New Materials Based on Cationic Porphyrins Conjugated to Chitosan or Titanium Dioxide: Synthesis, Characterization and Antimicrobial Efficacy
Abstract
1. Introduction
2. Results and Discussion
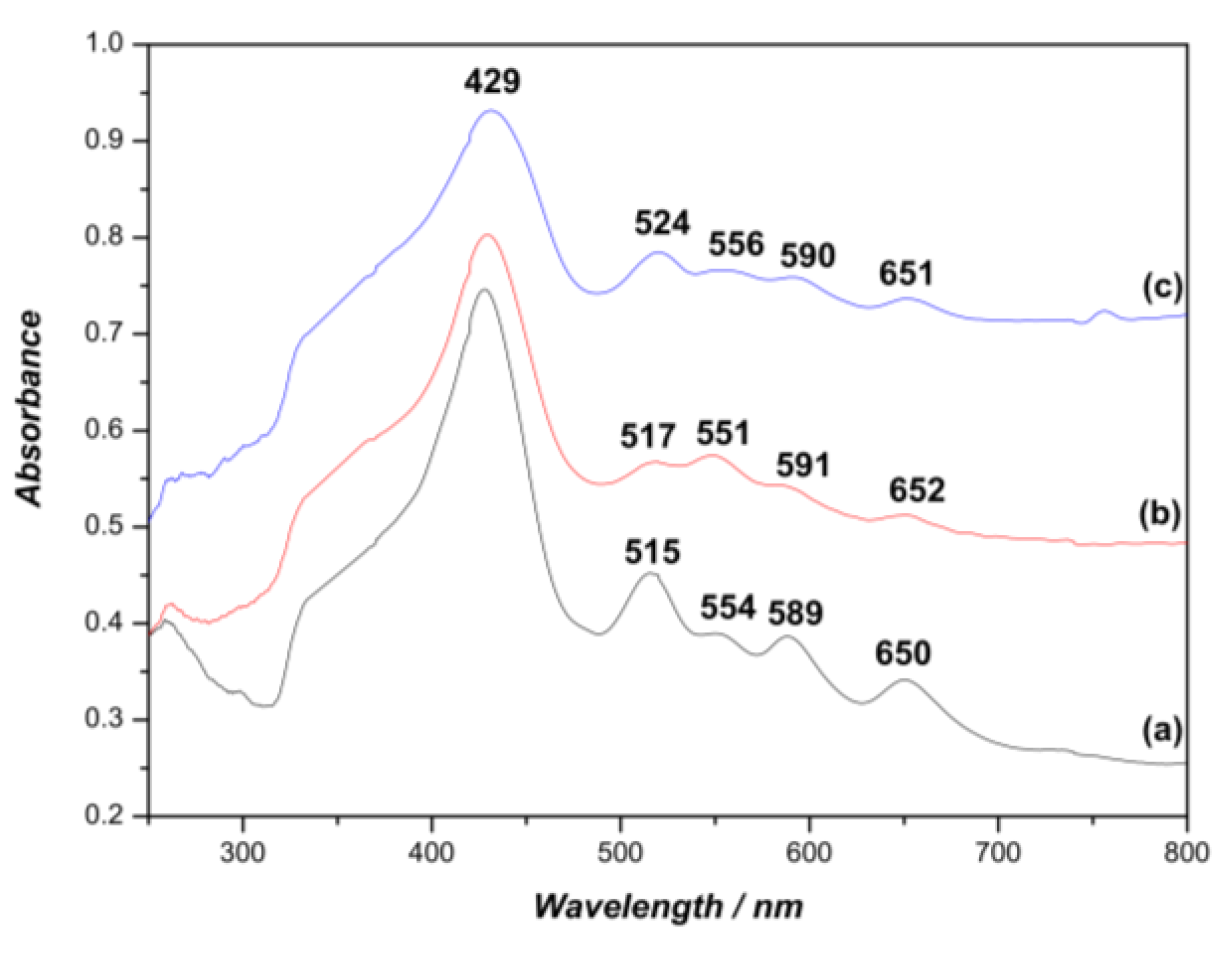
2.1. Synthesis of Porphyrin Derivatives
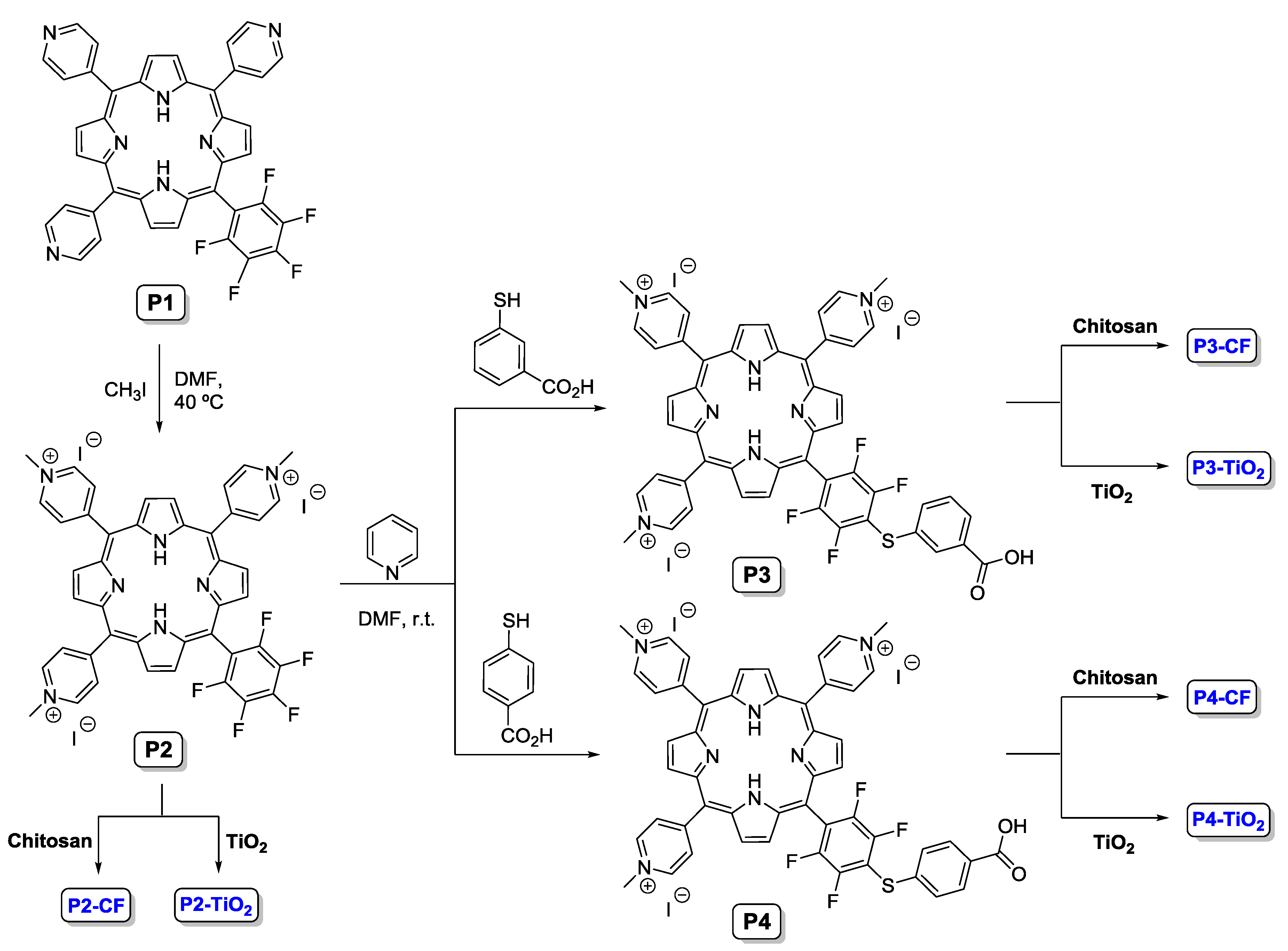
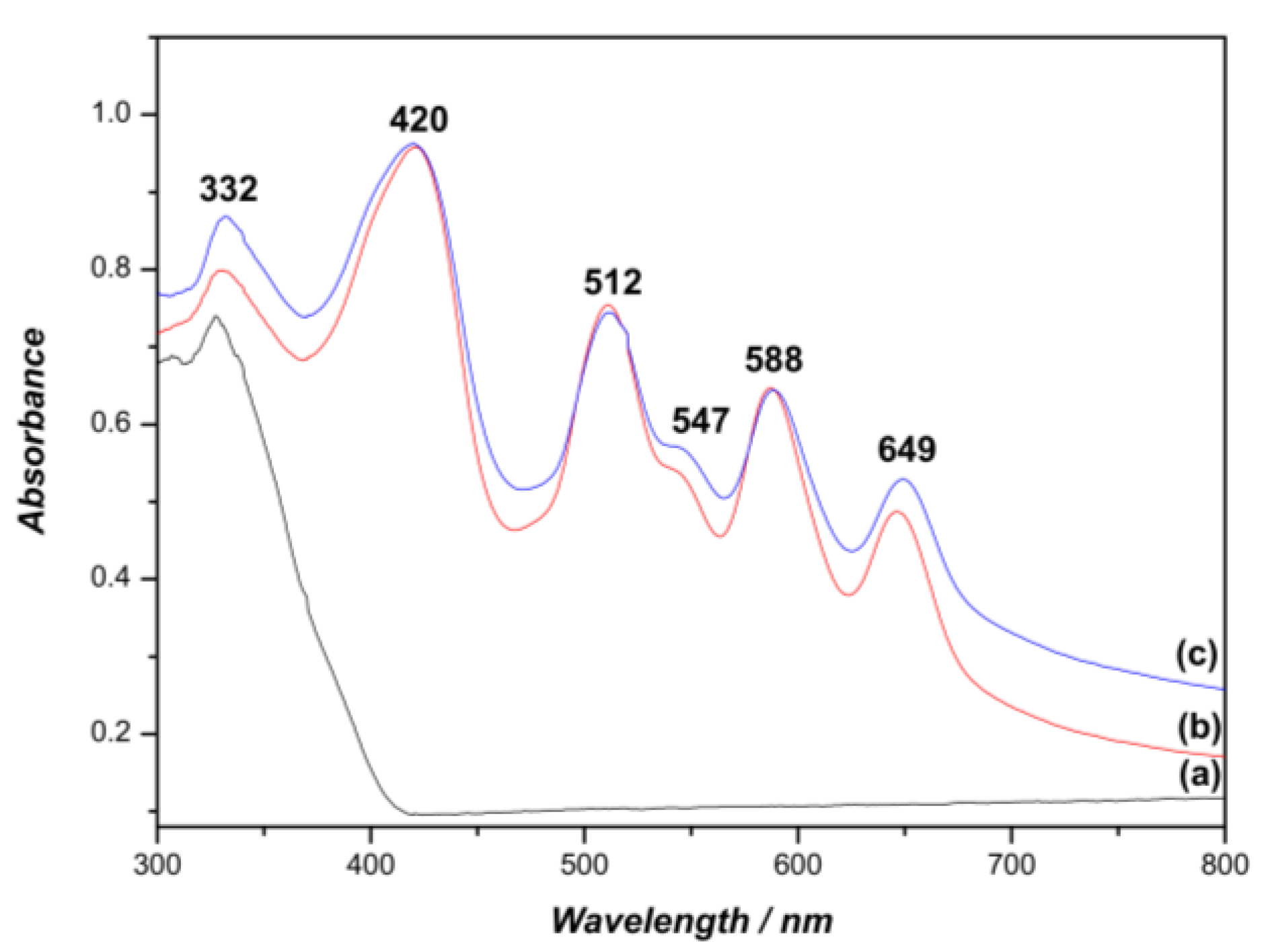
2.2. Immobilization of Porphyrins on Chitosan
2.3. Immobilization of Porphyrins on Titanium Dioxide
2.4. Singlet Oxygen Generation and Photostability
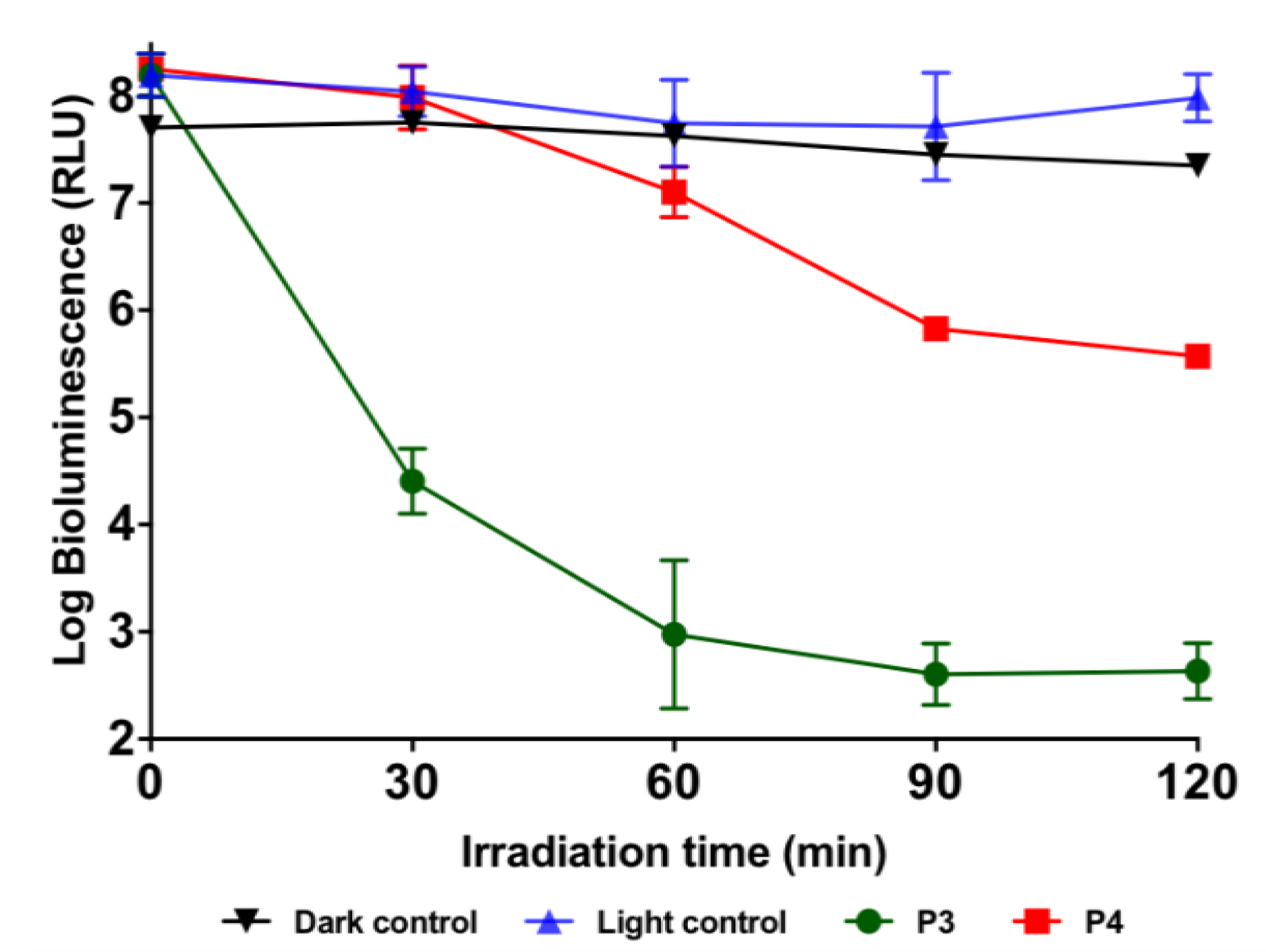
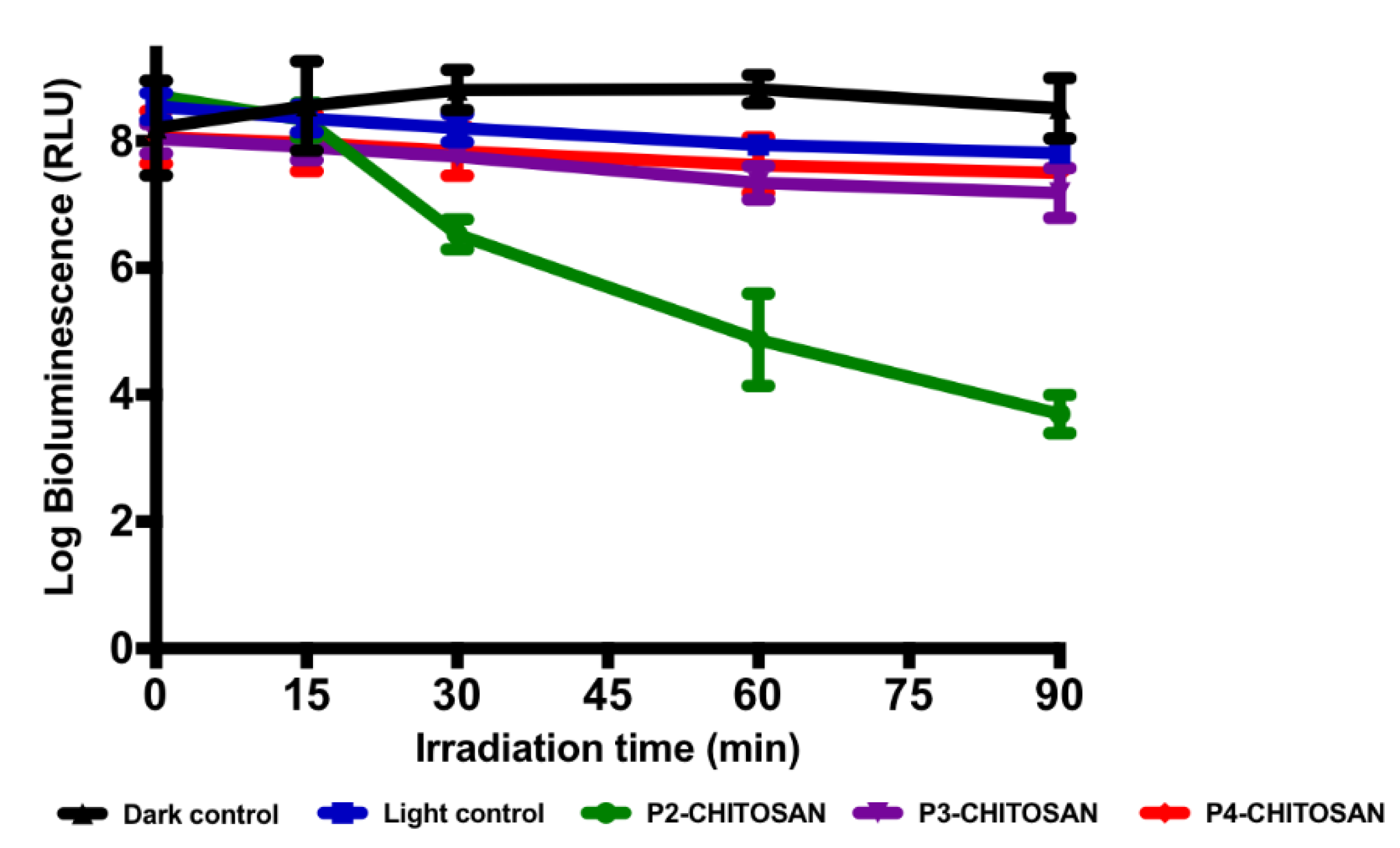
2.5. Photoinactivation Studies
3. Materials and Methods
3.1. Synthesis of Porphyrin Derivatives
3.2. Immobilization of Porphyrins on Chitosan
3.3. Immobilization of Porphyrins on Titanium Dioxide
3.4. Singlet Oxygen Generation and Photostability
3.5. Bacterial Strain and Culture Growth Conditions
3.6. Irradiation Conditions for Photosensitization
3.7. Photosensitization Procedure
