Disturbed Cardiorespiratory Adaptation in Preeclampsia: Return to Normal Stress Regulation Shortly after Delivery?
Abstract
1. Introduction
2. Results
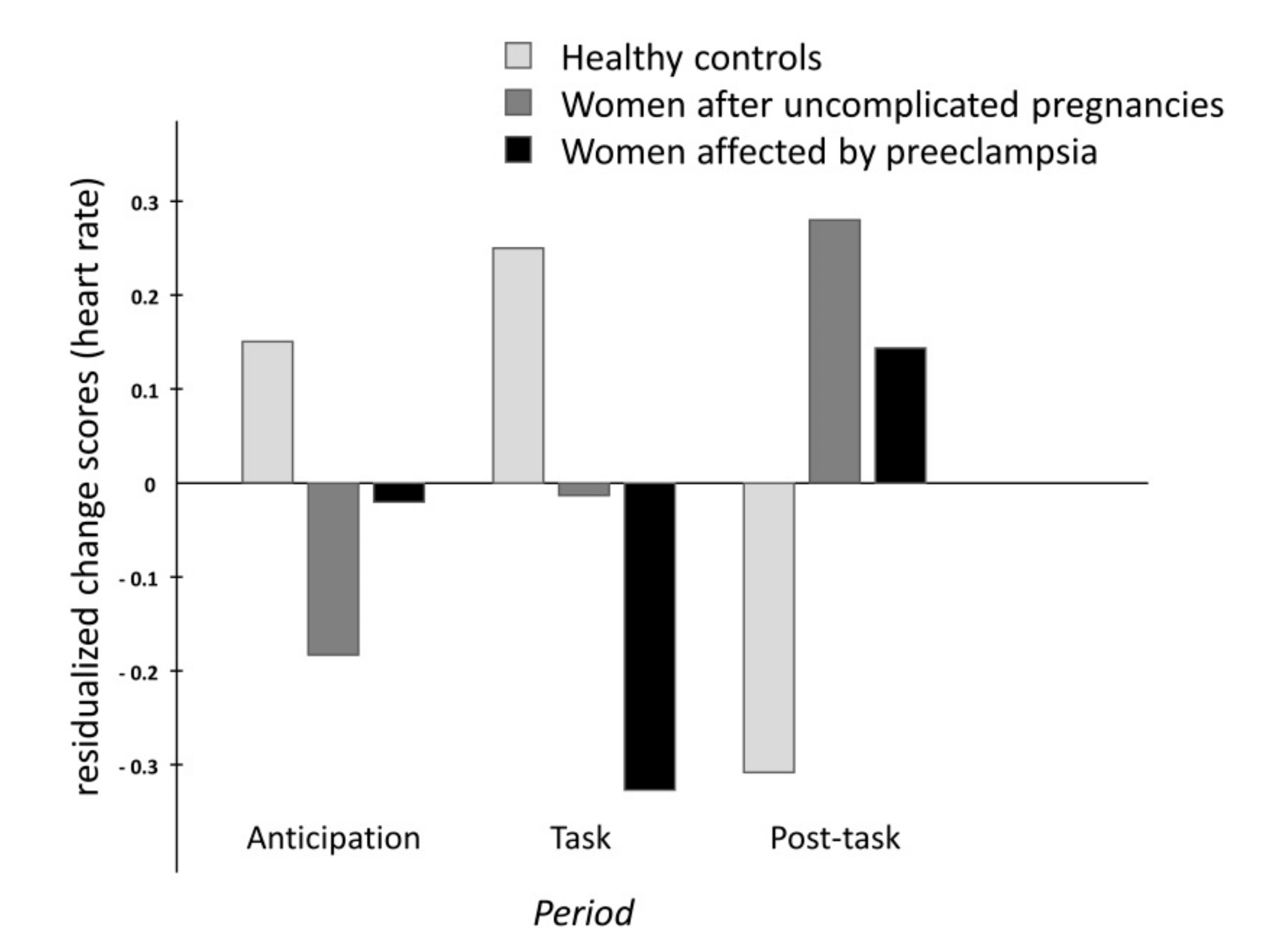
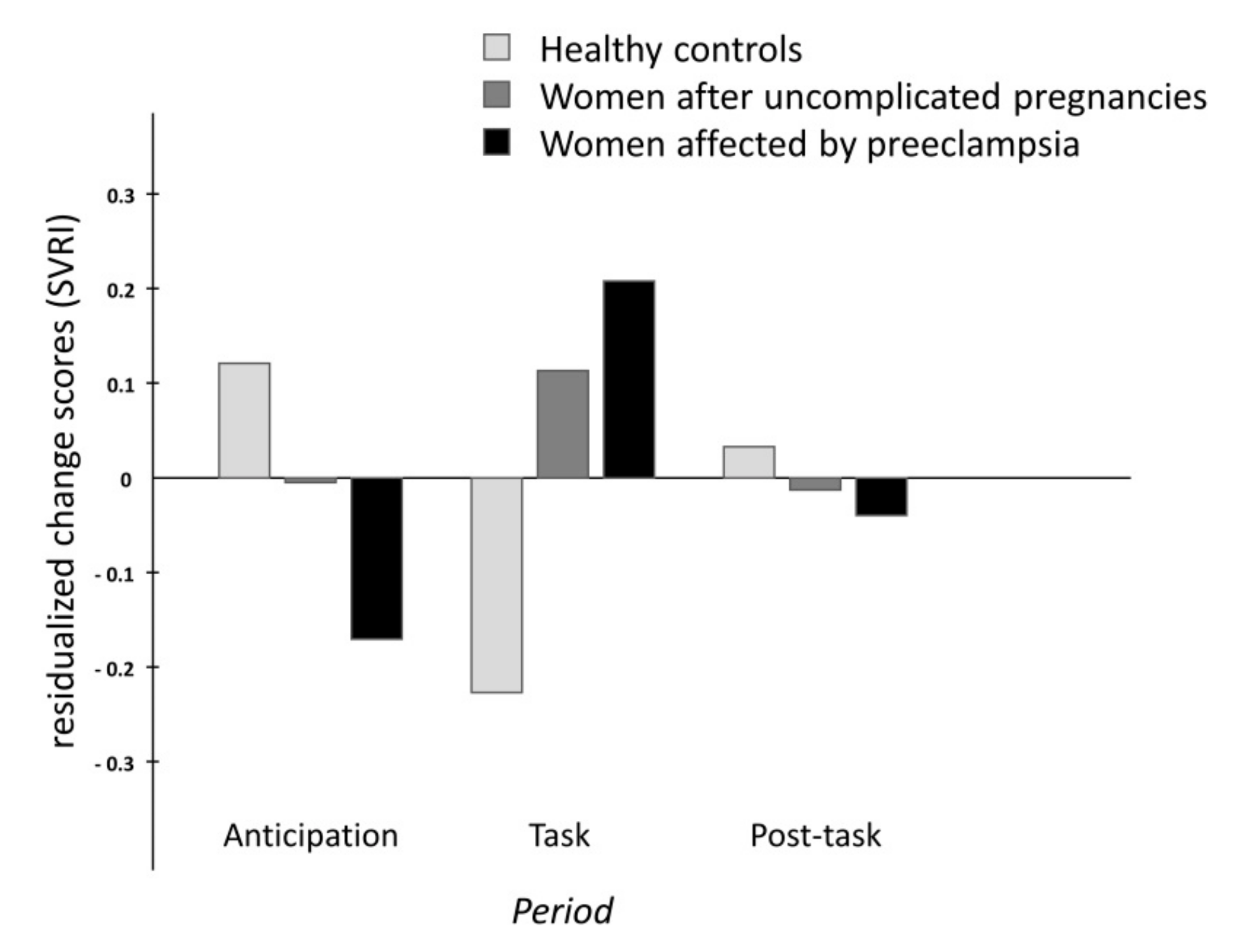
2.1. Cardiovascular and Hemodynamic Variables
2.2. Respiration Rate and Baroreflex Sensitivity
2.3. Adjustment of Blood Pressure, R–R Intervals, and Respiration
2.3.1. Low-Frequency Components
2.3.2. High-Frequency Components
2.4. Supplementary Analyses
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Experimental Procedure
4.3. Data Acquisition and Preprocessing
4.4. Analysis Procedure Using Phase Synchronization
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| BRS | Baroreflex reflex sensitivity |
| CO | Women without gestation during the last three years |
| DBP | Diastolic blood pressure |
| MAP | Mean arterial pressure |
| PE | Women with a history of preeclampsia |
| SBP | Systolic blood pressure |
| SI | Stroke index |
| SVRI | Systemic vascular resistance index |
| UP | Women with uncomplicated pregnancies |
References
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Keyes, K.M.; Wapner, R.J. Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. BMJ 2013, 347, f6564. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Bell, M.J. If we know so much about preeclampsia, why haven’t we cured the disease? J. Reprod Immunol. 2013, 99, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Ziadie, M.S.; McIntire, D.D.; Rogers, B.B.; Leveno, K.J. Placental pathology suggesting that preeclampsia is more than one disease. Am. J. Obstet. Gynecol. 2014, 210, 66.e1–66.e7. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.A.; Schlitt, J.M.; Jackson, D.L.; Schulz, L.C.; Schust, D.J. Preeclampsia: Multiple approaches for a multifactorial disease. Dis. Model. Mech. 2012, 5, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lakovschek, I.C.; Ulrich, D.; Jauk, S.; Csapo, B.; Kolovetsiou-Kreiner, V.; Mayer-Pickel, K.; Stern, C.; Lang, U.; Obermayer-Pietsch, B.; Cervar-Zivkovic, M. Risk assessment for preterm preeclampsia in first trimester: Comparison of three calculation algorithms. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Nzelu, D.; Dumitrascu-Biris, D.; Nicolaides, K.H.; Kametas, N.A. Chronic hypertension: First-trimester blood pressure control and likelihood of severe hypertension, preeclampsia, and small for gestational age. Am. J. Obstet. Gynecol. 2018, 218, 337.e1–337.e7. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef]
- Palei, A.C.; Spradley, F.T.; Warrington, J.P.; George, E.M.; Granger, J.P. Pathophysiology of hypertension in pre-eclampsia: A lesson in integrative physiology. Acta Physiol. 2013, 208, 224–233. [Google Scholar] [CrossRef]
- Sohlberg, S.; Mulic-Lutvica, A.; Lindgren, P.; Ortiz-Nieto, F.; Wikström, A.K.; Wikström, J. Placental perfusion in normal pregnancy and early and late preeclampsia: A magnetic resonance imaging study. Placenta 2014, 35, 202–206. [Google Scholar] [CrossRef]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Perry, H.; Khalil, A.; Thilaganathan, B. Preeclampsia and the cardiovascular system: An update. Trends Cardiovasc. Med. 2018, 28, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, K.; Sutherland, G.R.; Baltabaeva, A.; Liberati, M.; Thilaganathan, B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension 2011, 57, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Duhig, K.E.; Myers, J.; Seed, P.T.; Sparkes, J.; Lowe, J.; Hunter, R.M.; Shennan, A.H.; Chappell, L.C.; PARROT trial group. Placental growth factor testing to assess women with suspected pre-eclampsia: A multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 2019, 393, 1807–1818. [Google Scholar] [CrossRef]
