Neuropharmacology of the Neuropsychiatric Symptoms of Dementia and Role of Pain: Essential Oil of Bergamot as a Novel Therapeutic Approach
Abstract
1. Introduction: Neuropsychiatric Symptoms of Dementia
2. Neuropharmacology of NPSs
Glutamatergic Transmission and NPSs
- -
- patients affected by AD showed a positive correlation of the ratio homovanillic acid/5-hydroxyindoleacetic acid with the cluster anxieties/phobias as assessed through the BEHAVE-AD;
- -
- patients with dementia with Lewy bodies were found to show a negative correlation between homovanillic acid and the cluster hallucinations at BEHAVE-AD;
- -
- taurine was inversely correlated with the Cornell Scale for Depression and BEHAVE-AD;
- -
- patients suffering from frontotemporal dementia presented an inverse correlation of glutamate with the cluster verbally agitated behavior at the Cohen–Mansfield Agitation Inventory [44].
- -
- an increase of the binding affinity to glycine recognition site;
- -
- a reduction of NR2A subunits compared to NR2B of N-methyl-D-aspartate (NMDA) receptors in the postmortem orbitofrontal cortex of AD patient subgroups with higher anxiety [45].
3. Novel Pharmacological Mechanisms for NPSs of Dementia Clinical Management: The Essential Oil of Bergamot
- -
- antagonist at 5-HT2A receptors;
- -
- partial agonist at presynaptic D2 receptors, while antagonist at postsynaptic receptors;
- -
- enhancer of NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activity through the pathway of the mammalian target of rapamycin (mTOR).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| BPSDs | Behavioral and Psychological Symptoms of Dementia |
| BA | Brodmann area |
| BEO | Bergamot essential oil |
| CNS | Central Nervous System |
| GABA | γ-aminobutyric acid |
| mTOR | mammalian target of rapamycin |
| MCI | Mild cognitive impairment |
| MMSE | Mini-Mental State Examination |
| NAADP | Nicotinic acid adenine dinucleotide phosphate |
| NMDA | N-methyl-D-aspartate |
| NPI | Neuropsychiatric Inventory |
| NPSs | Neuropsychiatric Symptoms |
| QoL | Quality of life |
| 5-HT | Serotonin |
| ZnT3 | Synaptic vesicle zinc transporter |
| TRPV1 | Type 1 vanilloid receptor |
| UCLA-ADRC | University of California, Los Angeles Alzheimer Disease Research Center |
References
- Patterson, C. World Alzheimer Report 2018: The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International (ADI): London, UK, 2018. [Google Scholar]
- Cummings, J.; Lee, G.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s Dement. 2018, 4, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lin, C.H.; Lane, H.Y.; Tsai, G.E. Nmda neurotransmission dysfunction in behavioral and psychological symptoms of alzheimer’s disease. Curr. Neuropharmacol. 2012, 10, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Jacoby, R.; Levy, R. Psychiatric phenomena in alzheimer’s disease. I: Disorders of thought content. Br. J. Psychiatry J. Ment. Sci. 1990, 157, 72–76. [Google Scholar] [CrossRef]
- Burns, A.; Jacoby, R.; Levy, R. Psychiatric phenomena in alzheimer’s disease. II: Disorders of perception. Br. J. Psychiatry J. Ment. Sci. 1990, 157, 76–81. [Google Scholar] [CrossRef]
- Burns, A.; Jacoby, R.; Levy, R. Psychiatric phenomena in alzheimer’s disease. III: Disorders of mood. Br. J. Psychiatry J. Ment. Sci. 1990, 157, 81–86. [Google Scholar] [CrossRef]
