MtBZR1 Plays an Important Role in Nodule Development in Medicago truncatula
Abstract
1. Introduction
2. Results
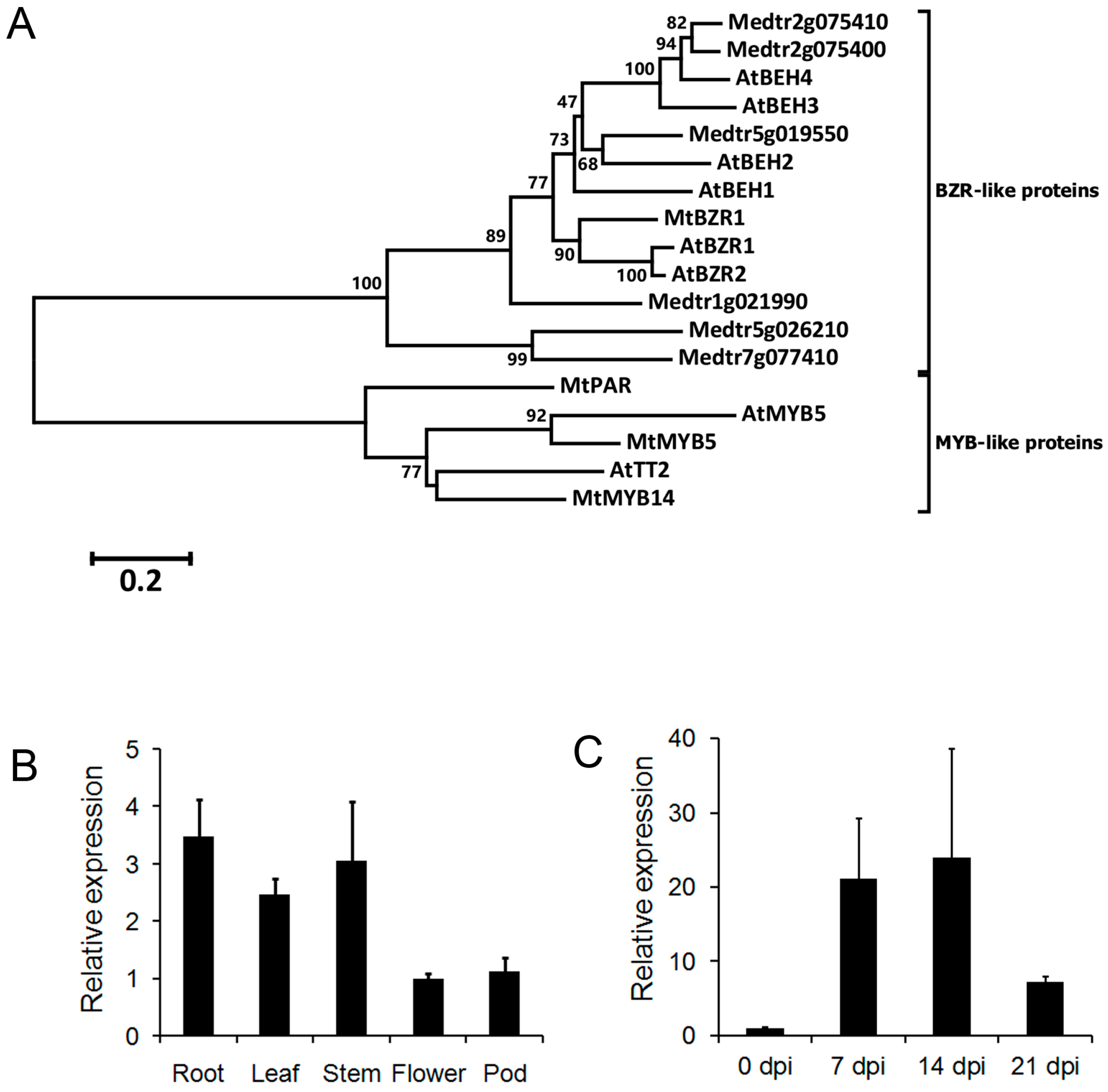
2.1. Search for AtBZR1 Homologs in M. truncatula
2.2. Induction of MtBZR1 upon S. meliloti 1021 Inoculation
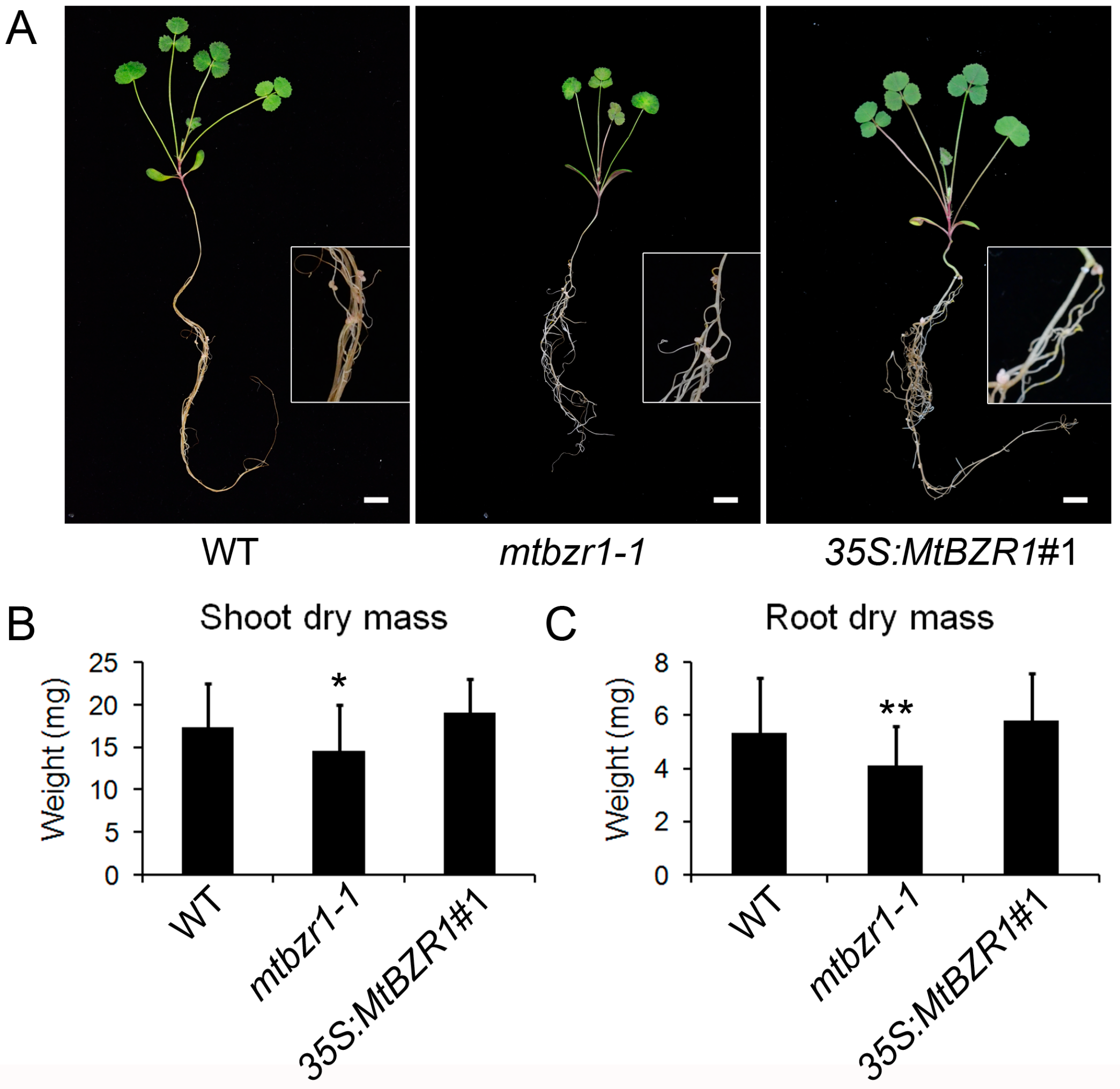
2.3. Identification of the mtbzr1-1 Null Mutant
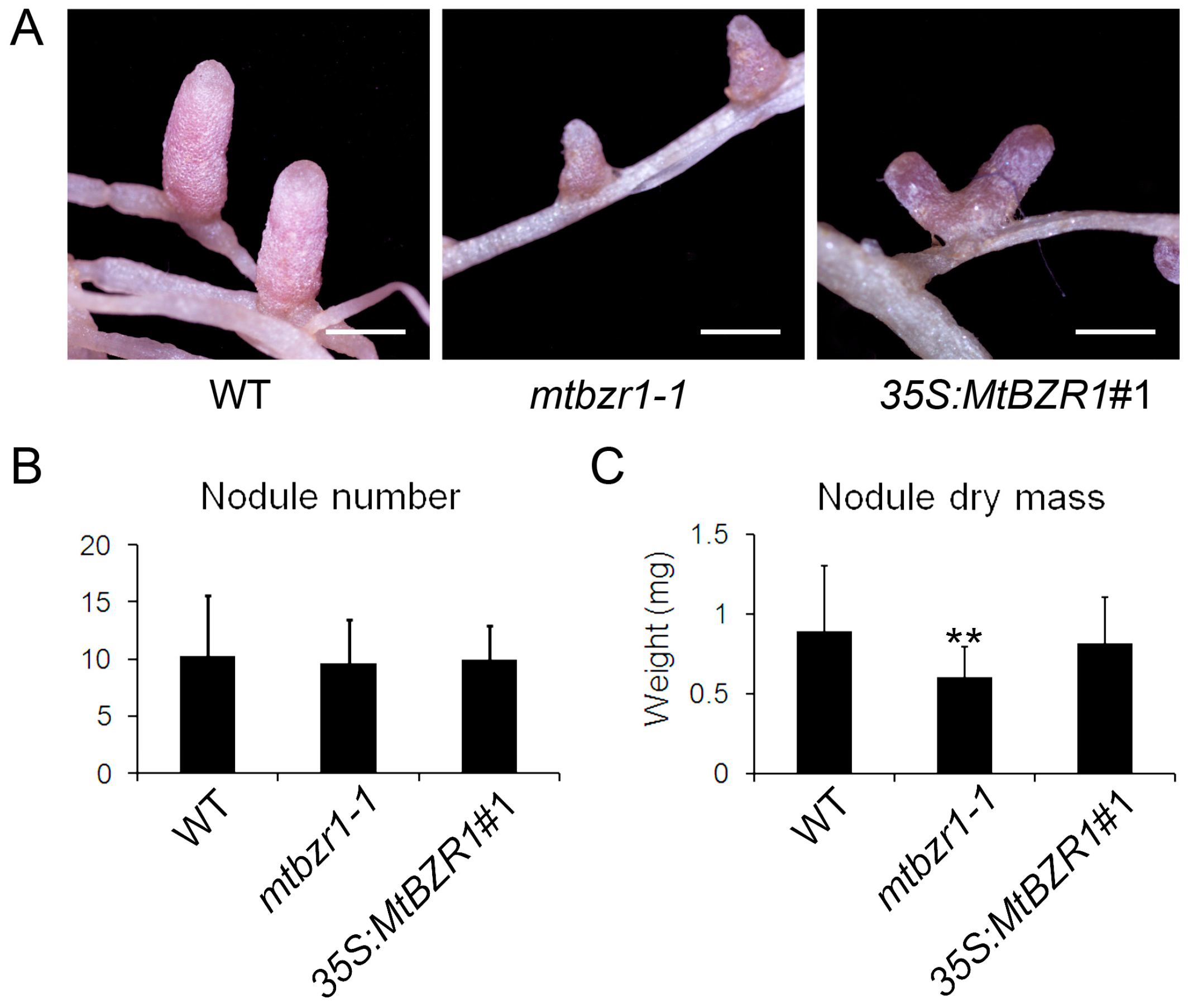
2.4. Loss of Function in MtBZR1 Leads to a Decrease in Plant Dry Mass in S. meliloti 1021 Nodulation
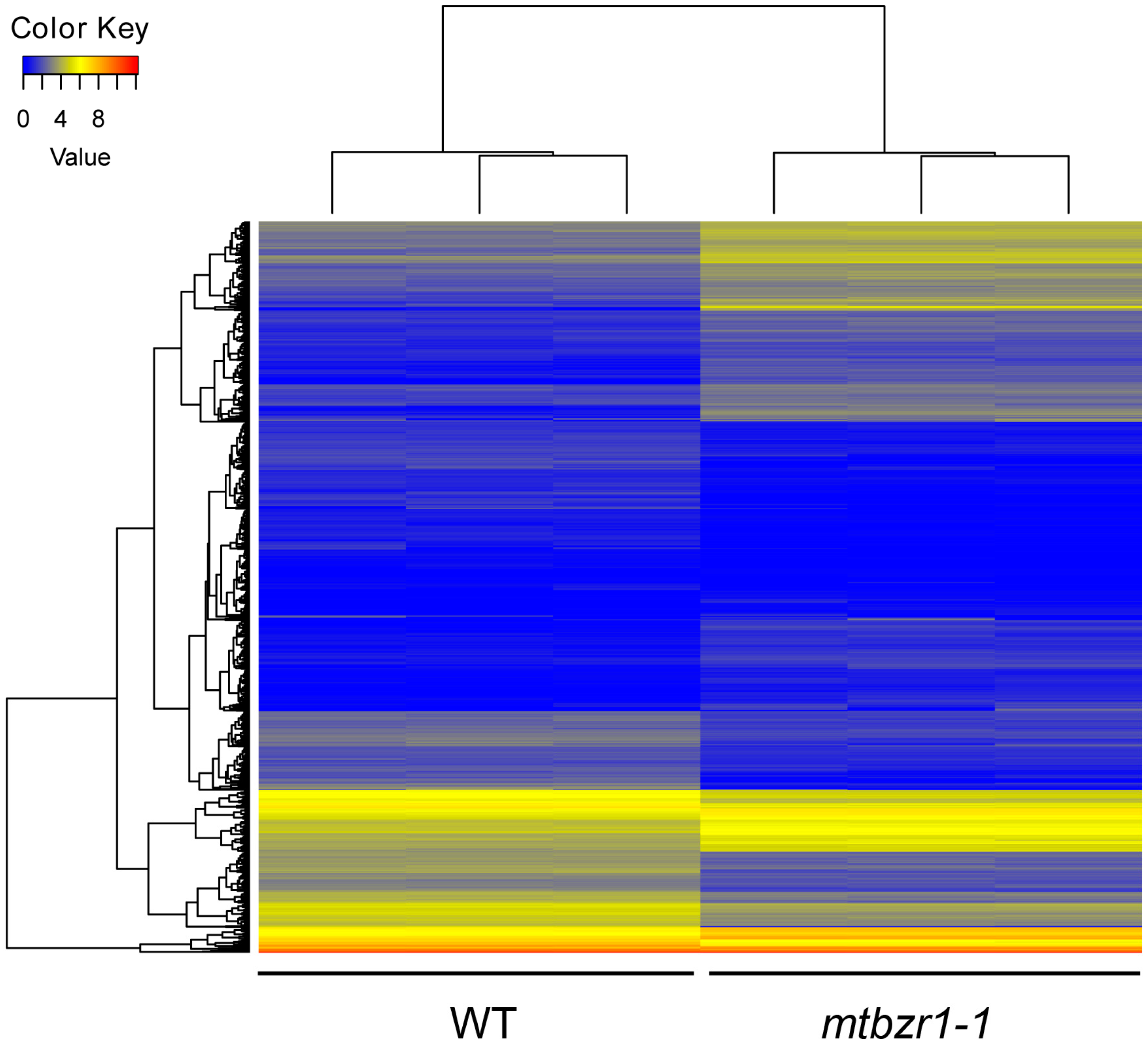
2.5. Transcriptomic Profiles of Nodules in mtbzr1-1
2.6. Differential Expression of Genes Involved in Flavonoid Biosynthesis in mtbzr1-1
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Root Nodule Induction
4.3. Identification of MtBZR1 Gene and Phylogenetic Analysis
4.4. Plasmid Construction and Plant Transformation
4.5. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.6. Transcriptomic Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ARF | AUXIN RESPONSE FACTOR |
| BES1 | BRASSINOSTEROID INSENSITIVE 1-EMS SUPPRESSOR 1 |
| BR | brassinosteroid |
| BZR | BRASSINAZOLE RESISTANT 1 |
| CDS | coding sequence |
| DEG | differentially expressed gene |
| DPI | days post inoculation |
| FDR | false discovery rate |
| FLS | flavonol synthase |
| FPKM | fragments per kilobase of transcript per million reads mapped |
| GFP | green fluorescence protein |
| GO | gene ontology |
| IOMT | isoflavone O-methyltransferase |
| JA | jasmonic acid |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LCR | low-molecular-weight cysteine-rich |
| NCR | nodule-specific cysteine-rich |
| qRT-PCR | quantitative real-time PCR |
| RSEM | RNA-seq by expectation maximization |
| TIR1 | TRANSPORT INHIBITOR RESPONSE 1 |
| TT2 | TRANSPARENT TESTA 2 |
| TY | tryptone, yeast extract, and sodium chloride |
| WT | wild type |
References
