New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity
Abstract
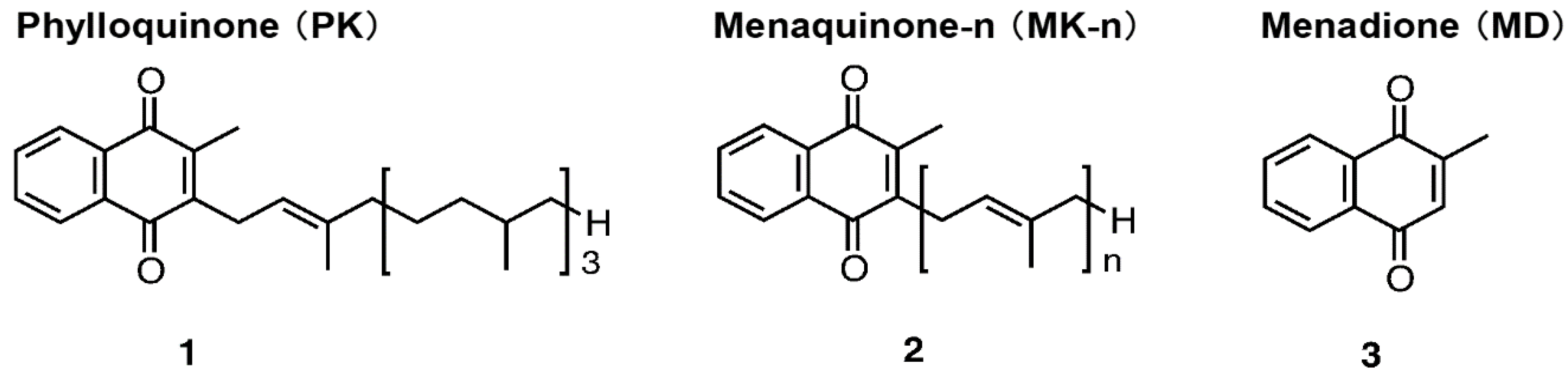
1. Introduction
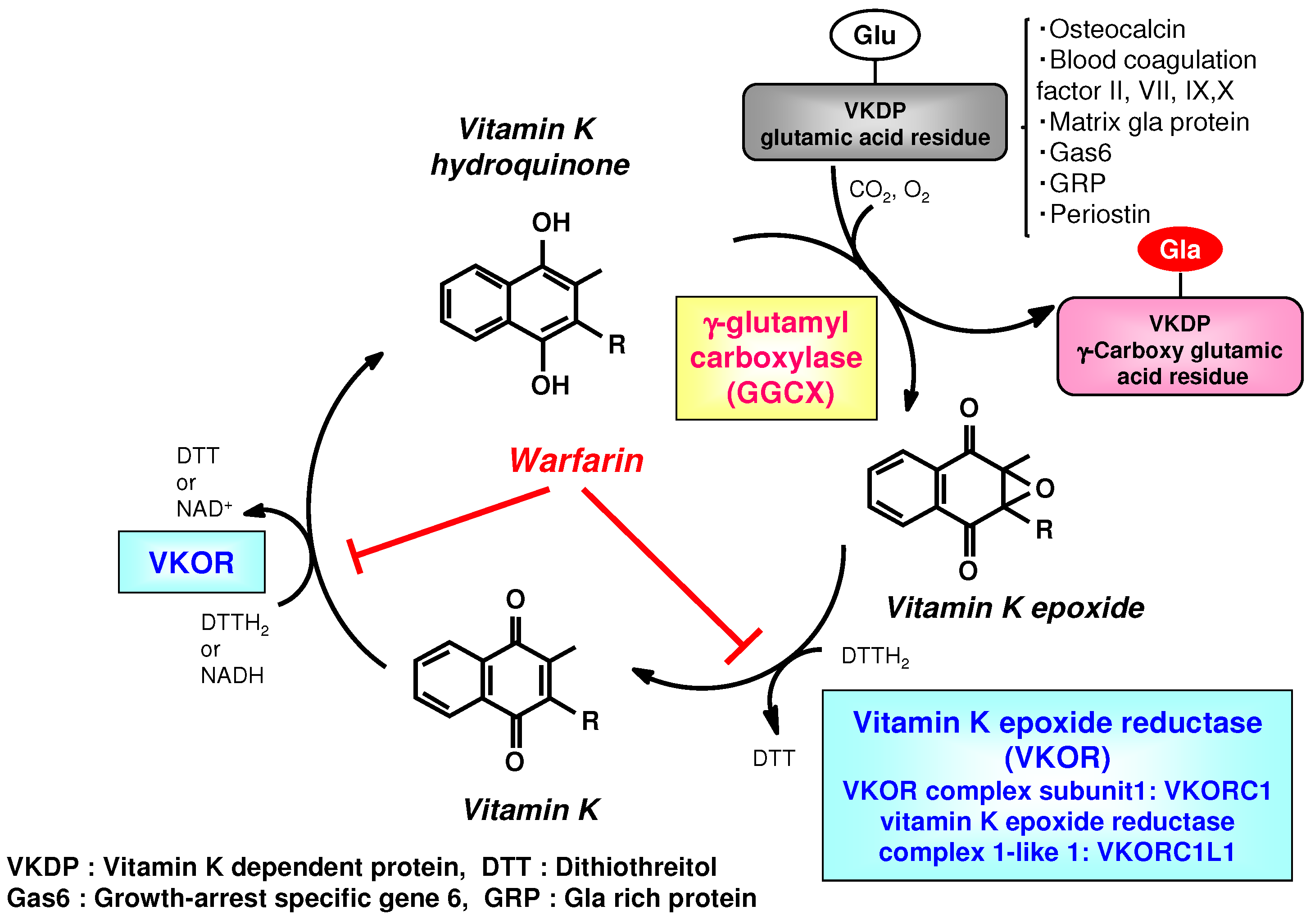
2. Physiological Effects Identified in Studies of the Vitamin K Cycle and Derivatives Targeting γ-Glutamyl Carboxylase (GGCX)
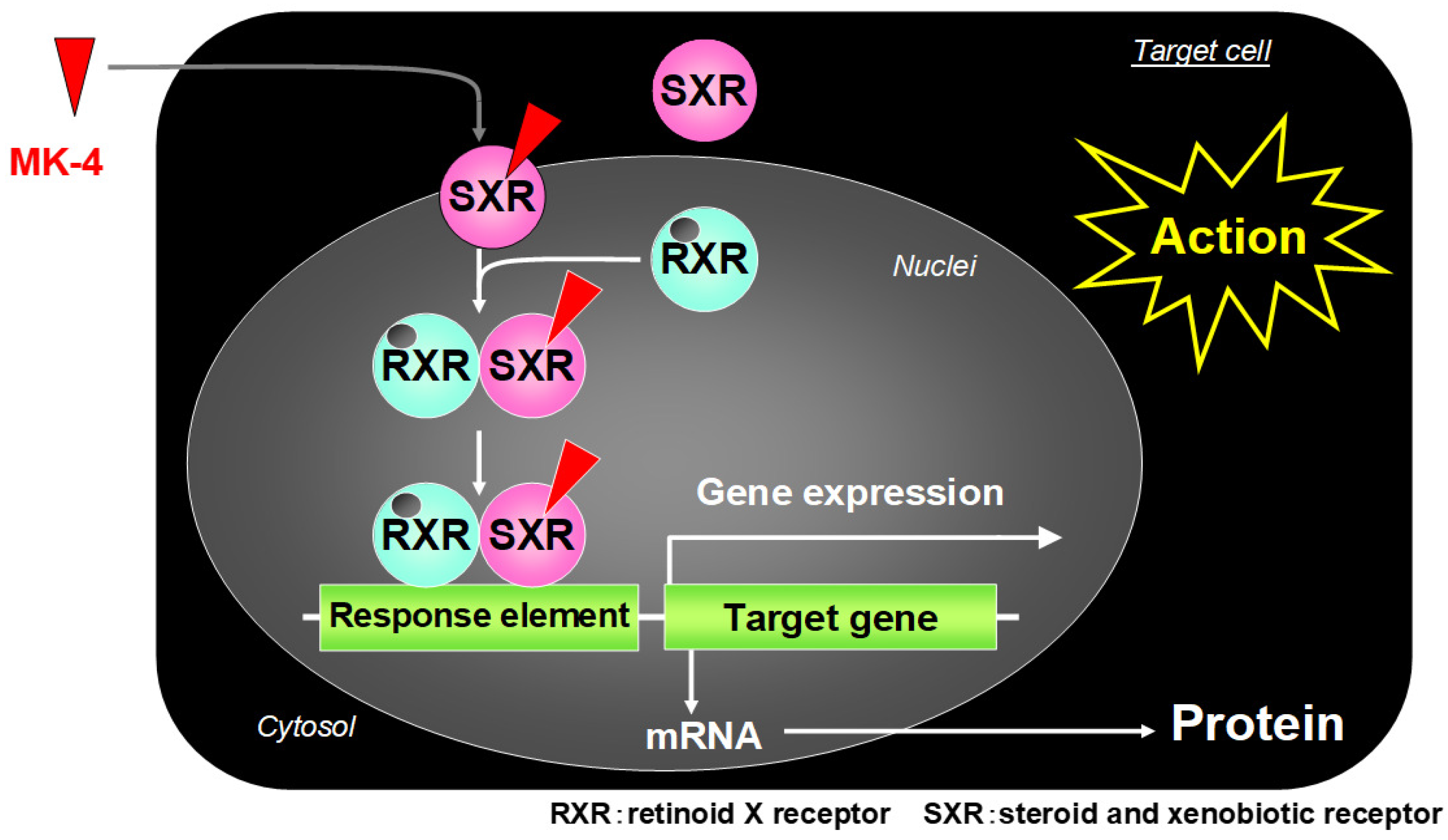
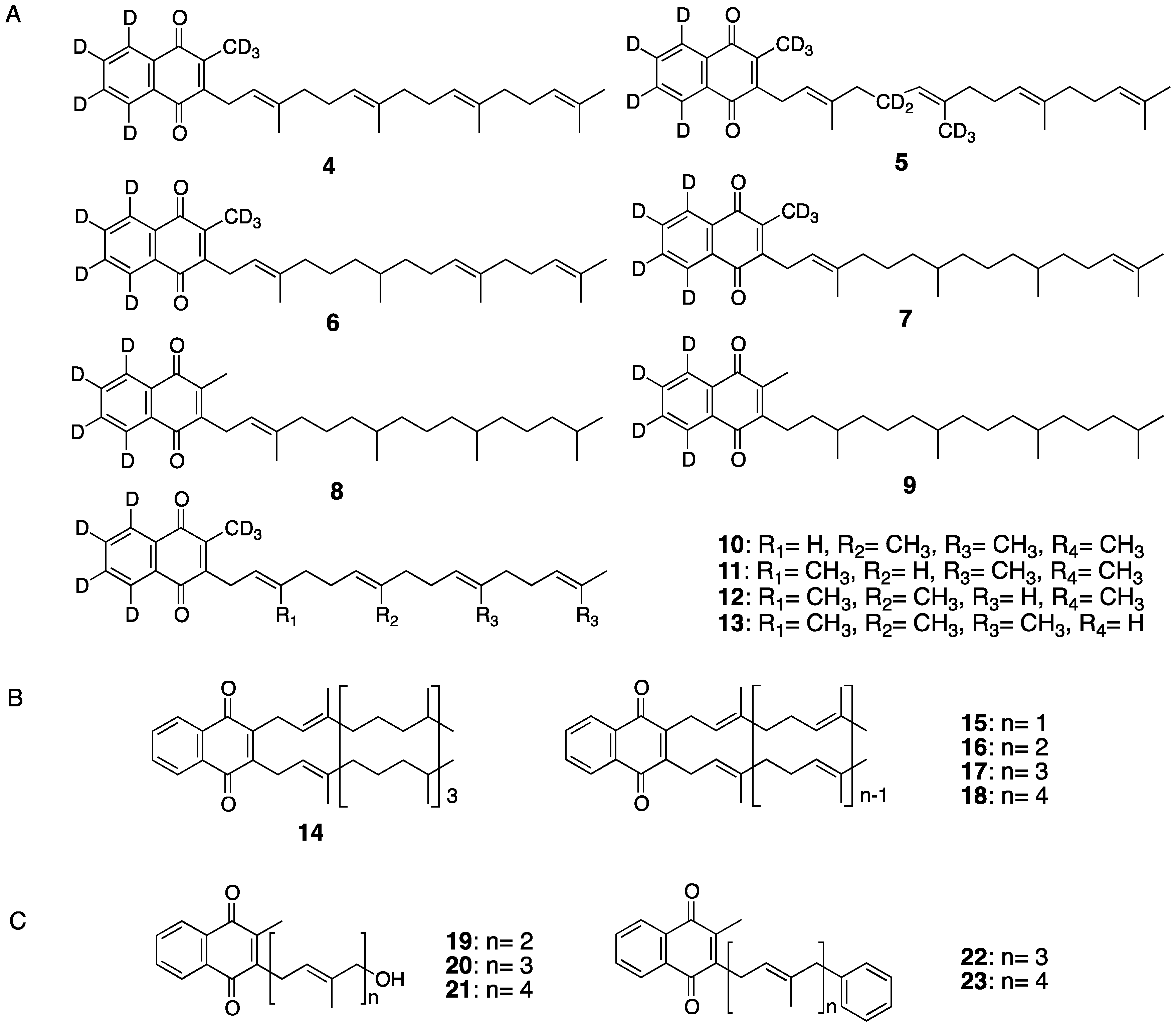
3. Vitamin K Derivatives Targeting Steroid and Xenobiotic Receptor (SXR) to Mediate Transcriptional Activity
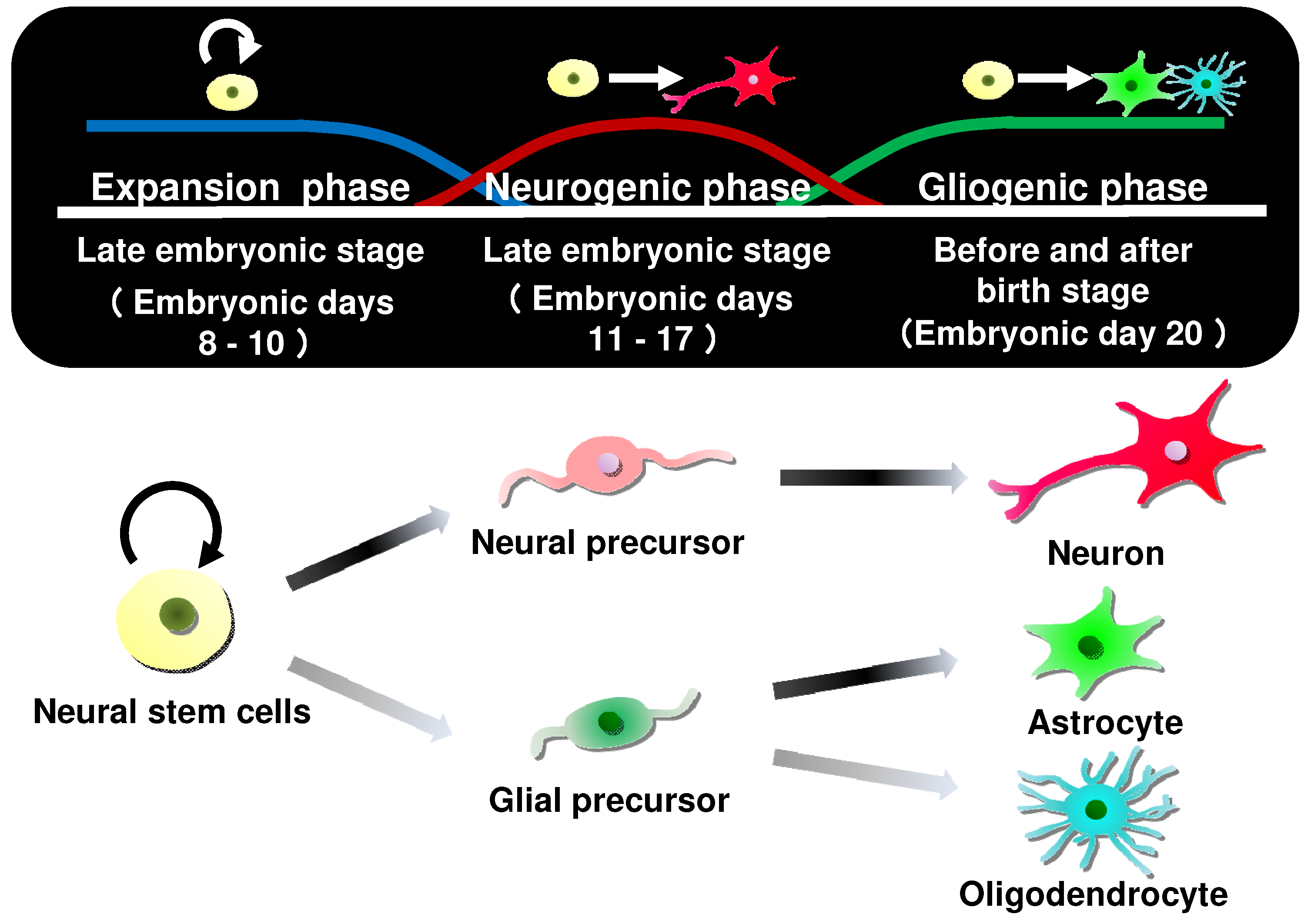
4. Differentiation-Inducing Action of Neural Stem Cells by Vitamin K and Derivatives
5. Conclusions
Funding
Conflicts of Interest
References
- Dam, H.; Schønheyder, F.; Tage-Hansen, E. Studies on the mode of action of vitamin K. Biochem. J. 1936, 30, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Okano, T. Key Pathways and Regulators of Vitamin K Function and Intermediary Metabolism. Annu. Rev. Nutr. 2018, 38, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Thayer, S.A.; Maccorquodale, D.W.; Binkley, S.B.; Doisy, E.A. The Isolation of a Crystalline Compound with Vitamin K Activity. Science 1938, 88, 243. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.L.; Tucker, K.L.; Chen, H.; Hannan, M.T.; Gagnon, D.R.; Cupples, L.A.; Wilson, P.W.; Ordovas, J.; Schaefer, E.J.; Dawson-Hughes, B.; et al. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am. J. Clin. Nutr. 2000, 71, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Gallieni, M.; Rizzo, M.A.; Stucchi, A.; Delanaye, P.; Cavalier, E.; Moysés, R.M.A.; Jorgetti, V.; Iervasi, G.; Giannini, S.; et al. Vitamin