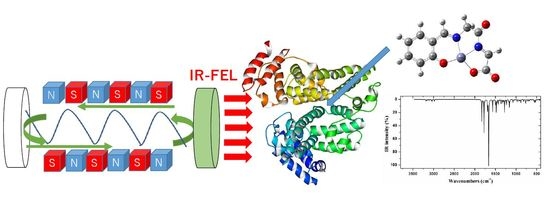
Investigation by DFT Methods of the Damage of Human Serum Albumin Including Amino Acid Derivative Schiff Base Zn(II) Complexes by IR-FEL Irradiation
Abstract
1. Introduction
2. Results and Discussion
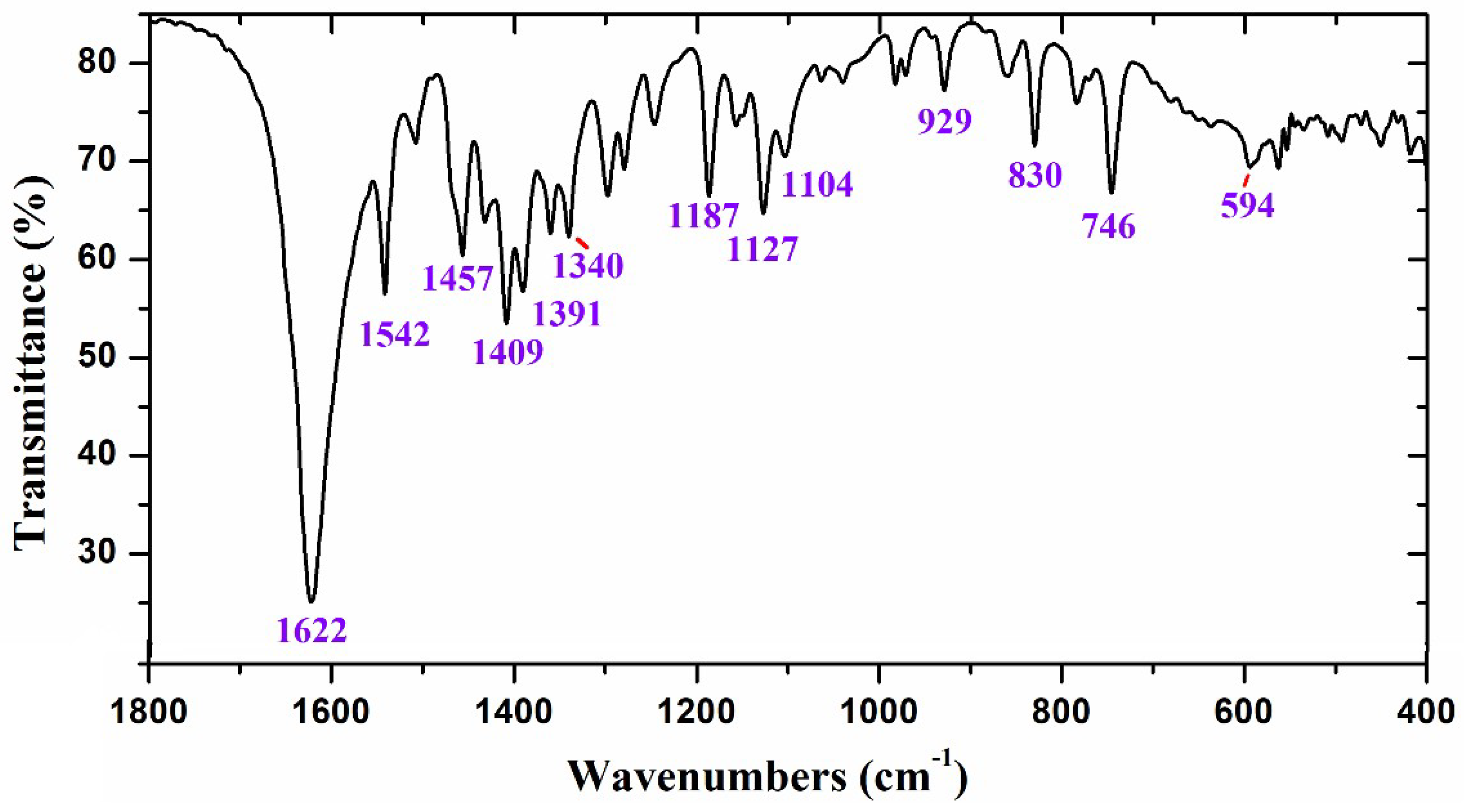
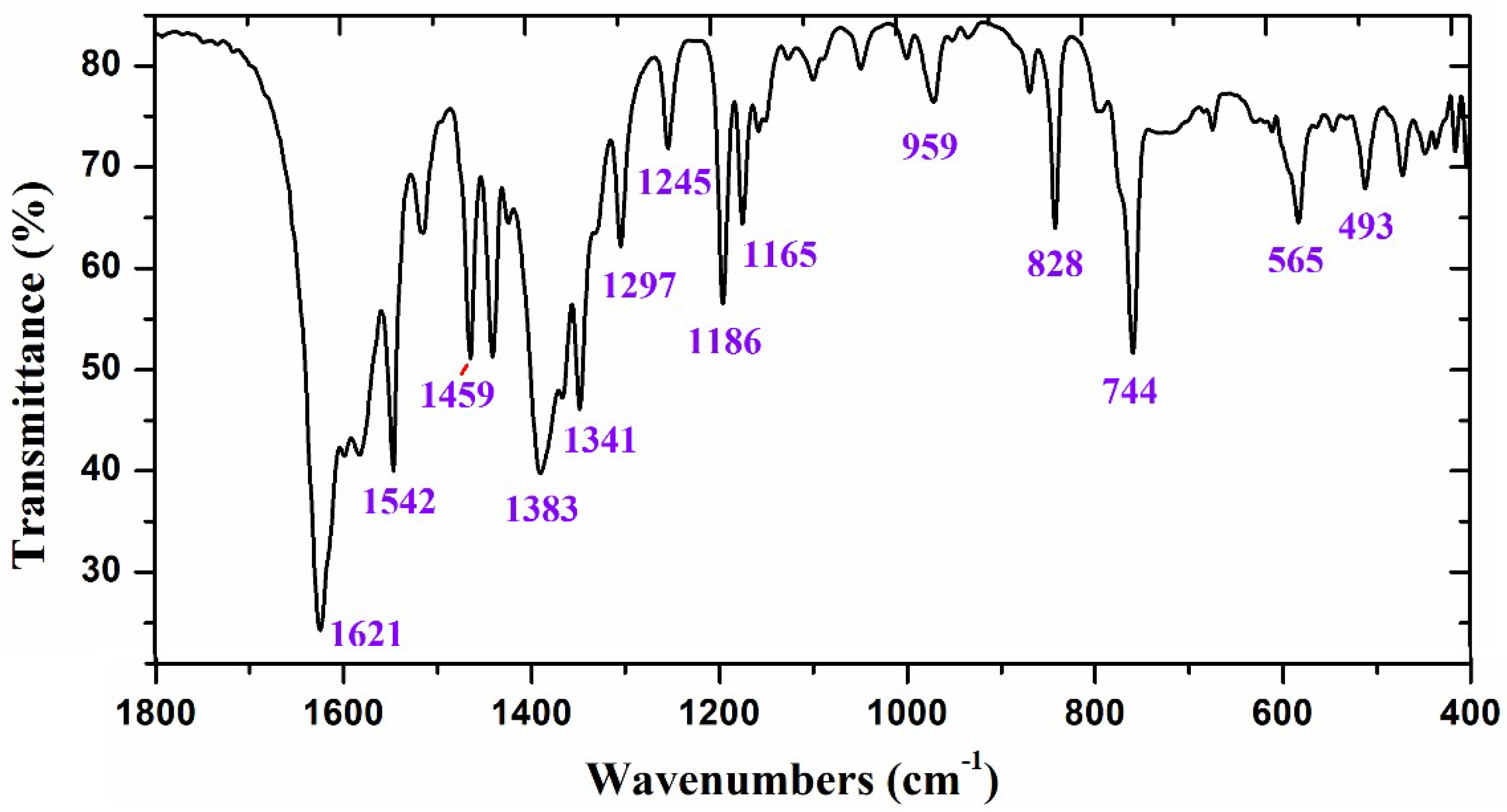
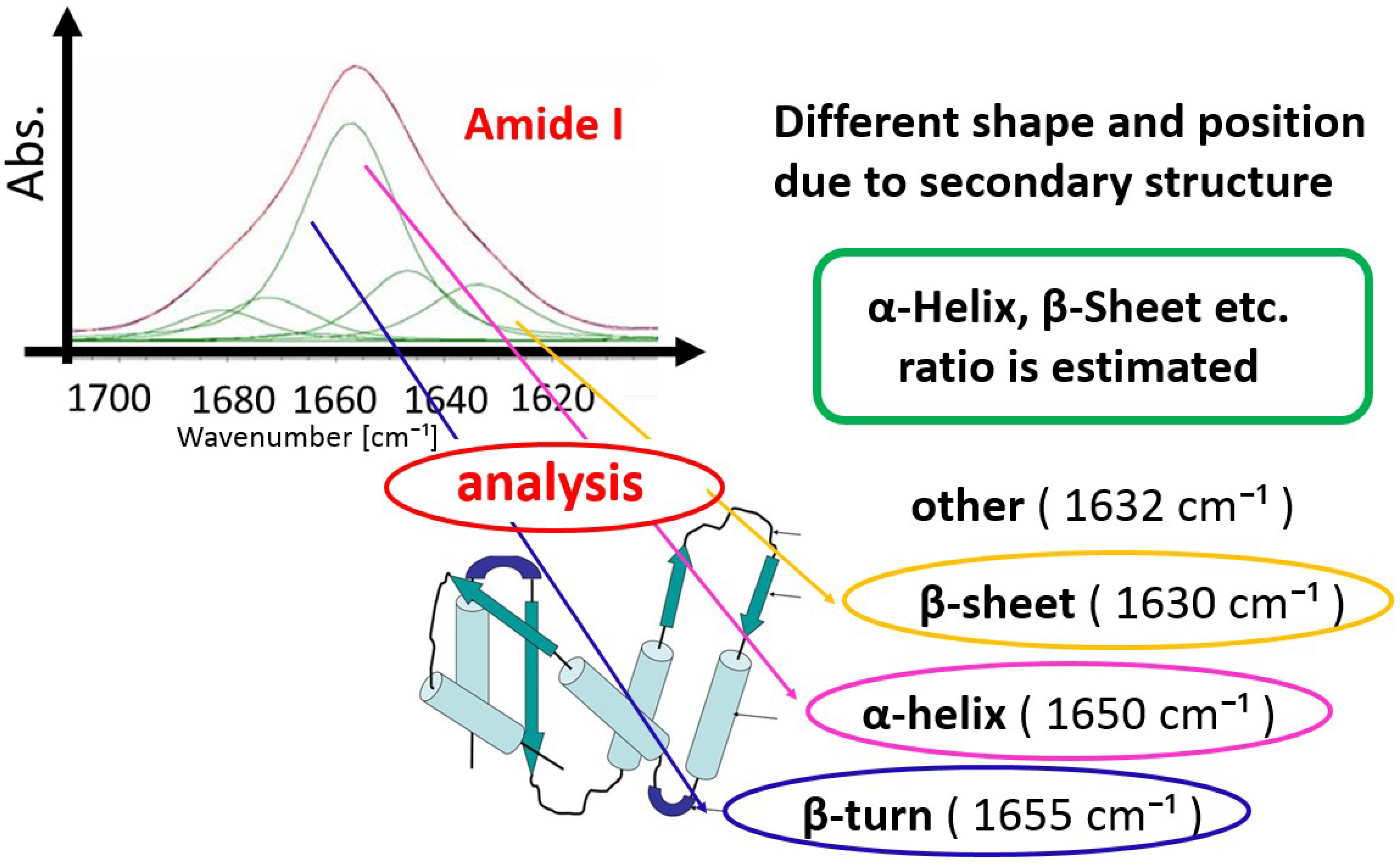
2.1. Experimental IR Spectra
2.2. DFT Calculations of Zn(II) Complexes
2.2.1. Geometry Optimization in the Isolated State
2.2.2. Other Molecular Properties
2.2.3. Scaling the Wavenumbers
2.2.4. Vibrational Wavenumbers
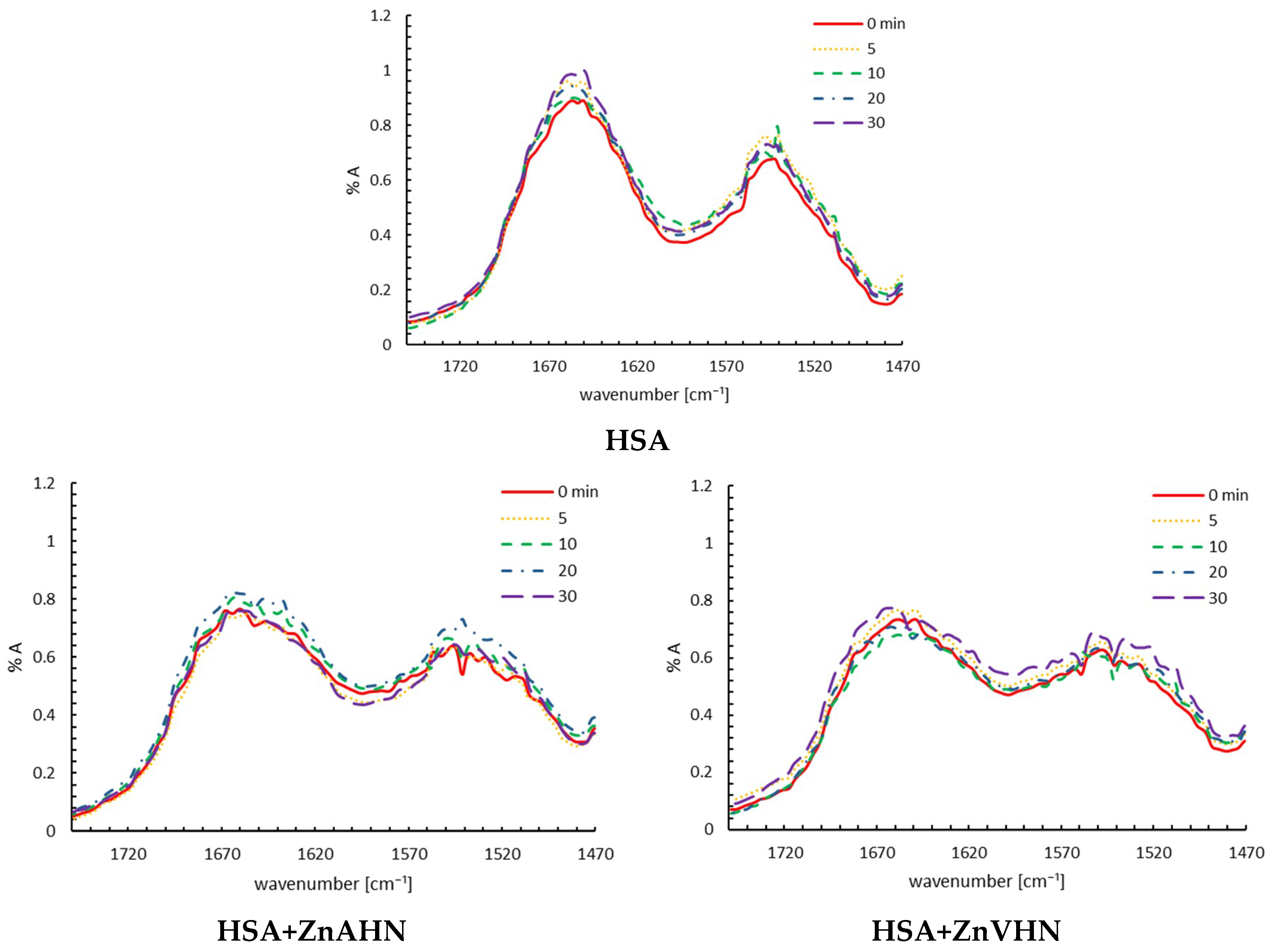
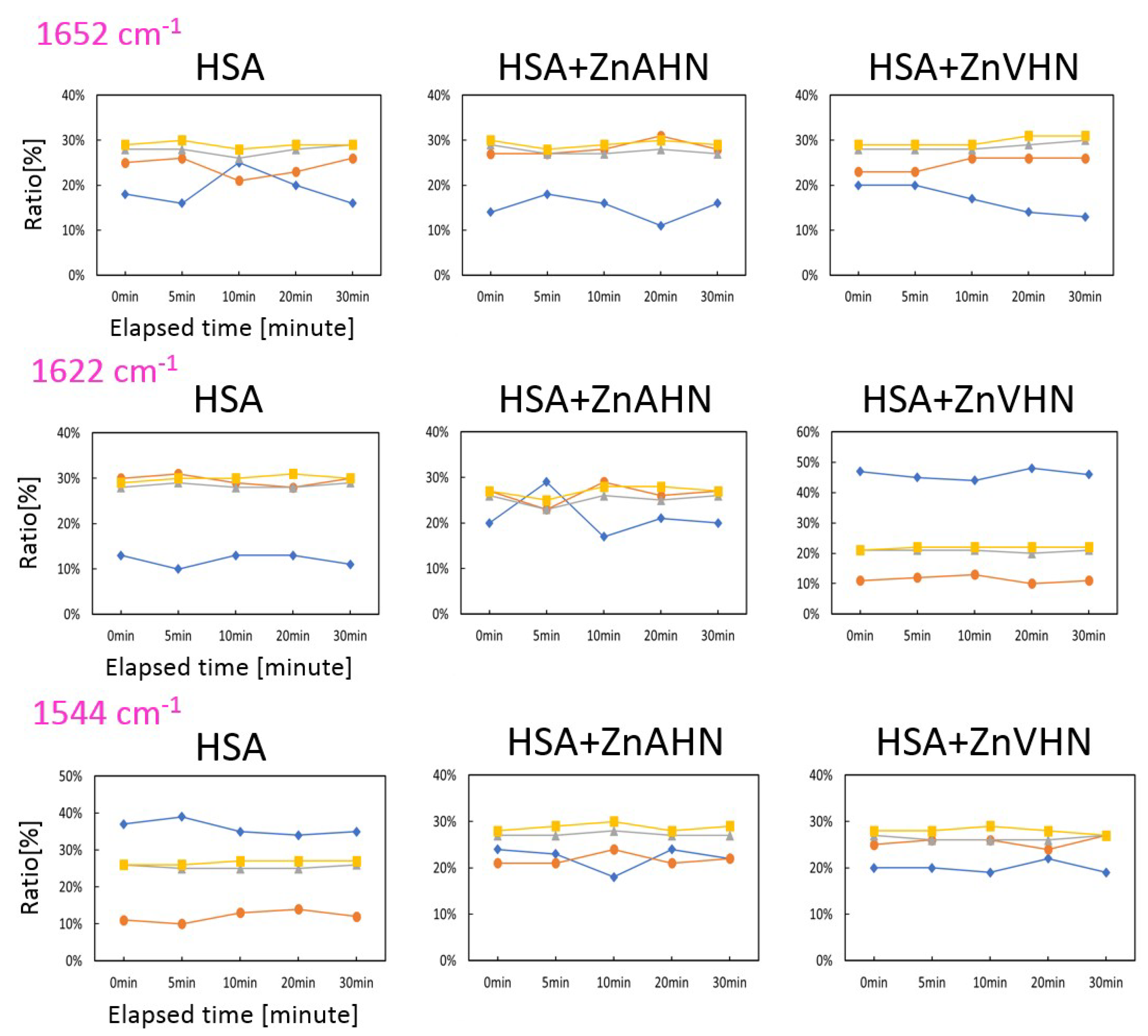
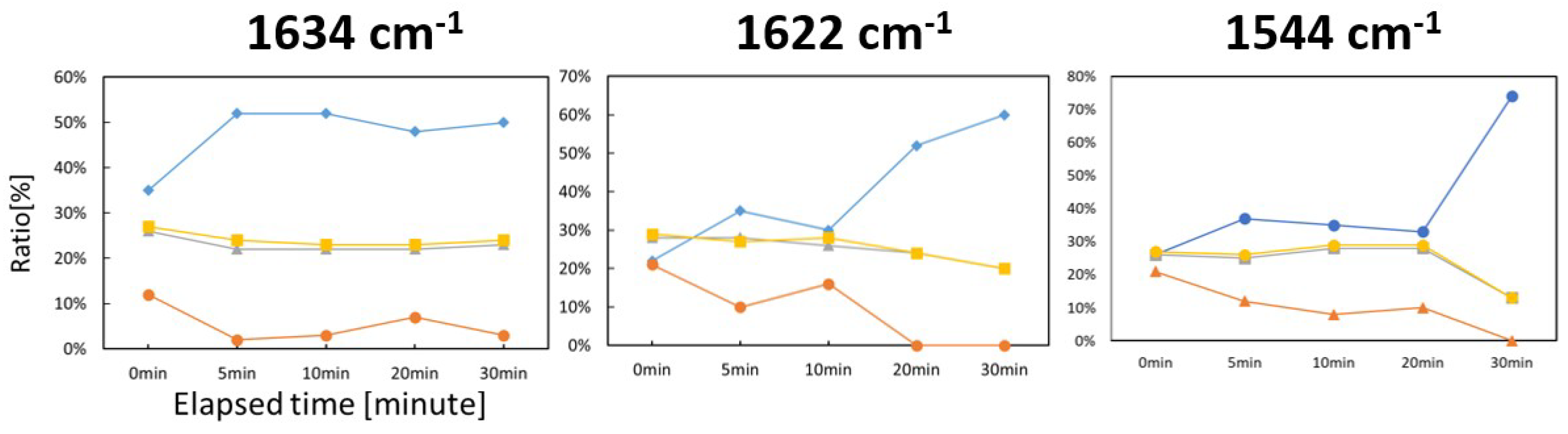
2.3. Damage of HSA (with Zn(II) Complex) by IR-FEL
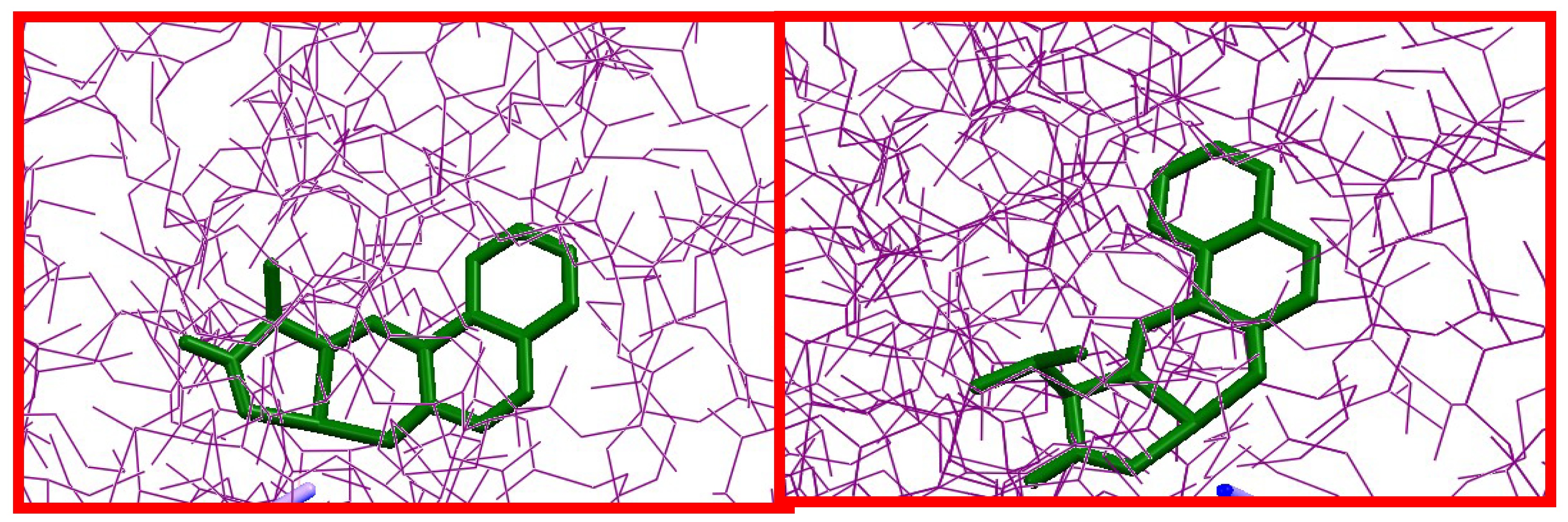
2.4. Docking Simulations
3. Materials and Methods
3.1. General Procedures
3.2. Preparations
3.3. Physical Measurements
3.4. Computational Methods
3.5. IR-FEL Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | Density Functional Theory |
| IR | Infrared |
| FEL | Free Electron Laser |
| HSA | Human Serum Albumin |
| TD | Time Dependent |
| UVA | Ultraviolet A |
| IRM | Infrared Microscopic analysis |
| ZPVE | Zero-Point Vibrational Energy |
| TLSE | Two Linear Scale Equations |
| FT | Fourier Transfer |
| Vis | Visible |
| CD | Circular Dichroism |
References
