Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance
Abstract
1. Introduction
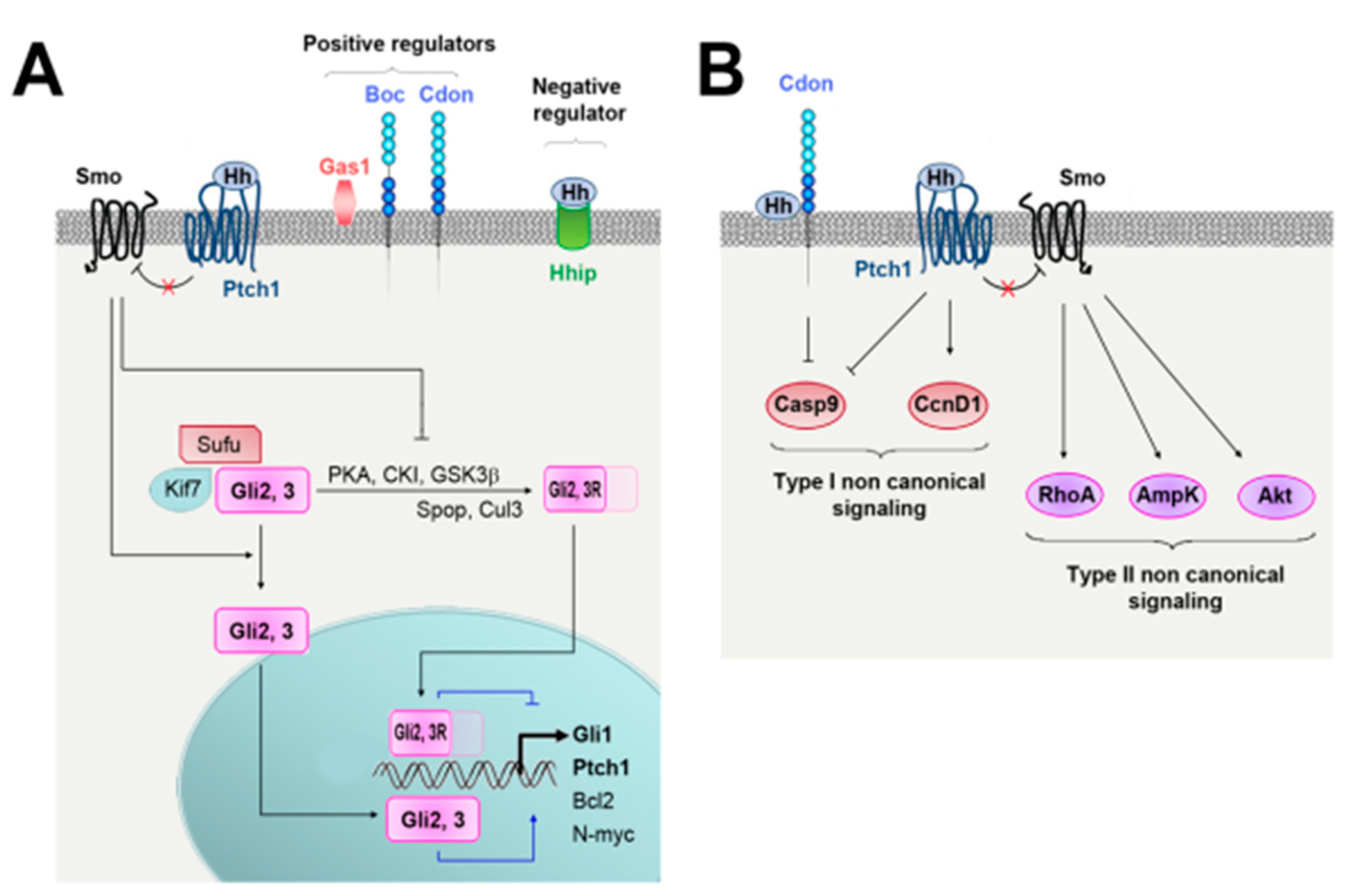
2. Hedgehog Signaling and Regulation
2.1. Regulation of Hh Ligand Secretion
2.2. Hh Signaling
2.3. Regulation of Hh Signaling
3. Vascular Development
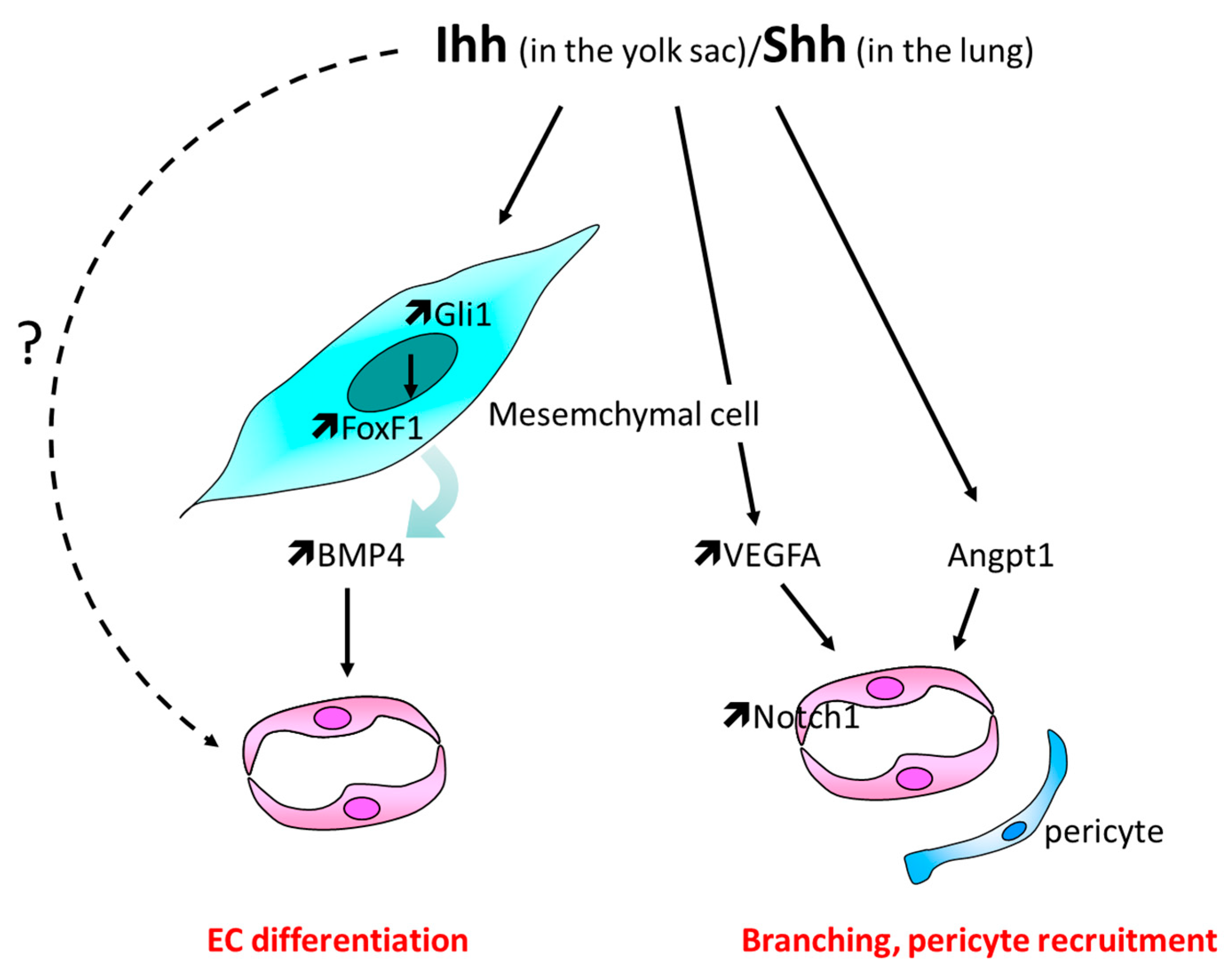
3.1. Yolk Sac Vascularization
3.2. Lungs Vasculature Development
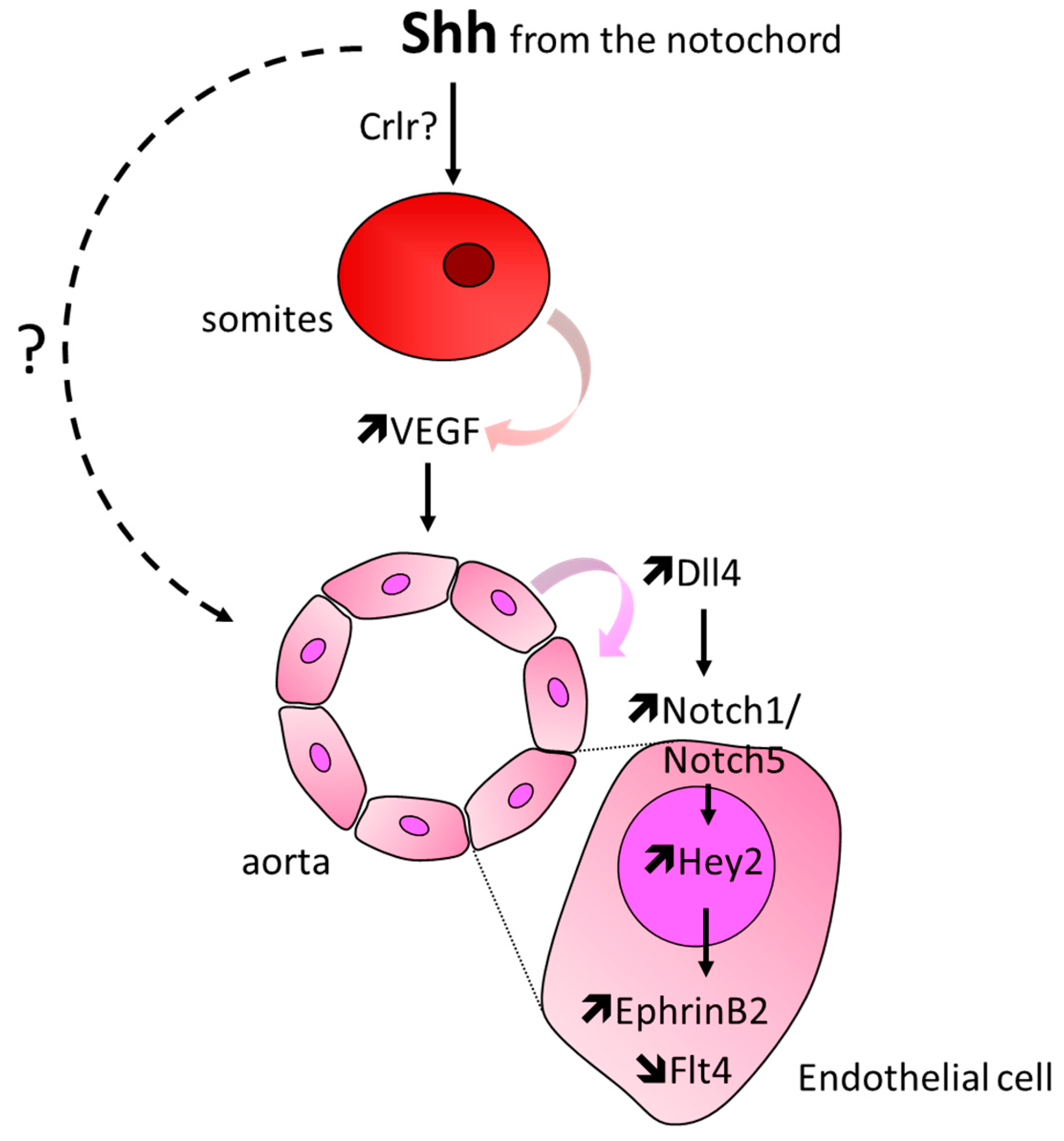
3.3. Formation of the Aorta and Intersomitic Vessels
3.4. Involvement of Hh Signaling in the Setting of Other Vascular Bed Development
3.5. Controversial Data
4. Postnatal Angiogenesis
4.1. In the Setting of Ischemia
4.1.1. Role of Hh-Signaling in Ischemia-Induced Angiogenesis in the Hindlimb
Controversial Data
4.1.2. Role of Hh Signaling in Other Ischemic Tissues
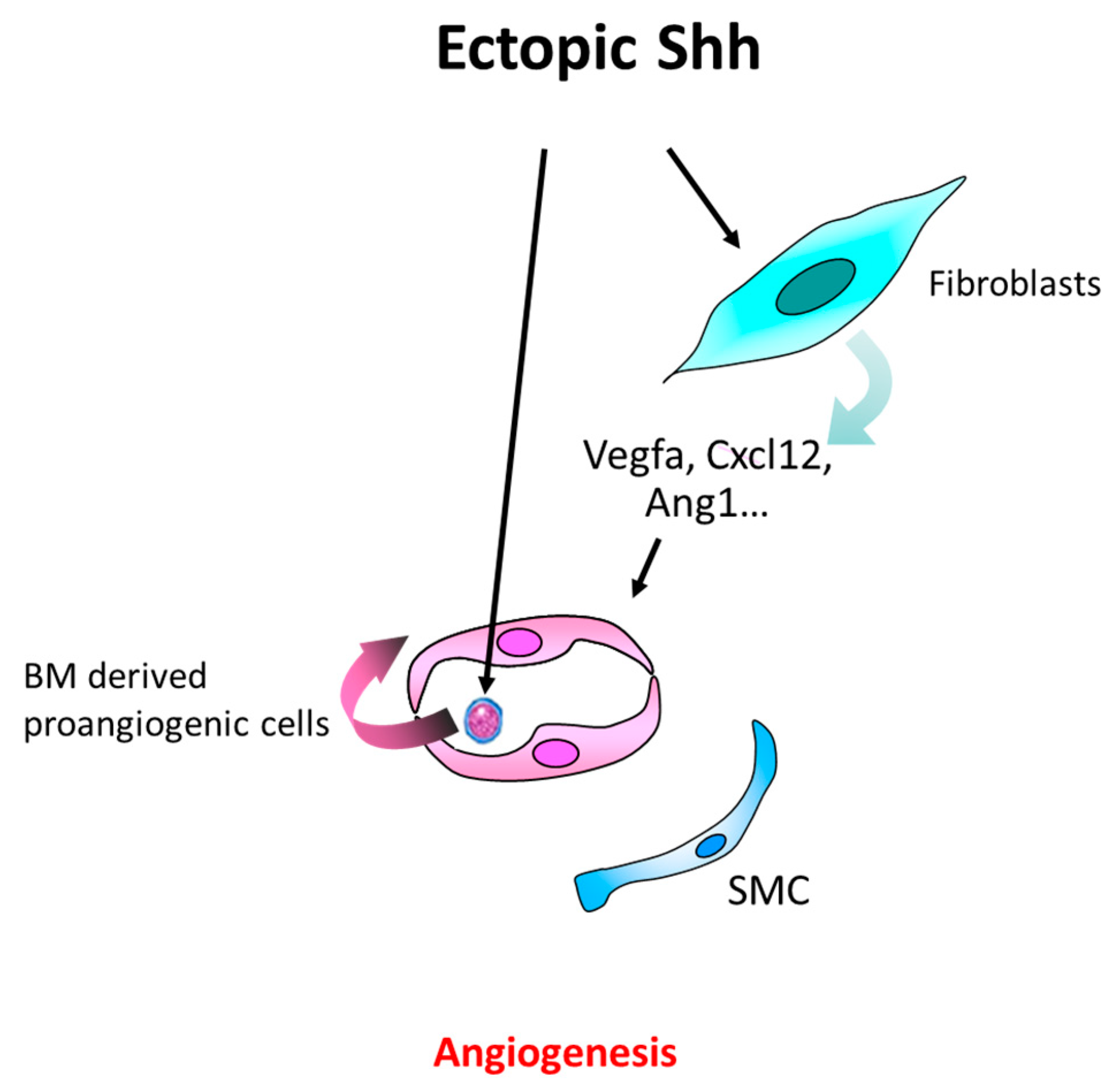
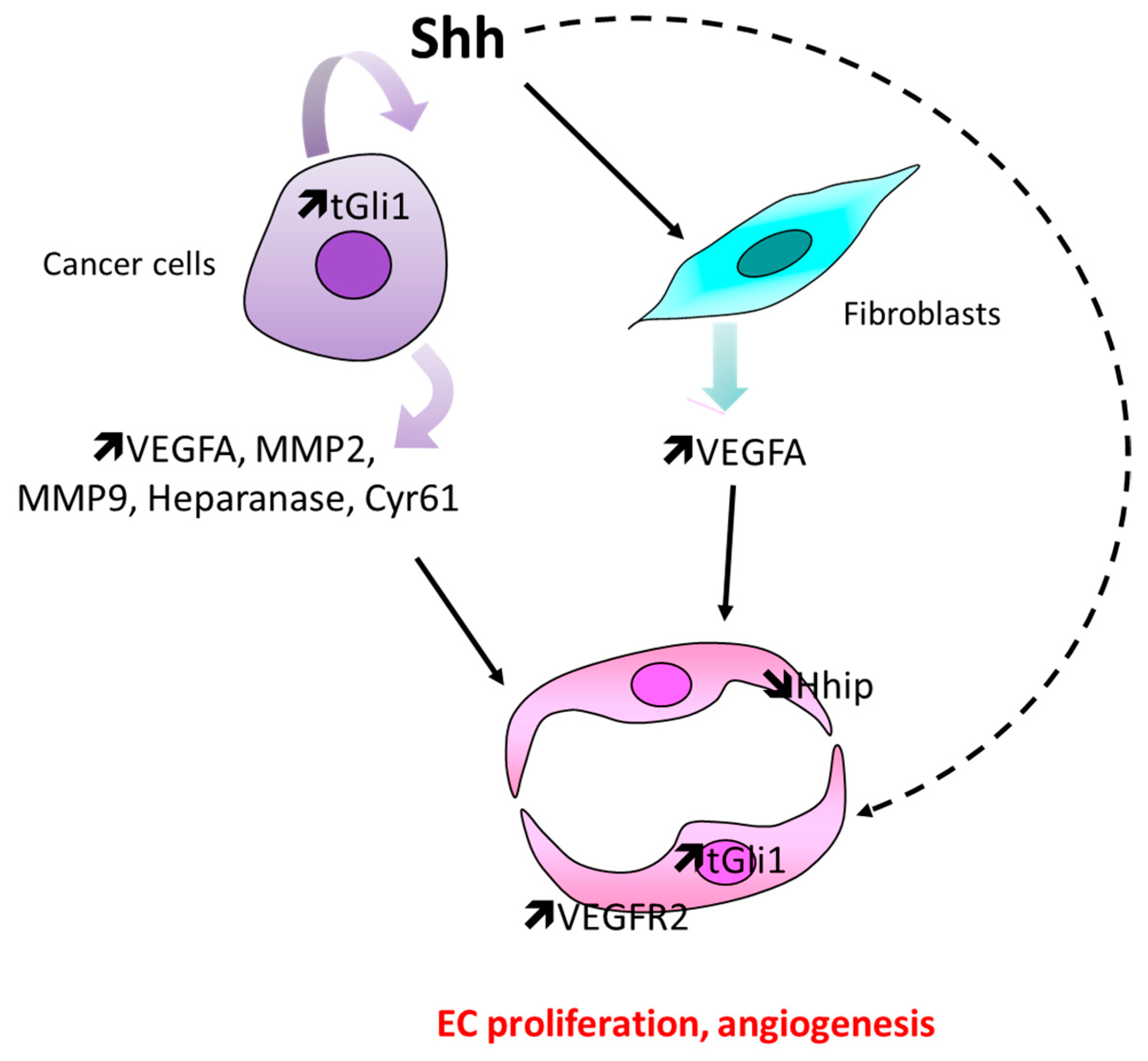
4.2. In the Setting of Cancer
Controversial Data
4.3. Other Pathological Angiogenesis
5. Maintenance of Blood Vessel Integrity and Quiescence
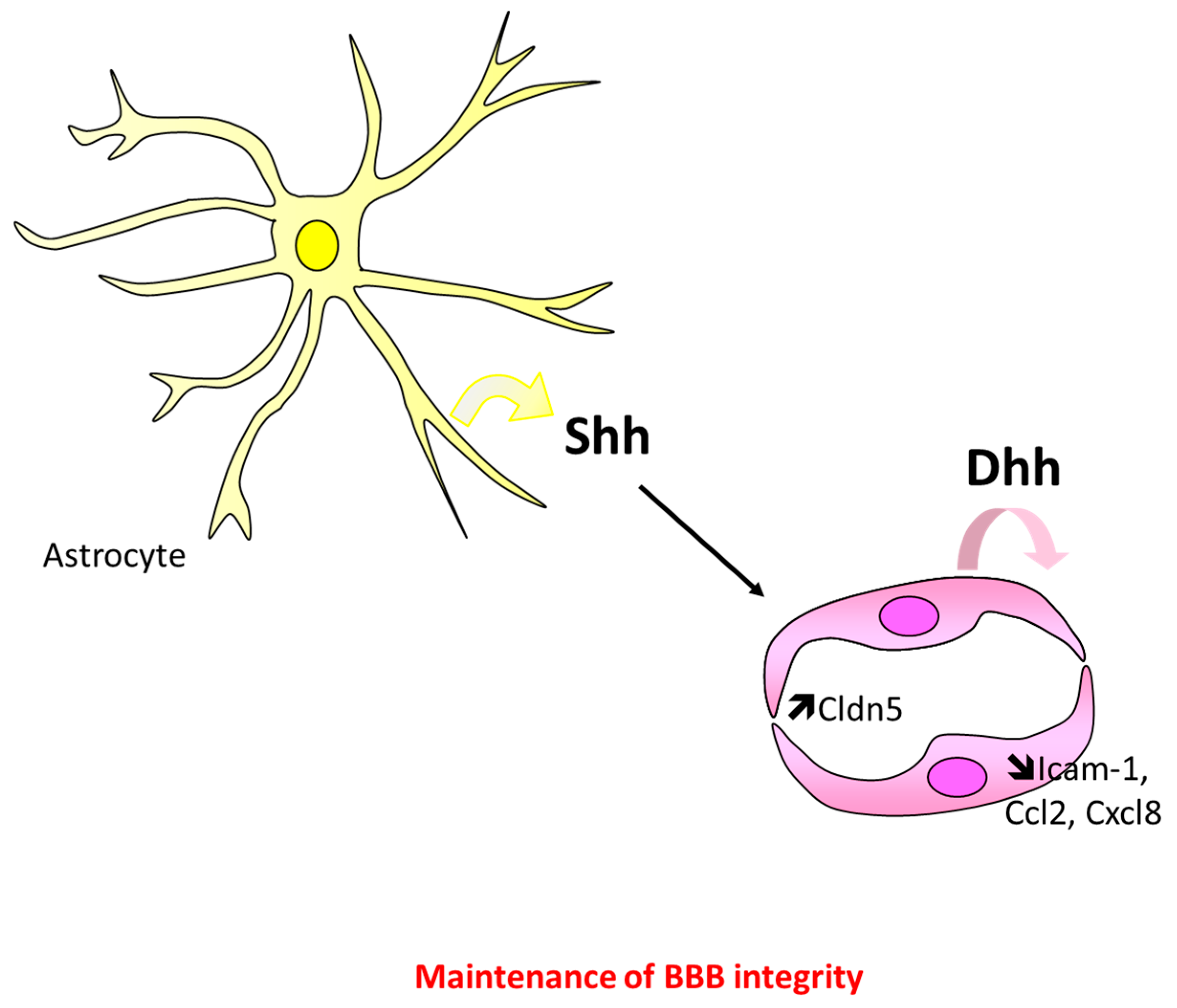
5.1. At the Blood–Brain Barrier
Controversial Data
5.2. At the Blood Nerve Barrier
5.3. Outside of the Nervous System
5.4. Molecular Mechanism Involved in Hh-Induced Maintenance of Endothelium Integrity
6. Blood Vessel Maturation
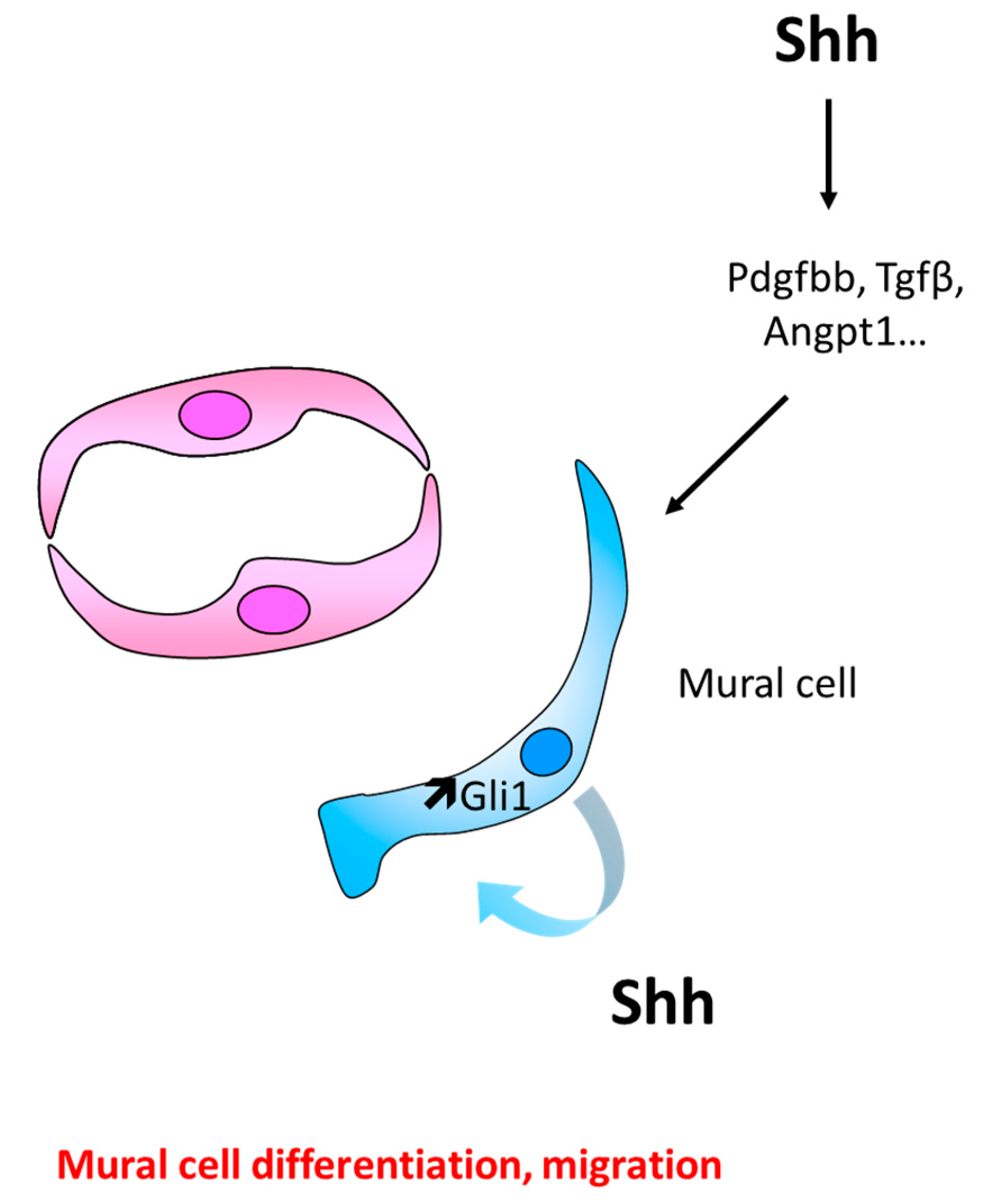
6.1. Role of Hh Signaling in Mural Cell Recruitment and Differentiation
Controversial Data
6.2. Role of Hh Signaling in Vascular Wall Remodeling
Discussion/Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Bitgood, M.J.; McMahon, A.P. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 1995, 172, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Santos, M.; Melton, D.A.; McMahon, A.P. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 2000, 127, 2763–2772. [Google Scholar] [PubMed]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Bitgood, M.J.; Shen, L.; McMahon, A.P. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 1996, 6, 298–304. [Google Scholar] [CrossRef]
- Parmantier, E.; Lynn, B.; Lawson, D.; Turmaine, M.; Namini, S.S.; Chakrabarti, L.; McMahon, A.P.; Jessen, K.R.; Mirsky, R. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 1999, 23, 713–724. [Google Scholar] [CrossRef]
- Petrova, R.; Joyner, A.L. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 2014, 141, 3445–3457. [Google Scholar] [CrossRef] [PubMed]
- Scales, S.J.; de Sauvage, F.J. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharm. Sci. 2009, 30, 303–312. [Google Scholar] [CrossRef]
- Pepicelli, C.V.; Lewis, P.M.; McMahon, A.P. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998, 8, 1083–1086. [Google Scholar] [CrossRef]
- Rowitch, D.H.S.; Jacques, B.; Lee, S.M.; Flax, J.D.; Snyder, E.Y.; McMahon, A.P. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J. Neurosci. 1999, 19, 8954–8965. [Google Scholar] [CrossRef]
- Pola, R.; Ling, L.E.; Silver, M.; Corbley, M.J.; Kearney, M.; Blake Pepinsky, R.; Shapiro, R.; Taylor, F.R.; Baker, D.P.; Asahara, T.; et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat. Med. 2001, 7, 706–711. [Google Scholar] [CrossRef]
- Renault, M.A.; Roncalli, J.; Tongers, J.; Thorne, T.; Klyachko, E.; Misener, S.; Volpert, O.V.; Mehta, S.; Burg, A.; Luedemann, C.; et al. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J. Mol. Cell. Cardiol. 2010, 49, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Miyamoto, T.; Saika, S. Sonic hedgehog: Its expression in a healing cornea and its role in neovascularization. Mol. Vis. 2009, 15, 1036–1044. [Google Scholar] [PubMed]
- Yao, Q.; Renault, M.A.; Chapouly, C.; Vandierdonck, S.; Belloc, I.; Jaspard-Vinassa, B.; Daniel-Lamaziere, J.M.; Laffargue, M.; Merched, A.; Desgranges, C.; et al. Sonic hedgehog mediates a novel pathway of PDGF-BB-dependent vessel maturation. Blood 2014, 123, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonniere, L.; Bernard, M.; et al. The Hedgehog Pathway Promotes Blood-Brain Barrier Integrity and CNS Immune Quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef]
- Lawson, N.D.; Vogel, A.M.; Weinstein, B.M. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell 2002, 3, 127–136. [Google Scholar] [CrossRef]
- Lavine, K.J.; Ornitz, D.M. Rebuilding the coronary vasculature: Hedgehog as a new candidate for pharmacologic revascularization. Trends Cardiovasc. Med. 2007, 17, 77–83. [Google Scholar] [CrossRef][Green Version]
- Cristofaro, B.; Emanueli, C. Possible novel targets for therapeutic angiogenesis. Curr. Opin. Pharmacol. 2009, 9, 102–108. [Google Scholar] [CrossRef]
- Pan, J.Y.; Zhou, S.H. The hedgehog signaling pathway, a new therapeutic target for treatment of ischemic heart disease. Pharmazie 2012, 67, 475–481. [Google Scholar]
- Wang, Y.; Lu, P.; Zhao, D.; Sheng, J. Targeting the hedgehog signaling pathway for cardiac repair and regeneration. Herz 2017, 42, 662–668. [Google Scholar] [CrossRef]