K plasma levels determination in human health. Clin. Chem. Lab. Med. 2017, 55, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Kaneki, M.; Hodges, S.J.; Hosoi, T.; Fujiwara, S.; Lyons, A.; Crean, S.J.; Ishida, N.; Nakagawa, M.; Takechi, M.; Sano, Y.; et al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: Possible implications for hip-fracture risk. Nutrition 2001, 17, 315–321. [Google Scholar] [CrossRef]
- Booth, S.L.; Suttie, J.W. Dietary intake and adequacy of vitamin K. J. Nutr. 1998, 128, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J. Vitamin K. Lancet 1995, 345, 229–234. [Google Scholar] [CrossRef]
- Suttie, J.W. The importance of menaquinones in human nutrition. Annu. Rev. Nutr. 1995, 15, 399–417. [Google Scholar] [CrossRef]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: Two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef]
- Thijssen, H.H.; Drittij-Reijnders, M.J. Vitamin K status in human tissues: Tissue-specific accumulation of phylloquinone and menaquinone-4. Br. J. Nutr. 1996, 75, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, H.H.; Drittij-Reijnders, M.J. Vitamin K distribution in rat tissues: Dietary phylloquinone is a source of tissue menaquinone-4. Br. J. Nutr. 1994, 72, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010, 468, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Tsugawa, N.; Nakagawa, K.; Suhara, Y.; Tanaka, K.; Uchino, Y.; Takeuchi, A.; Sawada, N.; Kamao, M.; Wada, A.; et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J. Biol. Chem. 2013, 288, 33071–33080. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Sawada, N.; Hirota, Y.; Uchino, Y.; Suhara, Y.; Hasegawa, T.; Amizuka, N.; Okamoto, T.; Tsugawa, N.; Kamao, M.; et al. Vitamin K2 biosynthetic enzyme, UBIAD1 is essential for embryonic development of mice. PLoS ONE 2014, 9, e104078. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Nakagawa, K.; Sawada, N.; Okuda, N.; Suhara, Y.; Uchino, Y.; Kimoto, T.; Funahashi, N.; Kamao, M.; Tsugawa, N.; et al. Functional characterization of the vitamin K2 biosynthetic enzyme UBIAD1. PLoS ONE 2015, 10, e0125737. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Yamanashi, Y.; Konishi, K.; Yamamoto, T.; Toyoda, Y.; Masuo, Y.; Yamamoto, H.; Suzuki, H. NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci. Transl. Med. 2015, 7, 275ra23. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Margier, M.; Roi, S.; Collet, X.; Niot, I.; Goupy, P.; Caris-Veyrat, C.; Reboul, E. Intestinal scavenger receptors are involved in vitamin K1 absorption. J. Biol. Chem. 2014, 289, 30743–30752. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S. Vitamin K-dependent gamma-glutamyl carboxylase activity in the chick embryonic chorioallantoic membrane. J. Biol. Chem. 1979, 254, 1356–1364. [Google Scholar]
- Azuma, K.; Shiba, S.; Hasegawa, T.; Ikeda, K.; Urano, T.; Horie-Inoue, K.; Ouchi, Y.; Amizuka, N.; Inoue, S. Osteoblast-Specific ã-Glutamyl Carboxylase-Deficient Mice Display Enhanced Bone Formation with Aberrant Mineralization. J. Bone Miner. Res. 2015, 30, 1245–1254. [Google Scholar] [CrossRef]
- Tie, J.K.; Stafford, D.W. Functional Study of the Vitamin K Cycle Enzymes in Live Cells. Methods Enzymol. 2017, 584, 349–394. [Google Scholar]
