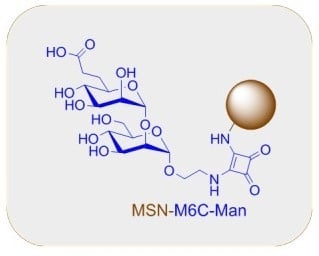
Efficient Photodynamic Therapy of Prostate Cancer Cells through an Improved Targeting of the Cation-Independent Mannose 6-Phosphate Receptor
Abstract
1. Introduction
2. Results and Discussion
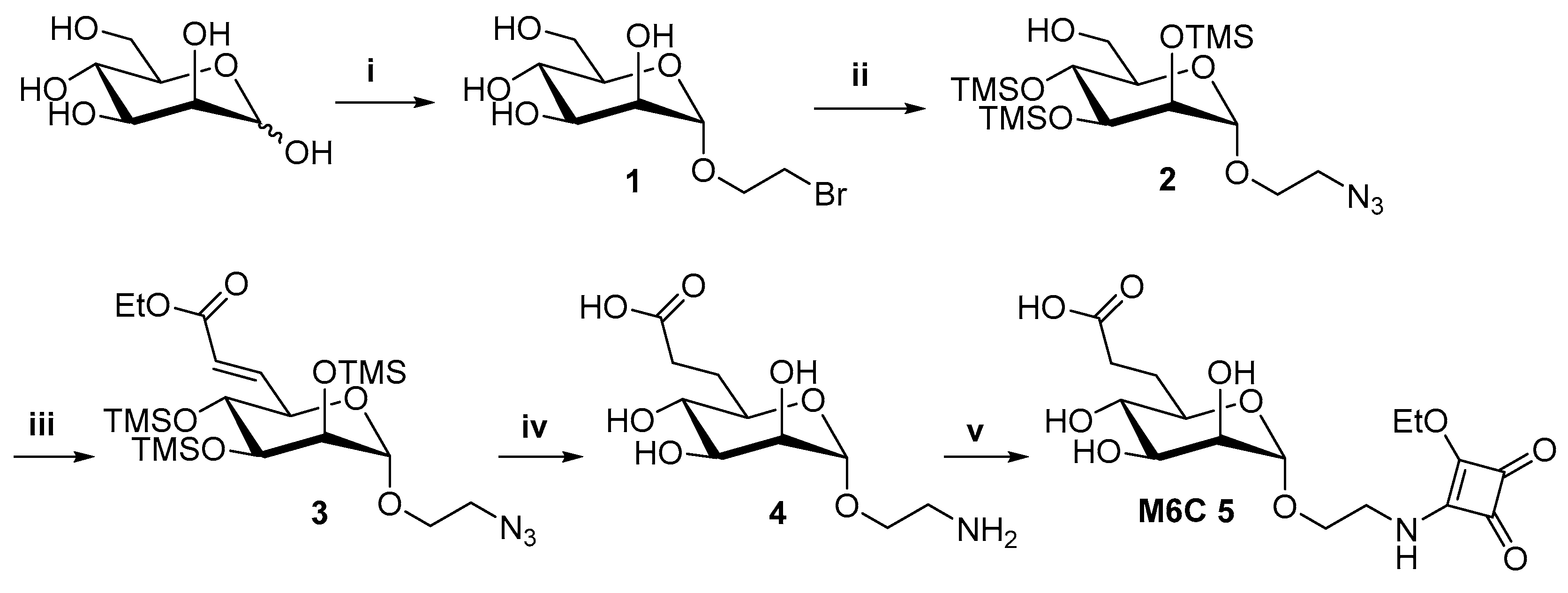
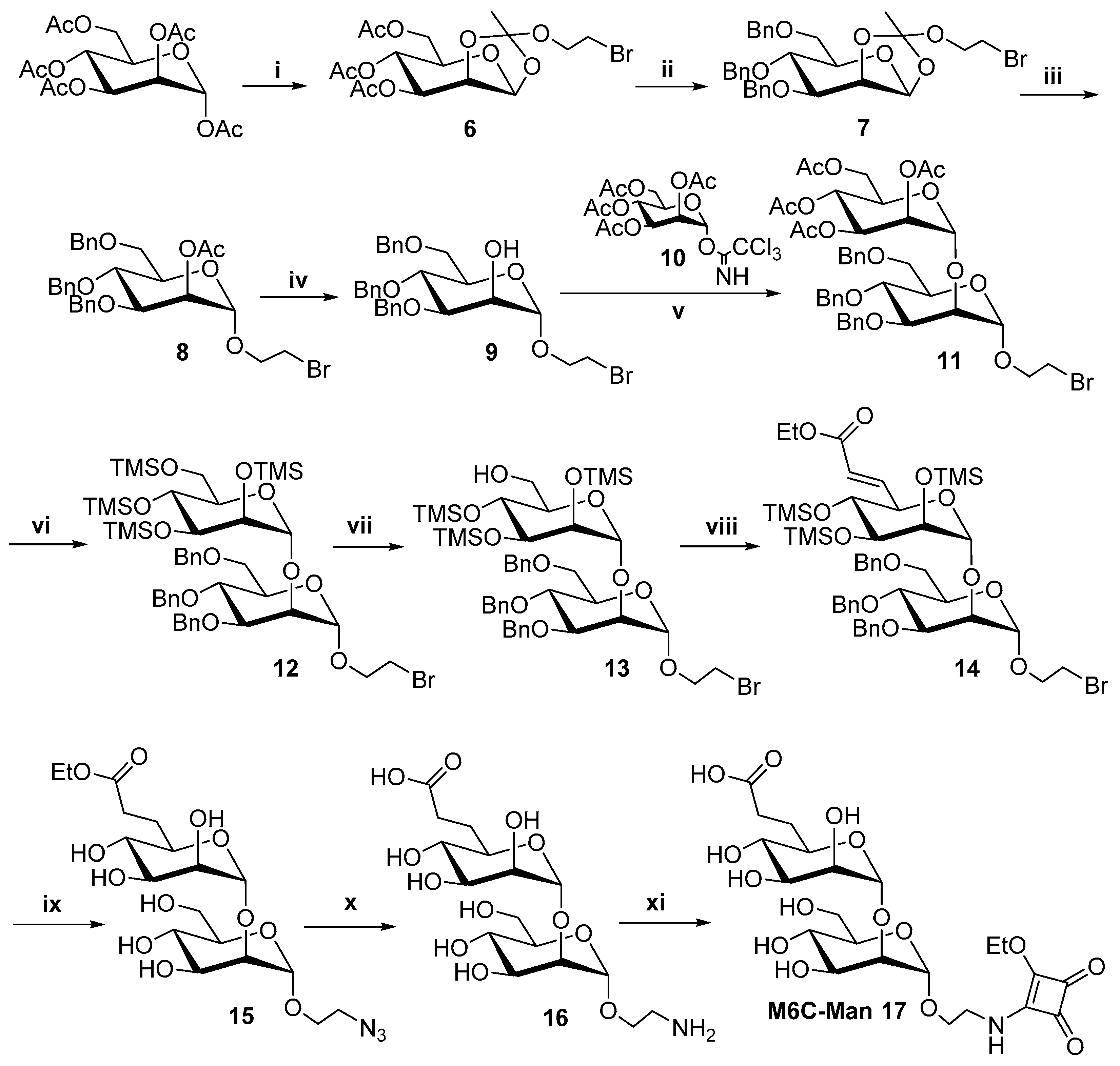
2.1. Synthesis of the M6C 5 and M6C-Man 17 Saccharidic Ligands
2.2. Affinity for CI-M6PR
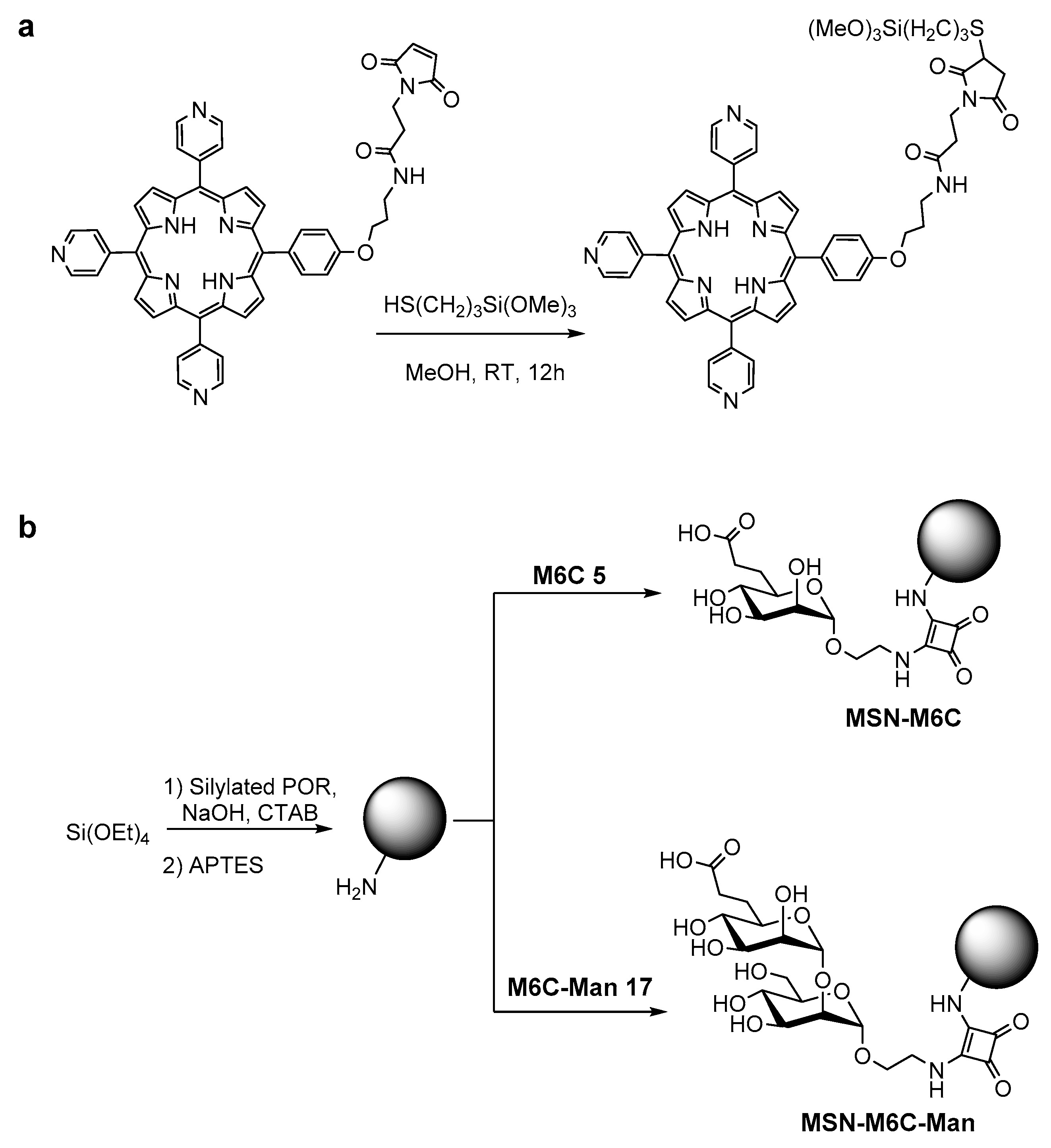
2.3. Synthesis of Mesoporous Silica Nanoparticles
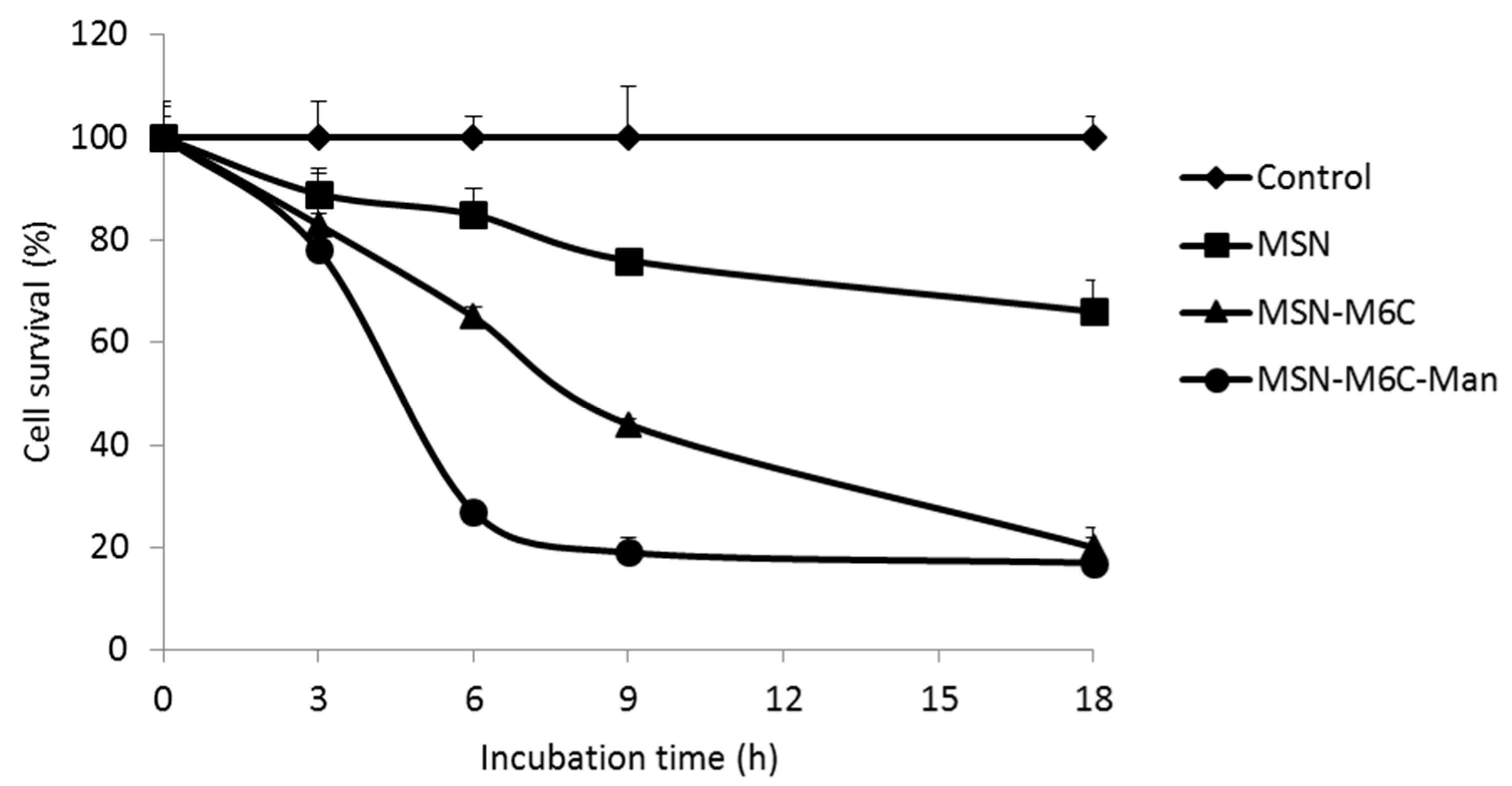
2.4. In Vitro PDT Experiments
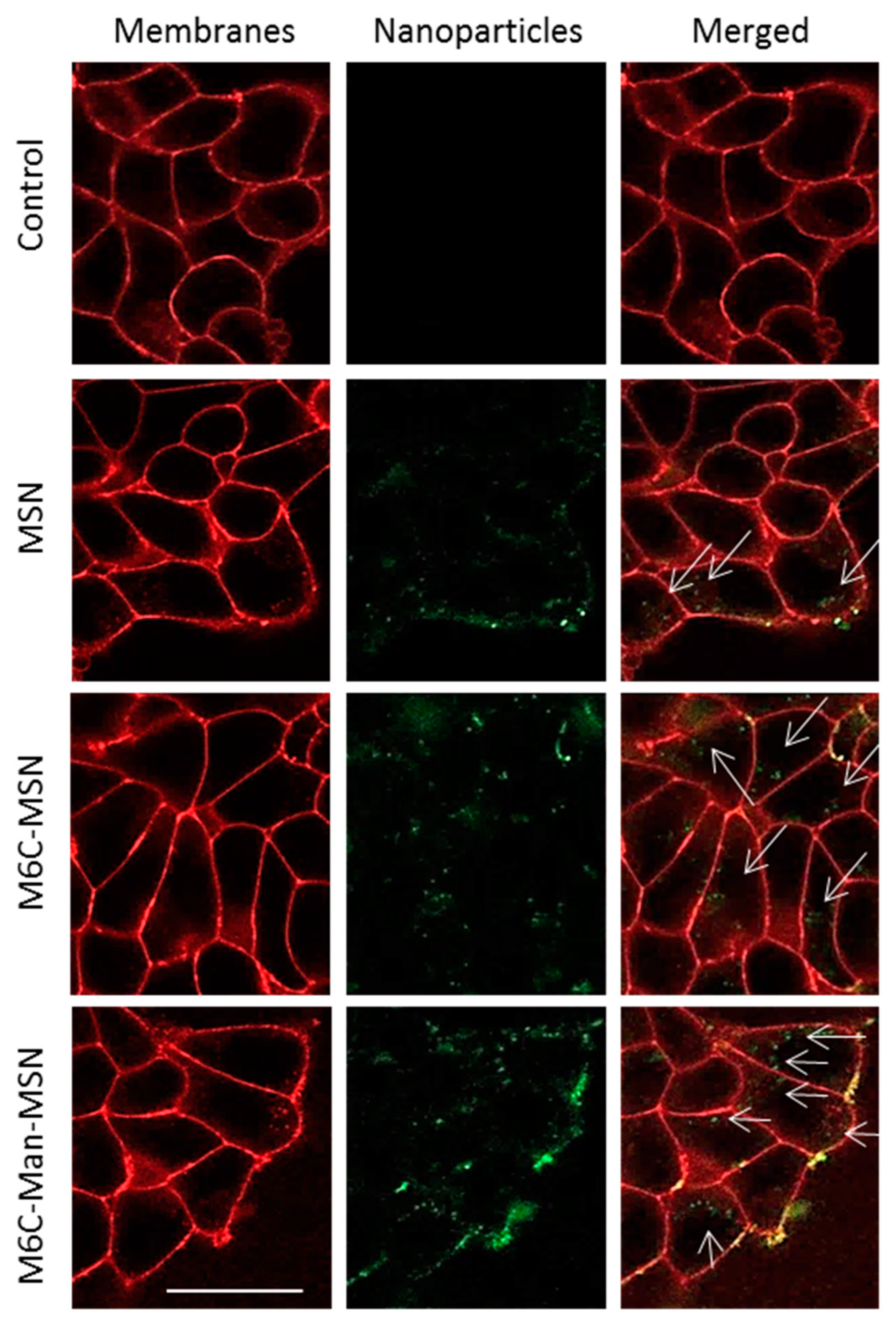
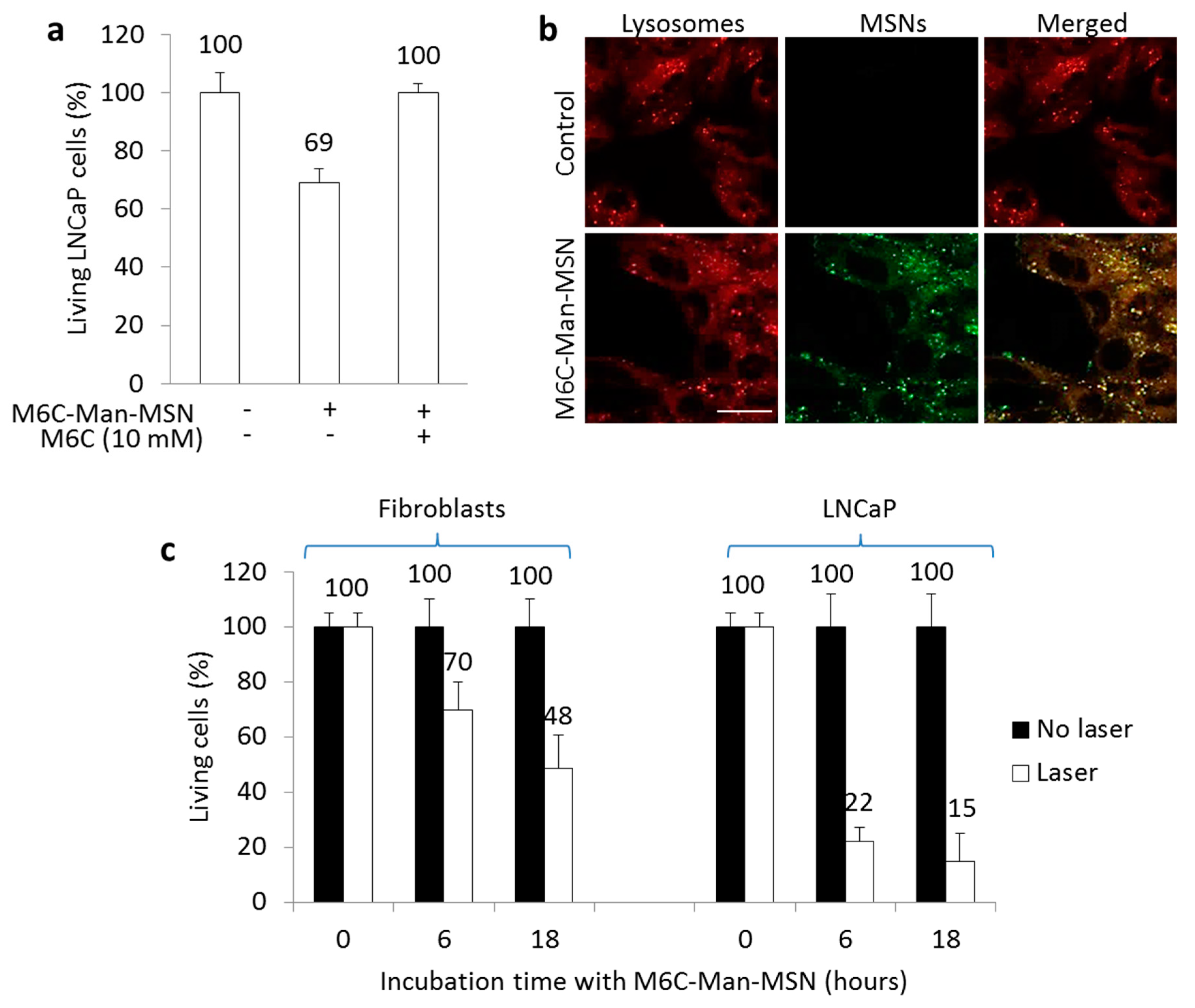
2.5. Endocytosis Mediated by CI-M6PR
3. Materials and Methods
3.1. In Vitro Photodynamic Therapy (PDT) Experimental Settings
3.2. In Vitro Fluorescence Imaging Experimental Settings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MSN | Mesoporous Silica Nanoparticles |
| PDT | Photodynamic Therapy |