- Dunaeva, M.; Waltenberger, J. Hh signaling in regeneration of the ischemic heart. Cell. Mol. Life Sci. 2017, 74, 3481–3490. [Google Scholar] [CrossRef] [PubMed]
- Salybekov, A.A.; Salybekova, A.K.; Pola, R.; Asahara, T. Sonic Hedgehog Signaling Pathway in Endothelial Progenitor Cell Biology for Vascular Medicine. Int. J. Mol. Sci. 2018, 19, 3040. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Singh, S.; Schilling, N.S.; Robbins, D.J. Hedgehog Processing and Biological Activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G844–G849. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, C.A.; Asp, E.; Emerson, C.P. A new role for Hedgehogs in juxtacrine signaling. Mech. Dev. 2013, 131, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Tukachinsky, H.; Kuzmickas, R.P.; Jao, C.Y.; Liu, J.; Salic, A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2012, 2, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.J.; Fei, D.L.; Riobo, N.A. The Hedgehog signal transduction network. Sci. Signal. 2012, 5, re6. [Google Scholar] [CrossRef] [PubMed]
- Ramsbottom, S.A.; Pownall, M.E. Regulation of Hedgehog Signalling Inside and Outside the Cell. J. Dev. Biol. 2016, 4, 23. [Google Scholar] [CrossRef]
- Bangs, F.; Anderson, K.V. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harb. Perspect Biol. 2017, 9, a028175. [Google Scholar] [CrossRef]
- Byrd, N.; Becker, S.; Maye, P.; Narasimhaiah, R.; St-Jacques, B.; Zhang, X.; McMahon, J.; McMahon, A.; Grabel, L. Hedgehog is required for murine yolk sac angiogenesis. Development 2002, 129, 361–372. [Google Scholar]
- Dyer, M.A.; Farrington, S.M.; Mohn, D.; Munday, J.R.; Baron, M.H. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development 2001, 128, 1717–1730. [Google Scholar]
- Nagase, M.; Nagase, T.; Koshima, I.; Fujita, T. Critical time window of hedgehog-dependent angiogenesis in murine yolk sac. Microvasc. Res. 2006, 71, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Astorga, J.; Carlsson, P. Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development 2007, 134, 3753–3761. [Google Scholar] [CrossRef] [PubMed]
- Hochman, E.; Castiel, A.; Jacob-Hirsch, J.; Amariglio, N.; Izraeli, S. Molecular pathways regulating pro-migratory effects of Hedgehog signaling. J. Biol. Chem. 2006, 281, 33860–33870. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.-A.D.; Wert, S.E.; Clark, J.C.; Xu, Y.; Perl, A.-K.T.; Whitsett, J.A. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev. Dyn. 2004, 231, 57–71. [Google Scholar] [CrossRef] [PubMed]
- White, A.C.; Lavine, K.J.; Ornitz, D.M. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development 2007, 134, 3743–3752. [Google Scholar] [CrossRef]
- van Tuyl, M.; Groenman, F.; Wang, J.; Kuliszewski, M.; Liu, J.; Tibboel, D.; Post, M. Angiogenic factors stimulate tubular branching morphogenesis of sonic hedgehog-deficient lungs. Dev. Biol. 2007, 303, 514–526. [Google Scholar] [CrossRef]
- Seo, H.; Kim, J.; Park, G.-H.; Kim, Y.; Cho, S.-W. Long-range enhancers modulate Foxf1 transcription in blood vessels of pulmonary vascular network. Histochem. Cell Biol. 2016, 146, 289–300. [Google Scholar] [CrossRef]
- Vokes, S.A.; Yatskievych, T.A.; Heimark, R.L.; McMahon, J.; McMahon, A.P.; Antin, P.B.; Krieg, P.A. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development 2004, 131, 4371–4380. [Google Scholar] [CrossRef]
- Kolesova, H.; Roelink, H.; Grim, M. Sonic hedgehog is required for the assembly and remodeling of branchial arch blood vessels. Dev. Dyn 2008, 237, 1923–1934. [Google Scholar] [CrossRef]
- Moran, C.M.; Salanga, M.C.; Krieg, P.A. Hedgehog signaling regulates size of the dorsal aortae and density of the plexus during avian vascular development. Dev. Dyn. 2011, 240, 1354–1364. [Google Scholar] [CrossRef]
- Coultas, L.; Nieuwenhuis, E.; Anderson, G.A.; Cabezas, J.; Nagy, A.; Henkelman, R.M.; Hui, C.C.; Rossant, J. Hedgehog regulates distinct vascular patterning events through VEGF-dependent and -independent mechanisms. Blood 2010, 116, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Rodaway, A.R.; Schilling, T.F.; Jowett, T.; Ingham, P.W.; Patient, R.K.; Sharrocks, A.D. Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech. Dev. 2000, 90, 237–252. [Google Scholar] [CrossRef]
- Williams, C.; Kim, S.-H.; Ni, T.T.; Mitchell, L.; Ro, H.; Penn, J.S.; Baldwin, S.H.; Solnica-Krezel, L.; Zhong, T.P. Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev. Biol. 2010, 341, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Nagase, T.; Nagase, M.; Yoshimura, K.; Machida, M.; Yamagishi, M. Defects in aortic fusion and craniofacial vasculature in the holoprosencephalic mouse embryo under inhibition of sonic hedgehog signaling. J. Craniofac. Surg. 2006, 17, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, S.; Tobia, C.; Gualandi, L.; De Sena, G.; Presta, M. Calcitonin receptor-like receptor guides arterial differentiation in zebrafish. Blood 2008, 111, 4965–4972. [Google Scholar] [CrossRef]