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Huang, L.; El-Hussein, A.; Xuan, W.; Hamblin, M.R. Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J. Photochem. Photobiol. B 2018, 178, 277–286. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Gois, M.M.; Kurachi, C.; Santana, E.J.; Mima, E.G.; Spolidorio, D.M.; Pelino, J.E.; Salvador Bagnato, V. Susceptibility of Staphylococcus aureus to porphyrin-mediated photodynamic antimicrobial chemotherapy: an in vitro study. Lasers Med. Sci. 2010, 25, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Mamone, L.; Ferreyra, D.D.; Gandara, L.; Di Venosa, G.; Vallecorsa, P.; Saenz, D.; Calvo, G.; Batlle, A.; Buzzola, F.; Durantini, E.N.; Casas, A. Photodynamic inactivation of planktonic and biofilm growing bacteria mediated by a meso-substituted porphyrin bearing four basic amino groups. J. Photochem. Photobiol. B 2016, 161, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Almeida, A.; Carvalho, C.M.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â. Porphyrin derivatives as photosensitizers for the inactivation of Bacillus cereus endospores. J. Appl. Microbiol. 2009, 106, 1986–1995. [Google Scholar] [CrossRef]
- Rossi, G.; Goi, D.; Comuzzi, C. The photodynamic inactivation of Staphylococcus aureus in water using visible light with a new expanded porphyrin. J. Water Health 2012, 10, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Xu, Q.; Tang, H.; Liu, L.; Wang, S. Conjugated polymer/porphyrin complexes for efficient energy transfer and improving light-activated antibacterial activity. J. Am. Chem. Soc. 2009, 131, 13117–13124. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Cao, X.; Yang, F.; Wang, J.; Zhou, X.; Zhang, X.L. Synthesis and antibacterial study of 10, 15, 20-triphenyl-5-(4-hydroxy-3-(trimethylammonium)methyl)phenylporphyrin as models for combination of porphyrin and alkylating agent. Bioorg. Med. Chem. Lett. 2003, 13, 1097–1100. [Google Scholar] [CrossRef]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, Â. Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Dias, C.J.; Gamelas, S.; Fardilha, M.; Neves, M.G.P.M.S.; Faustino, M.A.F. An insight on the role of photosensitizer nanocarriers for Photodynamic Therapy. An. Acad. Bras. Cienc. 2018, 90, 1101–1130. [Google Scholar] [CrossRef]