- Holmes, A.M.; Song, Z.; Moghimi, H.R.; Roberts, M.S. Relative Penetration of Zinc Oxide and Zinc Ions into Human Skin after Application of Different Zinc Oxide. ACS Nano 2016, 10, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Shimada, T.; Hiroki, H.; Takase, M.; Akitsu, T. L-valine and salicylaldehyde derivative Schiff base Zn(II) complexes as UVA sunscreen. Jacob J. Inorg. Chem. 2016, 1, 6. [Google Scholar]
- Jankov II, M.R.; Coskunseven, E.; Hafezi, F. Contralateral Eye Study of Corneal Collagen Cross-linking With Riboflavin and UVA Irradiation in Patients With Keratoconus. J. Refractive Surgery 2009, 25, 371–376. [Google Scholar]
- Johnson, R.; Maciunas, M.; Mendenhall, R.; Ossoff, J.; Tribble, J.W.; O’Day, D. Tissue ablation by a free-electron laser tuned to the amide II band. Nature 1994, 371, 416–419. [Google Scholar]
- Kawasaki, T.; Fujioka, J.; Imai, T.; Torigoe, K.; Tsukiyama, K. Mid-infrared free-electron laser tuned to the amide I band for converting insoluble amyloid-like protein fibrils into the soluble monomeric form. Lasers Med. Sci. 2014, 29, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Yaji, T.; Ohta, T.; Tsukiyama, K. Application of mid-infrared free-electron laser tuned to amide bands for dissociation of aggregate structure of protein. J. Synchrotron Rad. 2016, 23, 152–157. [Google Scholar] [CrossRef]
- Tsuda, E.; Mitsumoto, Y.; Takakura, K.; Sunaga, N.; Akitsu, T.; Konomi, T.; Katoh, M. Electrochemical tuning by polarized UV light induced molecular orientation of chiral salen-type Mn(II) and Co(II) complexes in an albumin matrix. J. Chem. Chem. Eng. 2016, 2, 53–59. [Google Scholar]