- Ciobanu, A.; Wright, A.; Panaitescu, A.; Syngelaki, A.; Wright, D.; Nicolaides, K.H. Prediction of imminent preeclampsia at 35–37 weeks gestation. Am. J. Obstet. Gynecol. 2019. [Google Scholar] [CrossRef]
- Sattar, N.; Greer, I.A. Pregnancy complications and maternal cardiovascuar risk: Opportunities for intervention and prevention. BMJ 2002, 325, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes. 2017, 10. [Google Scholar] [CrossRef]
- Sattar, N.; Ramsay, J.; Crawford, L.; Cheyne, H.; Greer, I.A. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension 2003, 42, 39–42. [Google Scholar] [CrossRef]
- Thilaganathan, B.; Kalafat, E. Cardiovascular System in Preeclampsia and Beyond. Hypertension 2019, 73, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Catov, J.M.; Ouyang, P. Hypertensive Disorders of Pregnancy and Future Maternal Cardiovascular Risk. J. Am. Heart Assoc. 2018, 7, e009382. [Google Scholar] [CrossRef] [PubMed]
- Lugue, O.C.; George, E.M.; Bidwell, G.L. Preeclampsia and the brain: Neural control of cardiovascular changes during pregnancy and neurological outcomes of preeclampsia. Clin. Sci. 2016, 130, 1417–1434. [Google Scholar] [CrossRef] [PubMed]
- Ekholm, E.M.; Erkkola, R.U. Autonomic cardiovascular control in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 64, 29–36. [Google Scholar] [CrossRef]
- Fu, Q.; Levine, B.D. Autonomic circulatory control during pregnancy in humans. Semin. Reprod. Med. 2009, 27, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, A.C.; Stigter, R.H.; Bruinse, H.W. Cardiac output in normal pregnancy: A critical review. Obstet. Gynecol. 1996, 87, 310–318. [Google Scholar] [CrossRef]
- Schobel, H.; Fischer, T.; Heuszer, K.; Geiger, H.; Schmieder, R. Preeclampsia—A state of sympathetic overactivity. N. Engl. J. Med. 1996, 335, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chao, T.; Kuo, T.; Yin, C.; Chen, H. Preeclamptic pregnancy is associated with increased sympathetic and decreased parasympathetic control of HR. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, 1269–1273. [Google Scholar] [CrossRef]
- Kolovetsiou-Kreiner, V.; Moertl, M.G.; Papousek, I.; Schmid-Zalaudek, K.; Lang, U.; Schlembach, D.; Cervar-Zivkovic, M.; Lackner, H.K. Maternal cardiovascular and endothelial function from first trimester to postpartum. PLoS ONE 2018, 13, e0197748. [Google Scholar] [CrossRef]
- Moertl, M.G.; Lackner, H.K.; Papousek, I.; Roessler, A.; Hinghofer-Szalkay, H.; Lang, U.; Kolovetsiou-Kreiner, V.; Schlembach, D. Phase synchronization of hemodynamic variables at rest and after deep breathing measured during the course of pregnancy. PLoS ONE 2013, 8, e60675. [Google Scholar] [CrossRef]
- Voss, A.; Malberg, H.; Schumann, A.; Wessel, N.; Walther, T.; Stepan, H.; Faber, R. Baroreflex sensitivity, heart rate, and blood pressure variability in normal pregnancy. Am. J. Hypertens. 2000, 13, 1218–1225. [Google Scholar] [CrossRef]
- Weber, T.M.; Lackner, H.K.; Roessler, A.; Papousek, I.; Kolovetsiou-Kreiner, V.; Lucovnik, M.; Schmid-Zalaudek, K.; Lang, U.; Moertl, M.G. Heart rate variability and baroreceptor reflex sensitivity in early- versus late-onset preeclampsia. PLoS ONE 2017, 12, e0186521. [Google Scholar] [CrossRef] [PubMed]