- Cardote, T.A.F.; Barata, J.F.B.; Amador, C.; Alves, E.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A.; Faustino, M.A.F. Evaluation of meso-substituted cationic corroles as potential antibacterial agents. An. Acad. Bras. Cienc. 2018, 90, 1175–1185. [Google Scholar] [CrossRef]
- Gomes, M.C.; Silva, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A.; Cavaleiro, J.A.S.; Tomé, J.P.C.; Cunha, Â. Cationic galactoporphyrin photosensitisers against UV-B resistant bacteria: oxidation of lipids and proteins by 1O2. Photochem. Photobiol. Sci. 2013, 12, 262–271. [Google Scholar] [CrossRef]
- Costa, D.C.; Gomes, M.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Cavaleiro, J.A.; Almeida, A.; Tomé, J.P.C. Comparative photodynamic inactivation of antibiotic resistant bacteria by first and second generation cationic photosensitizers. Photochem. Photobiol. Sci. 2012, 11, 1905–1913. [Google Scholar] [CrossRef]
- Spesia, M.B.; Lazzeri, D.; Pascual, L.; Rovera, M.; Durantini, E.N. Photoinactivation of Escherichia coli using porphyrin derivatives with different number of cationic charges. FEMS Immunol. Med. Microbiol. 2005, 44, 289–295. [Google Scholar] [CrossRef]