| CI-M6PR | Cation Independent Mannose 6-Phosphate Receptor |
| M6C | Mannose 6-Carboxylate |
| M6P | Mannose 6-Phosphate |
| Man | Mannose |
References
- Arora, A.; Scholar, E.M. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.M.; Veeramani, S.; Weiner, G.J. Complement in monoclonal antibody therapy of cancer. Immunol. Res. 2014, 59, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.M.; Bernardes, N.; Fialho, A.M. Bacterial proteins and peptides in cancer therapy: Today and tomorrow. Bioengineered 2014, 5, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv. Drug Deliv. Rev. 2013, 65, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Bojarova, P.; Kren, V. Sugared biomaterial binding lectins: Achievements and perspectives. Biomater. Sci. 2016, 4, 1142–1160. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Han, H.S.; Kim, K.; Park, J.H.; Lee, S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.K.; Li, B.Y.; Lin, C.C. Advances in multifunctional glycosylated nanomaterials: Preparation and applications in glycoscience. Carbohydr. Res. 2015, 405, 2–12. [Google Scholar] [CrossRef]
- Yilmaz, G.; Becer, C.R. Glyconanoparticles and their interactions with lectins. Polym. Chem. 2015, 6, 5503–5514. [Google Scholar] [CrossRef]
- Liu, K.G.; Jiang, X.H.; Hunziker, P. Carbohydrate-based amphiphilic nano delivery systems for cancer therapy. Nanoscale 2016, 8, 16091–16156. [Google Scholar] [CrossRef]
- Marradi, M.; Chiodo, F.; Garcia, I.; Penades, S. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. 2013, 42, 4728–4745. [Google Scholar] [CrossRef]
- Vicent, M.J.; Duncan, R. Polymer conjugates: Nanosized medicines for treating cancer. Trends Biotechnol. 2006, 24, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ramstrom, O.; Yan, M. Glyconanomaterials: Synthesis, characterization, and ligand presentation. Adv. Mater. 