Whole Body Vibration-Induced Omental Macrophage Polarization and Fecal Microbiome Modification in a Murine Model
Abstract
1. Introduction
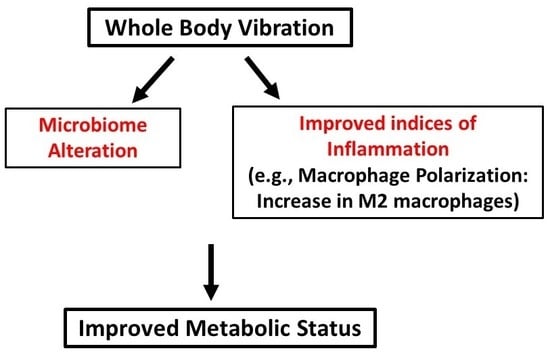
- M1 predominates in the abdominal and blood macrophages in T2DM mice;
- WBV can cause macrophage polarization from M1 towards M2, decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines;
- WBV causes alterations in both the alpha and beta diversity of fecal microbiome.
2. Results
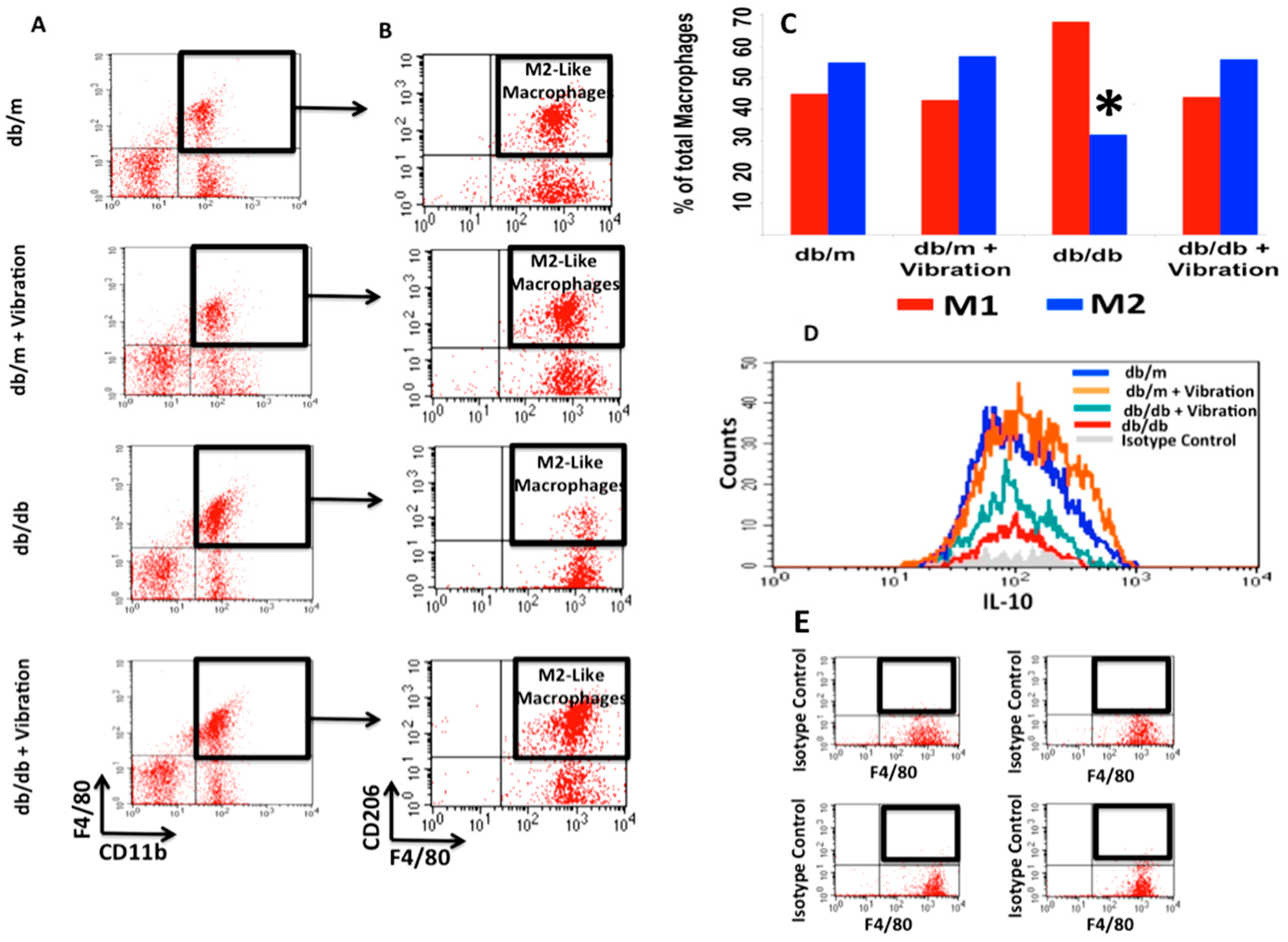
2.1. Macrophage Polarization
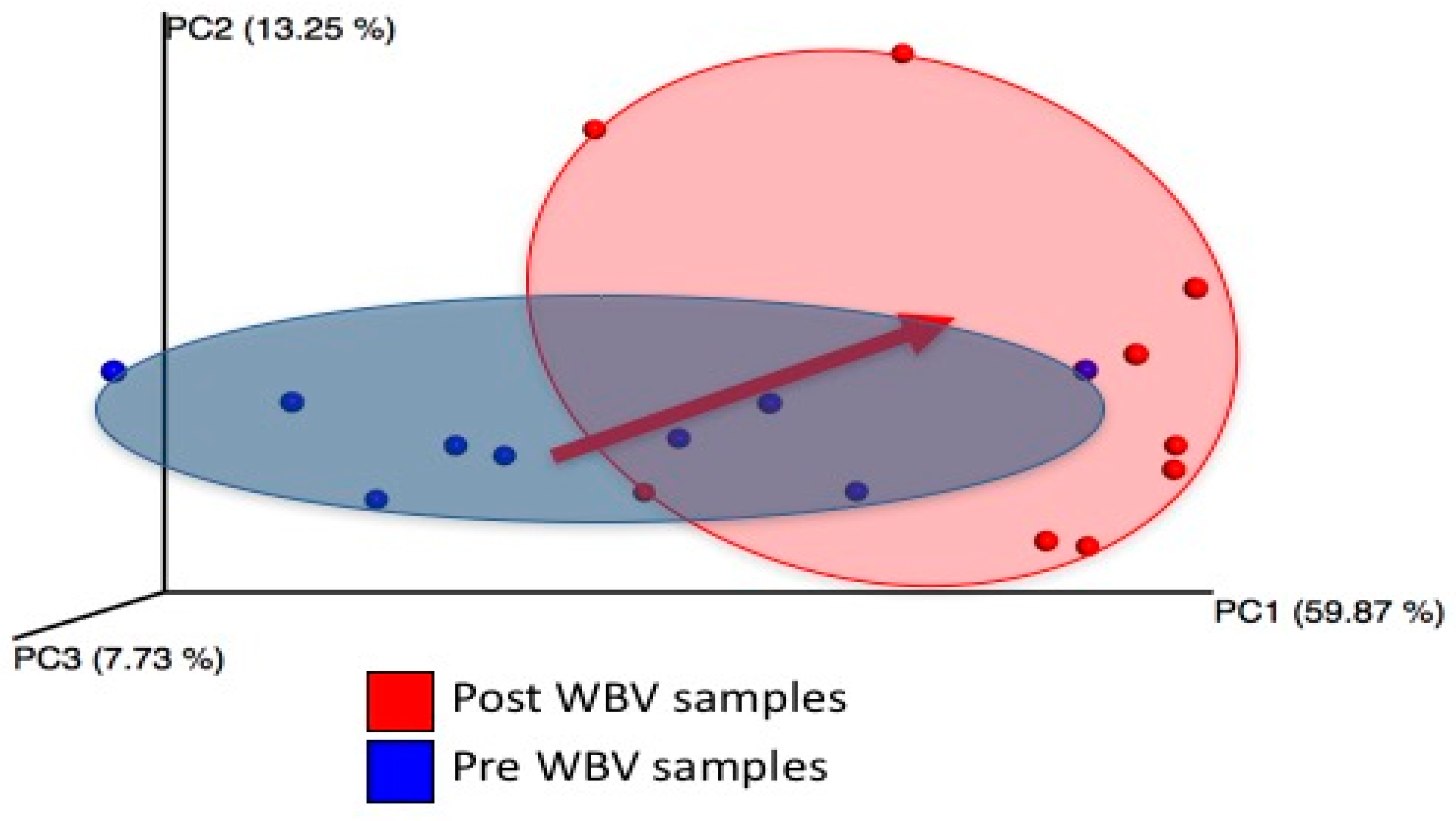
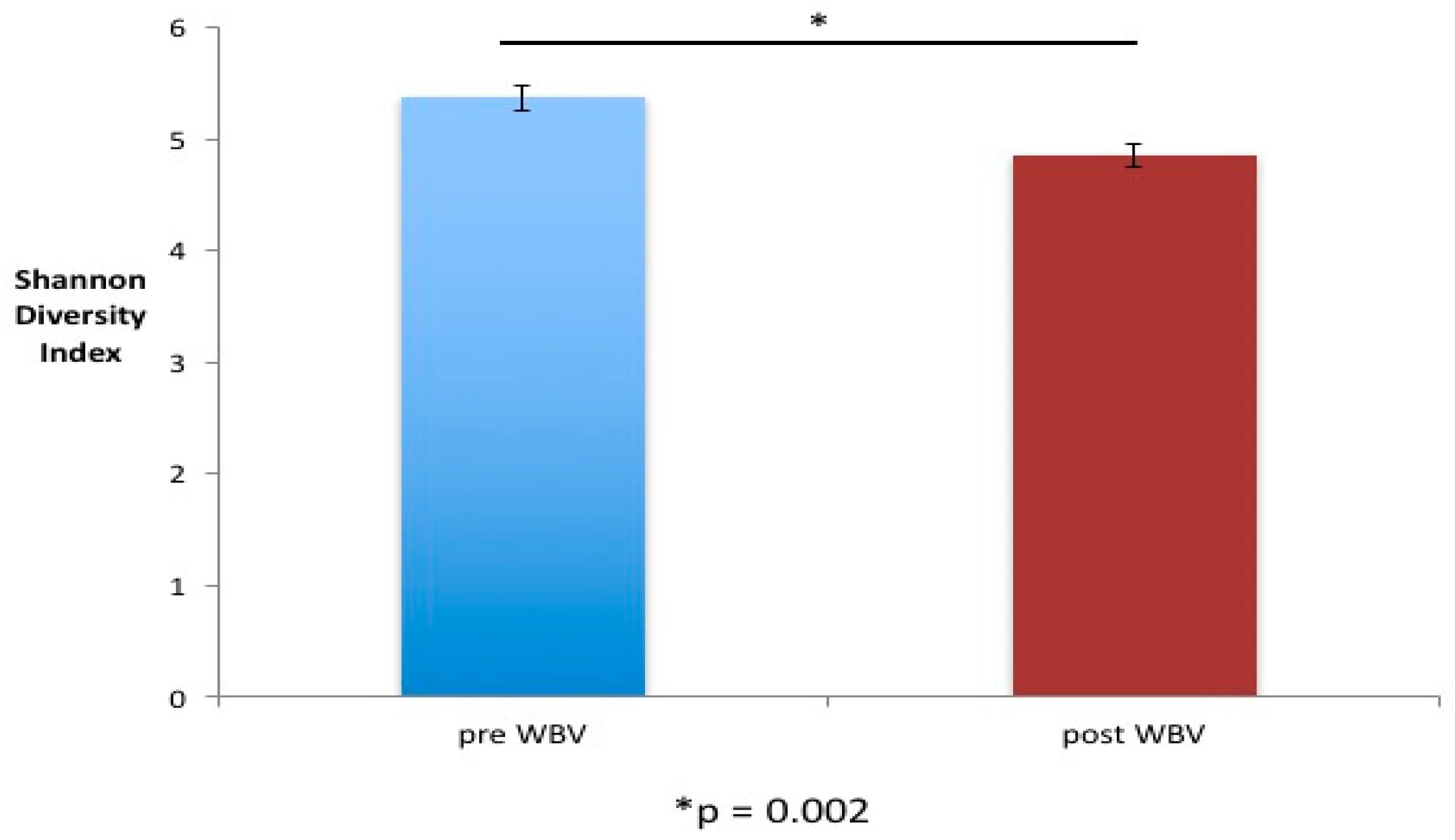
2.2. WBV Induced Changes in db/db Microbiome
3. Discussion
4. Materials and Methods
4.1. Macrophage Polarization Experiments
4.2. Analytic Flow Cytometry
4.3. Fecal Microbiome Changes
4.4. DNA Extraction, Library Preparation, Sequencing
4.5. Statistics
5. Conclusions
- The baseline ratio of omental M1 to M2 macrophages in T2DM mice is 2:1;
- WBV can cause M1 to M2 polarization in both control and T2DM mice;
- WBV restores M2 levels in T2DM to near baseline levels of the normal control;
- WBV alters the fecal microbiome in T2DM mice, increasing bacteroides, especially those belonging to the genus Alistipes of the Rikenellaceae family, which increased by 17.75 times.