- Carvalho, C.M.B.; Gomes, A.T.P.C.; Fernandes, S.C.; Prata, A.C.C.; Almeida, M.A.; Cunha, M.A.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Lin, Z.; Rainho, J.P.; Rocha, J. Photoinactivation of bacteria in wastewater by porphyrins: Bacterial β-galactosidase activity and leucine-uptake as methods to monitor the process. J. Photochem. Photobiol. B 2007, 88, 112–118. [Google Scholar] [CrossRef]
- Merchat, M.; Bertolini, G.; Giacomini, P.; Villanueva, A.; Jori, G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Pereira, M.A.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Influence of external bacterial structures on the efficiency of photodynamic inactivation by a cationic porphyrin. Photochem. Photobiol. Sci. 2014, 13, 680–690. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A.; Hackbarth, S.; Roder, B.; Faustino, M.A.F. Photodynamic inactivation of bioluminescent Escherichia coli by neutral and cationic pyrrolidine-fused chlorins and isobacteriochlorins. Bioorg. Med. Chem. Lett. 2014, 24, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Alves, E.; Costa, L.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Cunha, Â.; Lin, Z.; Rocha, J. Functional cationic nanomagnet-porphyrin hybrids for the photoinactivation of microorganisms. ACS Nano 2010, 4, 7133–7140. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Rodrigues, J.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Lin, Z.; Cunha, Â.; Nadais, M.H.; Tomé, J.P.C.; Almeida, A. A new insight on nanomagnet-porphyrin hybrids for photodynamic inactivation of microorganisms. Dyes Pigments 2014, 110, 80–88. [Google Scholar] [CrossRef]
- Almeida, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef]
- Coppellotti, O.; Fabris, C.; Soncin, M.; Magaraggia, M.; Camerin, M.; Jori, G.; Guidolin, L. Porphyrin photosensitised processes in the prevention and treatment of water- and vector-borne diseases. Curr. Med. Chem. 2012, 19, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Costa, L.; Alves, E.; Oliveira, A.; Cunha, A.; Almeida, A. Antimicrobial photodynamic activity of porphyrin derivatives: potential application on medical and water disinfection. J. Porphyrins Phthalocyanines 2009, 13, 574–577. [Google Scholar] [CrossRef]