- Burns, A.; Jacoby, R.; Levy, R. Psychiatric phenomena in alzheimer’s disease. IV: Disorders of behaviour. Br. J. Psychiatry J. Ment. Sci. 1990, 157, 86–94. [Google Scholar] [CrossRef]
- Steinberg, M.; Shao, H.; Zandi, P.; Lyketsos, C.G.; Welsh-Bohmer, K.A.; Norton, M.C.; Breitner, J.C.; Steffens, D.C.; Tschanz, J.T.; Cache County, I. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The cache county study. Int. J. Geriatr. Psychiatry 2008, 23, 170–177. [Google Scholar] [CrossRef]
- Wise, E.A.; Rosenberg, P.B.; Lyketsos, C.G.; Leoutsakos, J.M. Time course of neuropsychiatric symptoms and cognitive diagnosis in national alzheimer’s coordinating centers volunteers. Alzheimers Dement. (Amst) 2019, 11, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Vik-Mo, A.O.; Giil, L.M.; Ballard, C.; Aarsland, D. Course of neuropsychiatric symptoms in dementia: 5-year longitudinal study. Int. J. Geriatr. Psychiatry 2018, 33, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Mintzer, J.; Brodaty, H.; Sano, M.; Banerjee, S.; Devanand, D.P.; Gauthier, S.; Howard, R.; Lanctot, K.; Lyketsos, C.G.; et al. Agitation in cognitive disorders: International psychogeriatric association provisional consensus clinical and research definition. Int. Psychogeriatr. 2015, 27, 7–17. [Google Scholar] [CrossRef]
- Sennik, S.; Schweizer, T.A.; Fischer, C.E.; Munoz, D.G. Risk factors and pathological substrates associated with agitation/aggression in alzheimer’s disease: A preliminary study using nacc data. J. Alzheimer’s Dis. JAD 2017, 55, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M.M. The basis for behavioural disturbances in dementia. J. Neurol. Neurosurg. Psychiatry 1996, 61, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Tsang, S.W.; Gil-Bea, F.J.; Francis, P.T.; Lai, M.K.; Marcos, B.; Chen, C.P.; Ramirez, M.J. Involvement of the gabaergic system in depressive symptoms of alzheimer’s disease. Neurobiol. Aging 2006, 27, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Lanctot, K.L.; Amatniek, J.; Ancoli-Israel, S.; Arnold, S.E.; Ballard, C.; Cohen-Mansfield, J.; Ismail, Z.; Lyketsos, C.; Miller, D.S.; Musiek, E.; et al. Neuropsychiatric signs and symptoms of alzheimer’s disease: New treatment paradigms. Alzheimer’s Dement. 2017, 3, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Kromer Vogt, L.J.; Hyman, B.T.; Van Hoesen, G.W.; Damasio, A.R. Pathological alterations in the amygdala in alzheimer’s disease. Neuroscience 1990, 37, 377–385. [Google Scholar] [CrossRef]
- Sassin, I.; Schultz, C.; Thal, D.R.; Rub, U.; Arai, K.; Braak, E.; Braak, H. Evolution of alzheimer’s disease-related cytoskeletal changes in the basal nucleus of meynert. Acta Neuropathol. 2000, 100, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, L.T.; Rub, U.; Ferretti, R.E.; Nitrini, R.; Farfel, J.M.; Polichiso, L.; Gierga, K.; Jacob-Filho, W.; Heinsen, H.; Brazilian Brain Bank Study Group. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in alzheimer’s disease. A precocious onset? Neuropathol. Appl. Neurobiol. 2009, 35, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Grudzien, A.; Shaw, P.; Weintraub, S.; Bigio, E.; Mash, D.C.; Mesulam, M.M. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early alzheimer’s disease. Neurobiol. Aging 2007, 28, 327–335. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.; Cummings, J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol. 2005, 4, 735–742. [Google Scholar] [CrossRef]