- Cain, D.; Hutson, S.M.; Wallin, R. Assembly of the warfarin-sensitive vitamin K 2,3-epoxide reductase enzyme complex in the endoplasmic reticulum membrane. J. Biol. Chem. 1997, 272, 29068–29075. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.H.; Huang, T.Y.; Williams, J.; Stafford, D.W. Purified vitamin K epoxide reductase alone is sufficient for conversion of vitamin K epoxide to vitamin K and vitamin K to vitamin KH2. Proc. Natl. Acad. Sci. USA 2006, 103, 19308–19313. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Schulman, S.; Dutton, R.J.; Boyd, D.; Beckwith, J.; Rapoport, T.A. Structure of a bacterial homologue of vitamin K epoxide reductase. Nature 2010, 463, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Westhofen, P.; Watzka, M.; Marinova, M.; Hass, M.; Kirfel, G.; Müller, J.; Bevans, C.G.; Müller, C.R.; Oldenburg, J. Human vitamin K 2,3-epoxide reductase complex subunit 1-like 1 (VKORC1L1) mediates vitamin K-dependent intracellular antioxidant function. J. Biol. Chem. 2011, 286, 15085–15094. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Rishavy, M.A.; Berkner, K.L.; Ferron, M. VKOR paralog VKORC1L1 supports vitamin K-dependent protein carboxylation in vivo. JCI Insight 2018, 3, 96501. [Google Scholar] [CrossRef] [PubMed]
- Wajih, N.; Sane, D.C.; Hutson, S.M.; Wallin, R. The inhibitory effect of calumenin on the vitamin K-dependent gamma-carboxylation system. Characterization of the system in normal and warfarin-resistant rats. J. Biol. Chem. 2004, 279, 25276–25283. [Google Scholar] [CrossRef]