- Rey, T.; Nars, A.; Bonhomme, M.; Bottin, A.; Huguet, S.; Balzergue, S.; Jardinaud, M.F.; Bono, J.J.; Cullimore, J.; Dumas, B.; et al. Nfp, a lysm protein controlling nod factor perception, also intervenes in medicago truncatula resistance to pathogens. New Phytol. 2013, 198, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Q.; Fedorova, E.; Liu, J.; Qin, Q.; Zheng, Q.; Price, P.A.; Pan, H.; Wang, D.; Griffitts, J.S.; et al. Microsymbiont discrimination mediated by a host-secreted peptide in medicago truncatula. Proc. Natl. Acad. Sci. USA 2017, 114, 6848–6853. [Google Scholar] [CrossRef] [PubMed]
- Guefrachi, I.; Nagymihaly, M.; Pislariu, C.I.; Van de Velde, W.; Ratet, P.; Mars, M.; Udvardi, M.K.; Kondorosi, E.; Mergaert, P.; Alunni, B. Extreme specificity of ncr gene expression in medicago truncatula. BMC Genom. 2014, 15, 712. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; McAdam, E.L.; Weller, J.L.; Reid, J.B. Interactions between ethylene, gibberellins, and brassinosteroids in the development of rhizobial and mycorrhizal symbioses of pea. J. Exp. Bot. 2016, 67, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Ross, J.J.; Reid, J.B. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant. Physiol. 2005, 138, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Gifford, I.; Battenberg, K.; Vaniya, A.; Wilson, A.; Tian, L.; Fiehn, O.; Berry, A.M. Distinctive patterns of flavonoid biosynthesis in roots and nodules of datisca glomerata and medicago spp. Revealed by metabolomic and gene expression profiles. Front. Plant Sci. 2018, 9, 1463. [Google Scholar] [CrossRef] [PubMed]
- Besseau, S.; Hoffmann, L.; Geoffroy, P.; Lapierre, C.; Pollet, B.; Legrand, M. Flavonoid accumulation in arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant. Cell 2007, 19, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Murray, J.D. The role of flavonoids in nodulation host-range specificity: An update. Plants 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant. Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef]