- Sultzer, D.L.; Mahler, M.E.; Mandelkern, M.A.; Cummings, J.L.; Van Gorp, W.G.; Hinkin, C.H.; Berisford, M.A. The relationship between psychiatric symptoms and regional cortical metabolism in alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 1995, 7, 476–484. [Google Scholar]
- Hirao, K.; Pontone, G.M.; Smith, G.S. Molecular imaging of neuropsychiatric symptoms in alzheimer’s and parkinson’s disease. Neurosci. Biobehav. Rev. 2015, 49, 157–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cummings, J.L. Toward a molecular neuropsychiatry of neurodegenerative diseases. Ann. Neurol. 2003, 54, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, D.R.; Francis, P.T.; Ballard, C.; Williams, G. Associations between znt3, tau pathology, agitation, and delusions in dementia. Int. J. Geriatr. Psychiatry 2018, 33, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Back, C. The cholinergic hypothesis of neuropsychiatric symptoms in alzheimer’s disease. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 1998, 6, S64–S78. [Google Scholar] [CrossRef]
- Cummings, J.L.; Kaufer, D. Neuropsychiatric aspects of alzheimer’s disease: The cholinergic hypothesis revisited. Neurology 1996, 47, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Russo-Neustadt, A.; Cotman, C.W. Adrenergic receptors in alzheimer’s disease brain: Selective increases in the cerebella of aggressive patients. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 5573–5580. [Google Scholar] [CrossRef]
- Sharp, S.I.; Ballard, C.G.; Chen, C.P.; Francis, P.T. Aggressive behavior and neuroleptic medication are associated with increased number of alpha1-adrenoceptors in patients with alzheimer disease. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2007, 15, 435–437. [Google Scholar] [CrossRef]
- Trillo, L.; Das, D.; Hsieh, W.; Medina, B.; Moghadam, S.; Lin, B.; Dang, V.; Sanchez, M.M.; De Miguel, Z.; Ashford, J.W.; et al. Ascending monoaminergic systems alterations in alzheimer’s disease. Translating basic science into clinical care. Neurosci. Biobehav. Rev. 2013, 37, 1363–1379. [Google Scholar] [CrossRef]
- Matthews, K.L.; Chen, C.P.; Esiri, M.M.; Keene, J.; Minger, S.L.; Francis, P.T. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol. Psychiatry 2002, 51, 407–416. [Google Scholar] [CrossRef]
- Lanari, A.; Amenta, F.; Silvestrelli, G.; Tomassoni, D.; Parnetti, L. Neurotransmitter deficits in behavioural and psychological symptoms of alzheimer’s disease. Mech. Ageing Dev. 2006, 127, 158–165. [Google Scholar] [CrossRef]
- Storga, D.; Vrecko, K.; Birkmayer, J.G.; Reibnegger, G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of alzheimer patients. Neurosci. Lett. 1996, 203, 29–32. [Google Scholar] [CrossRef]