Author Contributions
Funding
Conflicts of Interest
References
- Yin, H.; Berdel, H.O.; Moore, D.; Davis, F.; Liu, J.; Mozaffari, M.; Yu, J.C.; Baban, B. Whole body vibration therapy: A novel potential treatment for type 2 diabetes mellitus. SpringerPlus 2015, 4, 578. [Google Scholar] [CrossRef]
- McGee-Lawrence, M.E.; Wenger, K.H.; Misra, S.; Davis, C.L.; Pollock, N.K.; Elsalanty, M.; Ding, K.; Isales, C.M.; Hamrick, M.W.; Wosiski-Kuhn, M.; et al. Whole-Body Vibration Mimics the Metabolic Effects of Exercise in Male Leptin Receptor-Deficient Mice. Endocrinology 2017, 158, 1160–1171. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Interleukin-3 facilitates glucose transport in a myeloid cell line by regulating the affinity of the glucose transporter for glucose: Involvement of protein phosphorylation in transporter activation. Biochem. J. 1995, 305, 843–851. [Google Scholar] [CrossRef]
- Chan, A.; Shinde, R.; Chow, C.C.; Cockram, C.S.; Swaminathan, R. Hyperinsulinaemia and Na+, K(+)-ATPase activity in thyrotoxic periodic paralysis. Clin. Endocrinol. 1994, 41, 213–216. [Google Scholar] [CrossRef]
- Chen, W.L.; Wang, J.H.; Zhao, A.H.; Xu, X.; Wang, Y.H.; Chen, T.L.; Li, J.M.; Mi, J.Q.; Zhu, Y.M.; Liu, Y.F.; et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 2014, 124, 1645–1654. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Yu, J.C.; Khodadadi, H.; Baban, B. Innate immunity and oral microbiome: A personalized, predictive, and preventive approach to the management of oral diseases. EPMA J. 2019, 10, 43–50. [Google Scholar] [CrossRef]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; Salles, E.; Bhatia, J.; Hale, V.L.; Baban, B. Innate Immunity of Neonates and Infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef]
- Park, S.Y.; Son, W.M.; Kwon, O.S. Effects of whole body vibration training on body composition, skeletal muscle strength, and cardiovascular health. JER 2015, 11, 289–295. [Google Scholar] [CrossRef]
- Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Collado, P.S.; Almar, M.; Martinez-Florez, S.; de Paz, J.A.; Gonzalez-Gallego, J.; Cuevas, M.J. Whole-body vibration improves the anti-inflammatory status in elderly subjects through toll-like receptor 2 and 4 signaling pathways. Mech. Ageing Dev. 2015, 150, 12–19. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Schloss, P.D.; Schubert, A.M.; Zackular, J.P.; Iverson, K.D.; Young, V.B.; Petrosino, J.F. Stabilization of the murine gut microbiome following weaning. Gut microbes 2012, 3, 383–393. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Carey, H.V.; Assadi-Porter, F.M. The Hibernator Microbiome: Host-Bacterial Interactions in an Extreme Nutritional Symbiosis. Ann. Rev. Nutr. 2017, 37, 477–500. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef]
