Tumor-Associated Protein Profiles in Kaposi Sarcoma and Mimicking Vascular Tumors, and Their Pathological Implications
Abstract
1. Introduction
2. Results
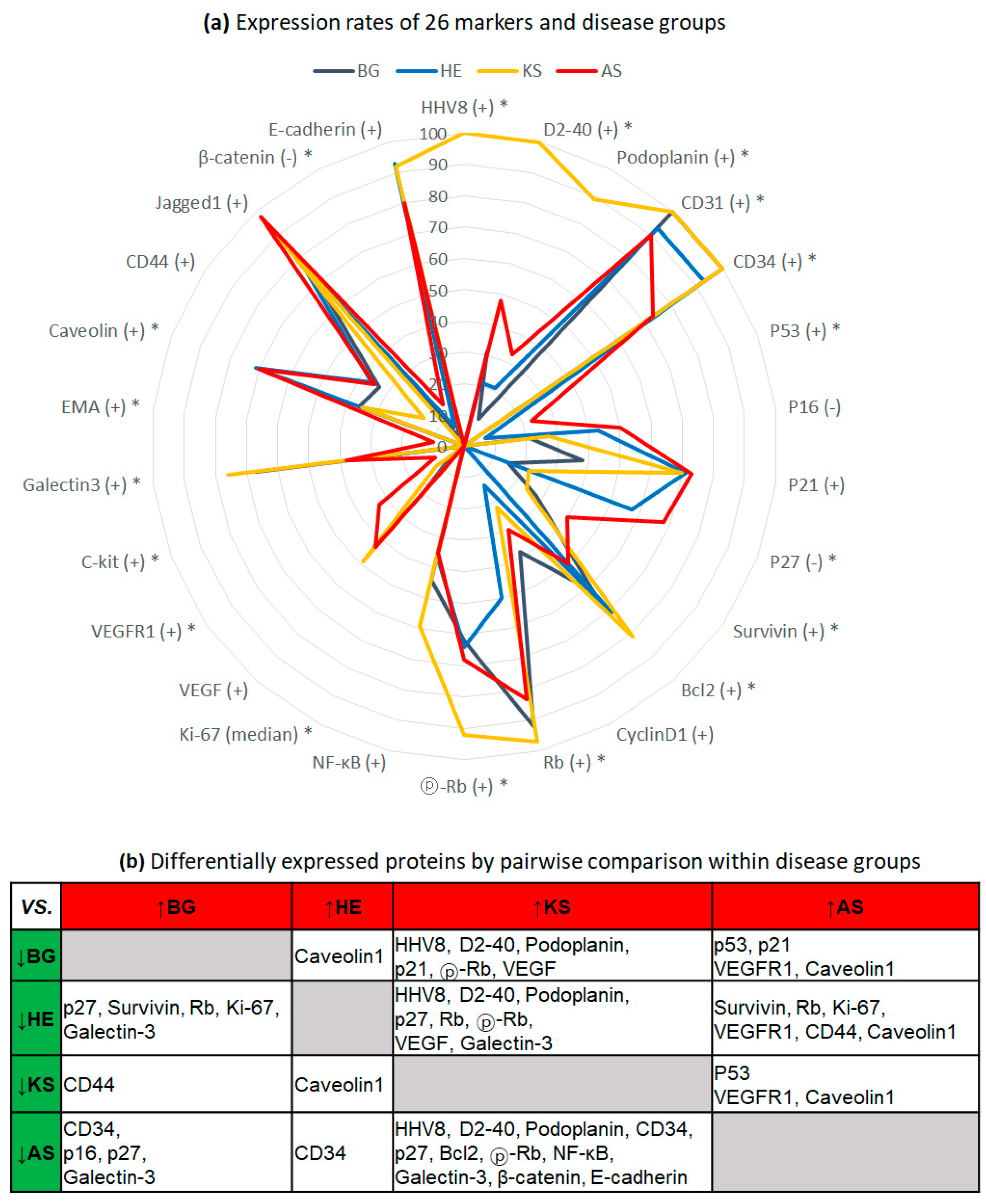
2.1. Exclusive Expression of HHV8 and Lymphatic Differentiation of KS
2.2. Low Proliferative Activity in HE
2.3. Activation of Rb Signaling in KS, and Inactivation of Cell Cycle Inhibitors and Aberrant p53 in AS
2.4. Expression of VEGFR1 and C-Kit in AS
2.5. Inverse Correlation of Galectin-3 and Caveolin-1 Expression between BG/KS and AS/HE
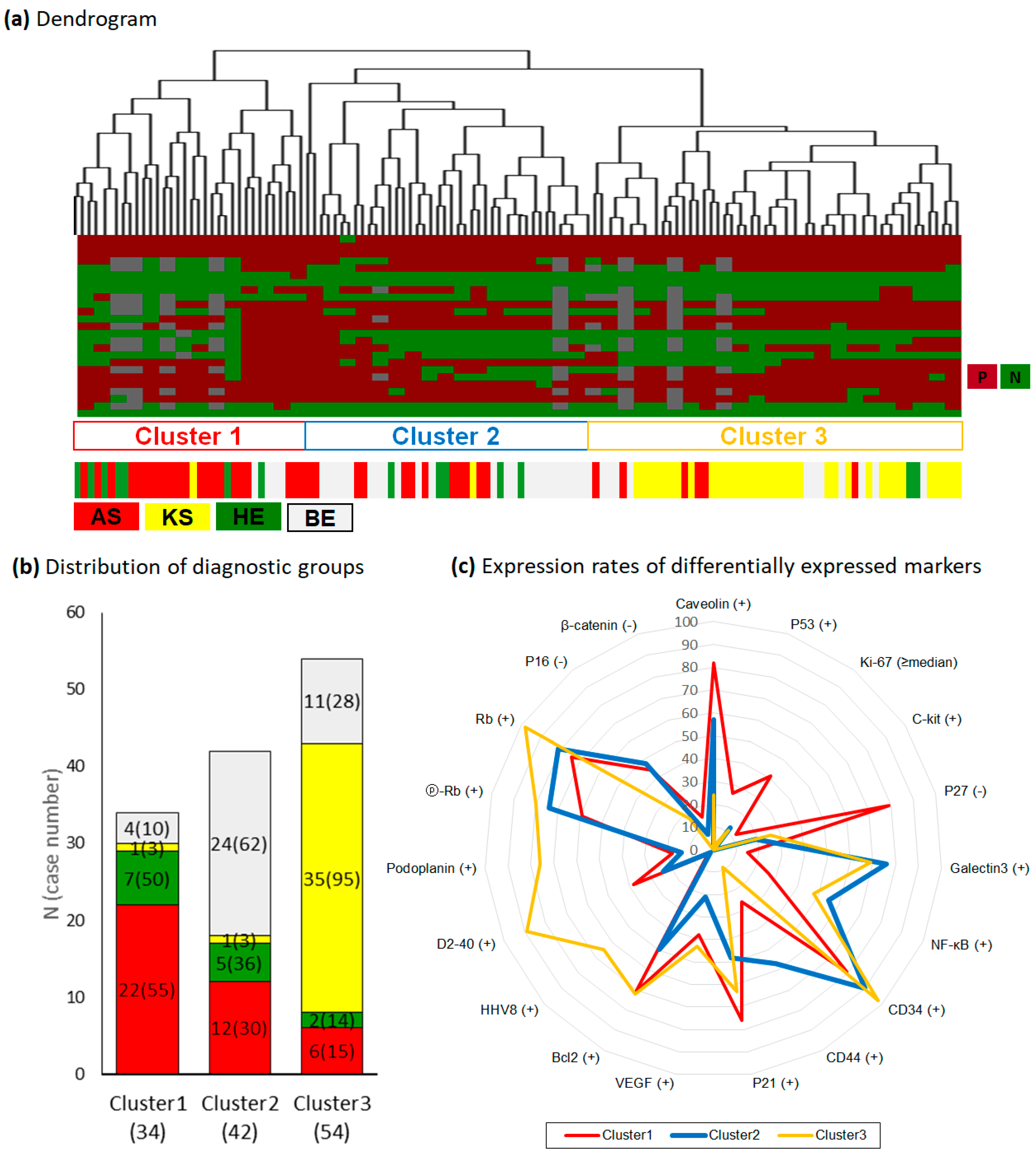
2.6. Differential Power and Protein Profiles of Hierarchical Clusters
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemical Staining
4.3. Evaluation of Immunohistochesmical Staining
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AS | Angiosarcoma |
| BG | Benign vascular lesion |
| HE | Hemangioendothelioma |
| HHV8 | Human herpesvirus 8 |
| KS | Kaposi sarcoma |
| LNA-1 | Latent nuclear antigen-1 |
| pRb | Phosphorylated Rb |
| RBC | Red blood cell |
References
- Gramolelli, S.; Schulz, T.F. The role of Kaposi sarcoma-associated herpesvirus in the pathogenesis of Kaposi sarcoma. J. Pathol. 2015, 235, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Lee, H.S.; Lee, H.E.; Park, S.Y.; Chung, J.H.; Choe, G.; Kim, W.H.; Kye, Y.S. Immunohistochemical characteristics of Kaposi sarcoma and its mimicries. Korean J. Pathol. 2006, 40, 361–367. [Google Scholar]
- Fletcher, C.D.M.; Bridge, J.A.; Hogendoorn, P.C.W.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; IARC: Lyon, France, 2013. [Google Scholar]
- David, E.E.; Daniela, M.; Richard, A.S.; Rein, W. WHO Classification of Skin Tumours, 4th ed.; IARC: Lyon, France, 2018. [Google Scholar]
- Radkov, S.A.; Kellam, P.; Boshoff, C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 2000, 6, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.M.; Biddolph, S.; Lucas, S.B.; Howells, D.D.; Picton, S.; McGee, J.O.; Silva, I.; Uhlmann, V.; Luttich, K.; O’Leary, J.J. Cyclin D1 expression and HHV8 in Kaposi sarcoma. J. Clin. Pathol. 