- Tohgi, H.; Ueno, M.; Abe, T.; Takahashi, S.; Nozaki, Y. Concentrations of monoamines and their metabolites in the cerebrospinal fluid from patients with senile dementia of the alzheimer type and vascular dementia of the binswanger type. J. Neural Transm. Park. Dis. Dement. Sect. 1992, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Meguro, K.; Yamaguchi, S.; Ishii, H.; Watanuki, S.; Funaki, Y.; Yamaguchi, K.; Yamadori, A.; Iwata, R.; Itoh, M. Decreased striatal d2 receptor density associated with severe behavioral abnormality in alzheimer’s disease. Ann. Nuclear Med. 2003, 17, 567–573. [Google Scholar] [CrossRef]
- Mann, D.M.; Yates, P.O. Serotonin nerve cells in alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1983, 46, 96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanctot, K.L.; Herrmann, N.; Mazzotta, P. Role of serotonin in the behavioral and psychological symptoms of dementia. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Gil-Bea, F.J.; Diez-Ariza, M.; Chen, C.P.; Francis, P.T.; Lasheras, B.; Ramirez, M.J. Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in alzheimer’s disease. Neuropsychologia 2005, 43, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Ueno, H.; Sato, N.; Shinjo, H.; Morita, Y. Serotonin transporter gene polymorphism and bpsd in mild alzheimer’s disease. J. Alzheimer’s Dis. JAD 2007, 12, 245–253. [Google Scholar] [CrossRef]
- Galimberti, D.; Scarpini, E. Behavioral genetics of neurodegenerative disorders. Curr. Top. Behav. Neurosci. 2012, 12, 615–631. [Google Scholar]
- Norton, N.; Owen, M.J. Htr2a: Association and expression studies in neuropsychiatric genetics. Ann. Med. 2005, 37, 121–129. [Google Scholar] [CrossRef]
- Polesskaya, O.O.; Sokolov, B.P. Differential expression of the “c” and “t” alleles of the 5-ht2a receptor gene in the temporal cortex of normal individuals and schizophrenics. J. Neurosci. Res. 2002, 67, 812–822. [Google Scholar] [CrossRef]
- Serretti, A.; Drago, A.; De Ronchi, D. Htr2a gene variants and psychiatric disorders: A review of current literature and selection of snps for future studies. Curr. Med. Chem. 2007, 14, 2053–2069. [Google Scholar] [PubMed]
- Flirski, M.; Sobow, T.; Kloszewska, I. Behavioural genetics of alzheimer’s disease: A comprehensive review. Arch. Med. Sci. AMS 2011, 7, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, Y.; Le Bastard, N.; Van Hemelrijck, A.; Drinkenburg, W.H.; Engelborghs, S.; De Deyn, P.P. Behavioral correlates of cerebrospinal fluid amino acid and biogenic amine neurotransmitter alterations in dementia. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2013, 9, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.W.; Vinters, H.V.; Cummings, J.L.; Wong, P.T.; Chen, C.P.; Lai, M.K. Alterations in nmda receptor subunit densities and ligand binding to glycine recognition sites are associated with chronic anxiety in alzheimer’s disease. Neurobiol. Aging 2008, 29, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Alexander, R.; Smith, M.A.; Pathak, S.; Kanes, S.; Lee, C.M.; Sanacora, G. Glutamate-based depression gbd. Med. Hypotheses 2012, 78, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Rombolà, L.; Berliocchi, L.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Aging brain: In search for better neurotherapeutics. Confin. Cephalalalgica Neurol. 2017, 27, 65–71. [Google Scholar]