- Faber, R.; Baumert, M.; Stepan, H.; Wessel, N.; Voss, A.; Walther, T. Baroreflex sensitivity, heart rate, and blood pressure variability in hypertensive pregnancy disorders. J. Hum. Hypertens. 2004, 18, 707–712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walther, T.; Voss, A.; Baumert, M.; Truebner, S.; Till, H.; Stepan, H.; Wessel, N.; Faber, R. Cardiovascular variability before and after delivery: Recovery from arterial stiffness in women with preeclampsia 4 days post partum. Hypertens. Pregnancy 2014, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Visontai, Z.; Lenard, Z.; Studinger, P.; Rigo Jr, J.; Kollai, M. Impaired baroreflex function during pregnancy is associated with stiffening of the carotid artery. Ultrasound Obstet Gynecol. 2002, 20, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Lackner, H.K.; Papousek, I.; Batzel, J.J.; Roessler, A.; Scharfetter, H.; Hinghofer-Szalkay, H. Phase synchronization of hemodynamic variables and respiration during mental challenge. Int. J. Psychophysiol. 2011, 79, 401–409. [Google Scholar] [CrossRef]
- Schaefer, C.; Rosenblum, M.G.; Abel, H.H.; Kurths, J. Synchronization in the human cardiorespiratory system. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 1999, 60, 857–870. [Google Scholar] [CrossRef]
- Drobnjak, T.; Gizurarson, S.; Gokina, N.I.; Meiri, H.; Mandalá, M.; Huppertz, B.; Osol, G. Placental protein 13 (PP13)-induced vasodilation of resistance arteries from pregnant and nonpregnant rats occurs via endothelial-signaling pathways. Hypertens. Pregnancy 2017, 36, 186–195. [Google Scholar] [CrossRef]
- Benschop, L.; Schalekamp-Timmermans, S.; Broere-Brown, Z.A.; Roeters van Lennep, J.E.; Jaddoe, V.W.V.; Roos-Hesselink, J.W.; Ikram, M.K.; Steegers, E.A.P.; Roberts, J.M.; Gandley, R.E. Placental Growth Factor as an Indicator of Maternal Cardiovascular Risk After Pregnancy. Circulation 2019, 139, 1698–1709. [Google Scholar] [CrossRef]
- Huppertz, B.; Sammar, M.; Chefetz, I.; Neumaier-Wagner, P.; Bartz, C.; Meiri, H. Longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn. Ther. 2008, 24, 230–236. [Google Scholar] [CrossRef]
- Shapiro, P.A.; Loan, R.P.; Horn, E.M.; Myers, M.M.; Gorman, J.M. Effect of innervation on heart rate response to mental stress. Arch. Gen. Psychiatry 1993, 50, 275–279. [Google Scholar] [CrossRef]
- Lucovnik, M.; Lackner, H.K.; Papousek, I.; Schmid-Zalaudek, K.; Schulter, G.; Roessler, A.; Moertl, M.G. Systemic vascular resistance and endogenous inhibitors of nitric oxide synthesis in early- compared to lateonset preeclampsia: Preliminary findings. Hypertens. Pregnancy 2017, 36, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Herse, F.; Youpeng, B.; Staff, A.C.; Yong-Meid, J.; Dechend, R.; Rong, Z. Circulating and uteroplacental adipocytokine concentrations in preeclampsia. Reprod. Sci. 2009, 16, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.D.; Ness, R.B.; Olsen, J.; Hougaard, D.M.; Skogstrand, K.; Roberts, J.M.; Haggerty, C.L. Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women. Hypertension 2015, 65, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.; Guild, S.J.; Barrett, C.J.; Chen, Q.; McCowan, L.; Jordan, V.; Chamley, L.W. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: A systematic review and meta-analysis. Am. J. Reprod. Immunol. 2013, 70, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Peraçoli, M.T.; Bannwart, C.F.; Cristofalo, R.; Borges, V.T.; Costa, R.A.; Witkin, S.S.; Peraçoli, J.C. Increased Reactive Oxygen Species and Tumor Necrosis Factor-Alpha Production by Monocytes are Associated with Elevated Levels of Uric Acid in Pre-Eclamptic Women. Am. J. Reprod. Immunol. 