2010, 22, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.C.; Martin-Lomas, M.; Penades, S. Glyconanotechnology. Chem. Soc. Rev. 2013, 42, 4358–4376. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, O.; El Cheikh, K.; Warther, D.; Brevet, D.; Maynadier, M.; Bouffard, E.; Salgues, F.; Jeanjean, A.; Puche, P.; Mazerolles, C.; et al. Mannose-6-phosphate receptor: A target for theranostics of prostate cancer. Angew. Chem. Int. Ed. Engl. 2015, 54, 5952–5956. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, S. Structure and function of the mannose 6-phosphate/insulin-like growth factor II receptors. Annu. Rev. Biochem. 1992, 61, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Garcia, M.; Montero, J.L.; Morere, A. Synthesis and biological evaluation of new mannose 6-phosphate analogues. Bioorg. Med. Chem. 2002, 10, 4051–4056. [Google Scholar] [CrossRef]
- Jeanjean, A.; Garcia, M.; Leydet, A.; Montero, J.L.; Morere, A. Synthesis and receptor binding affinity of carboxylate analogues of the mannose 6-phosphate recognition marker. Bioorg. Med. Chem. 2006, 14, 3575–3582. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, D.B.; Maiti, G.; Charette, B.D.; Dreis, C.D.; MacDonald, R.G. Mono- and bivalent ligands bearing mannose 6-phosphate (M6P) surrogates: Targeting the M6P/insulin-like growth factor II receptor. Org. Lett. 2004, 6, 4921–4924. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Vidil, C.; Morere, A.; Garcia, M.; Montero, J.L. Synthesis and biological evaluation of two mannose 6-phosphate analogs. Eur. J. Org. Chem. 2000, 3433–3437. [Google Scholar] [CrossRef]
- Jeanjean, A.; Gary-Bobo, M.; Nirde, P.; Leiris, S.; Garcia, M.; Morere, A. Synthesis of new sulfonate and phosphonate derivatives for cation-independent mannose 6-phosphate receptor targeting. Bioorg. Med. Chem. Lett. 2008, 18, 6240–6243. [Google Scholar] [CrossRef]