- Clouse, S.D.; Langford, M.; McMorris, T.C. A brassinosteroid-insensitive mutant in arabidopsis thaliana exhibits multiple defects in growth and development. Plant. Physiol. 1996, 111, 671–678. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, X.Y.; Cao, D.M.; Tang, W.; He, K.; Zhu, J.Y.; He, J.X.; Bai, M.Y.; Zhu, S.; Oh, E.; et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in arabidopsis. Dev. Cell 2010, 19, 765–777. [Google Scholar] [CrossRef] [PubMed]
- He, J.X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.Y. The gsk3-like kinase bin2 phosphorylates and destabilizes bzr1, a positive regulator of the brassinosteroid signaling pathway in arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 10185–10190. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yuan, M.; Wang, R.; Yang, Y.; Wang, C.; Oses-Prieto, J.A.; Kim, T.W.; Zhou, H.W.; Deng, Z.; Gampala, S.S.; et al. Pp2a activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating bzr1. Nat. Cell Biol. 2011, 13, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-localized bzr1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2002, 2, 505–513. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. Bes1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef]
- Cheng, X.; Gou, X.; Yin, H.; Mysore, K.S.; Li, J.; Wen, J. Functional characterisation of brassinosteroid receptor mtbri1 in medicago truncatula. Sci. Rep. 2017, 7, 9327. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Liu, J.; Terecskei, K.; Abraham, E.; Gombar, A.; Domonkos, A.; Szucs, A.; Kormoczi, P.; Wang, T.; et al. Host-secreted antimicrobial peptide enforces symbiotic selectivity in medicago truncatula. Proc. Natl. Acad. Sci. USA 2017, 114, 6854–6859. [Google Scholar] [CrossRef]
- Alunni, B.; Kevei, Z.; Redondo-Nieto, M.; Kondorosi, A.; Mergaert, P.; Kondorosi, E. Genomic organization and evolutionary insights on grp and ncr genes, two large nodule-specific gene families in medicago truncatula. Mol. Plant Microbe Interact. MPMI 2007, 20, 1138–1148. [Google Scholar] [CrossRef]