- Tamano, H.; Ide, K.; Adlard, P.A.; Bush, A.I.; Takeda, A. Involvement of hippocampal excitability in amyloid beta-induced behavioral and psychological symptoms of dementia. J. Toxicol. Sci. 2016, 41, 449–457. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Loureiro, J.C.; Pais, M.V.; Stella, F. Recent advances in the management of neuropsychiatric symptoms in dementia. Curr. Opin. Psychiatry 2017, 30, 151–158. [Google Scholar] [CrossRef]
- Ballard, C.; Corbett, A. Agitation and aggression in people with alzheimer’s disease. Curr. Opin. Psychiatry 2013, 26, 252–259. [Google Scholar] [CrossRef]
- Kumar, B.; Kuhad, A.; Kuhad, A. Lumateperone: A new treatment approach for neuropsychiatric disorders. Drugs Today 2018, 54, 713–719. [Google Scholar] [CrossRef]
- Ballard, C.; Banister, C.; Khan, Z.; Cummings, J.; Demos, G.; Coate, B.; Youakim, J.M.; Owen, R.; Stankovic, S.; Investigators, A.D.P. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with alzheimer’s disease psychosis: A phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018, 17, 213–222. [Google Scholar] [CrossRef]
- Ballard, C.G.; Gauthier, S.; Cummings, J.L.; Brodaty, H.; Grossberg, G.T.; Robert, P.; Lyketsos, C.G. Management of agitation and aggression associated with alzheimer disease. Nat. Rev. Neurol. 2009, 5, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G.; O’Brien, J.T.; Reichelt, K.; Perry, E.K. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: The results of a double-blind, placebo-controlled trial with melissa. J. Clin. Psychiatry 2002, 63, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Rombola, L.; Tridico, L.; Scuteri, D.; Sakurada, T.; Sakurada, S.; Mizoguchi, H.; Avato, P.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Bergamot essential oil attenuates anxiety-like behaviour in rats. Molecules 2017, 22, 614. [Google Scholar] [CrossRef] [PubMed]
- Rombolà, L.; Scuteri, D.; Adornetto, A.; Straface, A.; Sakurada, T.; Sakurada, S.; Mizoguchi, H.; Corasaniti, M.T.; Bagetta, G.; Tonin, P.; et al. Anxiolytic-like effects of bergamot essential oil are insensitive to flumazenil in rat. Evid. Based Complement. Altern. Med. 2019. submitted. [Google Scholar]
- Rombola, L.; Corasaniti, M.T.; Rotiroti, D.; Tassorelli, C.; Sakurada, S.; Bagetta, G.; Morrone, L.A. Effects of systemic administration of the essential oil of bergamot (BEO) on gross behaviour and eeg power spectra recorded from the rat hippocampus and cerebral cortex. Funct. Neurol. 2009, 24, 107–112. [Google Scholar] [PubMed]
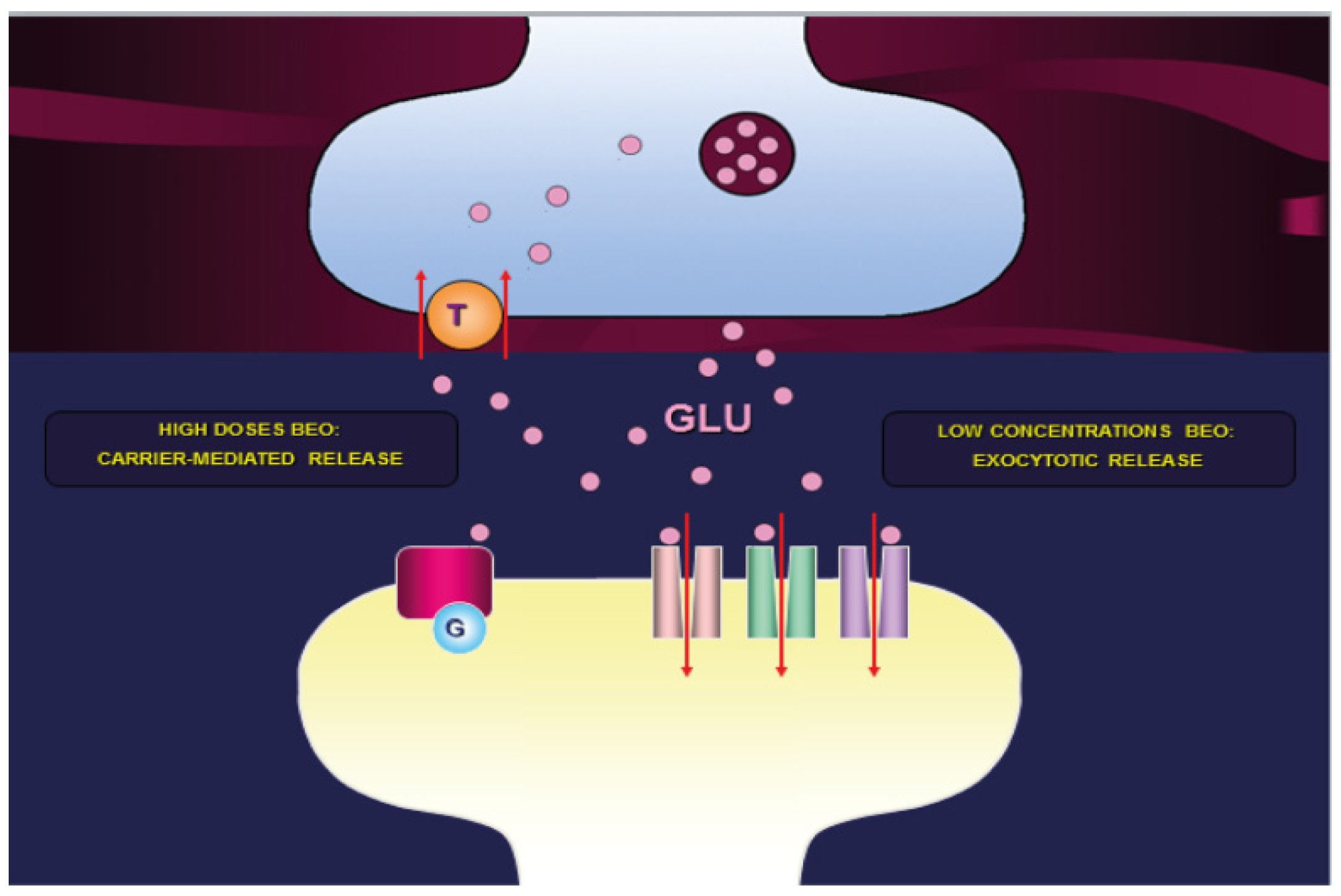
- Morrone, L.A.; Rombola, L.; Pelle, C.; Corasaniti, M.T.; Zappettini, S.; Paudice, P.; Bonanno, G.; Bagetta, G. The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: Implication of monoterpene hydrocarbons. Pharmacol. Res. 2007, 55, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Pittaluga, A. Presynaptic release-regulating mglu1 receptors in central nervous system. Front. Pharmacol. 2016, 7, 295. [Google Scholar] [CrossRef]
- Scuteri, D.; Rombola, L.; Tridico, L.; Mizoguchi, H.; Watanabe, C.; Sakurada, T.; Sakurada, S.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Neuropharmacological properties of the essential oil of bergamot for the clinical management of pain-related bpsds. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Morrone, L.A.; Scuteri, D.; Rombola, L.; Mizoguchi, H.; Bagetta, G. Opioids resistance in chronic pain management. Curr. Neuropharmacol. 2017, 15, 444–456. [Google Scholar] [CrossRef]