- Hayashi, T.; Akitsu, T. Fluorescence, UV-vis, and CD Spectroscopic Study on Docking of Chiral Salen-Type Zn(II) Complexes and Lysozyme and HSA Proteins. In Threonine: Food Sources, Functions and Health Benefits; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 49–72. [Google Scholar]
- Watanabe, Y.; Akitsu, T. Preparations of hybrid systems of L-amino acid derivatives of Schiff base Cu(II) and Zn(II) complexes and polyoxometalates. Asian Chem. Lett. 2012, 16, 9–18. [Google Scholar]
- Awazu, K.; Fukami, Y. Selective removal of cholesteryl oleate through collagen films by MIR FEL. Nucl. Instrum. Methods Phys. Res. 2001, 475, 650–655. [Google Scholar] [CrossRef]
- Akao, K. Development and Application of Secondary Structural Estimation Program of Protein for FTIR (IR-SSE). Jasco Rep. 2002, 44, 54–57. [Google Scholar]
- Tang, J.; Luan, F.; Chen, X. Binding analysis of glycyrrhetinic acid to human serum albumin: Fluorescence spectroscopy, FTIR, and molecular modeling. Bioorg. Med. Chem. 2006, 14, 3210–3217. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Xie, M.-X.; Li, S.; Jiang, M.; Wang, Y.-D. Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Biophys. Acta (BBA) General Subjects. 2004, 1674, 205–214. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, Y.; Xue, C.; Hu, Z.; Chen, X.; Sheng, F. Effect of Chinese medicine alpinetin on the structure of human serum albumin. Bioorg. Med. Chem. 2005, 13, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Awazu, K.; Nagai, A.; Aizawa, K. Selective removal of cholesterol esters in an arteriosclerotic region of blood vessels with a free-electron laser. Laser Surgery Med. 1998, 23, 233–237. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshihashi-Suzuki, S.; Ishii, K.; Kanai, T.; Awazu, K. Matrix-assisted laser desorption/ionization of protein samples containing a denaturant at high concentration using a mid-infrared free-electron laser (MIR-FEL). Int. J. Mass. Spectr. 2005, 241, 49–56. [Google Scholar] [CrossRef]
- Leroy, G.; Sana, M.; Wilante, C.; Temsamani, D.R. Determination of bond energy terms in silicon-containing compounds. J. Mol. Struct. (Theochem) 1992, 259, 369–381. [Google Scholar] [CrossRef]
- Alcolea Palafox, M. Scaling factors for the prediction of vibrational spectra. I. Benzene molecule. Int. J. Quantum Chem. 2000, 77, 661–684. [Google Scholar] [CrossRef]
- Alcolea Palafox, M.; Rastogi, V.K. Quantum chemical predictions of the vibrational spectra of polyatomic molecules. The uracil molecule and two derivatives. Spectrochim. Acta 2002, 58A, 411–440. [Google Scholar] [CrossRef]
- Alcolea Palafox, M. DFT computations on vibrational spectra: Scaling procedures to improve the wavenumbers. Phys. Sci. Rev. 2018, 3, 1–30. [Google Scholar]