- Lamont, R.E.; Vu, W.; Carter, A.D.; Serluca, F.C.; MacRae, C.A.; Childs, S.J. Hedgehog signaling via angiopoietin1 is required for developmental vascular stability. Mech. Dev. 2010, 127, 159–168. [Google Scholar] [CrossRef]
- Surace, E.M.; Balaggan, K.S.; Tessitore, A.; Mussolino, C.; Cotugno, G.; Bonetti, C.; Vitale, A.; Ali, R.R.; Auricchio, A. Inhibition of ocular neovascularization by hedgehog blockade. Mol. Ther. 2006, 13, 573–579. [Google Scholar] [CrossRef]
- Walshe, T.E.; Connell, P.; Cryan, L.; Ferguson, G.; Gardiner, T.; Morrow, D.; Redmond, E.M.; O’Brien, C.; Cahill, P.A. Microvascular retinal endothelial and pericyte cell apoptosis in vitro: Role of hedgehog and Notch signaling. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4472–4483. [Google Scholar] [CrossRef]
- Berrios-Otero, C.A.; Wadghiri, Y.Z.; Nieman, B.J.; Joyner, A.L.; Turnbull, D.H. Three-dimensional micro-MRI analysis of cerebral artery development in mouse embryos. Magn. Reson. Med. 2009, 62, 1431–1439. [Google Scholar] [CrossRef]
- Nielsen, C.M.; Dymecki, S.M. Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev. Biol. 2010, 340, 430–437. [Google Scholar] [CrossRef]
- Colnot, C.; de la Fuente, L.; Huang, S.; Hu, D.; Lu, C.; St-Jacques, B.; Helms, J.A. Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development. Development 2005, 132, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; Fuchs, S.; Kolbe, M.; Hofmann, A.; Schmidt, H.; Kirkpatrick, C.J. Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng. Part A 2010, 16, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Murata, Y.; Liu, Y.; Nicolae, C.; Olsen, B.R.; Berendsen, A.D. Vegfa regulates perichondrial vascularity and osteoblast differentiation in bone development. Development 2015, 142, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; White, A.C.; Park, C.; Smith, C.S.; Choi, K.; Long, F.; Hui, C.C.; Ornitz, D.M. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006, 20, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Long, F.; Choi, K.; Smith, C.; Ornitz, D.M. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development 2008, 135, 3161–3171. [Google Scholar] [CrossRef]
- Moran, C.M.; Myers, C.T.; Lewis, C.M.; Krieg, P.A. Hedgehog regulates angiogenesis of intersegmental vessels through the VEGF signaling pathway. Dev. Dyn. 2012, 241, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Cowan, R.G.; Migone, F.F.; Quirk, S.M. Overactivation of hedgehog signaling alters development of the ovarian vasculature in mice. Biol. Reprod. 2012, 86, 174. [Google Scholar] [CrossRef] [PubMed]
- Weiss, O.; Kaufman, R.; Mishani, E.; Inbal, A. Ocular vessel patterning in zebrafish is indirectly regulated by Hedgehog signaling. Int. J. Dev. Biol. 2017, 61, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; James, J.; Paton, I.R.; Burt, D.W.; Tickle, C. Analysis of talpid3 and wild-type chicken embryos reveals roles for Hedgehog signalling in development of the limb bud vasculature. Dev. Biol. 2007, 301, 155–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, W.; Tang, T.; Eastham-Anderson, J.; Dunlap, D.; Alicke, B.; Nannini, M.; Gould, S.; Yauch, R.; Modrusan, Z.; DuPree, K.J.; et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc. Natl. Acad. Sci. USA 2011, 108, 9589–9594. [Google Scholar] [CrossRef]
- Pola, R.; Ling, L.E.; Aprahamian, T.R.; Barban, E.; Bosch-Marce, M.; Curry, C.; Corbley, M.; Kearney, M.; Isner, J.M.; Losordo, D.W. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation 2003, 108, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Renault, M.A.; Chapouly, C.; Yao, Q.; Larrieu-Lahargue, F.; Vandierdonck, S.; Reynaud, A.; Petit, M.; Jaspard-Vinassa, B.; Belloc, I.; Traiffort, E.; et al. Desert hedgehog promotes ischemia-induced angiogenesis by ensuring peripheral nerve survival. Circ. Res. 2013, 112, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Palladino, M.; Gatto, I.; Neri, V.; Straino, S.; Silver, M.; Tritarelli, A.; Piccioni, A.; Smith, R.C.; Gaetani, E.; Losordo, D.W.; et al. Pleiotropic beneficial effects of sonic hedgehog gene therapy in an experimental model of peripheral limb ischemia. Mol. Ther. 2011, 19, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Renault, M.A.; Robbesyn, F.; Chapouly, C.; Yao, Q.; Vandierdonck, S.; Reynaud, A.; Belloc, I.; Traiffort, E.; Ruat, M.; Desgranges, C.; et al. Hedgehog-dependent regulation of angiogenesis and myogenesis is impaired in aged mice. Arter. Thromb. Vasc. Biol. 2013, 33, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Renault, M.A.; Roncalli, J.; Tongers, J.; Misener, S.; Thorne, T.; Jujo, K.; Ito, A.; Clarke, T.; Fung, C.; Millay, M.; et al. The Hedgehog transcription factor Gli3 modulates angiogenesis. Circ. Res. 2009, 105, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Caradu, C.; Guy, A.; James, C.; Reynaud, A.; Gadeau, A.-P.; Renault, M.-A. Endogenous Sonic Hedgehog limits inflammation and angiogenesis in the ischaemic skeletal muscle of mice. Cardiovasc. Res. 2018, 114, 759–770. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Porro, C.; Andriantsitohaina, R.; Martínez, M.C. Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS ONE 2010, 5, e12688. [Google Scholar] [CrossRef]