- Scuteri, D.; Adornetto, A.; Rombola, L.; Naturale, M.D.; Morrone, L.A.; Bagetta, G.; Tonin, P.; Corasaniti, M.T. New trends in migraine pharmacology: Targeting calcitonin gene-related peptide (cgrp) with monoclonal antibodies. Front. Pharmacol. 2019, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Adornetto, A.; Rombolà, L.; Naturale, M.D.; De Francesco, A.E.; Esposito, S.; Zito, M.; Morrone, L.A.; Bagetta, G.; Tonin, P.; et al. Pattern of prescription of triptans in calabria region. Front. Neurol. 2019. submitted. [Google Scholar]
- Sengstaken, E.A.; King, S.A. The problems of pain and its detection among geriatric nursing home residents. J. Am. Geriatr. Soc. 1993, 41, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Sampson, E.L.; White, N.; Lord, K.; Leurent, B.; Vickerstaff, V.; Scott, S.; Jones, L. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: A longitudinal cohort study. Pain 2015, 156, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Scherder, E.; Herr, K.; Pickering, G.; Gibson, S.; Benedetti, F.; Lautenbacher, S. Pain in dementia. Pain 2009, 145, 276–278. [Google Scholar] [CrossRef]
- Husebo, B.S.; Ballard, C.; Aarsland, D. Pain treatment of agitation in patients with dementia: A systematic review. Int. J. Geriatr. Psychiatry 2011, 26, 1012–1018. [Google Scholar] [CrossRef]
- Ballard, C.; Smith, J.; Husebo, B.; Aarsland, D.; Corbett, A. The role of pain treatment in managing the behavioural and psychological symptoms of dementia (BPSD). Int. J. Palliat. Nurs. 2011, 17, 420–424. [Google Scholar] [CrossRef]
- Scuteri, D.; Piro, B.; Morrone, L.A.; Corasaniti, M.T.; Vulnera, M.; Bagetta, G. The need for better access to pain treatment: Learning from drug consumption trends in the USA. Funct. Neurol. 2017, 22, 229–230. [Google Scholar] [CrossRef]
- Scuteri, D.; Garreffa, M.R.; Esposito, S.; Bagetta, G.; Naturale, M.D.; Corasaniti, M.T. Evidence for accuracy of pain assessment and painkillers utilization in neuropsychiatric symptoms of dementia in calabria region, italy. Neural Regen. Res. 2018, 13, 1619–1621. [Google Scholar]
- Husebo, B.S.; Ballard, C.; Sandvik, R.; Nilsen, O.B.; Aarsland, D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: Cluster randomised clinical trial. BMJ 2011, 343, d4065. [Google Scholar] [CrossRef]
- Scuteri, D.; Morrone, L.A.; Rombola, L.; Avato, P.R.; Bilia, A.R.; Corasaniti, M.T.; Sakurada, S.; Sakurada, T.; Bagetta, G. Aromatherapy and aromatic plants for the treatment of behavioural and psychological symptoms of dementia in patients with alzheimer’s disease: Clinical evidence and possible mechanisms. Evid.-Based complement. Altern. Med. eCAM 2017, 2017, 9416305. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Kuwahata, H.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S. Intraplantar injection of bergamot essential oil into the mouse hindpaw: Effects on capsaicin-induced nociceptive behaviors. Int. Rev. Neurobiol. 2009, 85, 237–248. [Google Scholar] [PubMed]