- Seminario, J.M.; Politzer, P. (Eds.) Modern Density Functional Theory: A Tool for Chemistry; Elsevier: Amsterdam, The Netherlands, 1995; vol. 2. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. 3. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev. 1988, B37, 785–789. [Google Scholar] [CrossRef]
- Alcolea Palafox, M.; Iza, N.; Gil, M. The hydration effect on the uracil wavenumbers: An experimental and quantum chemical study. J. Molec. Struct. (Theochem) 2002, 585, 69–92. [Google Scholar] [CrossRef]
- Singh, C.; Alcolea Palafox, M.; Bali, N.P.; Bagchi, S.S.; Shrivastava, P.K. Synthesis and characterisation of complex of Cu(II) with 5-carboxy-2-thiouracil. Asian Chem. Letts. 1999, 3, 248–249. [Google Scholar]
- Gaussian 09, Revision, D.01: (Gaussian, Inc). Available online: http://gaussian.com/ (accessed on 9 June 2019).
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- GOLD: (CCDC). Available online: https://www.ccdc.cam.ac.uk/solutions/csd-discovery/Components/Gold/ (accessed on 9 June 2019).
- Yokoyama, M.; Oda, F.; Nomaru, K.; Koike, H.; Sobajima, M.; Miura, H.; Hattori, H.; Kawai, M.; Kuroda, H. First lasing of KHI FEL device at FEL-SUT. Nucl. Instrum. Methods Phys. Res 2001, 475, 38–42. [Google Scholar] [CrossRef]
- Sarver, R.W., Jr.; Krueger, W.C. Protein secondary structure from Fourier transform infrared spectroscopy: A data base analysis. Anal. Biochem. 1991, 194, 89–100. [Google Scholar] [CrossRef]
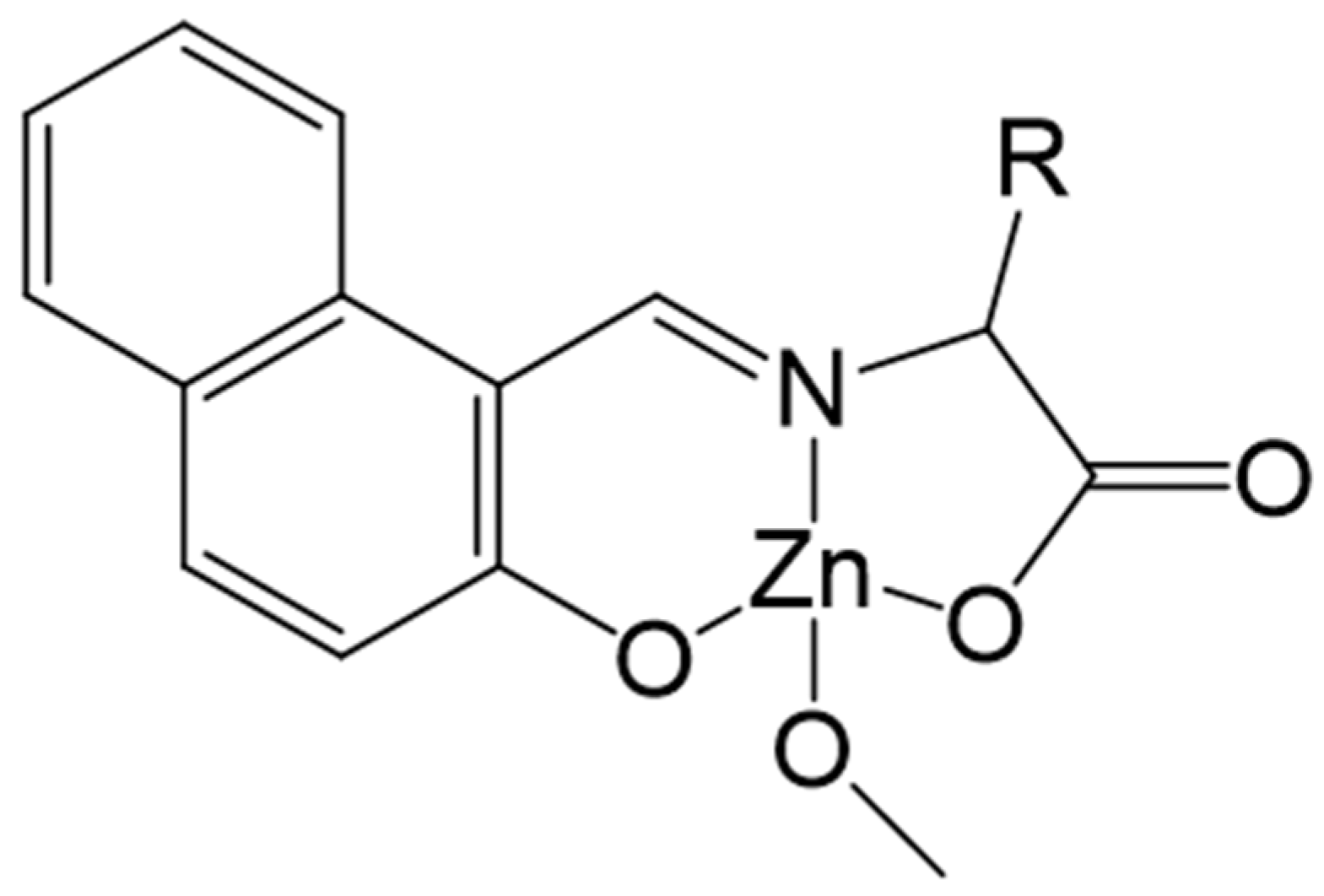

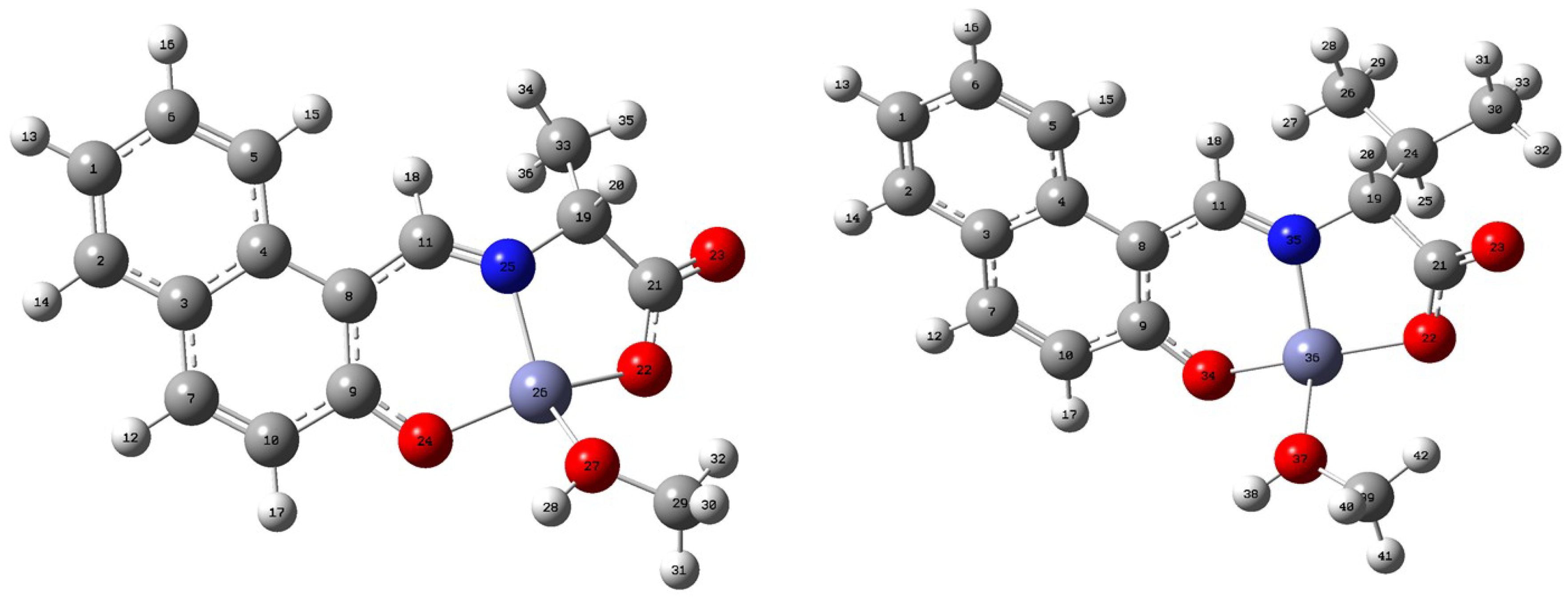
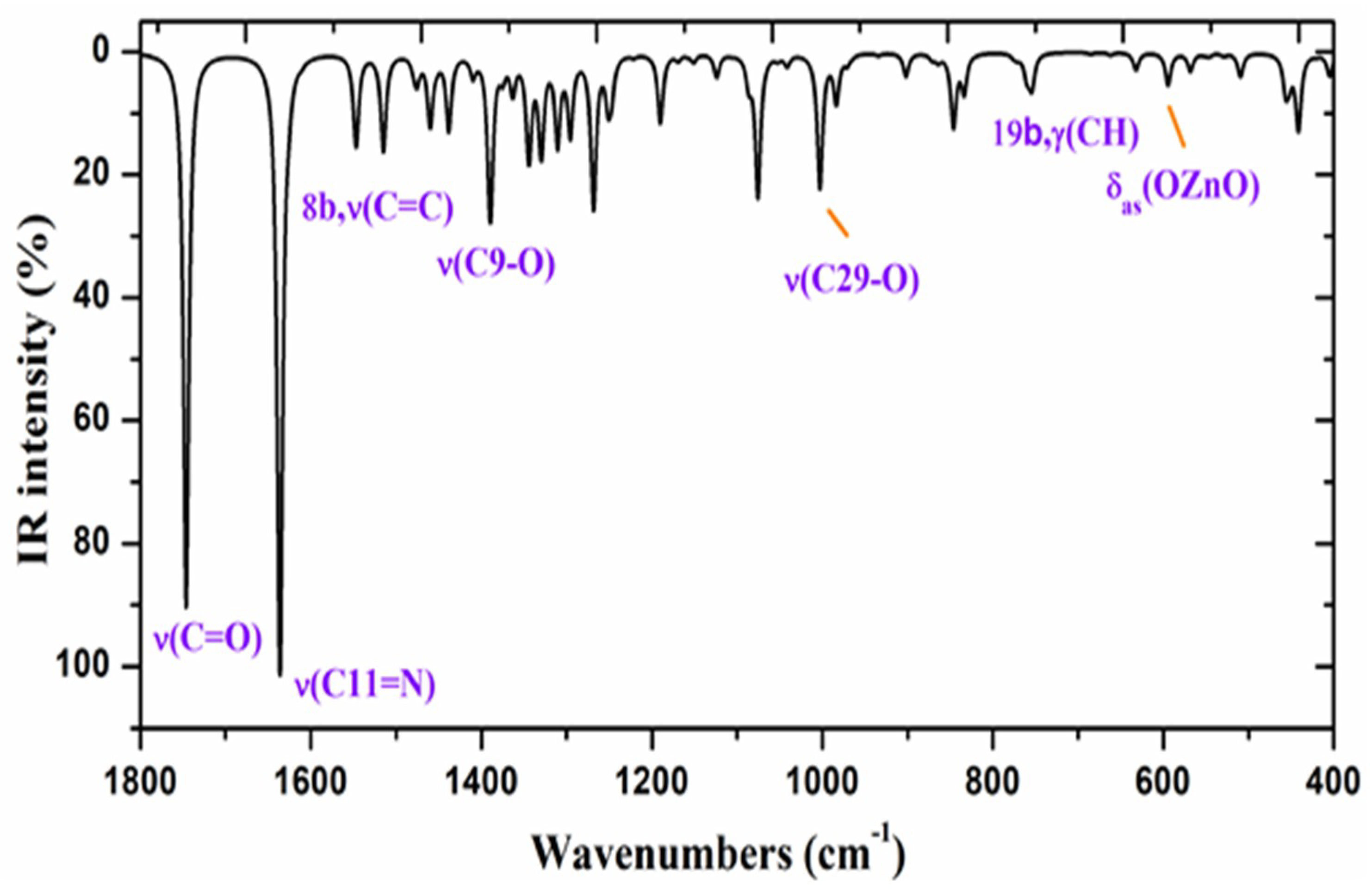