- Wajih, N.; Owen, J.; Wallin, R. Enhanced functional recombinant factor VII production by HEK 293 cells stably transfected with VKORC1 where the gamma-carboxylase inhibitor calumenin is stably suppressed by shRNA transfection. Thromb. Res. 2008, 122, 405–410. [Google Scholar] [CrossRef][Green Version]
- Thijssen, H.H.; Vervoort, L.M.; Schurgers, L.J.; Shearer, M.J. Menadione is a metabolite of oral vitamin K. Br. J. Nutr. 2006, 95, 260–266. [Google Scholar] [CrossRef]
- Vermeer, C.; van’t Hoofd, C.; Knapen, M.H.J.; Xanthoulea, S. Synthesis of 2-methyl-1,4-naphthoquinones with higher gamma-glutamyl carboxylase activity than MK-4 both in vitro and in vivo. Bioorg. Med. Chem. Lett. 2017, 27, 208–211. [Google Scholar] [CrossRef]
- Fujii, S.; Shimizu, A.; Takeda, N.; Oguchi, K.; Katsurai, T.; Shirakawa, H.; Komai, M.; Kagechika, H. Systematic synthesis and anti-inflammatory activity of ω-carboxylated menaquinone derivatives- Investigations on identified and putative vitamin K2 metabolites. Bioorg. Med. Chem. 2015, 23, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grün, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Horie-Inoue, K.; Ikeda, K.; Blumberg, B.; Inoue, S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J. Biol. Chem. 2006, 281, 16927–16934. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Barwick, J.L.; Downes, M.; Blumberg, B.; Simon, C.M.; Nelson, M.C.; Neuschwander-Tetri, B.A.; Brunt, E.M.; Guzelian, P.S.; Evans, R.M. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 2000, 406, 435–439. [Google Scholar] [CrossRef]