- Qin, Y.; He, Y.-H.; Hou, N.; Zhang, G.-S.; Cai, Y.; Zhang, G.-P.; Xiao, Q.; He, L.-S.; Li, S.-J.; Yi, Q.; et al. Sonic hedgehog improves ischemia-induced neovascularization by enhancing endothelial progenitor cell function in type 1 diabetes. Mol. Cell. Endocrinol. 2016, 423, 30–39. [Google Scholar] [CrossRef]
- Renault, M.A.; Vandierdonck, S.; Chapouly, C.; Yu, Y.; Qin, G.; Metras, A.; Couffinhal, T.; Losordo, D.W.; Yao, Q.; Reynaud, A.; et al. Gli3 regulation of myogenesis is necessary for ischemia-induced angiogenesis. Circ. Res. 2013, 113, 1148–1158. [Google Scholar] [CrossRef]
- Gupta, R.; Mackie, A.R.; Misener, S.; Liu, L.; Losordo, D.W.; Kishore, R. Endothelial smoothened-dependent hedgehog signaling is not required for sonic hedgehog induced angiogenesis or ischemic tissue repair. Lab. Investig. 2018, 98, 682–691. [Google Scholar] [CrossRef]
- Straface, G.; Aprahamian, T.; Flex, A.; Gaetani, E.; Biscetti, F.; Smith, R.C.; Pecorini, G.; Pola, E.; Angelini, F.; Stigliano, E.; et al. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J. Cell. Mol. Med. 2009, 13, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Gaetani, E.; Neri, V.; Gatto, I.; Palladino, M.; Silver, M.; Smith, R.C.; Giarretta, I.; Pola, E.; Hlatky, L.; et al. Sonic Hedgehog Therapy in a Mouse Model of Age-Associated Impairment of Skeletal Muscle Regeneration. J. Gerontol. 2013, 69, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kusano, K.F.; Pola, R.; Murayama, T.; Curry, C.; Kawamoto, A.; Iwakura, A.; Shintani, S.; Ii, M.; Asai, J.; Tkebuchava, T.; et al. Sonic hedgehog myocardial gene therapy: Tissue repair through transient reconstitution of embryonic signaling. Nat. Med. 2005, 11, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.P.H.; Haider, K.H.; Shujia, J.; Afzal, M.R.; Ashraf, M. Sonic Hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway. PLoS ONE 2010, 5, e8576. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, Y.; Barnett, A.; Zhang, Y.; Yu, X.; Luo, Y. Poststroke Sonic Hedgehog Agonist Treatment Improves Functional Recovery by Enhancing Neurogenesis and Angiogenesis. Stroke 2017, 48, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Huang, M.; He, Q.-W.; Zhang, Y.; Opoku, E.N.; Yang, H.; Jin, H.-J.; Xia, Y.-P.; Hu, B. Administration of sonic hedgehog protein induces angiogenesis and has therapeutic effects after stroke in rats. Neuroscience 2017, 352, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Chechneva, O.V.; Mayrhofer, F.; Daugherty, D.J.; Krishnamurty, R.G.; Bannerman, P.; Pleasure, D.E.; Deng, W. A Smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis. 2014, 5, e1481. [Google Scholar] [CrossRef] [PubMed]
- Asai, J.; Takenaka, H.; Kusano, K.F.; Ii, M.; Luedemann, C.; Curry, C.; Eaton, E.; Iwakura, A.; Tsutsumi, Y.; Hamada, H.; et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation 2006, 113, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Kusano, K.F.; Allendoerfer, K.L.; Munger, W.; Pola, R.; Bosch-Marce, M.; Kirchmair, R.; Yoon, Y.S.; Curry, C.; Silver, M.; Kearney, M.; et al. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arter. Thromb. Vasc. Biol. 2004, 24, 2102–2107. [Google Scholar] [CrossRef]
- Bueno-Betí, C.; Novella, S.; Soleti, R.; Mompeón, A.; Vergori, L.; Sanchís, J.; Andriantsitohaina, R.; Martínez, M.C.; Hermenegildo, C. Microparticles Harboring Sonic Hedgehog Morphogen Improve the Vasculogenesis Capacity of Endothelial Progenitor Cells Derived from Myocardial Infarction Patients. Cardiovasc. Res. 2018, 115, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Kovacs, A.; Ornitz, D.M. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J. Clin. Investig. 2008, 118, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, M.F.; Leenders, P.J.; Janssen, B.J.; Peppelenbosch, M.P.; Ten Cate, H.; Spek, C.A. Endogenous hedgehog expression contributes to myocardial ischemia-reperfusion-induced injury. Exp. Biol. Med. 2008, 233, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Kurio, N.; Shimo, T.; Matsumoto, K.; Masui, M.; Takabatake, K.; Okui, T.; Ibaragi, S.; Kunisada, Y.; Obata, K.; et al. Oral Squamous Cell Carcinoma-derived Sonic Hedgehog Promotes Angiogenesis. Anticancer Res. 2017, 37, 6731–6737. [Google Scholar] [PubMed]
- Geng, L.; Cuneo, K.C.; Cooper, M.K.; Wang, H.; Sekhar, K.; Fu, A.; Hallahan, D.E. Hedgehog signaling in the murine melanoma microenvironment. Angiogenesis 2007, 10, 259–267. [Google Scholar] [CrossRef]
- Agrawal, V.; Kim, D.Y.; Kwon, Y.G. Hhip regulates tumor-stroma-mediated upregulation of tumor angiogenesis. Exp. Mol. Med. 2017, 49, e289. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Chen, X.; Yin, J.; Wang, W.; Lou, M.; Gu, S. Aberrant activation of Hedgehog/Gli1 pathway on angiogenesis in gliomas. Neurol India 2012, 60, 589–596. [Google Scholar] [PubMed]