1999, 52, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Trotter Matthew, W.B.; Lagos, D.; Bourboulia, D.; Henderson, S.; Mäkinen, T.; Elliman, S.; Flanagan, A.M.; Alitalo, K.; Boshoff, C. Kaposi sarcoma herpesvirus–induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 2004, 36, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Jarviluoma, A.; Child, E.S.; Sarek, G.; Sirimongkolkasem, P.; Peters, G.; Ojala, P.M.; Mann, D.J. Phosphorylation of the cyclin-dependent kinase inhibitor p21Cip1 on serine 130 is essential for viral cyclin-mediated bypass of a p21Cip1-imposed G1 arrest. Mol. Cell Biol. 2006, 26, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.Y.; Wen, V.W.; Pasquier, E.; Jankowski, K.; Chang, M.; Richards, L.A.; Kavallaris, M.; MacKenzie, K.L. Endothelial cell dysfunction and cytoskeletal changes associated with repression of p16(INK4a) during immortalization. Oncogene 2012, 31, 4815–4827. [Google Scholar] [CrossRef]
- Weihrauch, M.; Markwarth, A.; Lehnert, G.; Wittekind, C.; Wrbitzky, R.; Tannapfel, A. Abnormalities of the ARF-p53 pathway in primary angiosarcomas of the liver. Hum. Pathol. 2002, 33, 884–892. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Yoshida, A.; Guo, T.; Chang, N.E.; Zhang, L.; Agaram, N.P.; Qin, L.X.; Brennan, M.F.; Singer, S.; Maki, R.G. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 2009, 69, 7175–7179. [Google Scholar] [CrossRef]
- Verbeke, S.L.; Bertoni, F.; Bacchini, P.; Oosting, J.; Sciot, R.; Krenacs, T.; Bovée, J.V. Active TGF-beta signaling and decreased expression of PTEN separates angiosarcoma of bone from its soft tissue counterpart. Mod. Pathol. 2013, 26, 1211–1221. [Google Scholar] [CrossRef]
- Hong, Y.K.; Foreman, K.; Shin, J.W.; Hirakawa, S.; Curry, C.L.; Sage, D.R.; Libermann, T.; Dezube, B.J.; Fingeroth, J.D.; Detmar, M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 2004, 36, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Lesser, M.; Rosen, P.P. Hemangiomas and angiosarcomas of the breast: Diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch. Pathol Lab. Med. 2007, 131, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Laken, P.A. Infantile hemangiomas. Adv. Neonatal Care 2016, 16, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Marchuk, D.A. Pathogenesis of hemangioma. J. Clin. Investig. 