- Azuma, K.; Urano, T.; Ouchi, Y.; Inoue, S. Vitamin K2 suppresses proliferation and motility of hepatocellular carcinoma cells by activating steroid and xenobiotic receptor. Endocr. J. 2009, 56, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Sada, E.; Abe, Y.; Ohba, R.; Tachikawa, Y.; Nagasawa, E.; Shiratsuchi, M.; Takayanagi, R. Vitamin K2 modulates differentiation and apoptosis of both myeloid and erythroid lineages. Eur. J. Haematol. 2010, 85, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Kortagere, S.; Iyer, M.; Reschly, E.J.; Lill, M.A.; Redinbo, M.R.; Krasowski, M.D. Challenges predicting ligand-receptor interactions of promiscuous proteins: The nuclear receptor PXR. PLoS Comput. Biol. 2009, 5, e1000594. [Google Scholar] [CrossRef] [PubMed]
- Chrencik, J.E.; Orans, J.; Moore, L.B.; Xue, Y.; Peng, L.; Collins, J.L.; Wisely, G.B.; Lambert, M.H.; Kliewer, S.A.; Redinbo, M.R. Structural disorder in the complex of human pregnane X receptor and the macrolide antibiotic rifampicin. Mol. Endocrinol. 2005, 9, 1125–1134. [Google Scholar] [CrossRef]
- Suhara, Y.; Hanada, N.; Okitsu, T.; Sakai, M.; Watanabe, M.; Nakagawa, K.; Wada, A.; Takeda, K.; Takahashi, K.; Tokiwa, H.; et al. Structure-activity relationship of novel menaquinone-4 analogues: Modification of the side chain affects their biological activities. J. Med. Chem. 2012, 55, 1553–1558. [Google Scholar] [CrossRef]
- Suhara, Y.; Watanabe, M.; Motoyoshi, S.; Nakagawa, K.; Wada, A.; Takeda, K.; Takahashi, K.; Tokiwa, H.; Okano, T. Synthesis of new vitamin K analogues as steroid xenobiotic receptor (SXR) agonists: Insights into the biological role of the side chain part of vitamin, K.J. Med. Chem. 2011, 54, 4918–4922. [Google Scholar] [CrossRef]