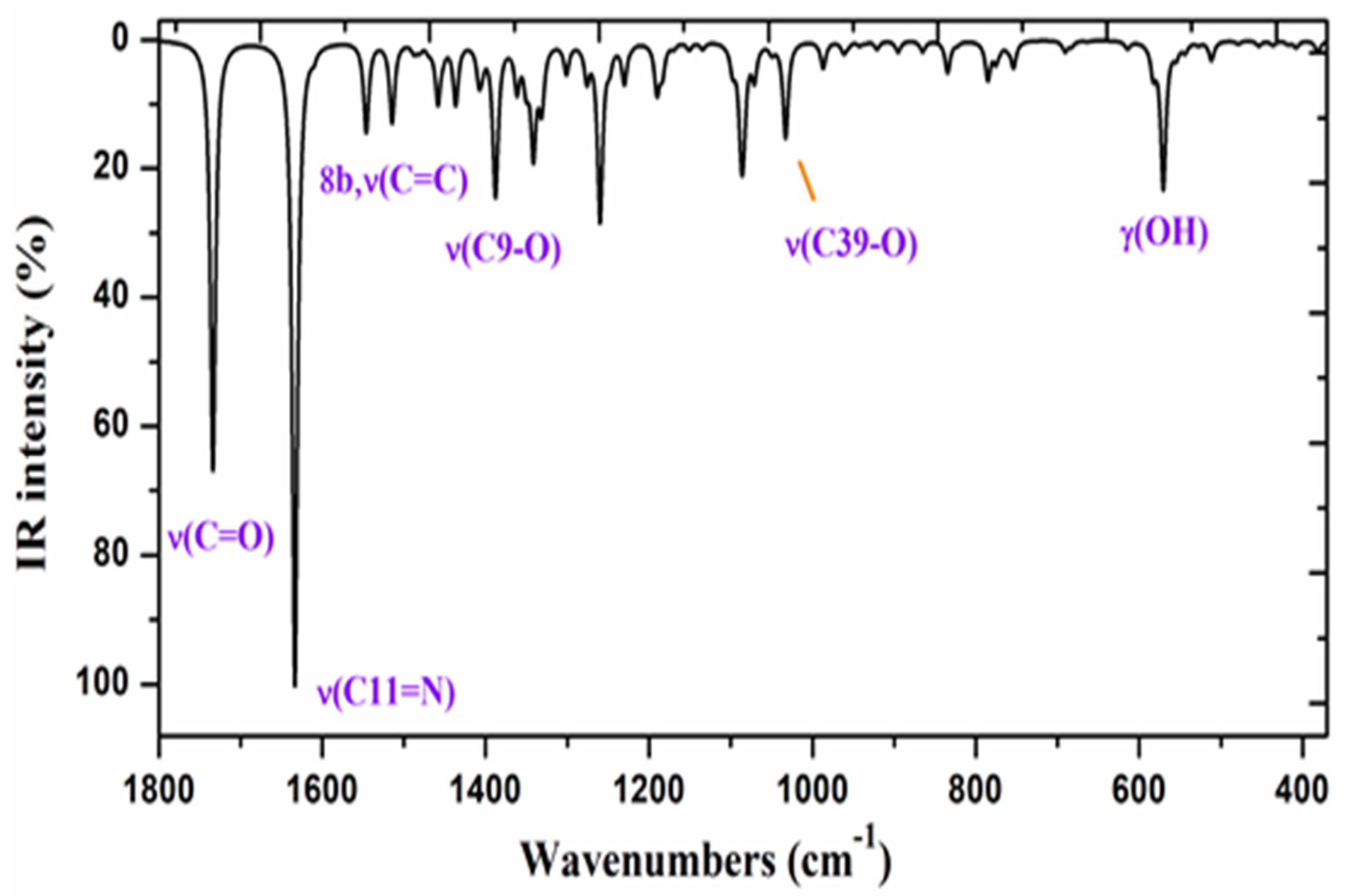
| Parameters | ZnAHN | ZnVHN | |||
|---|---|---|---|---|---|
| Conf. 1 | Conf. 2 | Conf. 1 | Conf. 2 | Conf. 3 | |
| C8-C9-O24-Zn | 5.7 | 4.9 | 9.1 | 9.6 | 8.2 |
| C9-O24-Zn-O22 | −108.3 | −106.4 | −117.2 | −117.4 | −114.2 |
| C9-O24-Zn-O27 | 133.6 | 130.1 | 118.6 | 114.8 | 124.0 |
| C9-O24-Zn-N | −8.4 | −7.4 | −19.8 | −20.9 | −15.6 |
| O22-Zn-O27-C29 | −134.3 | −13.7 | −39.4 | −4.1 | 149.0 |
| C9-C8-C11-N | −20.5 | −20.4 | −9.5 | −9.8 | −16.0 |
| O24-Zn-O22-C21 | 108.3 | 107.8 | 113.2 | 112.4 | 113.1 |
| Theoretical conf 1 | Theoretical conf 2 | Experimental | Characterization | |||||
|---|---|---|---|---|---|---|---|---|
| νcal | Aa | µ | f | νscal, b | νcal | νscal, b | νexp | |
| 1215 | 12 | 1.9 | 1.68 | 1196 | 1215 | 1196 | 1187 s | 83%, 9b δ(CC, C-H) + 15% δ(C11-H) |
| 1247 | 0 | 2.0 | 1.84 | 1227 | 1247 | 1227 | 1222 vw | 88%, δ(CC, C-H) in benzene ring |
| 1275 | 5 | 2.2 | 2.07 | 1254 | 1275 | 1254 | 1247 w | 55%, 3 δ(C-H) + 32%, ν(C-O) |
| 1280 | 8 | 1.6 | 1.51 | 1259 | 1279 | 1258 | 82%, δ(C19-H) | |
| 1297 | 26 | 3.3 | 3.26 | 1276 | 1296 | 1275 | 1280 m | 60%, ν(C21-O) + 20%, δ(C19-H) |
| 1324 | 13 | 1.7 | 1.81 | 1303 | 1325 | 1304 | 1298 m | 70%, δ(C20-H) + 25%, 19b ν(CC, CH) |
| 1339 | 14 | 1.9 | 2.06 | 1317 | 1340 | 1318 | 70%, δ(C20-H) + 25%, 19b ν(CC, CH) | |
| 1368 | 6 | 1.3 | 1.46 | 1346 | 1360 | 1338 | 1340 m | 70%, δ(O-H) + 25% δ(C-H) in C29H3 |