2001, 107, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Deyrup, A.T.; Tighiouart, M.; Montag, A.G.; Weiss, S.W. Epithelioid hemangioendothelioma of soft tissue: A proposal for risk stratification based on 49 cases. Am. J. Surg. Pathol. 2008, 32, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Poliani, P.L.; Missale, C.; Monti, E.; Fanzani, A. Caveolins in rhabdomyosarcoma. J. Cell Mol. Med. 2011, 15, 2553–2568. [Google Scholar] [CrossRef] [PubMed]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3--a jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef]
- Shankar, J.; Wiseman, S.M.; Meng, F.; Kasaian, K.; Strugnell, S.; Mofid, A.; Gown, A.; Jones, S.J.; Nabi, I.R. Coordinated expression of galectin-3 and caveolin-1 in thyroid cancer. J. Pathol. 2012, 228, 56–66. [Google Scholar] [CrossRef]
- Cueni, L.N.; Detmar, M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J. Investig. Dermatol. 2006, 126, 2167–2177. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, C.; Liu, D.; Yu, W.; Chen, W.; Wang, Y.; Shi, S.; Yuan, Y. Evidence for Kaposi sarcoma originating from mesenchymal stem cell through KSHV-induced mesenchymal-to-endothelial transition. Cancer Res. 2018, 78, 230–245. [Google Scholar] [CrossRef]
- Kim, S.W.; Roh, J.; Chan-Sik Park, C.S. Immunohistochemistry for pathologists: Protocols, pitfalls, and tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.d.; Imoto, S.; Miyano, S. Gene Cluster 2002. Available online: http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm (accessed on 1 March 2016).
| N | BG | HE | KS | AS | ||
|---|---|---|---|---|---|---|
| N (%) | 130 | 39 (30) | 14 (11) | 37 (28) | 40 (31) | p-Value |
| Viral Protein | ||||||
| HHV8 (+) | 37 (28) | 0 (0) | 0 (0) | 37 (100) | 0 (0) | <0.001 * |
| Endothelial Differentiation | ||||||
| CD31 (+) | 125 (96) | 39 (100) | 13 (93) | 37 (100) | 36 (90) | 0.027 * |
| CD34 (+) | 118 (91) | 39 (100) | 13 (93) | 37 (100) | 29 (73) | <0.001 * |
| D2-40 (+) | 71 (55) | 12 (31) | 3 (21) | 37 (100) | 19 (48) | <0.001 * |
| Podoplanin (+) | 53 (41) | 4 (10) | 3 (21) | 33 (89) | 13 (33) | <0.001 * |
| Cell Cycle, Apoptosis, or Proliferation | ||||||