- Sakurada, T.; Mizoguchi, H.; Kuwahata, H.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S. Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol. Biochem. Behav. 2011, 97, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, S.; Otowa, A.; Kamio, S.; Sato, K.; Yagi, T.; Kishikawa, Y.; Komatsu, T.; Bagetta, G.; Sakurada, T.; Nakamura, H. Effect of plantar subcutaneous administration of bergamot essential oil and linalool on formalin-induced nociceptive behavior in mice. Biomed. Res. 2015, 36, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bagetta, G.; Morrone, L.A.; Rombola, L.; Amantea, D.; Russo, R.; Berliocchi, L.; Sakurada, S.; Sakurada, T.; Rotiroti, D.; Corasaniti, M.T. Neuropharmacology of the essential oil of bergamot. Fitoterapia 2010, 81, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kuwahata, H.; Komatsu, T.; Katsuyama, S.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S.; Sakurada, T.; Takahama, K. Peripherally injected linalool and bergamot essential oil attenuate mechanical allodynia via inhibiting spinal erk phosphorylation. Pharmacol. Biochem. Behav. 2013, 103, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Crudo, M.; Rombola, L.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Sakurada, T.; Greco, R.; Corasaniti, M.T.; Morrone, L.A.; et al. Antinociceptive effect of inhalation of the essential oil of bergamot in mice. Fitoterapia 2018, 129, 20–24. [Google Scholar] [CrossRef]
- Vance, D. Considering olfactory stimulation for adults with age-related dementia. Percept. Motor Skills 1999, 88, 398–400. [Google Scholar] [CrossRef]
- Gratteri, S.; Scuteri, D.; Gaudio, R.M.; Monteleone, D.; Ricci, P.; Avato, F.M.; Bagetta, G.; Morrone, L.A. Benefits and risks associated with cannabis and cannabis derivatives use. Confin. Cephalalalgica Neurol. 2017, 27, 109–116. [Google Scholar]
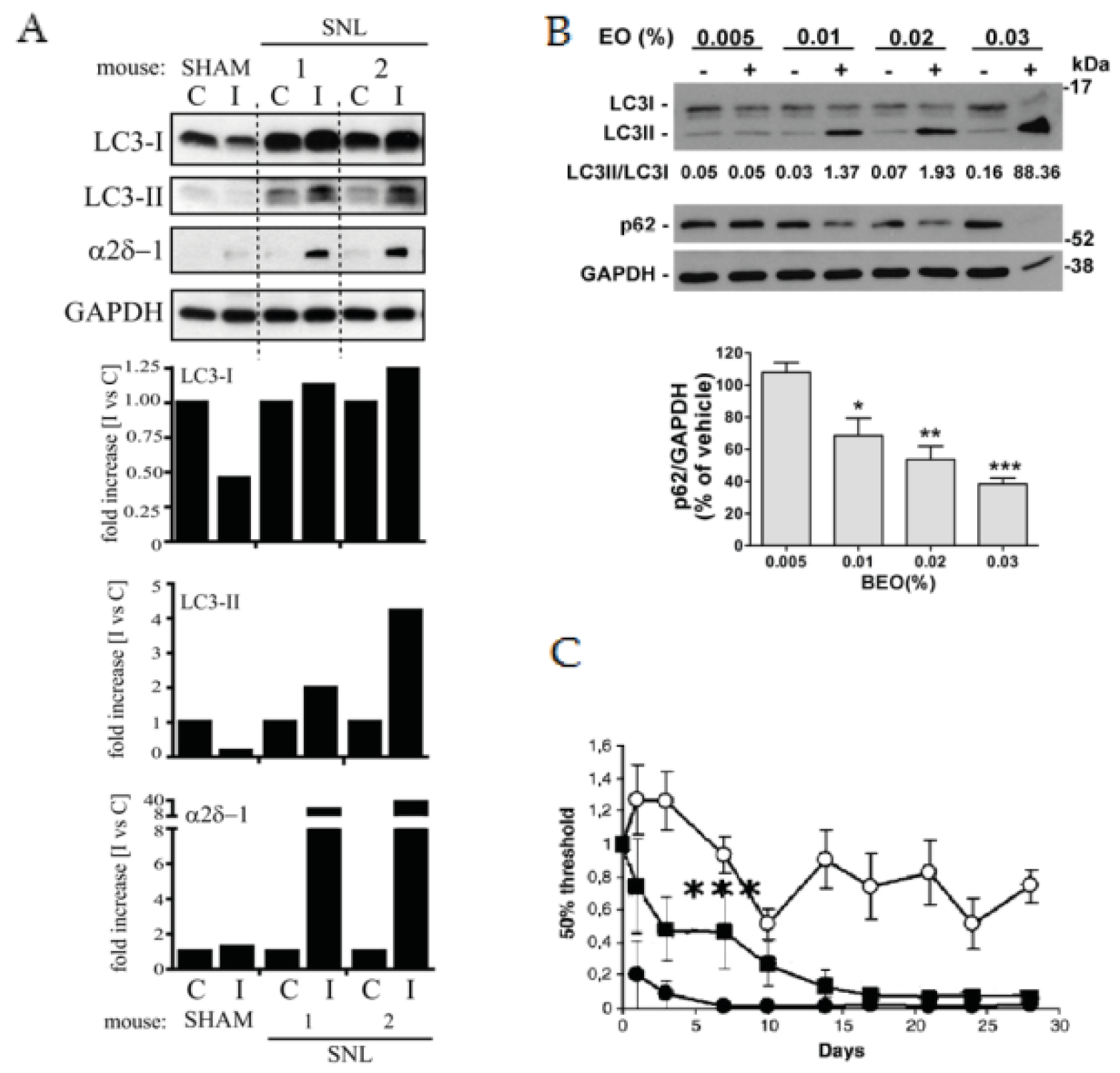
- Berliocchi, L.; Russo, R.; Maiaru, M.; Levato, A.; Bagetta, G.; Corasaniti, M.T. Autophagy impairment in a mouse model of neuropathic pain. Mol. Pain 2011, 7, 83. [Google Scholar] [CrossRef]
- Pereira, G.J.; Antonioli, M.; Hirata, H.; Ureshino, R.P.; Nascimento, A.R.; Bincoletto, C.; Vescovo, T.; Piacentini, M.; Fimia, G.M.; Smaili, S.S. Glutamate induces autophagy via the two-pore channels in neural cells. Oncotarget 2017, 8, 12730–12740. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Chuang, C.C.; Lewis, A.M.; Aley, P.K.; Brailoiu, E.; Dun, N.J.; Churchill, G.C.; Patel, S. Recruitment of naadp-sensitive acidic Ca2+ stores by glutamate. Biochem. J. 2009, 422, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Cassiano, M.G.; Ciociaro, A.; Adornetto, A.; Varano, G.P.; Chiappini, C.; Berliocchi, L.; Tassorelli, C.; Bagetta, G.; Corasaniti, M.T. Role of d-limonene in autophagy induced by bergamot essential oil in sh-sy5y neuroblastoma cells. PLoS ONE 2014, 9, e113682. [Google Scholar] [CrossRef] [PubMed]
- Zucchella, C.; Sinforiani, E.; Tamburin, S.; Federico, A.; Mantovani, E.; Bernini, S.; Casale, R.; Bartolo, M. The multidisciplinary approach to alzheimer’s disease and dementia. A narrative review of non-pharmacological treatment. Front. Neurol. 2018, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Rombolà, L.; Morrone, L.A.; Monteleone, D.; Corasaniti, M.T.; Sakurada, T.; Sakurada, S.; Bagetta, G. Exploitation of aromatherapy in dementia—Impact on pain and neuropsychiatric symptoms. In The Neuroscience of Dementia: Diagnosis and Management; Preedy, V.R., Martin, C.R., Eds.; Academic Press: San Diego, CA, USA, 2019; in press. [Google Scholar]