| 1375 | 19 | 3.7 | 4.14 | 1353 | 1375 | 1353 | 1360 m | 52%, 14 ν(C=C) + 28% δ(C11-H) |
| 1395 | 6 | 3.1 | 3.57 | 1372 | 1395 | 1372 | 70%, 14 ν(C=C) + 20% δ(C11-H) | |
| 1408 | 3 | 1.3 | 1.55 | 1385 | 1408 | 1385 | 1391 m | 80% δs(C-H) in C33H3 |
| 1421 | 31 | 4.9 | 5.82 | 1398 | 1423 | 1400 | 1409 s | 60%, ν(C9-O) + 30%, δ(CC, C-H) |
| 1443 | 3 | 1.8 | 2.17 | 1419 | 1444 | 1420 | 60%, δ(C11-H) +25%, δ(C11=N) | |
| 1473 | 12 | 2.5 | 3.23 | 1424 | 1473 | 1424 | 1432 w | 60%,19b ν(C=C, C-H) +25%, δ(C11-H) |
| 1495 | 13 | 2.2 | 2.84 | 1444 | 1496 | 1445 | 1457 s | 68%,19b ν(C=C, C-H) + 25%, δas(C-H) |
| 1506 | 1 | 1.1 | 1.42 | 1455 | 1509 | 1458 | 92%, δas(C-H) in C29H3 | |
| 1514 | 3 | 1.0 | 1.42 | 1462 | 1513 | 1461 | 88%, δas(C-H) in C33H3 | |
| 1519 | 1 | 1.0 | 1.42 | 1467 | 1517 | 1465 | 85%, δas(C-H) in C29H3 | |
| 1554 | 16 | 3.3 | 4.73 | 1500 | 1554 | 1500 | 1508 w | 85%, 19a ν(C=C) + δ(C-H) in benzene |
| 1587 | 15 | 5.7 | 8.51 | 1531 | 1587 | 1531 | 1542 s | 85%, 19a ν(C=C) |
| 1654 | 1 | 6.1 | 9.77 | 1594 | 1654 | 1594 | 80%, 8b ν(C=C) in benzene rings | |
| 1670 | 4 | 6.0 | 9.85 | 1609 | 1670 | 1609 | 85%, 8b ν(C=C) in benzene rings | |
| 1680 | 100 | 6.6 | 10.91 | 1619 | 1681 | 1620 | 1622 vs | 70%, ν(C11=N) + 20% ν(C-C) in ring I |
