c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer
Abstract
1. Introduction
2. Results
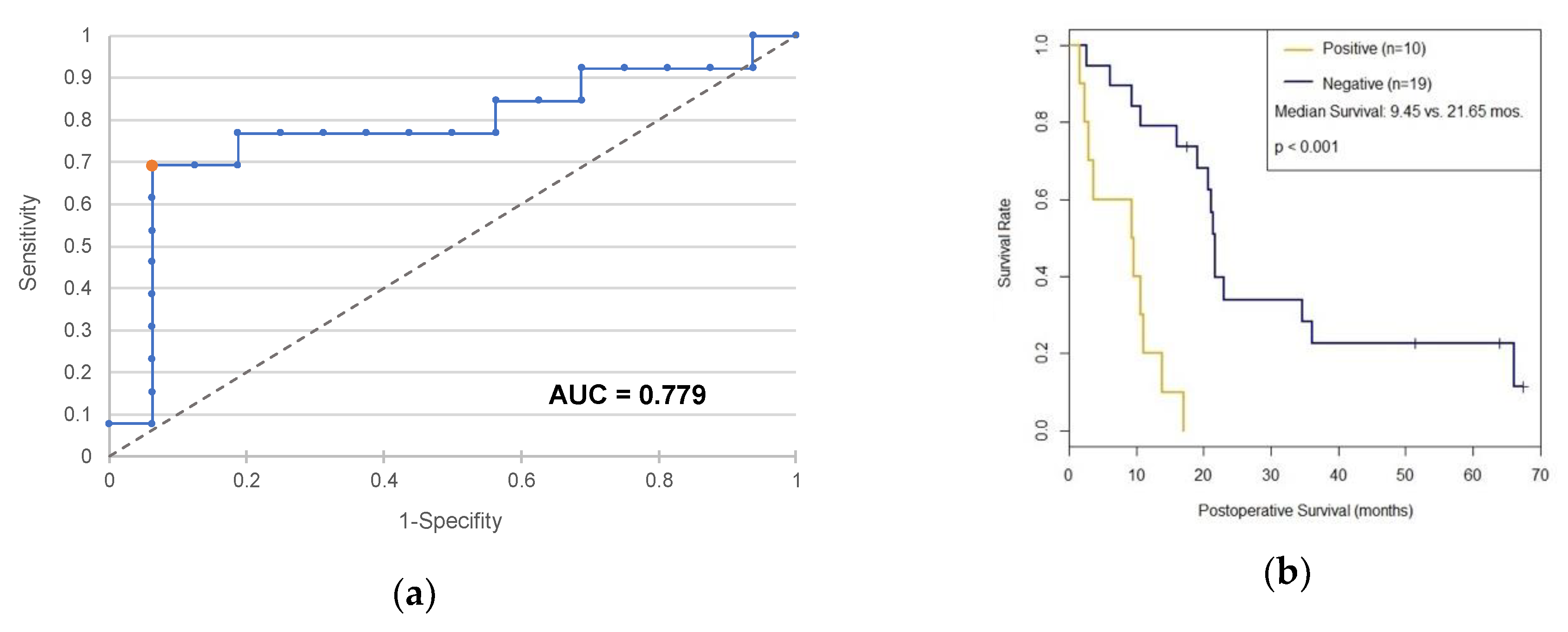
2.1. c-Met as a Diagnostic and Prognostic Marker
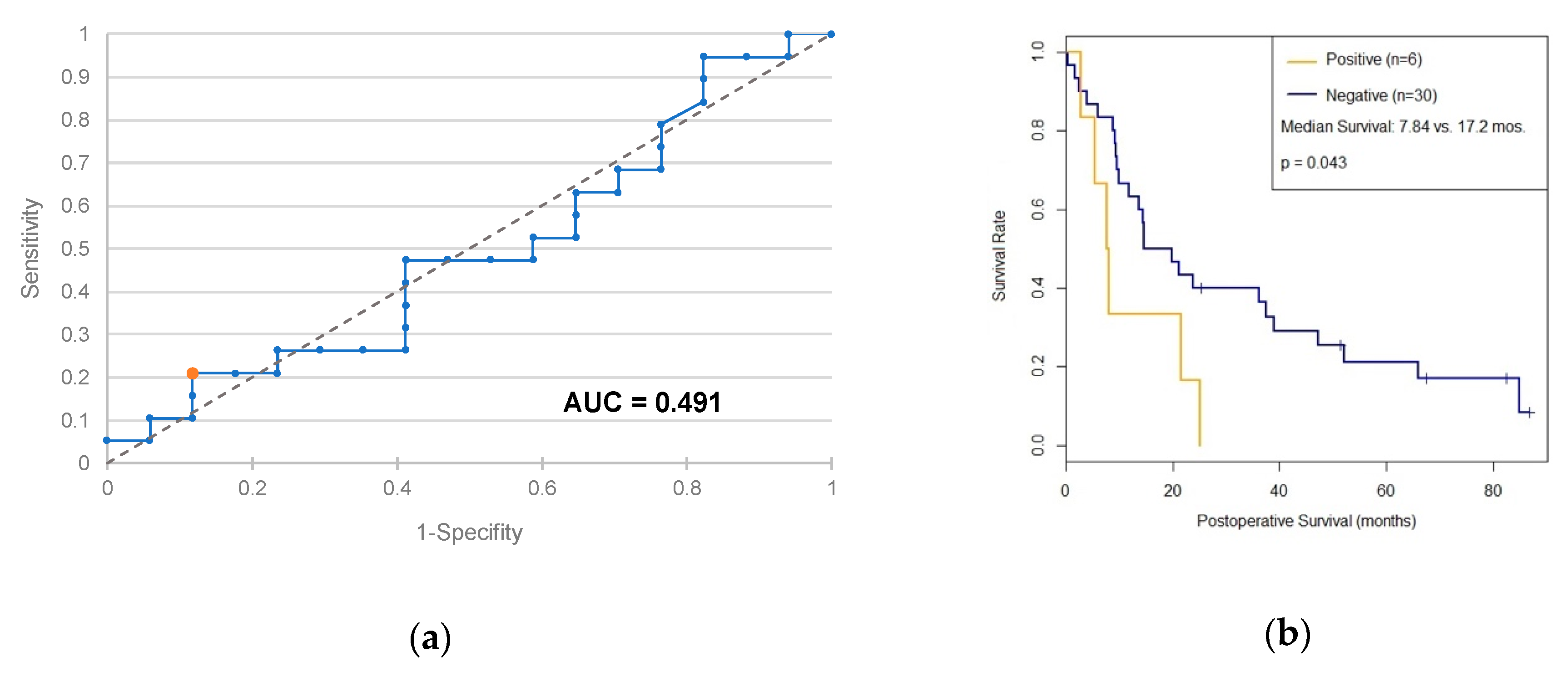
2.2. PD-L1 as a Diagnostic and Prognostic Marker
3. Discussion
3.1. c-Met
3.2. PD-L1
4. Materials and Methods
4.1. Cell Culture Media
4.2. Patient Sera Collection and Processing
4.3. Exosome Isolation
4.4. Exosome Staining and Flow Cytometry Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| AUC | Area Under the Curve |
| BSA | Bovine Serum Albumin |
| CA 19-9 | Carbohydrate Antigen 19-9 |
| c-Met | Proto-oncogene Mesenchymal-epithelial Transition Factor |
| CP | Chronic Pancreatitis |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DPBS | Dulbecco’s Phosphate Buffered Saline |
| EV | Extracellular Vesicles |
| FBS | Fetal Bovine Serum |
| HEPES | 4-(2-Hydroxyethyl)-1-Piperazineethanesulfonic Acid |
| HGFR | Hepatocyte Growth Factor Receptor |
| HPDE | Human Pancreatic Duct Epithelial Cell Line |
| KSFM | Keratinocyte Serum-free Medium |
| MAPK | Mitogen-activated Protein Kinase |
| (m)RNA | (Messenger) Ribonucleic Acid |
| NF-κB | Nuclear Factor “Kappa Light Chain Enhancer” of Activated B-Cells |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Cell Death 1 Ligand 1 |
| PI3K | Phosphoinositide-3-Kinase |
| ROC | Receiver Operator Characteristics |
| RPMI | Roswell Park Memorial Institute |
| SCA | Serous Cyst Adenoma |
| STAT | Signal Transducers and Activators of Transcription |
References
- Kaatsch, P.; Spix, C.; Katalinic, A.; Hentschel, S.; Luttmann, S.; Stegmaier, C.; Waldeyer-Sauerland, M.; Waldmann, A.; Caspritz, S.; Chris, M.; et al. Krebs in Deutschland für 2013/2014, 11th ed.; Robert Koch-Institut: Berlin, Germany, 2017. [Google Scholar]
- Rahbari, M.; Rahbari, N.; Reissfelder, C.; Weitz, J.; Kahlert, C. Exosomes: Novel implications in diagnosis and treatment of gastrointestinal cancer. Langenbecks Arch. Surg. 2016, 401, 1097–1110. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Bottaro, D.P.; Rubin, J.S.; Faletto, D.L.; Chan, A.M.; Kmiecik, T.E.; Vande Woude, G.F.; Aaronson, S.A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991, 251, 802–804. [Google Scholar] [CrossRef]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef]