2011, 66, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Brassard, P.; Amiri, F.; Schiffrin, E.L. Combined angiotensin II type 1 and type 2 receptor blockade on vascular remodeling and matrix metalloproteinases in resistance arteries. Hypertension 2005, 46, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Elliott, K.J.; Kawai, T.; Obama, T.; Boyer, M.J.; Preston, K.J.; Yan, Z.; Eguchi, S.; Rizzo, V. Caveolin-1 Deletion Prevents Hypertensive Vascular Remodeling Induced by Angiotensin II. Hypertension 2017, 69, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Zhong, J.C.; Fan, D.; Basu, R.; Morton, J.S.; Parajuli, N.; McMurtry, M.S.; Davidge, S.T.; Kassiri, Z.; Oudit, G.Y. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension 2014, 64, 157–164. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Cheng, Y.W.; Jin, H.Y.; Chang, Q.; Shang, Q.H.; Xu, Y.L.; Chen, L.X.; Xu, R.; Song, B.; Zhong, J.C. The sirtuin 6 prevents angiotensin II-mediated myocardial fibrosis and injury by targeting AMPK-ACE2 signaling. Oncotarget 2017, 8, 72302–72314. [Google Scholar] [CrossRef]
- Cui, N.; Li, W.; Mazzuca, M.Q.; Khalil, R.A.; Mata, K.M. Increased vascular and uteroplacental matrix metalloproteinase-1 and -7 levels and collagen type I deposition in hypertension in pregnancy: Role of TNF-α. Am. J. Physiol. Circ. Physiol. 2017, 313, H491–H507. [Google Scholar] [CrossRef]
- Herse, F.; Lamarca, B.; Hubel, C.A.; Kaartokallio, T.; Lokki, A.I.; Ekholm, E.; Laivuori, H.; Gauster, M.; Huppertz, B.; Sugulle, M.; et al. Cytochrome P450 subfamily 2J polypeptide 2 expression and circulating epoxyeicosatrienoic metabolites in preeclampsia. Circulation 2012, 126, 2990–2999. [Google Scholar] [CrossRef] [PubMed]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef] [PubMed]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv. Pharmacol. 2016, 77, 361–431. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A. Preventing Preeclampsia by Silencing Soluble Flt-1? N. Engl. J. Med. 2019, 380, 1080–1082. [Google Scholar] [CrossRef]
- Meher, S.; Duley, L. Nitric oxide for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2007, CD006490. [Google Scholar] [CrossRef] [PubMed]
- Groten, T.; Fitzgerald, J.; Lehmann, T.; Schneider, U.; Kähler, C.; Schleussner, E. Reduction of preeclampsia related complications with with the NO-donor penterythriltetranitrat (petn) in risk pregnancies—A prospective randomized doubleblind placebo pilot study. Pregnancy Hypertens. 2012, 2, 181. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Fu, H.; Zhang, L.; Huang, H.; Luo, F.; Wu, W.; Guo, Y.; Liu, X. Angiotensin II upregulates the expression of placental growth factor in human vascular endothelial cells and smooth muscle cells. BMC Cell Biol. 2010, 11, 36. [Google Scholar] [CrossRef]
- Zhou, C.C.; Ahmad, S.; Mi, T.; Xia, L.; Abbasi, S.; Hewett, P.W.; Sun, C.; Ahmed, A.; Kellems, R.E.; Xia, Y. Angiotensin II induces soluble fms-like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ. Res. 2007, 100, 88–95. [Google Scholar] [CrossRef]
- Xia, Y.; Kellems, R.E. Angiotensin receptor agonistic autoantibodies and hypertension: Preeclampsia and beyond. Circ. Res. 2013, 113, 78–87. [Google Scholar] [CrossRef]
- Parrish, M.R.; Wallace, K.; Tam Tam, K.B.; Herse, F.; Weimer, A.; Wenzel, K.; Wallukat, G.; Ray, L.F.; Arany, M.; Cockrell, K.; et al. Hypertension in response to AT1-AA: Role of reactive oxygen species in pregnancy-induced hypertension. Am. J. Hypertens. 2011, 24, 835–840. [Google Scholar] [CrossRef]