- Di Mauro, C.; Rosa, R.; D’Amato, V.; Ciciola, P.; Servetto, A.; Marciano, R.; Orsini, R.C.; Formisano, L.; De Falco, S.; Cicatiello, V.; et al. Hedgehog signalling pathway orchestrates angiogenesis in triple-negative breast cancers. Br. J. Cancer 2017, 116, 1425–1435. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, G.; Wang, Y.; Guo, L. Cooperation of Indian Hedgehog and Vascular Endothelial Growth Factor in Tumor Angiogenesis and Growth in Human Hepatocellular Carcinomas, an Immunohistochemical Study. Appl. Immunohistochem. Mol. Morphol. 2018. [Google Scholar] [CrossRef]
- Cao, X.; Geradts, J.; Dewhirst, M.W.; Lo, H.W. Upregulation of VEGF-A and CD24 gene expression by the tGLI1 transcription factor contributes to the aggressive behavior of breast cancer cells. Oncogene 2012, 31, 104–115. [Google Scholar] [CrossRef]
- Liao, X.; Siu, M.K.; Au, C.W.; Wong, E.S.; Chan, H.Y.; Ip, P.P.; Ngan, H.Y.; Cheung, A.N. Aberrant activation of hedgehog signaling pathway in ovarian cancers: Effect on prognosis, cell invasion and differentiation. Carcinogenesis 2009, 30, 131–140. [Google Scholar] [CrossRef]
- Zhu, H.; Carpenter, R.L.; Han, W.; Lo, H.W. The GLI1 splice variant TGLI1 promotes glioblastoma angiogenesis and growth. Cancer Lett. 2014, 343, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.G.; Pannell, L.K.; Singh, S.; Samant, R.S.; Shevde, L.A. Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61. Oncogene 2012, 31, 3370–3380. [Google Scholar] [CrossRef] [PubMed]
- de Faro Valverde, L.; de Almeida Pereira, T.; Dias, R.B.; Guimaraes, V.S.; Ramos, E.A.; Santos, J.N.; Gurgel Rocha, C.A. Macrophages and endothelial cells orchestrate tumor-associated angiogenesis in oral cancer via hedgehog pathway activation. Tumour Biol. 2016, 37, 9233–9241. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.L.; Hsu, P.P.; Glienke, J.; Rubanyi, G.M.; Brooks, A.R. Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC Cancer 2004, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Nakamura, K.; Mizukami, Y.; Ii, M.; Sasajima, J.; Sugiyama, Y.; Nishikawa, T.; Nakano, Y.; Yanagawa, N.; Sato, K.; et al. Sonic hedgehog derived from human pancreatic cancer cells augments angiogenic function of endothelial progenitor cells. Cancer Sci. 2008, 99, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Becher, O.J.; Hambardzumyan, D.; Fomchenko, E.I.; Momota, H.; Mainwaring, L.; Bleau, A.M.; Katz, A.M.; Edgar, M.; Kenney, A.M.; Cordon-Cardo, C.; et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008, 68, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.N.; Yang, L.; Lv, Y.F.; Shi, Y.; Shen, L.L.; Yao, X.H.; Guo, Q.N.; Zhang, P.; Cui, Y.H.; Zhang, X.; et al. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J. Pathol. 2014, 234, 11–22. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Liu, C.-Y.; Kannagi, R.; Yang, R.-B. Inhibition of Endothelial SCUBE2 (Signal Peptide-CUB-EGF Domain-Containing Protein 2), a Novel VEGFR2 (Vascular Endothelial Growth Factor Receptor 2) Coreceptor, Suppresses Tumor Angiogenesis. Arter. Thromb. Vasc. Biol. 2018, 38, 1202–1215. [Google Scholar] [CrossRef]
- Huaitong, X.; Yuanyong, F.; Yueqin, T.; Peng, Z.; Wei, S.; Kai, S. Microvesicles releasing by oral cancer cells enhance endothelial cell angiogenesis via Shh/RhoA signaling pathway. Cancer Biol. 2017, 18, 783–791. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Roy Chaudhuri, T.; Straubinger, N.L.; Pitoniak, R.F.; Hylander, B.L.; Repasky, E.A.; Ma, W.W.; Straubinger, R.M. Tumor-Priming Smoothened Inhibitor Enhances Deposition and Efficacy of Cytotoxic Nanoparticles in a Pancreatic Cancer Model. Mol. Cancer 2016, 15, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, F.-F.; Shang, Y.-Y.; Li, Y.; Wang, Z.-H.; Han, L.; Li, Y.-H.; Zhang, L.; Ti, Y.; Zhang, W.; et al. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE-/-mice. Int. J. Cardiol. 2018, 265, 181–187. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, H.; Li, B.; Li, G.; Wang, Z. Blockade of the sonic hedgehog signalling pathway inhibits choroidal neovascularization in a laser-induced rat model. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Choi, S.S.; Syn, W.-K.; Michelotti, G.A.; Swiderska, M.; Karaca, G.; Chan, I.S.; Chen, Y.; Diehl, A.M. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut 2013, 62, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhu, Y.; Jia, Y.; Jiang, H.; Zheng, X.; Liu, D.; Gao, S.; Sun, M.; Hu, B.; Jiao, B.; et al. The Annexin a2 Promotes Development in Arthritis through Neovascularization by Amplification Hedgehog Pathway. PLoS ONE 2016, 11, e0150363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, S.; Sonobe, Y.; Cheng, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Interleukin-1β Induces Blood–Brain Barrier Disruption by Downregulating Sonic Hedgehog in Astrocytes. PLoS ONE 2014, 9, e110024. [Google Scholar] [CrossRef] [PubMed]