| Analgesic Effect | Pain Model | Route of Administration | Main Results of the Research | Study |
|---|---|---|---|---|
| Antinociceptive effect on licking/biting response | Capsaicin test [73,74] | Intraplantar [73] | BEO (5, 10 and 20 mg) exerted antinociceptive effect in the capsaicin test (50 µg) [73]. | Sakurada et al., 2009 [73] |
| Subcutaneous into the plantar surface [74] | BEO (20 μg) produced significant antinociception in capsaicin test (1.6 μg), only in the ipsilateral side, reverted by naloxone hydrochloride and methiodide, suggesting a role of peripheral opioid system [74] | Sakurada et al., 2011 [74] | ||
| Formalin test [75,78]. | Plantar subcutaneous [75] | BEO (10 μg) significantly inhibited the nociceptive response to 2% formalin, only in the ipsilateral side, and this effect was antagonized by naloxone hydrochloride and methiodide [75] | Katsuyama et al., 2015 [75] | |
| Inhalatory [78] | A filter paper disc soaked with different volumes of BEO (100, 200, 400, 800 μL) to the edge of the cage allowed inhalation of BEO in different experimental settings, showing its antinociceptive activity in formalin test (2%) in a volume and time of exposure dependent manner [78] | Scuteri et al., 2018 [78] | ||
| Antiallodynic effect | Spinal nerve ligation [76] | Subcutaneous into the plantar surface [76] | BEO (1 mL/kg) subcutaneously administered daily for 7 days attenuated mechanical allodynia [76] | Bagetta et al., 2010 [76] |
| Partial sciatic nerve ligation [77] | Subcutaneous into the plantar surface [77] | On post-operative day 7, BEO (5.0, 10.0 and 20.0 μg) dose-dependently increased ipsilateral hindpaw withdrawal thresholds and blocked spinal ERK activation [77]. | Kuwahata et al., 2013 [77] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuteri, D.; Rombolà, L.; Morrone, L.A.; Bagetta, G.; Sakurada, S.; Sakurada, T.; Tonin, P.; Corasaniti, M.T. Neuropharmacology of the Neuropsychiatric Symptoms of Dementia and Role of Pain: Essential Oil of Bergamot as a Novel Therapeutic Approach. Int. J. Mol. Sci. 2019, 20, 3327. https://doi.org/10.3390/ijms20133327
Scuteri D, Rombolà L, Morrone LA, Bagetta G, Sakurada S, Sakurada T, Tonin P, Corasaniti MT. Neuropharmacology of the Neuropsychiatric Symptoms of Dementia and Role of Pain: Essential Oil of Bergamot as a Novel Therapeutic Approach. International Journal of Molecular Sciences. 2019; 20(13):3327. https://doi.org/10.3390/ijms20133327
Chicago/Turabian StyleScuteri, Damiana, Laura Rombolà, Luigi Antonio Morrone, Giacinto Bagetta, Shinobu Sakurada, Tsukasa Sakurada, Paolo Tonin, and Maria Tiziana Corasaniti. 2019. "Neuropharmacology of the Neuropsychiatric Symptoms of Dementia and Role of Pain: Essential Oil of Bergamot as a Novel Therapeutic Approach" International Journal of Molecular Sciences 20, no. 13: 3327. https://doi.org/10.3390/ijms20133327
APA StyleScuteri, D., Rombolà, L., Morrone, L. A., Bagetta, G., Sakurada, S., Sakurada, T., Tonin, P., & Corasaniti, M. T. (2019). Neuropharmacology of the Neuropsychiatric Symptoms of Dementia and Role of Pain: Essential Oil of Bergamot as a Novel Therapeutic Approach. International Journal of Molecular Sciences, 20(13), 3327. https://doi.org/10.3390/ijms20133327