- Cunningham, M.W., Jr.; Castillo, J.; Ibrahim, T.; Cornelius, D.C.; Campbell, N.; Amaral, L.; Vaka, V.R.; Usry, N.; Williams, J.M.; LaMarca, B. AT1-AA (angiotensin II type 1 receptor agonistic autoantibody) blockade prevents preeclamptic symptoms in placental ischemic rats. Hypertension 2018, 71, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Wang, X.; Zhang, N.; Yang, H.; Bai, R.; Liu, M.; Bian, Y.; Xiao, C.; Yang, Z. Angiotensin-(1-7) attenuates angiotensin II-induced ICAM-1, VCAM-1, and MCP-1 expression via the MAS receptor through suppression of P38 and NF-κB pathways in HUVECs. Cell Physiol. Biochem. 2015, 35, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Hayes, E.M.; Lehoux, S.; Jeremy, J.Y.; Horrevoets, A.J.; Newby, A.C. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J. Cell Physiol. 2011, 226, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Ramkhelawon, B.; Rivas, D.; Lehoux, S. Shear stress activates extracellular signal-regulated kinase 1/2 via the angiotensin II type 1 receptor. FASEB J. 2013, 27, 3008–3016. [Google Scholar] [CrossRef] [PubMed]
- Planas-Rigol, E.; Terrades-Garcia, N.; Corbera-Bellalta, M.; Lozano, E.; Alba, M.A.; Segarra, M.; Espígol-Frigolé, G.; Prieto-González, S.; Hernández-Rodríguez, J.; Preciado, S.; et al. Endothelin-1 promotes vascular smooth muscle cell migration across the artery wall: A mechanism contributing to vascular remodelling and intimal hyperplasia in giant-cell arteritis. Ann. Rheum. Dis. 2017, 76, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Atef, M.E.; Anand-Srivastava, M.B. Enhanced expression of Gqα and PLC-β1 proteins contributes to vascular smooth muscle cell hypertrophy in SHR: Role of endogenous angiotensin II and endothelin-1. Am. J. Physiol. Cell Physiol. 2014, 307, C97–C106. [Google Scholar] [CrossRef] [PubMed]
- Azahri, N.S.; Di Bartolo, B.A.; Khachigian, L.M.; Kavurma, M.M. Sp1, acetylated histone-3 and p300 regulate TRAIL transcription: Mechanisms of PDGF-BB-mediated VSMC proliferation and migration. J. Cell Biochem. 2012, 113, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Zhang, E.; Senapati, P.; Amaram, V.; Reddy, M.A.; Stapleton, K.; Leung, A.; Lanting, L.; Wang, M.; Chen, Z.; et al. A novel angiotensin II-induced long noncoding RNA giver regulates oxidative stress, inflammation, and proliferation in vascular smooth muscle cells. Circ. Res. 2018, 123, 1298–1312. [Google Scholar] [CrossRef]
- Jover, E.; Silvente, A.; Marín, F.; Martínez-González, J.; Orriols, M.; Martinez, C.M.; Puche, C.M.; Valdés, M.; Rodriguez, C.; Hernández-Romero, D. Inhibition of enzymes involved in collagen cross-linking reduces vascular smooth muscle cell calcification. FASEB J. 2018, 32, 4459–4469. [Google Scholar] [CrossRef]
- Spradley, F.T. Sympathetic nervous system control of vascular function and blood pressure during pregnancy and preeclampsia. J. Hypertens. 2019, 37, 476–487. [Google Scholar] [CrossRef]
- Riedl, M.; Suhrbier, A.; Stepan, H.; Kurths, J.; Wessel, N. Short-term couplings of the cardiovascular system in pregnant women suffering from pre-eclampsia. Philos. Transact. A Math. Phys. Eng. Sci. 2010, 368, 2237–2250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haug, E.B.; Horn, J.; Markovitz, A.R.; Fraser, A.; Macdonald-Wallis, C.; Tilling, K.; Romundstad, P.R.; Rich-Edwards, J.W.; Åsvold, B.O. The impact of parity on life course blood pressure trajectories: The HUNT study in Norway. Eur. J. Epidemiol. 2018, 33, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Ditisheim, A.; Wuerzner, G.; Ponte, B.; Vial, Y.; Irion, O.; Burnier, M.; Boulvain, M.; Pechère-Bertschi, A. Prevalence of Hypertensive Phenotypes After Preeclampsia: A Prospective Cohort Study. Hypertension 2018, 71, 103–109. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Fliege, H.; Rose, M.; Arck, P.; Walter, O.B.; Kocalevent, R.D.; Weber, C.; Klapp, B.F. The Perceived Stress Questionnaire (PSQ) reconsidered: Validation and reference values from different clinical and healthy adult samples. Psychosom. Med. 2005, 67, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Hautzinger, M.; Bailer, M. ADS: Allgemeine Depressions Skala; Beltz: Weinheim, Germany, 1993. [Google Scholar] [CrossRef]