- Brilha, S.; Ong, C.W.M.; Weksler, B.; Romero, N.; Couraud, P.-O.; Friedland, J.S. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci. Rep. 2017, 7, 16031. [Google Scholar] [CrossRef] [PubMed]
- Podjaski, C.; Alvarez, J.I.; Bourbonniere, L.; Larouche, S.; Terouz, S.; Bin, J.M.; Lécuyer, M.-A.; Saint-Laurent, O.; Larochelle, C.; Darlington, P.J.; et al. Netrin 1 regulates blood–brain barrier function and neuroinflammation. Brain 2015, 138, 1598–1612. [Google Scholar] [CrossRef]
- Caradu, C.; Couffinhal, T.; Chapouly, C.; Guimbal, S.; Hollier, P.-L.; Ducasse, E.; Bura-Rivière, A.; Dubois, M.; Gadeau, A.-P.; Renault, M.-A. Restoring Endothelial Function by Targeting Desert Hedgehog Downstream of Klf2 Improves Critical Limb Ischemia in Adults. Circ. Res. 2018, 123, 1053–1065. [Google Scholar] [CrossRef]
- Xia, Y.; He, Q.; Li, Y.; Chen, S.; Huang, M.; Wang, Y.; Gao, Y.; Huang, Y.; Wang, M.; Mao, L.; et al. Recombinant Human Sonic Hedgehog Protein Regulates the Expression of ZO-1 and Occludin by Activating Angiopoietin-1 in Stroke Damage. PLoS ONE 2013, 8, e68891. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.B.; Singh, M.V.; Gorantla, S.; Poluektova, L.Y.; Maggirwar, S.B. Smoothened Agonist Reduces Human Immunodeficiency Virus Type-1-Induced Blood-Brain Barrier Breakdown in Humanized Mice. Sci. Rep. 2016, 6, 26876. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.B.; Singh, M.V.; Piekna-Przybylska, D.; Gorantla, S.; Poluektova, L.Y.; Maggirwar, S.B. Sonic Hedgehog mimetic prevents leukocyte infiltration into the CNS during acute HIV infection. Sci. Rep. 2017, 7, 9578. [Google Scholar] [CrossRef] [PubMed]
- Allahyari, R.V.; Clark, K.L.; Shepard, K.A.; Garcia, A.D.R. Sonic hedgehog signaling is negatively regulated in reactive astrocytes after forebrain stab injury. Sci Rep. 2019, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, J.; Liu, R.Y.; Lian, Z.G.; Chen, X.L.; Ma, L.; Sun, H.M.; Zhao, Y.L. The role of the sonic hedgehog signaling pathway in early brain injury after experimental subarachnoid hemorrhage in rats. Neurosci. Lett. 2013, 552, 81–86. [Google Scholar] [CrossRef]
- Zhen, H.; Zhao, L.; Ling, Z.; Kuo, L.; Xue, X.; Feng, J. Wip1 regulates blood-brain barrier function and neuro-inflammation induced by lipopolysaccharide via the sonic hedgehog signaling signaling pathway. Mol. Immunol. 2018, 93, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-L.; Chen, T.; Zhang, X. Activation of sonic hedgehog signaling attenuates oxidized low-density lipoprotein-stimulated brain microvascular endothelial cells dysfunction in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 12820–12828. [Google Scholar]
- Azzi, S.; Treps, L.; Leclair, H.M.; Ngo, H.-M.; Harford-Wright, E.; Gavard, J. Desert Hedgehog/Patch2 Axis Contributes to Vascular Permeability and Angiogenesis in Glioblastoma. Front. Pharm. 2015, 6, 281. [Google Scholar] [CrossRef]
- Soleti, R.; Benameur, T.; Porro, C.; Panaro, M.A.; Andriantsitohaina, R.; Martínez, M.C. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 2009, 30, 580–588. [Google Scholar] [CrossRef]
- Chapouly, C.; Yao, Q.; Vandierdonck, S.; Larrieu-Lahargue, F.; Mariani, J.N.; Gadeau, A.-P.; Renault, M.-A. Impaired Hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: Implication in diabetes. Cardiovasc. Res. 2016, 109, 217–227. [Google Scholar] [CrossRef]
- Sharghi-Namini, S.; Turmaine, M.; Meier, C.; Sahni, V.; Umehara, F.; Jessen, K.R.; Mirsky, R. The Structural and Functional Integrity of Peripheral Nerves Depends on the Glial-Derived Signal Desert Hedgehog. J. Neurosci. 2006, 26, 6364–6376. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.; Mauborgne, A.; Bourgoin, S.; Couraud, P.-O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain 2016, 157, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.; Dieb, W.; Mauborgne, A.; Bourgoin, S.; Villanueva, L.; Pohl, M.; Boucher, Y. Hedgehog Pathway-Mediated Vascular Alterations Following Trigeminal Nerve Injury. J. Dent. Res. 2017, 96, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Q.; Deng, Z.; Zhang, Z.; Xu, J.; Qian, G.; Wang, G. Protection from lipopolysaccharide-induced pulmonary microvascular endothelial cell injury by activation of hedgehog signaling pathway. Mol. Biol. Rep. 2011, 38, 3615–3622. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, J.; Johnson, D.; Wei, Y.; Liu, Y.; Wang, S.; Lutty, G.A.; Duh, E.J.; Semba, R.D. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1α-VEGF pathway inhibition. Diabetes 2015, 64, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Agouni, A.; Mostefai, H.A.; Porro, C.; Carusio, N.; Favre, J.; Richard, V.; Henrion, D.; Martínez, M.C.; Andriantsitohaina, R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007, 21, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Marrachelli, V.G.; Mastronardi, M.L.; Sarr, M.; Soleti, R.; Leonetti, D.; Martínez, M.C.; Andriantsitohaina, R. Sonic hedgehog carried by microparticles corrects angiotensin II-induced hypertension and endothelial dysfunction in mice. PLoS ONE 2013, 8, e72861. [Google Scholar] [CrossRef]