- Nallu, S.; Silverstein, K.A.; Samac, D.A.; Bucciarelli, B.; Vance, C.P.; VandenBosch, K.A. Regulatory patterns of a large family of defensin-like genes expressed in nodules of medicago truncatula. PLoS ONE 2013, 8, e60355. [Google Scholar] [CrossRef]
- Chaiwanon, J.; Wang, Z.Y. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in arabidopsis roots. Curr. Biol. CB 2015, 25, 1031–1042. [Google Scholar] [CrossRef]
- Wang, W.; Bai, M.Y.; Wang, Z.Y. The brassinosteroid signaling network-a paradigm of signal integration. Curr. Opin. Plant Biol. 2014, 21, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Wasson, A.P.; Pellerone, F.I.; Mathesius, U. Silencing the flavonoid pathway in medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant. Cell 2006, 18, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Vega, J.C.; Cady, B.; Kayanja, G.; Mauriello, A.; Cervantes, N.; Gillespie, A.; Lavia, L.; Trujillo, J.; Alkio, M.; Colon-Carmona, A. Detoxification of polycyclic aromatic hydrocarbons (pahs) in arabidopsis thaliana involves a putative flavonol synthase. J. Hazard. Mater. 2017, 321, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cardoza, V.; Mitchell, D.M.; Bright, L.; Oldroyd, G.; Harris, J.M. Crosstalk between jasmonic acid, ethylene and nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant. J. Cell Mol. Biol. 2006, 46, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, T.; Nagymihaly, M.; Lamouche, F.; Barriere, Q.; Guefrachi, I.; Alunni, B.; Ouadghiri, M.; Ibijbijen, J.; Kondorosi, E.; Mergaert, P.; et al. Specific host-responsive associations between medicago truncatula accessions and sinorhizobium strains. Mol. Plant Microbe Interact. MPMI 2017, 30, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Terpolilli, J.J.; O’Hara, G.W.; Tiwari, R.P.; Dilworth, M.J.; Howieson, J.G. The model legume medicago truncatula a17 is poorly matched for n2 fixation with the sequenced microsymbiont sinorhizobium meliloti 1021. New Phytol. 2008, 179, 62–66. [Google Scholar] [CrossRef]