- Alpers, C.E.; Hudkins, K.L. Mouse models of diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2011, 20, 278–284. [Google Scholar] [CrossRef]
- Vaibhav, K.; Braun, M.; Khan, M.B.; Fatima, S.; Saad, N.; Shankar, A.; Khan, Z.T.; Harris, R.B.S.; Yang, Q.; Huo, Y.; et al. Remote ischemic post-conditioning promotes hematoma resolution viaAMPK-dependent immune regulation. J. Exp. Med. 2018, 215, 2636–2654. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
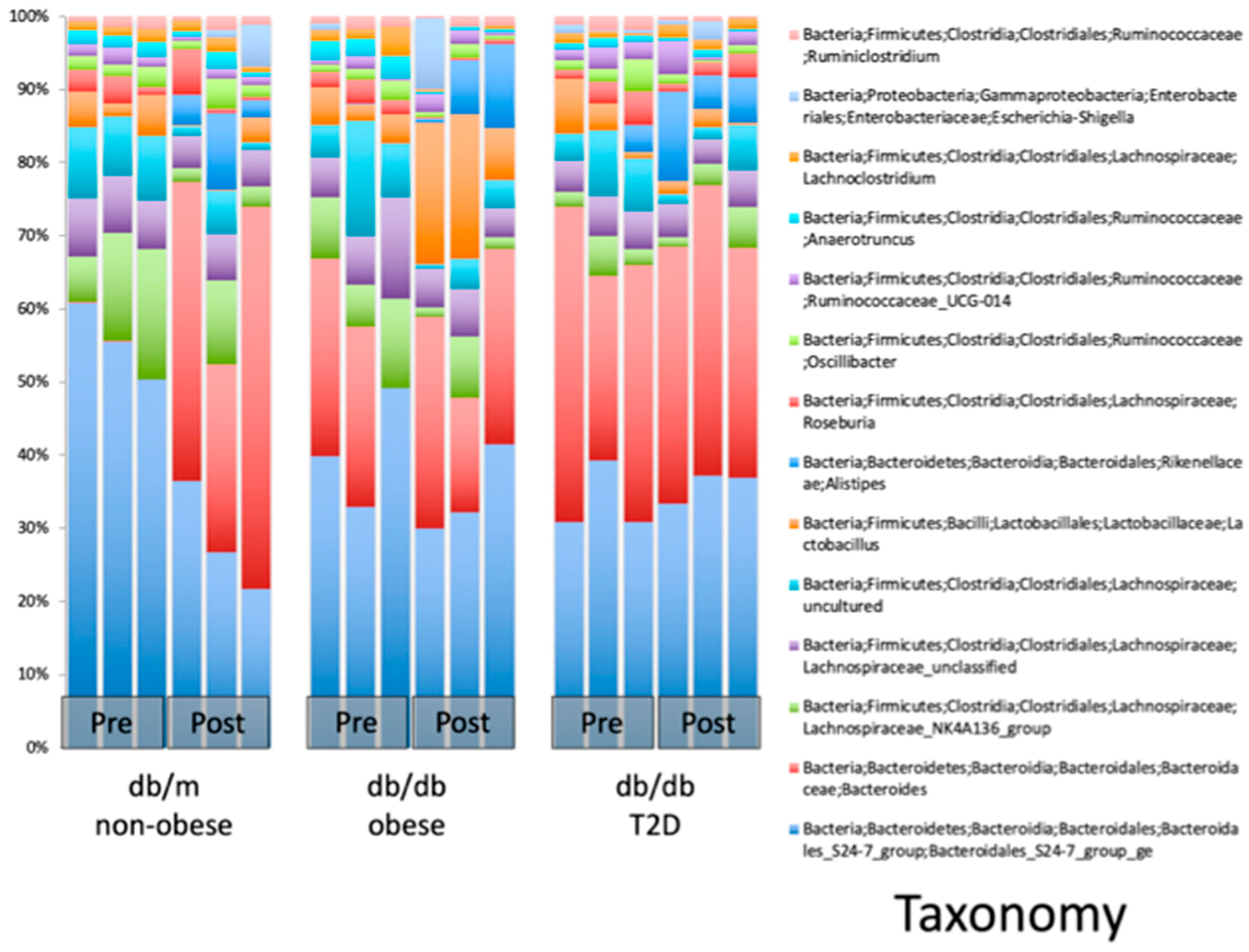
| Pre WBV Mean Reads | Post WBV Mean Reads | FDR p Value | Taxonomy |
|---|---|---|---|
| 703.22 | 12479.56 | 0.02 | Bacteria; Bacteroidetes; Bacteroidia; Rikenellaceae; Alistipes |
| 943.33 | 3724.56 | 0.03 | Bacteria; Bacteroidetes; Bacteroidia; Bacteroidales; Bacteroidales_S24-7_group; Bacteroidales_S24-7_group_ge |
| 2746.78 | 11404.44 | 0.03 | Bacteria; Bacteroidetes; Bacteroidia; Bacteroidales; Bacteroidales_S24-7_group; Bacteroidales_S24-7_group_ge |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.C.; Hale, V.L.; Khodadadi, H.; Baban, B. Whole Body Vibration-Induced Omental Macrophage Polarization and Fecal Microbiome Modification in a Murine Model. Int. J. Mol. Sci. 2019, 20, 3125. https://doi.org/10.3390/ijms20133125
Yu JC, Hale VL, Khodadadi H, Baban B. Whole Body Vibration-Induced Omental Macrophage Polarization and Fecal Microbiome Modification in a Murine Model. International Journal of Molecular Sciences. 2019; 20(13):3125. https://doi.org/10.3390/ijms20133125
Chicago/Turabian StyleYu, Jack C., Vanessa L. Hale, Hesam Khodadadi, and Babak Baban. 2019. "Whole Body Vibration-Induced Omental Macrophage Polarization and Fecal Microbiome Modification in a Murine Model" International Journal of Molecular Sciences 20, no. 13: 3125. https://doi.org/10.3390/ijms20133125
APA StyleYu, J. C., Hale, V. L., Khodadadi, H., & Baban, B. (2019). Whole Body Vibration-Induced Omental Macrophage Polarization and Fecal Microbiome Modification in a Murine Model. International Journal of Molecular Sciences, 20(13), 3125. https://doi.org/10.3390/ijms20133125