| P53 (+) | 10 (8) | 0 (0) | 1 (7) | 0 (0) | 9 (23) | <0.001 * |
| P16 (−) | 44 (34) | 8 (21) | 6 (43) | 10 (27) | 20 (50) | 0.057 |
| P21 (+) | 80 (62) | 15 (38) | 10 (71) | 26 (70) | 29 (73) | 0.065 |
| P27 (−) | 49 (38) | 6 (15) | 8 (57) | 8 (22) | 27 (68) | <0.001 * |
| Survivin (+) | 36 (28) | 11 (28) | 0 (0) | 9 (24) | 16 (40) | 0.036 * |
| Bcl2 (+) | 84 (65) | 24 (62) | 10 (71) | 30 (81) | 20 (50) | 0.027 * |
| CyclinD1 (+) | 37 (28) | 15 (38) | 2 (14) | 8 (22) | 12 (30) | 0.26 |
| Rb (+) | 112 (86) | 36 (92) | 7 (50) | 36 (97) | 33 (83) | <0.001 * |
| ⓟ-Rb (+) | 94 (72) | 24 (62) | 9 (64) | 34 (92) | 27 (68) | 0.009 * |
| NF-κB (+) | 58 (45) | 17 (44) | 5 (36) | 22 (59) | 14 (35) | 0.072 |
| Ki-67 (mean ± SD) | 23.3 ± 23.7 | 23.2 ± 22.1 | 5.0 ± 5.9 | 19.5 ± 18.3 | 33.4 ± 28.5 | 0.001* |
| Growth Factor or Protein Kinase | ||||||
| VEGF (+) | 45 (35) | 8 (21) | 2 (14) | 18 (49) | 17 (43) | 0.063 |
| VEGFR1 (+) | 19 (15) | 2 (5) | 0 (0) | 4 (11) | 13 (33) | 0.003 * |
| C-kit (+) | 4 (3) | 0 (0) | 0 (0) | 0 (0) | 4 (10) | 0.026 * |
| Cell Adhesion and Motility | ||||||
| Galectin-3 (+) | 74 (57) | 26 (67) | 5 (36) | 28 (76) | 15 (38) | <0.001 * |
| EMA (+) | 4 (3) | 0 (0) | 0 (0) | 0 (0) | 4 (10) | 0.027 * |
| Caveolin1 (+) | 65 (50) | 14 (36) | 10 (71) | 13 (35) | 28 (70) | 0.005 * |
| CD44 (+) | 38 (29) | 13 (33) | 5 (36) | 6 (16) | 14 (35) | 0.086 |
| Jagged1 (+) | 112 (86) | 28 (72) | 11 (79) | 34 (92) | 39 (98) | 0.063 |
| β-catenin (−) | 8 (6) | 1 (3) | 1 (7) | 0 (0) | 6 (15) | 0.033 * |
| E-cadherin (+) | 108 (83) | 29 (74) | 13 (93) | 34 (92) | 32 (80) | 0.095 |
| N | Cluster 1 | Cluster 2 | Cluster 3 | ||
|---|---|---|---|---|---|
| N (%) | 130 | 34 (26) | 42 (32) | 54 (42) | p-Value |
| Caveolin-1 (+) | 65 (50) | 28 (82) **,†,‡ | 24 (57) | 13 (24) | <0.001 * |
| P53 (+) | 10 (8) | 9 (26) **,‡ | 0 (0) | 1 (2) | <0.001 * |
| Ki-67 (≥median) | 25 (19) | 14 (41) **,‡ | 5 (12) | 6 (11) | 0.001 * |
| C-kit (+) | 4 (3) | 4 (12) **,‡ | 0 (0) | 0 (0) | 0.003 * |
| P27 (−) | 49 (38) | 27 (79) **,‡ | 8 (19) | 14 (26) | <0.001 * |
| Galectin-3 (+) | 74 (57) | 5 (15) **,‡ | 32 (76) | 37 (69) | <0.001 * |