- Tadege, M.; Wen, J.; He, J.; Tu, H.; Kwak, Y.; Eschstruth, A.; Cayrel, A.; Endre, G.; Zhao, P.X.; Chabaud, M. Large-scale insertional mutagenesis using the tnt1 retrotransposon in the model legume medicago truncatula. Plant J. 2008, 54, 335–347. [Google Scholar] [CrossRef]
- Beringer, J.E. R factor transfer in rhizobium leguminosarum. J. Gen. Microbiol. 1974, 84, 188–198. [Google Scholar] [CrossRef]
- Saldanha, A.J. Java treeview—Extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef]
- Earley, K.W.; Haag, J.R.; Pontes, O.; Opper, K.; Juehne, T.; Song, K.; Pikaard, C.S. Gateway-compatible vectors for plant functional genomics and proteomics. Plant. J. Cell Mol. Biol. 2006, 45, 616–629. [Google Scholar] [CrossRef]
- Cosson, V.; Durand, P.; d’Erfurth, I.; Kondorosi, A.; Ratet, P. Medicago truncatula transformation using leaf explants. Methods Mol. Biol. 2006, 343, 115–127. [Google Scholar] [PubMed]
- Tian, Y.; Fan, M.; Qin, Z.; Lv, H.; Wang, M.; Zhang, Z.; Zhou, W.; Zhao, N.; Li, X.; Han, C.; et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the brassinazole-resistant1 transcription factor. Nat. Commun. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Cheng, X.; Li, G.; Tang, Y.; Wen, J. Dissection of genetic regulation of compound inflorescence development in Medicago truncatula. Development 2018, 145, 158766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Han, L.; Hou, C.; Metelli, A.; Qi, L.; Tadege, M.; Mysore, K.S.; Wang, Z.Y. Developmental analysis of a medicago truncatula smooth leaf margin1 mutant reveals context-dependent effects on compound leaf development. Plant. Cell 2011, 23, 2106–2124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. Soapnuke: A mapreduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience 2018, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. Rsem: Accurate transcript quantification from rna-seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. Degseq: An R package for identifying differentially expressed genes from rna-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]

| GO_Term_ID | GO_Term | q-Value |