- Nie, D.M.; Wu, Q.L.; Zheng, P.; Chen, P.; Zhang, R.; Li, B.B.; Fang, J.; Xia, L.H.; Hong, M. Endothelial microparticles carrying hedgehog-interacting protein induce continuous endothelial damage in the pathogenesis of acute graft-versus-host disease. Am. J. Physiol. Cell Physiol. 2016, 310, C821–C835. [Google Scholar] [CrossRef]
- Farahani, R.M.; Sarrafpour, B.; Simonian, M.; Li, Q.; Hunter, N. Directed glia-assisted angiogenesis in a mature neurosensory structure: Pericytes mediate an adaptive response in human dental pulp that maintains blood-barrier function. J. Comp. Neurol. 2012, 520, 3803–3826. [Google Scholar] [CrossRef]
- Dakubo, G.D.; Mazerolle, C.; Furimsky, M.; Yu, C.; St-Jacques, B.; McMahon, A.P.; Wallace, V.A. Indian hedgehog signaling from endothelial cells is required for sclera and retinal pigment epithelium development in the mouse eye. Dev. Biol. 2008, 320, 242–255. [Google Scholar] [CrossRef][Green Version]
- Chinchilla, P.; Xiao, L.; Kazanietz, M.G.; Riobo, N.A. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle 2010, 9, 570–579. [Google Scholar] [CrossRef] [PubMed]
- He, Q.-W.; Xia, Y.-P.; Chen, S.-C.; Wang, Y.; Huang, M.; Huang, Y.; Li, J.-Y.; Li, Y.-N.; Gao, Y.; Mao, L.; et al. Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol. Neurobiol. 2013, 47, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Polizio, A.H.; Chinchilla, P.; Chen, X.; Kim, S.; Manning, D.R.; Riobo, N.A. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J. Biol. Chem. 2011, 286, 19589–19596. [Google Scholar] [CrossRef] [PubMed]
- Kanda, S.; Mochizuki, Y.; Suematsu, T.; Miyata, Y.; Nomata, K.; Kanetake, H. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J. Biol. Chem. 2003, 278, 8244–8249. [Google Scholar] [CrossRef] [PubMed]
- Kallakuri, S.; Yu, J.A.; Li, J.; Li, Y.; Weinstein, B.M.; Nicoli, S.; Sun, Z. Endothelial cilia are essential for developmental vascular integrity in zebrafish. J. Am. Soc. Nephrol. 2015, 26, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Roncalli, J.; Renault, M.A.; Tongers, J.; Misener, S.; Thorne, T.; Kamide, C.; Jujo, K.; Tanaka, T.; Ii, M.; Klyachko, E.; et al. Sonic hedgehog-induced functional recovery after myocardial infarction is enhanced by AMD3100-mediated progenitor-cell mobilization. J. Am. Coll. Cardiol. 2011, 57, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Frontini, M.J.; Nong, Z.; Gros, R.; Drangova, M.; O’Neil, C.; Rahman, M.N.; Akawi, O.; Yin, H.; Ellis, C.G.; Pickering, J.G. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat. Biotechnol. 2011, 29, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Sicklick, J.K.; Li, Y.X.; Choi, S.S.; Qi, Y.; Chen, W.; Bustamante, M.; Huang, J.; Zdanowicz, M.; Camp, T.; Torbenson, M.S.; et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab. Investig. 2005, 85, 1368–1380. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Xu, Z.; Yin, H.; Bai, L.; Ma, Z.; Decoster, M.A.; Qian, G.; Wu, G. Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia. Biochim. Biophys. Acta 2010, 1803, 1359–1367. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Li, Y.; Singh, P.; Cao, L.; Xu, L.J.; Li, D.; Wang, Y.; Xie, Z.; Gui, Y.; et al. Sonic hedgehog promotes autophagy of vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1319–H1331. [Google Scholar] [CrossRef]
- Morrow, D.; Sweeney, C.; Birney, Y.A.; Guha, S.; Collins, N.; Cummins, P.M.; Murphy, R.; Walls, D.; Redmond, E.M.; Cahill, P.A. Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. Am. J. Physiol. Cell Physiol. 2007, 292, C488–C496. [Google Scholar] [CrossRef]
- Li, F.; Duman-Scheel, M.; Yang, D.; Du, W.; Zhang, J.; Zhao, C.; Qin, L.; Xin, S. Sonic hedgehog signaling induces vascular smooth muscle cell proliferation via induction of the G1 cyclin-retinoblastoma axis. Arter. Thromb. Vasc. Biol. 2010, 30, 1787–1794. [Google Scholar] [CrossRef]
- Doyle, A.J.; Redmond, E.M.; Gillespie, D.L.; Knight, P.A.; Cullen, J.P.; Cahill, P.A.; Morrow, D.J. Differential expression of Hedgehog/Notch and transforming growth factor-beta in human abdominal aortic aneurysms. J. Vasc. Surg. 2015, 62, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.; Cullen, J.P.; Liu, W.; Guha, S.; Sweeney, C.; Birney, Y.A.; Collins, N.; Walls, D.; Redmond, E.M.; Cahill, P.A. Sonic Hedgehog induces Notch target gene expression in vascular smooth muscle cells via VEGF-A. Arter. Thromb. Vasc. Biol. 2009, 29, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, Q.J.; Wang, L.J.; Liu, Z.H.; Zhang, Q.; Yang, K.; Wang, H.B.; Yan, X.X.; Zhu, Z.B.; Du, R.; et al. Increment of HFABP Level in Coronary Artery In-Stent Restenosis Segments in Diabetic and Nondiabetic Minipigs: HFABP Overexpression Promotes Multiple Pathway-Related Inflammation, Growth and Migration in Human Vascular Smooth Muscle Cells. J. Vasc. Res. 2016, 53, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, C.; Zhang, R.Y.; Zhang, Q.; Shen, W.F.; Ding, F.H.; Lu, L. Association of increased serum CTRP5 levels with in-stent restenosis after coronary drug-eluting stent implantation: CTRP5 promoting inflammation, migration and proliferation in vascular smooth muscle cells. Int. J. Cardiol. 