- Di Renzo, M.F.; Poulsom, R.; Olivero, M.; Comoglio, P.M.; Lemoine, N.R. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995, 55, 1129–1138. [Google Scholar]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 2016, 9, 5023–5039. [Google Scholar]
- Nones, K.; Waddell, N.; Song, S.; Patch, A.-M.; Miller, D.; Johns, A.; Wu, J.; Kassahn, K.S.; Wood, D.; Bailey, P.; et al. Genome-wide DNA methylation patterns in pancreatic ductal adenocarcinoma reveal epigenetic deregulation of SLIT-ROBO, ITGA2 and MET signaling. Int. J. Cancer 2014, 135, 1110–1118. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.-M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Neuzillet, C.; Couvelard, A.; Tijeras-Raballand, A.; de Mestier, L.; de Gramont, A.; Bédossa, P.; Paradis, V.; Sauvanet, A.; Bachet, J.-B.; Ruszniewski, P.; et al. High c-Met expression in stage I-II pancreatic adenocarcinoma: Proposal for an immunostaining scoring method and correlation with poor prognosis. Histopathology 2015, 67, 664–676. [Google Scholar] [CrossRef]
- Maus, M.K.H.; Stephens, C.; Waldschmidt, D.; Roll, A.; Astrow, S.H.; Steffen, H.-M.; Hsiang, J.; Zeger, G.; Hölscher, A.H.; Grimminger, P.P. C-MET mRNA expression in pancreatic ductal adenocarcinoma and stromal tissue: Prognostic and therapeutic implications. J. Clin. Oncol. 2014, 32, e15199. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar]
- Wang, L.; Ma, Q.; Chen, X.; Guo, K.; Li, J.; Zhang, M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J. Surg. 2010, 34, 1059–1065. [Google Scholar] [CrossRef]
- Chen, X.-L.; Yuan, S.-X.; Chen, C.; Mao, Y.-X.; Xu, G.; Wang, X.-Y. Expression of B7-H1 protein in human pancreatic carcinoma tissues and its clinical significance. Ai Zheng Aizheng Chin. J. Cancer 2009, 28, 1328–1332. [Google Scholar] [CrossRef]
- Geng, L.; Huang, D.; Liu, J.; Qian, Y.; Deng, J.; Li, D.; Hu, Z.; Zhang, J.; Jiang, G.; Zheng, S. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J. Cancer Res. Clin. Oncol. 2008, 134, 1021–1027. [Google Scholar] [CrossRef]
- Kourembanas, S. Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef]
- Foley, K.; Kim, V.; Jaffee, E.; Zheng, L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016, 381, 244–251. [Google Scholar] [CrossRef]