|---|---|---|
| GO:0009878 | Nodule morphogenesis | 2.29 × 10−10 |
| GO:0044111 | Development involved in symbiotic interaction | 2.29 × 10−10 |
| GO:0051704 | Multi-organism process | 2.29 × 10−10 |
| GO:0009877 | Nodulation | 4.77 × 10−10 |
| GO:0044419 | Interspecies interaction between organisms | 4.77 × 10−10 |
| GO:0044403 | Symbiosis, encompassing mutualism through parasitism | 4.80 × 10−10 |
| GO:0009653 | Anatomical structure morphogenesis | 5.30 × 10−9 |
| GO:0032502 | Developmental process | 6.76 × 10−6 |
| GO:0048856 | Anatomical structure development | 1.40 × 10−5 |
| GO:0042737 | Drug catabolic process | 6.72 × 10−3 |
| GO:0005975 | Carbohydrate metabolic process | 7.35 × 10−3 |
| Gene | Annotation | log2FC | FDR |
|---|---|---|---|
| Medtr6g462060 | NCR secreted peptide | −1.03955 | 9.79 × 10−86 |
| Medtr4g065410 | Late nodulin | −1.30701 | 1.6 × 10−106 |
| Medtr4g065455 | NCR secreted peptide | −1.1222 | 3.27 × 10−24 |
| Medtr0337s0030 | NCR secreted peptide | −1.09355 | 4.47 × 10−64 |
| Medtr6g406350 | NCR secreted peptide | −1.66085 | 1.45 × 10−10 |
| Medtr1g037670 | NCR secreted peptide | −1.01068 | 2.46 × 10−36 |
| Medtr4g060370 | Late nodulin | −1.03975 | 1 × 10−136 |
| Medtr4g063740 | NCR secreted peptide | −1.30404 | 4.43 × 10−20 |
| Medtr5g063560 | Hypothetical protein | −1.29477 | 1.21 × 10−11 |
| Medtr0050s0100 | NCR secreted peptide | −1.10619 | 2.68 × 10−36 |
| Medtr6g445080 | NCR secreted peptide | −1.06689 | 0 |
| Medtr7g008130 | NCR secreted peptide | −1.28862 | 1.51 × 10−56 |
| Medtr7g056047 | NCR secreted peptide | −1.04601 | 1.2 × 10−11 |
| Medtr7g045410 | NCR secreted peptide | −1.16667 | 5.16 × 10−8 |
| Medtr5g459510 | NCR secreted peptide | −1.31551 | 1.5 × 10−111 |
| Medtr6g445020 | NCR secreted peptide | −1.34506 | 3.19 × 10−78 |
| Medtr6g461840 | NCR secreted peptide | −1.00809 | 1.7 × 10−108 |
| Medtr6g044700 | Unknown | −1.26281 | 1.1 × 10−113 |
| Medtr1g028980 | NCR secreted peptide | −1.0595 | 4.01 × 10−37 |