| 1797 | 90 | 12.5 | 23.74 | 1729 | 1797 | 1729 | 90%, ν(C21=O) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onami, Y.; Koya, R.; Kawasaki, T.; Aizawa, H.; Nakagame, R.; Miyagawa, Y.; Haraguchi, T.; Akitsu, T.; Tsukiyama, K.; Palafox, M.A. Investigation by DFT Methods of the Damage of Human Serum Albumin Including Amino Acid Derivative Schiff Base Zn(II) Complexes by IR-FEL Irradiation. Int. J. Mol. Sci. 2019, 20, 2846. https://doi.org/10.3390/ijms20112846
Onami Y, Koya R, Kawasaki T, Aizawa H, Nakagame R, Miyagawa Y, Haraguchi T, Akitsu T, Tsukiyama K, Palafox MA. Investigation by DFT Methods of the Damage of Human Serum Albumin Including Amino Acid Derivative Schiff Base Zn(II) Complexes by IR-FEL Irradiation. International Journal of Molecular Sciences. 2019; 20(11):2846. https://doi.org/10.3390/ijms20112846
Chicago/Turabian StyleOnami, Yuika, Ryousuke Koya, Takayasu Kawasaki, Hiroki Aizawa, Ryo Nakagame, Yoshito Miyagawa, Tomoyuki Haraguchi, Takashiro Akitsu, Koichi Tsukiyama, and Mauricio A. Palafox. 2019. "Investigation by DFT Methods of the Damage of Human Serum Albumin Including Amino Acid Derivative Schiff Base Zn(II) Complexes by IR-FEL Irradiation" International Journal of Molecular Sciences 20, no. 11: 2846. https://doi.org/10.3390/ijms20112846
APA StyleOnami, Y., Koya, R., Kawasaki, T., Aizawa, H., Nakagame, R., Miyagawa, Y., Haraguchi, T., Akitsu, T., Tsukiyama, K., & Palafox, M. A. (2019). Investigation by DFT Methods of the Damage of Human Serum Albumin Including Amino Acid Derivative Schiff Base Zn(II) Complexes by IR-FEL Irradiation. International Journal of Molecular Sciences, 20(11), 2846. https://doi.org/10.3390/ijms20112846