- Suhara, Y.; Hirota, Y.; Hanada, N.; Nishina, S.; Eguchi, S.; Sakane, R.; Nakagawa, K.; Wada, A.; Takahashi, K.; Tokiwa, H.; et al. Synthetic Small Molecules Derived from Natural Vitamin K Homologues that Induce Selective Neuronal Differentiation of Neuronal Progenitor Cells. J. Med. Chem. 2015, 58, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
- Suhara, Y.; Watanabe, M.; Nakagawa, K.; Wada, A.; Ito, Y.; Takeda, K.; Takahashi, K.; Okano, T. Synthesis of novel vitamin K2 analogues with modification at the ù-terminal position and their biological evaluation as potent steroid and xenobiotic receptor (SXR) agonists. J. Med. Chem. 2011, 54, 4269–4273. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, Y.; Gotoh, Y. Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci. Res. 2005, 51, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
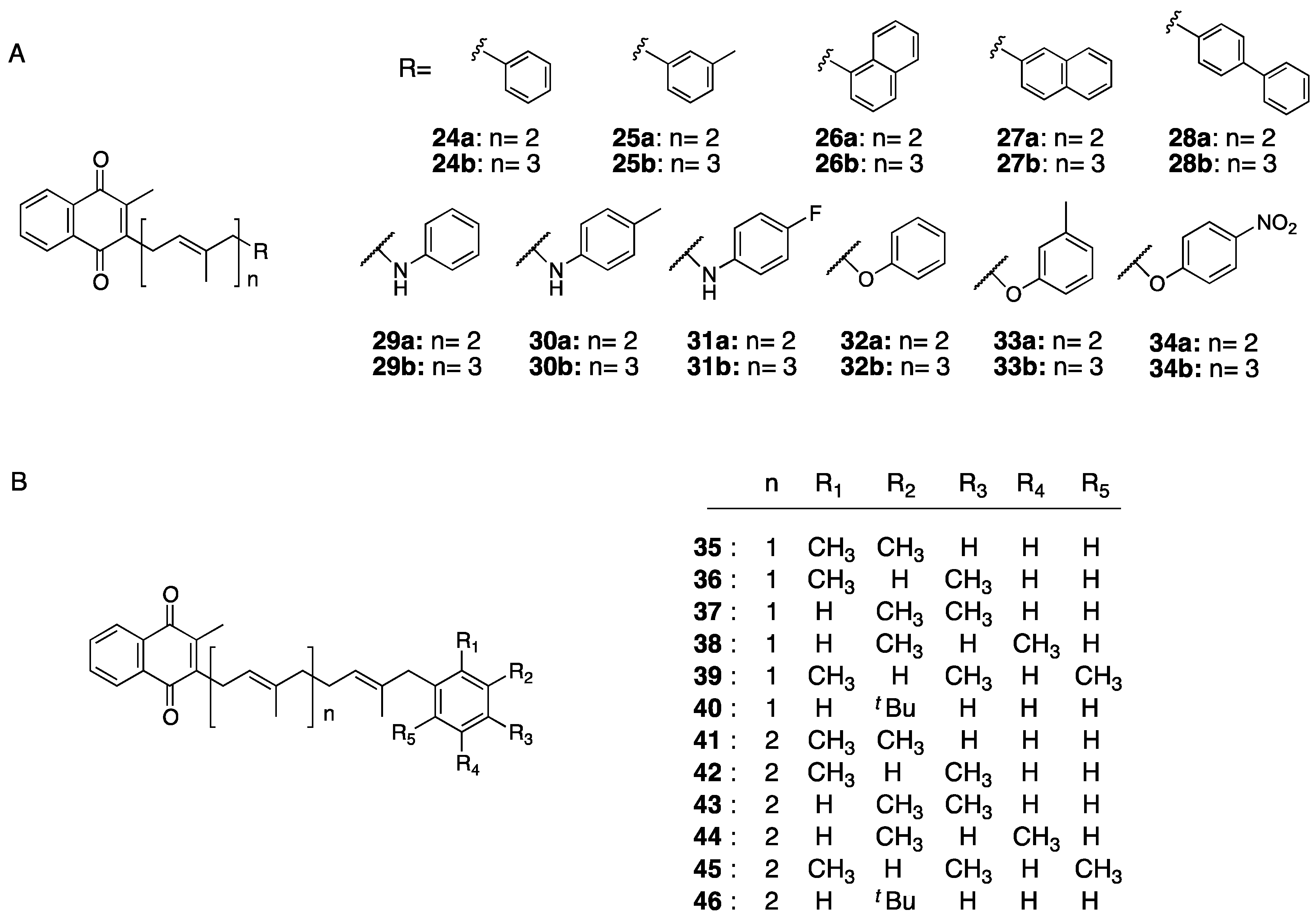
- Sakane, R.; Kimura, K.; Hirota, Y.; Ishizawa, M.; Takagi, Y.; Wada, A.; Kuwahara, S.; Makishima, M.; Suhara, Y. Synthesis of novel vitamin K derivatives with alkylated phenyl groups introduced at the ù-terminal side chain and evaluation of their neural differentiation activities. Bioorg. Med. Chem. Lett. 2017, 27, 4881–4884. [Google Scholar] [CrossRef]
- Kimura, K.; Hirota, Y.; Kuwahara, S.; Takeuchi, A.; Tode, C.; Wada, A.; Osakabe, N.; Suhara, Y. Synthesis of Novel Synthetic Vitamin K Analogues Prepared by Introduction of a Heteroatom and a Phenyl Group That Induce Highly Selective Neuronal Differentiation of Neuronal Progenitor Cells. J. Med. Chem. 2017, 60, 2591–2596. [Google Scholar] [CrossRef]