| Medtr7g071315 | NCR secreted peptide | −1.13168 | 1.4 × 10−121 |
| Medtr3g071360 | NCR secreted peptide | −1.08081 | 3.83 × 10−74 |
| Medtr2g066255 | Late nodulin | −1.06841 | 1.7 × 10−50 |
| Medtr7g045520 | NCR secreted peptide | −1.08055 | 0 |
| Medtr7g406940 | Late nodulin | −1.24693 | 1.01 × 10−28 |
| Medtr3g063450 | Late nodulin | −1.10333 | 1.25 × 10−48 |
| Medtr2g072970 | Late nodulin | −1.03982 | 1.13 × 10−9 |
| Medtr0330s0030 | NCR secreted peptide | −1.14577 | 1.01 × 10−4 |
| Medtr7g015880 | NCR secreted peptide | −1.21986 | 1.48 × 10−32 |
| Medtr2g450150 | NCR secreted peptide | −1.0796 | 2.94 × 10−24 |
| Medtr2g044310 | NCR secreted peptide | −1.08089 | 3.88 × 10−4 |
| Medtr7g102806 | NCR secreted peptide | −1.36812 | 1.68 × 10−33 |
| Medtr4g017790 | NCR secreted peptide | −1.28877 | 1.1 × 10−14 |
| Medtr5g059420 | NCR secreted peptide | 1.234336 | 1.19 × 10−11 |
| Medtr7g445930 | NCR secreted peptide | −1.09898 | 9.81 × 10−5 |
| Medtr7g008020 | NCR secreted peptide | −1.28923 | 4.52 × 10−6 |
| Medtr5g048335 | NCR secreted peptide | −1.22601 | 2.84 × 10−77 |
| Medtr2g044330 | NCR secreted peptide | −1.18661 | 0 |
| Medtr7g071585 | Late nodulin | −1.05481 | 2.36 × 10−12 |
| Medtr5g044135 | Late nodulin | −1.49748 | 4.23 × 10−66 |
| Medtr2g022740 | NCR secreted peptide | −1.08612 | 5.77 × 10−75 |
| Medtr7g065025 | NCR secreted peptide | −1.00326 | 1.49 × 10−98 |
| Medtr1g046020 | NCR secreted peptide | −1.03343 | 3.44 × 10−52 |
| Medtr6g060320 | NCR secreted peptide | −1.39606 | 1.5 × 10−5 |
| Medtr3g015940 | NCR secreted peptide | −1.32883 | 2.4 × 10−63 |
| Medtr7g045910 | NCR secreted peptide | −1.30758 | 3.5 × 10−21 |
| Medtr7g055933 | NCR secreted peptide | −1.08339 | 3.44 × 10−4 |
| Medtr6g027155 | NCR secreted peptide | −1.13114 | 6.91 × 10−69 |
| Medtr6g478110 | Kunitz-type trypsin inhibitor/miraculin | −1.15167 | 3.82 × 10−47 |
| Medtr6g038620 | NCR secreted peptide | −1.25415 | 1.84 × 10−16 |
| Medtr7g064970 | Late nodulin | 1.213179 | 1.85 × 10−6 |
| Medtr5g058510 | NCR secreted peptide | −1.10207 | 6.12 × 10−70 |