- Niemann, H.; Sturm, W.; Thöne-Otto, A.I.; Willmes, K. California Verbal Learning Test, German Adaptation; Hogrefe: Boston, MA, USA, 2008. [Google Scholar]
- Lackner, H.K.; Gramer, M.; Paechter, M.; Wimmer, S.; Hinghofer-Szalkay, H.; Papousek, I. Academic goal orientation and cardiovascular reactivity in a performance situation. Appl. Psychophysiol. Biofeedback 2015, 40, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Papousek, I.; Paechter, M.; Lackner, H.K. Delayed psychophysiological recovery after self-concept-inconsistent negative performance feedback. Int. J. Psychophysiol. 2011, 82, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Papousek, I.; Paechter, M.; Weiss, E.M.; Lackner, H.K. The tendency to ruminate and the dynamics of heart rate recovery after an ordinary, mildly stressful performance situation. Pers. Individ. Dif. 2017, 104, 150–154. [Google Scholar] [CrossRef]
- Fortin, J.; Marte, W.; Grüllenberger, R.; Hacker, A.; Habenbacher, W.; Heller, A.; Wagner, C.; Wach, P.; Skrabal, F. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput. Biol. Med. 2006, 36, 941–957. [Google Scholar] [CrossRef]
- Lackner, H.K.; Batzel, J.J.; Rössler, A.; Hinghofer-Szalkay, H.; Papousek, I. Multi-time scale perspective in analyzing cardiovascular data. Physiol. Res. 2014, 63, 439–456. [Google Scholar]
- Parati, G.; Omboni, S.T.; Frattola, A.; di Rienzo, M.; Zanchetti, A.G. Dynamic evaluation of the baroreflex in ambulant subject. In Blood Pressure and Heart Rate Variability; Rienzo, M., Mancia, G., Parati, G., Pedotti, A., Zanchetti, A., Eds.; IOS Press: Amsterdam, The Netherlands, 1992; pp. 123–137. [Google Scholar]
- Cysarz, D.; von Bonin, D.; Lackner, H.; Heusser, P.; Moser, M.; Bettermann, H. Oscillations of heart rate and respiration synchronize during poetry recitation. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H579–H587. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.M.; Rumrill, P.D. Pretest-posttest designs and measurement of change. Work 2003, 20, 159–165. [Google Scholar] [PubMed]
- Linden, W.; Earle, L.; Gerin, W.; Christenfeld, N. Physiological stress reactivity and recovery: Conceptual siblings separated at birth? J. Psychosom. Res. 1997, 42, 117–135. [Google Scholar] [CrossRef]
- Steketee, G.S.; Chambless, D.L. Methodological issues in prediction of treatment outcome. Clin. Psychol. Rev. 1992, 12, 387–400. [Google Scholar] [CrossRef]
- Papousek, I.; Weiss, E.M.; Schulter, G.; Fink, A.; Reiser, E.M.; Lackner, H.K. Prefrontal EEG alpha asymmetry changes while observing disaster happening to other people: Cardiac correlates and prediction of emotional impact. Biol. Psychol. 2014, 103, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Papousek, I.; Aydin, N.; Rominger, C.; Feyaerts, K.; Schmid-Zalaudek, K.; Lackner, H.K.; Fink, A.; Schulter, G.; Weiss, E.M. DSM-5 personality trait domains and withdrawal versus approach motivational tendencies in response to the perception of other people’s desperation and angry aggression. Biol. Psychol. 2018, 132, 106–115. [Google Scholar] [CrossRef]
| Baseline | Anticipation | Task | Post-Task | F-Statistics | ||
|---|---|---|---|---|---|---|
| Mean Arterial BP (mmHg) | ||||||
| CO | 86.9 ± 9.4 | 89.7 ± 9.7 | 94.8 ± 10.5 | 90.3 ± 9.3 | period | F(1.7,207.2) = 2.3, p = 0.108 |