| NF-κB (+) | 58 (45) | 9 (26) **,‡ | 23 (55) | 26 (48) | 0.013 * |
| CD34 (+) | 118 (91) | 27 (79) ‡ | 38 (90) | 53 (98) | 0.029 * |
| CD44 (+) | 38 (29) | 9 (26) | 24 (57) **,†,‡ | 5 (9) | <0.001 * |
| P21 (+) | 80 (62) | 26 (76) | 20 (48) **,† | 34 (63) | 0.015 * |
| VEGF (+) | 45 (35) | 13 (38) | 9 (21) † | 23 (43) | 0.025 * |
| Bcl2 (+) | 84 (65) | 24 (71) | 21 (50) **,† | 39 (72) | 0.044 * |
| HHV8 (+) | 37 (28) | 1 (3) | 1 (2) | 35 (65) †,‡ | <0.001 * |
| D2-40 (+) | 71 (55) | 13 (38) | 10 (24) | 48 (89) †,‡ | <0.001 * |
| Podoplanin (+) | 53 (41) | 6 (18) | 6 (14) | 41 (76) †,‡ | <0.001 * |
| ⓟ-Rb (+) | 94 (72) | 20 (59) | 31 (74) | 43 (80) †,‡ | 0.001 * |
| Rb (+) | 112 (86) | 25 (74) | 34 (81) | 53 (98) †,‡ | 0.002 * |
| P16 (−) | 44 (34) | 15 (44) | 20 (48) | 9 (17) †,‡ | 0.004 * |
| β-catenin (−) | 8 (6) | 5 (15) | 3 (7) | 0 (0) †,‡ | 0.019 * |
| Disease Groups | Numbers |
|---|---|
| Benign Vascular Lesion (BG) | 39 |
| Acroangiodermatitis | 1 |
| Angiofibroma | 2 |
| Capillary hemangioma | 10 |
| Cavernous hemangioma | 3 |
| Cherry angioma | 1 |
| Lobular capillary hemangioma | 18 |
| Hemangioma | 1 |
| Intravascular histiocytosis | 1 |
| Stasis dermatitis | 2 |
| Hemangioendothelioma (HE) | 14 |
| Kaposiform hemangioendothelioma | 9 |
| Spindle cell hemangioendothelioma | 5 |
| Kaposi sarcoma (KS) | 37 |
| Patch stage | 6 |
| Plaque stage | 12 |
| Nodular stage | 19 |
| Angiosarcoma (AS) | 40 |
| Total | BG | HE | KS | AS | ||
|---|---|---|---|---|---|---|
| N (%) | 107 | 39 (36) | 11 (10) | 29 (27) | 28 (26) | p-Value |
| Sex | ||||||
| Male | 69 (64) | 21 (54) | 7 (64) | 25 (86) | 16 (57) | 0.036 |
| Female | 38 (36) | 18 (46) | 4 (36) | 4 (14) | 12 (43) | |
| Age | 62 | 58 | 4.5 | 68 | 66 | <0.001 * |
| years, median (min–max) | (2–90) | (2–88) | (7–78) | (24–90) | (7–90) | |
| Sampling method | ||||||
| Biopsy | 74 (69) | 34 (87) | 6 (55) | 28 (97) | 6 (21) | <0.001 * |
| Resection | 33 (31) | 5 (13) | 5 (45) | 1 (3) | 22 (79) | |
| Organ | ||||||
| Skin | 82 (77) | 39 (100) | 6 (55) | 25 (86) | 13 (46) | <0.001 * |
| Non-skin | 25 (23) | 0 (0) | 5 (45) | 4 (14) | 15 (54) | |
| Skull | 3 | 0 | 2 | 0 | 1 | |
| Breast | 3 | 0 | 0 | 0 | 3 | |
| Heart | 2 | 0 | 0 | 0 | 2 | |