- Varki, A.; Kornfeld, S. Structural studies of phosphorylated high mannose-type oligosaccharides. J. Biol. Chem. 1980, 255, 10847–10858. [Google Scholar] [PubMed]
- Distler, J.J.; Guo, J.F.; Jourdian, G.W.; Srivastava, O.P.; Hindsgaul, O. The binding specificity of high and low molecular weight phosphomannosyl receptors from bovine testes. Inhibition studies with chemically synthesized 6-O-phosphorylated oligomannosides. J. Biol. Chem. 1991, 266, 21687–21692. [Google Scholar] [PubMed]
- Brevet, D.; Gary-Bobo, M.; Raehm, L.; Richeter, S.; Hocine, O.; Amro, K.; Loock, B.; Couleaud, P.; Frochot, C.; Morere, A.; et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem. Commun. 2009, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Hocine, O.; Gary-Bobo, M.; Brevet, D.; Maynadier, M.; Fontanel, S.; Raehm, L.; Richeter, S.; Loock, B.; Couleaud, P.; Frochot, C.; et al. Silicalites and mesoporous silica nanoparticles for photodynamic therapy. Int. J. Pharm. 2010, 402, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Vaillant, O.; Maynadier, M.; Basile, I.; Gallud, A.; El Cheikh, K.; Bouffard, E.; Morere, A.; Rebillard, X.; Puche, P.; et al. Targeting multiplicity: The key factor for anti-cancer nanoparticles. Curr. Med. Chem. 2013, 20, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouffard, E.; Mauriello Jimenez, C.; El Cheikh, K.; Maynadier, M.; Basile, I.; Raehm, L.; Nguyen, C.; Gary-Bobo, M.; Garcia, M.; Durand, J.-O.; et al. Efficient Photodynamic Therapy of Prostate Cancer Cells through an Improved Targeting of the Cation-Independent Mannose 6-Phosphate Receptor. Int. J. Mol. Sci. 2019, 20, 2809. https://doi.org/10.3390/ijms20112809
Bouffard E, Mauriello Jimenez C, El Cheikh K, Maynadier M, Basile I, Raehm L, Nguyen C, Gary-Bobo M, Garcia M, Durand J-O, et al. Efficient Photodynamic Therapy of Prostate Cancer Cells through an Improved Targeting of the Cation-Independent Mannose 6-Phosphate Receptor. International Journal of Molecular Sciences. 2019; 20(11):2809. https://doi.org/10.3390/ijms20112809
Chicago/Turabian StyleBouffard, Elise, Chiara Mauriello Jimenez, Khaled El Cheikh, Marie Maynadier, Ilaria Basile, Laurence Raehm, Christophe Nguyen, Magali Gary-Bobo, Marcel Garcia, Jean-Olivier Durand, and et al. 2019. "Efficient Photodynamic Therapy of Prostate Cancer Cells through an Improved Targeting of the Cation-Independent Mannose 6-Phosphate Receptor" International Journal of Molecular Sciences 20, no. 11: 2809. https://doi.org/10.3390/ijms20112809
APA StyleBouffard, E., Mauriello Jimenez, C., El Cheikh, K., Maynadier, M., Basile, I., Raehm, L., Nguyen, C., Gary-Bobo, M., Garcia, M., Durand, J.-O., & Morère, A. (2019). Efficient Photodynamic Therapy of Prostate Cancer Cells through an Improved Targeting of the Cation-Independent Mannose 6-Phosphate Receptor. International Journal of Molecular Sciences, 20(11), 2809. https://doi.org/10.3390/ijms20112809