- Feng, M.; Xiong, G.; Cao, Z.; Yang, G.; Zheng, S.; Song, X.; You, L.; Zheng, L.; Zhang, T.; Zhao, Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017, 407, 57–65. [Google Scholar] [CrossRef]
- Rückert, F.; Aust, D.; Böhme, I.; Werner, K.; Brandt, A.; Diamandis, E.P.; Krautz, C.; Hering, S.; Saeger, H.-D.; Grützmann, R.; et al. Five primary human pancreatic adenocarcinoma cell lines established by the outgrowth method. J. Surg. Res. 2012, 172, 29–39. [Google Scholar] [CrossRef]
- Rückert, F.; Werner, K.; Aust, D.; Hering, S.; Saeger, H.-D.; Grützmann, R.; Pilarsky, C. Establishment and Characterization of Six Primary Pancreatic Cancer Cell Lines. Austin J. Cancer Clin. Res. 2015, 2, 1055. [Google Scholar] [CrossRef]
- Herbert, R. Confidence Interval Calculator; Institute for Musculoskeletal Health: Sydney, Australia, 2013. [Google Scholar]
- Haker, S.; Wells, W.M.; Warfield, S.K.; Talos, I.-F.; Bhagwat, J.G.; Goldberg-Zimring, D.; Mian, A.; Ohno-Machado, L.; Zou, K.H. Combining Classifiers Using Their Receiver Operating Characteristics and Maximum Likelihood Estimation. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2005; Duncan, J.S., Gerig, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3749, pp. 506–514. ISBN 978-3-540-29327-9. [Google Scholar]



| Variable | PDAC | SCA | CP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | c-Met | PD-L1 | Total | c-Met | PD-L1 | Total | c-Met | PD-L1 | |
| Total | 55 | 30 | 36 | 10 | 10 | 0 | 24 | 10 | 17 |
| Male | 52.7% | 53.3% | 52.8% | 30% | 30% | - | 70.8% | 90% | 64.7% |
| Female | 47.3% | 46.7% | 47.2% | 70% | 70% | - | 29.2% | 10% | 35.3% |
| Median Age (yrs.) | 69 | 66.5 | 70.5 | 73 | 73 | - | 51 | 48 | 53 |
| Mean Age (yrs.) | 66.7 | 66.2 | 68.4 | 68.4 | 68.4 | - | 50.5 | 49.4 | 51.5 |
| Variable | T | N | M | Intention | ||||
|---|---|---|---|---|---|---|---|---|
| T1–2 | T3–4 | N0 | Npos | M0 | Mpos | Curative | Unresectable | |
| Total | 1.8% | 83.6% | 20% | 58.2% | 78.2% | 14.5% | 79.2% | 21.8% |
| c-Met | 3.3% | 90% | 23.3% | 70% | 93.3% | 6.7% | 93.3% | 6.7% |
| PD-L1 | 0% | 77.8% | 22.2% | 44.4% | 66.7% | 22.2% | 66.7% | 33.3% |
| PDAC | Non-malignant | SCA | CP | |
|---|---|---|---|---|
| Lower Endpoint | 629.2 | 175.7 | 45.4 | 153.8 |
| Mean Estimator | 889.4 | 356.8 | 228.8 | 487.7 |
| Upper Endpoint | 1149.6 | 537.8 | 412.2 | 815.6 |
| *** p < 0.001 | ||||