| Intestine | 1 | 0 | 0 | 0 | 1 | |
| Liver | 1 | 0 | 0 | 0 | 1 | |
| Lung | 2 | 0 | 0 | 1 | 1 | |
| Lymph node | 3 | 0 | 0 | 0 | 3 | |
| Oral cavity and tonsil | 3 | 0 | 0 | 3 | 0 | |
| Pancreas | 1 | 0 | 1 | 0 | 0 | |
| Soft tissue | 5 | 0 | 1 | 0 | 4 | |
| Bone | 1 | 0 | 1 | 0 | 0 | |
| Immune status | ||||||
| Competent | 78 (73) | 35 (90) | 9 (82) | 9 (31) | 25 (89) | <0.001 * |
| Compromised | 29 (27) | 4 (10) | 2 (18) | 20 (69) | 3 (11) | |
| HIV infection | 0 | 0 | 0 | 4 | 0 | |
| Chronic disease † | 0 | 0 | 0 | 5 | 2 | |
| Immunosuppressant user | 0 | 0 | 1 | 8 | 0 | |
| Diabetes mellitus | 0 | 4 | 1 | 2 | 1 | |
| Others ‡ | 0 | 0 | 0 | 1 | 0 | |
| Disease progression | ||||||
| No progress | 61 (57) | 37 (95) | 6 (55) | 11 (38) | 7 (25) | <0.001 * |
| Progress or deceased | 27 (25) | 1 (3) | 3 (27) | 7 (24) | 16 (57) | |
| Censored | 19 (18) | 1 (3) | 2 (18) | 11 (38) | 5 (18) |
| Name | Antibody Type (clone) | Dilution | Company (Cat. No.) | Cellular Location | Criteria of Abnormality |
|---|---|---|---|---|---|
| HHV8 (LANA-1) | Mouse monoclonal (13B10) | 1:100 | Cell Marque (CMC885) | N | ≥1+, ≥2 |
| CD31 | Mouse monoclonal (JC70A) | 1:100 | DAKO (M0823) | C | ≥1+, ≥5% |
| CD34 | Mouse monoclonal (QBEnd-10) | 1:300 | DAKO (M7165) | C | ≥1+, ≥5% |
| D2-40 | Mouse monoclonal (D2-40) | 1:100 | DAKO (M3619) | C ± M | ≥1+, ≥5% |
| Podoplanin | Mouse monoclonal (unspecified) | 1:100 | Angiobio Co. (11-003) | C ± M | ≥1+, ≥5% |
| P53 | Mouse monoclonal (DO-7) | 1:200 | DAKO (M7001) | N | ≥2+, ≥5% |
| P16 | Mouse monoclonal (G175-405) | 1:100 | BD Biosciences (551154) | C ± N | ≤1+, ≥95% |
| P21 | Mouse monoclonal (187) | 1:200 | Santa Cruz (sc-817) | N ± C | ≥ 2+, ≥5% |
| P27 | Rabbit polyclonal (unspecified) | 1:100 | Spring Bioscience (E2604) | N | ≤1+, ≥95% |
| Survivin | Rabbit polyclonal (unspecified) | 1:500 | R&D Systems (AF886) | N ± C | ≥1+, ≥5% |
| Bcl2 | Mouse monoclonal (124) | 1:100 | DAKO (M0887) | N ± C | ≥1+, ≥5% |
| CyclinD1 | Mouse monoclonal (DCS-6) | 1:100 | Santa Cruz (sc-20044) | N | ≥1+, ≥5% |
| Rb | Mouse monoclonal (G3-245) | 1:200 | BD Biosciences (554136) | N | ≥1+, ≥5% |
