, P. Long-term consequences of arsenic poisoning during infancy due to contaminated milk powder. Environ. Health 2006, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Doi, M.; Nishio, M.; Hojo, H.; Tanaka, M. Recent observations of Kyoto children poisoned by arsenic tainted “Morinaga Dry Milk”. Nippon Eiseigaku Zasshi 1972, 27, 364–399. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.C.; Rahman, M.M.; Chowdhury, U.K.; Sengupta, M.K.; Lodh, D.; Chanda, C.R.; Saha, K.C.; Chakraborti, D. Neuropathy in arsenic toxicity from groundwater arsenic contamination in West Bengal, India. J. Environ. Sci. Health Part A 2003, 38, 165–183. [Google Scholar] [CrossRef]
- Guha Mazumder, D.N. Chronic arsenic toxicity & human health. Indian J. Med. Res. 2008, 128, 436–447. [Google Scholar] [PubMed]
- Rahman, M.M.; Chowdhury, U.K.; Mukherjee, S.C.; Mondal, B.K.; Paul, K.; Lodh, D.; Biswas, B.K.; Chanda, C.R.; Basu, G.K.; Saha, K.C.; et al. Chronic arsenic toxicity in Bangladesh and West Bengal, India—A review and commentary. J. Toxicol. Clin. Toxicol. 2001, 39, 683–700. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Yoshida, T.; Yamauchi, H.; Fan Sun, G. Chronic health effects in people exposed to arsenic via the drinking water: Dose-response relationships in review. Toxicol. Appl. Pharmacol. 2004, 198, 243–252. [Google Scholar] [CrossRef]
- Islam, K.; Haque, A.; Karim, R.; Fajol, A.; Hossain, E.; Salam, K.A.; Ali, N.; Saud, Z.A.; Rahman, M.; Rahman, M.; et al. Dose-response relationship between arsenic exposure and the serum enzymes for liver function tests in the individuals exposed to arsenic: A cross sectional study in Bangladesh. Environ. Health 2011, 10, 64. [Google Scholar] [CrossRef]
- Moon, K.A.; Oberoi, S.; Barchowsky, A.; Chen, Y.; Guallar, E.; Nachman, K.E.; Rahman, M.; Sohel, N.; D’Ippoliti, D.; Wade, T.J.; et al. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int. J. Epidemiol. 2017, 46, 1924–1939. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shimoda, Y.; Wang, S.; Wang, Z.; Liu, J.; Liu, X.; Jin, H.; Gao, F.; Tong, J.; Yamanaka, K.; et al. Total arsenic and speciation analysis of saliva and urine samples from individuals living in a chronic arsenicosis area in China. Environ. Health Prev. Med. 2017, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Pottern, L.M.; Stone, B.J.; Fraumeni, J.F., Jr. Respiratory cancer in a cohort of copper smelter workers: Results from more than 50 years of follow-up. Am. J. Epidemiol. 2000, 151, 554–565. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, H.; Chen, B.; Zhang, H.; Tao, S. Association between lung cancer risk and inorganic arsenic concentration in drinking water: A dose-response meta-analysis. Toxicol. Res. 2018, 7, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, J.S.; Chang, E.T.; Gentry, P.R.; Clewell, H.J.; Boffetta, P.; Cohen, S.M. Dose-response for assessing the cancer risk of inorganic arsenic in drinking water: The scientific basis for use of a threshold approach. Rev. Toxicol. 2019, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Zierold, K.M.; Knobeloch, L.; Anderson, H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am. J. Public Health 2004, 94, 1936–1937. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wang, S.L.; Chiou, J.M.; Tseng, C.H.; Chiou, H.Y.; Hsueh, Y.M.; Chen, S.Y.; Wu, M.M.; Lai, M.S. Arsenic and diabetes and hypertension in human populations: A review. Toxicol. Appl. Pharmacol. 2007, 222, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Tolins, M.; Ruchirawat, M.; Landrigan, P. The developmental neurotoxicity of arsenic: Cognitive and behavioral consequences of early life exposure. Ann. Glob. Health 2014, 80, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Factor-Litvak, P.; Kline, J.; van Geen, A.; Slavkovich, V.; Loiacono, N.J.; Levy, D.; et al. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ. Health Perspect. 2007, 115, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Signes-Pastor, A.J.; Vioque, J.; Navarrete-Munoz, E.M.; Carey, M.; Garcia-Villarino, M.; Fernandez-Somoano, A.; Tardon, A.; Santa-Marina, L.; Irizar, A.; Casas, M.; et al. Inorganic arsenic exposure and neuropsychological development of children of 4-5 years of age living in Spain. Environ. Res. 2019, 174, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Von Ehrenstein, O.S.; Poddar, S.; Yuan, Y.; Mazumder, D.G.; Eskenazi, B.; Basu, A.; Hira-Smith, M.; Ghosh, N.; Lahiri, S.; Haque, R.; et al. Children’s intellectual function in relation to arsenic exposure. Epidemiology 2007, 18, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, U.K.; Rahman, M.M.; Sengupta, M.K.; Lodh, D.; Chanda, C.R.; Roy, S.; Quamruzzaman, Q.; Tokunaga, H.; Ando, M.; Chakraborti, D. Pattern of excretion of arsenic compounds [arsenite, arsenate, MMA(V), DMA(V)] in urine of children compared to adults from an arsenic exposed area in Bangladesh. J. Environ. Sci. Health Part A 2003, 38, 87–113. [Google Scholar] [CrossRef]
- Sun, G.; Xu, Y.; Li, X.; Jin, Y.; Li, B.; Sun, X. Urinary arsenic metabolites in children and adults exposed to arsenic in drinking water in Inner Mongolia, China. Environ. Health Perspect. 2007, 115, 648–652. [Google Scholar] [CrossRef]
- Yamauchi, H.; Kinoshita, J.; Nagai, N.; Shimazaki, K.; Kasamatsu, M. Nyotyuhisonoudo Karamita Jusyodobunrui Oyobi Hisobakuro to DNAsonsyohyouka ni Kansuru Kennkyu (Japanese); Wakayama Prefecture: Wakayama, Japan, 2002; pp. 32–49. [Google Scholar]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning--a review. J. Environ. Sci. Health Part A 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- Bacquart, T.; Frisbie, S.; Mitchell, E.; Grigg, L.; Cole, C.; Small, C.; Sarkar, B. Multiple inorganic toxic substances contaminating the groundwater of Myingyan Township, Myanmar: Arsenic, manganese, fluoride, iron, and uranium. Sci. Total Environ. 2015, 517, 232–245. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochizuki, H. Arsenic Neurotoxicity in Humans. Int. J. Mol. Sci. 2019, 20, 3418. https://doi.org/10.3390/ijms20143418
Mochizuki H. Arsenic Neurotoxicity in Humans. International Journal of Molecular Sciences. 2019; 20(14):3418. https://doi.org/10.3390/ijms20143418
Chicago/Turabian StyleMochizuki, Hitoshi. 2019. "Arsenic Neurotoxicity in Humans" International Journal of Molecular Sciences 20, no. 14: 3418. https://doi.org/10.3390/ijms20143418
APA StyleMochizuki, H. (2019). Arsenic Neurotoxicity in Humans. International Journal of Molecular Sciences, 20(14), 3418. https://doi.org/10.3390/ijms20143418