- Warashina, M.; Min, K.H.; Kuwabara, T.; Huynh, A.; Gage, F.H.; Schultz, P.G.; Ding, S. A synthetic small molecule that induces neuronal differentiation of adult hippocampal neural progenitor cells. Angew. Chem. Int. Ed. Engl. 2006, 45, 591–593. [Google Scholar] [CrossRef]
- Kakeya, H.; Takahashi, I.; Okada, G.; Isono, K.; Osada, H. Epolactaene, a novel neuritogenic compound in human neuroblastoma cells, produced by a marine fungus. J. Antibiot. (Tokyo) 1995, 48, 733–735. [Google Scholar] [CrossRef]
- Yu, S.; Levi, L.; Siegel, R.; Noy, N. Retinoic acid induces neurogenesis by activating both retinoic acid receptors (RARs) and peroxisome proliferator-activated receptor β/δ (PPARβ/δ). J. Biol. Chem. 2012, 287, 42195–42205. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirota, Y.; Suhara, Y. New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity. Int. J. Mol. Sci. 2019, 20, 3006. https://doi.org/10.3390/ijms20123006
Hirota Y, Suhara Y. New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity. International Journal of Molecular Sciences. 2019; 20(12):3006. https://doi.org/10.3390/ijms20123006
Chicago/Turabian StyleHirota, Yoshihisa, and Yoshitomo Suhara. 2019. "New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity" International Journal of Molecular Sciences 20, no. 12: 3006. https://doi.org/10.3390/ijms20123006
APA StyleHirota, Y., & Suhara, Y. (2019). New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity. International Journal of Molecular Sciences, 20(12), 3006. https://doi.org/10.3390/ijms20123006