| ⓟ-Rb | Rabbit polyclonal (Ser608) | 1:30 | Cell Signaling (#2181) | N | ≥1+, ≥5% |
| NF-κB | Rabbit polyclonal (unspecified) | 1:200 | Abcam (ab7970) | C | ≥1+, ≥5% |
| Ki-67 | Mouse monoclonal (MIB-1) | 1:100 | DAKO (M7240) | N | ≥1+, ≥5% |
| VEGF | Rabbit polyclonal (C-1) | 1:500 | Santa Cruz (sc-7269) | C | ≥2+, ≥5% |
| VEGFR1 (FLT1) | Rabbit polyclonal (C-17) | 1:50 | Santa Cruz (sc-316) | C | ≥2+, ≥5% |
| C-kit | Rabbit polyclonal (unspecified) | 1:200 | DAKO (A4502) | C ± M | ≥1+, ≥5% |
| Galectin-3 | Mouse monoclonal (9C4) | 1:300 | Novocastra (NCL-GAL3) | C | ≥1+, ≥5% |
| EMA | Mouse monoclonal (E29) | 1:200 | DAKO (M0613) | M | ≥1+, ≥5% |
| Caveolin-1 | Mouse monoclonal (pY14) | 1:300 | BD Biosciences (C37120) | C ± M | ≥2+, ≥5% |
| CD44 | Mouse monoclonal (DF1485) | 1:200 | Leica Microsystems (NCL-CD44-2) | M ± C | ≥1+, ≥5% |
| Jagged1 | Rabbit polyclonal (H-114) | 1:50 | Santa Cruz (sc-8303) | C ± M | ≥1+, ≥5% |
| β-catenin | Mouse monoclonal (14/Beta-Catenin) | 1:800 | BD Transduction (610153) | C ± M | ≤1+, ≥95% |
| E-cadherin | Mouse monoclonal (36/E-Cadherin) | 1:500 | BD Biosciences (610181) | N | ≥1+, ≥5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.B.; Lee, K.S.; Lee, H.S. Tumor-Associated Protein Profiles in Kaposi Sarcoma and Mimicking Vascular Tumors, and Their Pathological Implications. Int. J. Mol. Sci. 2019, 20, 3142. https://doi.org/10.3390/ijms20133142
Lee KB, Lee KS, Lee HS. Tumor-Associated Protein Profiles in Kaposi Sarcoma and Mimicking Vascular Tumors, and Their Pathological Implications. International Journal of Molecular Sciences. 2019; 20(13):3142. https://doi.org/10.3390/ijms20133142
Chicago/Turabian StyleLee, Kyoung Bun, Kyu Sang Lee, and Hye Seung Lee. 2019. "Tumor-Associated Protein Profiles in Kaposi Sarcoma and Mimicking Vascular Tumors, and Their Pathological Implications" International Journal of Molecular Sciences 20, no. 13: 3142. https://doi.org/10.3390/ijms20133142
APA StyleLee, K. B., Lee, K. S., & Lee, H. S. (2019). Tumor-Associated Protein Profiles in Kaposi Sarcoma and Mimicking Vascular Tumors, and Their Pathological Implications. International Journal of Molecular Sciences, 20(13), 3142. https://doi.org/10.3390/ijms20133142