2017, 228, 129–136. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Han, X.; Liu, W.; Corcoran, E.; Burtenshaw, D.; Morrow, D.; Helt, J.C.; Cahill, P.A.; Redmond, E.M. Alcohol Reduces Arterial Remodeling by Inhibiting Sonic Hedgehog-Stimulated Stem Cell Antigen-1 Positive Progenitor Stem Cell Expansion. Alcohol Clin. Exp. Res. 2017, 41, 2051–2065. [Google Scholar] [CrossRef]
- Mooney, C.J.; Hakimjavadi, R.; Fitzpatrick, E.; Kennedy, E.; Walls, D.; Morrow, D.; Redmond, E.M.; Cahill, P.A. Hedgehog and Resident Vascular Stem Cell Fate. Stem Cells Int. 2015, 2015, 468428. [Google Scholar] [CrossRef]
- Passman, J.N.; Dong, X.R.; Wu, S.P.; Maguire, C.T.; Hogan, K.A.; Bautch, V.L.; Majesky, M.W. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9349–9354. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wei, B.; Zhao, Y.; Wang, X.; Fu, Q.; Liu, H.; Li, F. Shh mediates PDGF-induced contractile-to-synthetic phenotypic modulation in vascular smooth muscle cells through regulation of KLF4. Exp. Cell Res. 2016, 345, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; Fuchs, S.; Kolbe, M.; Hofmann, A.; Schmidt, H.; Kirkpatrick, C.J. Comparative study assessing effects of sonic hedgehog and VEGF in a human co-culture model for bone vascularisation strategies. Eur. Cell Mater. 2011, 21, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Moskowitz, M.A.; Sims, J.R. Sonic hedgehog inversely regulates the expression of angiopoietin-1 and angiopoietin-2 in fibroblasts. Int. J. Mol. Med. 2007, 19, 445–451. [Google Scholar] [CrossRef] [PubMed]
- El-Hashash, A.H.; Al Alam, D.; Turcatel, G.; Rogers, O.; Li, X.; Bellusci, S.; Warburton, D. Six1 transcription factor is critical for coordination of epithelial, mesenchymal and vascular morphogenesis in the mammalian lung. Dev. Biol. 2011, 353, 242–258. [Google Scholar] [CrossRef] [PubMed]
- El-Hashash, A.H.; Al Alam, D.; Turcatel, G.; Bellusci, S.; Warburton, D. Eyes absent 1 (Eya1) is a critical coordinator of epithelial, mesenchymal and vascular morphogenesis in the mammalian lung. Dev. Biol. 2011, 350, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Emoto, N.; Yagi, K.; Vignon-Zellweger, N.; Nakayama, K.; Hatakeyama, K.; Asada, Y.; Rikitake, Y.; Hirata, K.-I. Localization and characterization of a novel secreted protein, SCUBE2, in the development and progression of atherosclerosis. Kobe J. Med. Sci. 2013, 59, E122–E131. [Google Scholar]
- Dutzmann, J.; Koch, A.; Weisheit, S.; Sonnenschein, K.; Korte, L.; Haertle, M.; Thum, T.; Bauersachs, J.; Sedding, D.G.; Daniel, J.M. Sonic hedgehog-dependent activation of adventitial fibroblasts promotes neointima formation. Cardiovasc. Res. 2017, 113, 1653–1663. [Google Scholar] [CrossRef]
- Herring, B.P.; Hoggatt, A.M.; Griffith, S.L.; McClintick, J.N.; Gallagher, P.J. Inflammation and vascular smooth muscle cell dedifferentiation following carotid artery ligation. Physiol. Genom. 2017, 49, 115–126. [Google Scholar] [CrossRef]
- Teng, H.; Chopp, M.; Hozeska-Solgot, A.; Shen, L.; Lu, M.; Tang, C.; Zhang, Z.G. Tissue plasminogen activator and plasminogen activator inhibitor 1 contribute to sonic hedgehog-induced in vitro cerebral angiogenesis. PLoS ONE 2012, 7, e33444. [Google Scholar] [CrossRef][Green Version]
- Maun, H.R.; Wen, X.; Lingel, A.; de Sauvage, F.J.; Lazarus, R.A.; Scales, S.J.; Hymowitz, S.G. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J. Biol. Chem. 2010, 285, 26570–26580. [Google Scholar] [CrossRef]
- Vanlandewijck, M.; He, L.; Mae, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapouly, C.; Guimbal, S.; Hollier, P.-L.; Renault, M.-A. Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance. Int. J. Mol. Sci. 2019, 20, 3076. https://doi.org/10.3390/ijms20123076
Chapouly C, Guimbal S, Hollier P-L, Renault M-A. Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance. International Journal of Molecular Sciences. 2019; 20(12):3076. https://doi.org/10.3390/ijms20123076
Chicago/Turabian StyleChapouly, Candice, Sarah Guimbal, Pierre-Louis Hollier, and Marie-Ange Renault. 2019. "Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance" International Journal of Molecular Sciences 20, no. 12: 3076. https://doi.org/10.3390/ijms20123076
APA StyleChapouly, C., Guimbal, S., Hollier, P.-L., & Renault, M.-A. (2019). Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance. International Journal of Molecular Sciences, 20(12), 3076. https://doi.org/10.3390/ijms20123076