| PDAC | CP | |
|---|---|---|
| Lower Endpoint | 107.4 | 90.1 |
| Mean Estimator | 233.1 | 287.0 |
| Upper Endpoint | 358.9 | 483.9 |
| Cut-Off Value | Sensitivity | Specificity | Odds Ratio |
|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | |
| c-Met >495 | 70% | 85% | 13.2 |
| (52.1%–83.3%) | (64.0%–94.8%) | (3.1–56.6) | |
| CA 19-9 >37 U/mL | 72.4% | 84.2% | 14.0 |
| (54.3%–85.3%) | (54.3%–94.5%) | (3.2–61.4) | |
| c-Met >543 or CA 19-9 >97 U/mL | 72.4% | 89.5% | 22.2 |
| (54.3%–85.3%) | (68.6%–97.1%) | (4.2–119.3) |
| Resectable | Unresectable | |
|---|---|---|
| PD-L1 pos. | 1 | 5 |
| PD-L1 neg. | 23 | 7 |
| * p = 0.0102 | ||
| Cell Line | Original Tissue | Provider | Cell Medium | Supplements |
|---|---|---|---|---|
| AsPc1 | ascites metastases | ATCC | RPMI1640 | 10% FBS 10 mM HEPES 1 mM sodium pyruvate 4.5 g/l Glucose |
| BxPc-3 | primary tumor | |||
| HPDE6-E6E7c7 | benign tissue | Ming-Sound Tsao, Toronto | KSFM | 10% FBS |
| MiaPaCa-2 | primary tumor | ATCC | DMEM | 10% FBS 2.5% horse serum |
| PaCaDD 119 | primary tumor | Surgical Laboratory, University Hospital Dresden [21,22] | 2/3 DMEM 1/3 KSFM | 20% FBS 1:100 Antibiotic/-mycotic |
| PaCaDD 137 | primary tumor | |||
| PaCaDD 161 | liver metastases | |||
| PaCaDD 165 | ascites metastases | |||
| PaCaDD 183 | ascites metastases | |||
| Panc-1 | primary tumor | ATCC | RPMI1640 | 10% FBS |
| Su.86.88 | liver metastases |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lux, A.; Kahlert, C.; Grützmann, R.; Pilarsky, C. c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 3305. https://doi.org/10.3390/ijms20133305
Lux A, Kahlert C, Grützmann R, Pilarsky C. c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. International Journal of Molecular Sciences. 2019; 20(13):3305. https://doi.org/10.3390/ijms20133305
Chicago/Turabian StyleLux, Alexander, Christoph Kahlert, Robert Grützmann, and Christian Pilarsky. 2019. "c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer" International Journal of Molecular Sciences 20, no. 13: 3305. https://doi.org/10.3390/ijms20133305
APA StyleLux, A., Kahlert, C., Grützmann, R., & Pilarsky, C. (2019). c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. International Journal of Molecular Sciences, 20(13), 3305. https://doi.org/10.3390/ijms20133305