| Medtr5g072205 | NCR secreted peptide | 1.301282 | 7.7 × 10−4 |
| Medtr3g071330 | NCR secreted peptide | −1.05226 | 1.49 × 10−54 |
| Medtr7g033855 | Low-molecular-weight cysteine-rich (LCR) protein | −1.05107 | 4.07 × 10−4 |
| Medtr4g060610 | NCR secreted peptide | −1.06185 | 1.25 × 10−25 |
| Medtr6g060370 | NCR secreted peptide | −5.53715 | 1.77 × 10−22 |
| KEGG_Term_ID | KEGG_Term | q-Value |
|---|---|---|
| ko00943 | Isoflavonoid biosynthesis | 5.91 × 10−7 |
| ko00902 | Monoterpenoid biosynthesis | 9.52 × 10−7 |
| ko00909 | Sesquiterpenoid and triterpenoid biosynthesis | 8.49 × 10−5 |
| ko00941 | Flavonoid biosynthesis | 8.84 × 10−4 |
| ko00940 | Phenylpropanoid biosynthesis | 4.34 × 10−3 |
| ko04712 | Circadian rhythm-plant | 7.15 × 10−3 |
| ko00620 | Pyruvate metabolism | 1.66 × 10−2 |
| ko00591 | Linoleic acid metabolism | 7.93 × 10−2 |
| ko00010 | Glycolysis/gluconeogenesis | 9.55 × 10−2 |
| ko00945 | Stilbenoid, diarylheptanoid, and gingerol biosynthesis | 9.55 × 10−2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.; Wang, H.; Hong, L.; Xu, Y.; Zhao, Y.; Zhou, C. MtBZR1 Plays an Important Role in Nodule Development in Medicago truncatula. Int. J. Mol. Sci. 2019, 20, 2941. https://doi.org/10.3390/ijms20122941
Cui C, Wang H, Hong L, Xu Y, Zhao Y, Zhou C. MtBZR1 Plays an Important Role in Nodule Development in Medicago truncatula. International Journal of Molecular Sciences. 2019; 20(12):2941. https://doi.org/10.3390/ijms20122941
Chicago/Turabian StyleCui, Can, Hongfeng Wang, Limei Hong, Yiteng Xu, Yang Zhao, and Chuanen Zhou. 2019. "MtBZR1 Plays an Important Role in Nodule Development in Medicago truncatula" International Journal of Molecular Sciences 20, no. 12: 2941. https://doi.org/10.3390/ijms20122941
APA StyleCui, C., Wang, H., Hong, L., Xu, Y., Zhao, Y., & Zhou, C. (2019). MtBZR1 Plays an Important Role in Nodule Development in Medicago truncatula. International Journal of Molecular Sciences, 20(12), 2941. https://doi.org/10.3390/ijms20122941