| UP | 84.9 ± 8.4 | 87.0 ± 8.5 | 94.0 ± 9.4 | 89.5 ± 8.2 | period x group | F(3.5,207.2) = 1.7, p = 0.149 |
| PE | 91.6 ± 10.0 | 92.7 ± 8.9 | 100.1 ± 10.7 | 95.5 ± 9.0 | ||
| Systolic BP (mmHg) | ||||||
| CO | 109.3 ± 11.0 | 112.8 ± 11.8 | 119.1 ± 12.7 | 113.3 ± 12.0 | period | F(1.2,200.7) = 1.2, p = 0.307 |
| UP | 107.0 ± 9.6 | 109.6 ± 10.2 | 118.7 ± 12.0 | 112.2 ± 9.5 | period x group | F(3.5,200.7) = 1.4, p = 0.243 |
| PE | 113.3 ± 11.8 | 115.2 ± 10.5 | 123.7 ± 12.6 | 118.2 ± 11.1 | ||
| Diastolic BP (mmHg) | ||||||
| CO | 70.9 ± 9.1 | 73.3 ± 8.8 | 77.5 ± 9.8 | 73.8 ± 8.5 | period | F(1.8,216.8) = 3.5, p < 0.05 |
| UP | 69.0 ± 8.2 | 71.2 ± 7.9 | 76.8 ± 8.6 | 73.2 ± 7.6 | period x group | F(3.6,216.8) = 1.9, p = 0.124 |
| PE | 75.4 ± 9.8 | 76.1 ± 8.9 | 82.6 ± 10.5 | 78.8 ± 8.3 | ||
| Heart Rate (bpm) | ||||||
| CO | 71.0 ± 10.9 | 73.6 ± 12.0 | 83.9 ± 15.0 | 72.1 ± 10.9 | period | F(1.3,153.2) = 1.6, p = 0.208 |
| UP | 72.6 ± 7.6 | 74.2 ± 8.8 | 83.5 ± 10.5 | 75.5 ± 8.7 | period x group | F(2.6,153.2) = 5.5, p < 0.01 |
| PE | 72.4 ± 9.3 | 74.5 ± 9.5 | 80.7 ± 11.7 | 74.9 ± 9.9 | ||
| Stroke Index (mL/m2) | ||||||
| CO | 43.0 ± 8.9 | 42.9 ± 8.6 | 42.4 ± 9.4 | 42.6 ± 8.2 | period | F(1.8,218.0) = 1.7, p = 0.183 |
| UP | 41.6 ± 6.4 | 41.1 ± 6.2 | 40.0 ± 6.2 | 40.1 ± 6.5 | period x group | F(3.6,218.0) = 0.8, p = 0.489 |
| PE | 38.2 ± 7.0 | 37.7 ± 7.0 | 37.3 ± 7.1 | 37.2 ± 6.9 | ||
| SVRI (dyn·s·m2/cm5) | ||||||
| CO | 2267 ± 541 | 2325 ± 548 | 2223 ± 589 | 2400 ± 561 | period | F(1.7,208.9) = 2.3, p = 0.438 |
| UP | 2251 ± 514 | 2292 ± 506 | 2292 ± 524 | 2377 ± 534 | period x group | F(3.5,208.9) = 1.7, p < 0.05 |
| PE | 2692 ± 650 | 2680 ± 614 | 2743 ± 660 | 2788 ± 625 | ||
| Baseline | Anticipation | Task | Post-Task | F-Statistics | ||
|---|---|---|---|---|---|---|
| Respiration Rate (breath/min) | ||||||
| CO | 14.7 ± 3.8 | 14.9 ± 3.5 | 17.4 ± 3.3 | 15.4 ± 4.1 | period | F(1.7,205.9) = 14.3, p < 0.001 |
| UP | 15.9 ± 3.2 | 16.4 ± 2.5 | 18.0 ± 2.8 | 16.0 ± 2.8 | period x group | F(3.4,205.9) = 0.7, p = 0.565 |
| PE | 16.4 ± 4.2 | 16.4 ± 2.6 | 17.8 ± 2.4 | 16.2 ± 2.6 | ||
| Baroreflex Sensitivity (ms/mmHg) | ||||||
| CO | 14.4 ± 4.0 | 14.1 ± 3.8 | 11.9 ± 3.4 | 13.4 ± 3.8 | period | F(2,240) = 1.7, p = 0.177 |
| UP | 13.4 ± 4.0 | 12.6 ± 3.1 | 11.8 ± 2.7 | 12.3 ± 3.2 | period x group | F(4,240) = 2.1, p = 0.088 |
| PE | 14.0 ± 4.0 | 13.3 ± 3.4 | 11.7 ± 2.9 | 12.2 ± 4.2 | ||
| Baseline | Anticipation | Task | Post-Task | F-Statistics | ||
|---|---|---|---|---|---|---|
| γSBPxRRI,LF (−) | ||||||
| CO | 0.41 ± 0.21 | 0.48 ± 0.18 | 0.34 ± 0.13 | 0.41 ± 0.19 | period | F(2,240) = 0.4, p = 0.675 |
| UP | 0.39 ± 0.17 | 0.41 ± 0.18 | 0.37 ± 0.15 | 0.42 ± 0.18 | period x group | F(4,240) = 2.3, p = 0.058 |
| PE | 0.36 ± 0.17 | 0.39 ± 0.16 | 0.37 ± 0.16 | 0.38 ± 0.16 | ||
| γDBPxRRI,LF (−) | ||||||
| CO | 0.40 ± 0.19 | 0.45 ± 0.17 | 0.32 ± 0.13 | 0.40 ± 0.17 | period | F(2,240) = 0.2, p = 0.821 |
| UP | 0.38 ± 0.15 | 0.38 ± 0.15 | 0.32 ± 0.13 | 0.40 ± 0.15 | period x group | F(4,240) = 1.0, p = 0.428 |
| PE | 0.36 ± 0.15 | 0.40 ± 0.16 | 0.31 ± 0.16 | 0.36 ± 0.14 | ||
| γSBPxDBP,LF (−) | ||||||
| CO | 0.75 ± 0.13 | 0.79 ± 0.11 | 0.73 ± 0.13 | 0.75 ± 0.12 | period | F(1.9,226.3) = 0.7, p = 0.481 |
| UP | 0.79 ± 0.12 | 0.78 ± 0.14 | 0.70 ± 0.17 | 0.78 ± 0.12 | period x group | F(3.8,226.3) = 1.7, p = 0.219 |
| PE | 0.75 ± 0.13 | 0.79 ± 0.11 | 0.68 ± 0.15 | 0.74 ± 0.12 | ||
| Baseline | Anticipation | Task | Post-Task | F-Statistics | ||
|---|---|---|---|---|---|---|
| γSBPxRRI,HF (−) | ||||||
| CO | 0.60 ± 0.19 | 0.50 ± 0.20 | 0.33 ± 0.17 | 0.50 ± 0.25 | period | F(2,240) = 0.6, p = 0.550 |
| UP | 0.63 ± 0.22 | 0.52 ± 0.21 | 0.33 ± 0.15 | 0.52 ± 0.22 | period x group | F(4,240) = 1.0, p = 0.408 |
| PE | 0.66 ± 0.17 | 0.53 ± 0.19 | 0.29 ± 0.13 | 0.54 ± 0.20 | ||
| γDBPxRRI,HF (−) | ||||||
| CO | 0.40 ± 0.26 | 0.37 ± 0.20 | 0.29 ± 0.12 | 0.36 ± 0.23 | period | F(2,240) = 8.4, p < 0.001 |
| UP | 0.36 ± 0.22 | 0.30 ± 0.20 | 0.29 ± 0.15 | 0.33 ± 0.17 | period x group | F(4,240) = 1.3, p = 0.258 |
| PE | 0.37 ± 0.21 | 0.31 ± 0.21 | 0.22 ± 0.13 | 0.31 ± 0.20 | ||
| γRESPxRRI,HF (−) | ||||||
| CO | 0.71 ± 0.19 | 0.62 ± 0.18 | 0.39 ± 0.19 | 0.62 ± 0.22 | period | F(2,240) = 0.5, p = 0.591 |
| UP | 0.69 ± 0.22 | 0.57 ± 0.20 | 0.33 ± 0.18 | 0.55 ± 0.26 | period x group | F(4,240) = 0.5, p = 0.736 |
| PE | 0.70 ± 0.23 | 0.57 ± 0.25 | 0.32 ± 0.18 | 0.58 ± 0.22 | ||
| γRESPxSBP,HF (−) | ||||||
| CO | 0.67 ± 0.22 | 0.55 ± 0.20 | 0.30 ± 0.18 | 0.58 ± 0.24 | period | F(1.9,223.4) = 1.1, p = 0.342 |
| UP | 0.70 ± 0.22 | 0.58 ± 0.19 | 0.27 ± 0.16 | 0.53 ± 0.26 | period x group | F(3.7,223.4) = 2.2, p = 0.080 |
| PE | 0.68 ± 0.23 | 0.54 ± 0.24 | 0.22 ± 0.15 | 0.57 ± 0.25 | ||
| γRESPxDBP,HF (-) | ||||||
| CO | 0.33 ± 0.25 | 0.26 ± 0.18 | 0.18 ± 0.13 | 0.24 ± 0.19 | period | F(2,240) = 3.0, p = 0.053 |
| UP | 0.33 ± 0.20 | 0.24 ± 0.15 | 0.17 ± 0.12 | 0.24 ± 0.16 | period x group | F(4,240) = 2.2, p = 0.065 |
| PE | 0.28 ± 0.18 | 0.20 ± 0.19 | 0.11 ± 0.09 | 0.24 ± 0.19 | ||
| PE (n = 35) | UP (n = 38) | CO (n = 51) | p-Value | |
|---|---|---|---|---|
| Age (years) | 33.7 ± 4.8, 25–42 | 32.4 ± 4.0, 26–44 | 32.4 ± 5.3, 25–44 | p = 0.41 |
| Height (cm) | 168.2 ± 6.8, 153–182 | 167.5 ± 5.8, 157.5–179 | 168.4 ± 5.6, 156–180 | p = 0.78 |
| Weight (kg) | 77.9 ± 15.7 3, 48.9–121.9 | 68.7 ± 11.5, 49.1–97.7 | 65.3 ± 10.5 1, 45–93 | p < 0.001 |
| BMI (kg/m2) | 27.8 ± 5.9 2,3, 20.7–44.2 | 24.6 ± 4.6 1, 17–36.3 | 23.0 ± 3.2 1, 16.9–30.9 | p < 0.001 |
| Delivery (day) | 253 ± 21, 197–287 | 278 ± 10, 254–291 | – | p < 0.001 |
| Baby height (cm) | 46.9 ± 5.1, 31–57 | 51.3 ± 1.8, 47–56 | – | p < 0.001 |
| Baby weight (g) | 2568 ± 853, 800–3940 | 3404 ± 341, 2780–4010 | – | p < 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lackner, H.K.; Papousek, I.; Schmid-Zalaudek, K.; Cervar-Zivkovic, M.; Kolovetsiou-Kreiner, V.; Nonn, O.; Lucovnik, M.; Pfniß, I.; Moertl, M.G. Disturbed Cardiorespiratory Adaptation in Preeclampsia: Return to Normal Stress Regulation Shortly after Delivery? Int. J. Mol. Sci. 2019, 20, 3149. https://doi.org/10.3390/ijms20133149
Lackner HK, Papousek I, Schmid-Zalaudek K, Cervar-Zivkovic M, Kolovetsiou-Kreiner V, Nonn O, Lucovnik M, Pfniß I, Moertl MG. Disturbed Cardiorespiratory Adaptation in Preeclampsia: Return to Normal Stress Regulation Shortly after Delivery? International Journal of Molecular Sciences. 2019; 20(13):3149. https://doi.org/10.3390/ijms20133149
Chicago/Turabian StyleLackner, Helmut K., Ilona Papousek, Karin Schmid-Zalaudek, Mila Cervar-Zivkovic, Vassiliki Kolovetsiou-Kreiner, Olivia Nonn, Miha Lucovnik, Isabella Pfniß, and Manfred G. Moertl. 2019. "Disturbed Cardiorespiratory Adaptation in Preeclampsia: Return to Normal Stress Regulation Shortly after Delivery?" International Journal of Molecular Sciences 20, no. 13: 3149. https://doi.org/10.3390/ijms20133149
APA StyleLackner, H. K., Papousek, I., Schmid-Zalaudek, K., Cervar-Zivkovic, M., Kolovetsiou-Kreiner, V., Nonn, O., Lucovnik, M., Pfniß, I., & Moertl, M. G. (2019). Disturbed Cardiorespiratory Adaptation in Preeclampsia: Return to Normal Stress Regulation Shortly after Delivery? International Journal of Molecular Sciences, 20(13), 3149. https://doi.org/10.3390/ijms20133149