- Rahimi, R.; Zargari, S.; Yousefi, A.; Berijani, M.Y.; Ghaffarinejad, A.; Morsali, A. Visible light photocatalytic disinfection of E. coli with TiO2-graphene nanocomposite sensitized with tetrakis(4-carboxyphenyl)porphyrin. Appl. Surf. Sci. 2015, 355, 1098–1106. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Moura, N.M.M.; Fernandes, A.; Faustino, M.A.F.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Nakagaki, S.; Almeida, A.; Cunha, A.; Silvestre, A.J.D.; Freire, C.S.R.; Pinto, R.J.B.; Neves, M.G.P.M.S. Control of Listeria innocua biofilms by biocompatible photodynamic antifouling chitosan based materials. Dyes Pigments 2017, 137, 265–276. [Google Scholar] [CrossRef]
- Jiang, L.; Gan, C.R.; Gao, J.; Loh, X.J. A Perspective on the Trends and Challenges Facing Porphyrin-Based Anti-Microbial Materials. Small 2016, 12, 3609–3644. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Lukseviciute, V.; Marsalka, A.; Reklaitis, I.; Luksiene, Z. Effective photosensitization-based inactivation of Gram (-) food pathogens and molds using the chlorophyllin-chitosan complex: towards photoactive edible coatings to preserve strawberries. Photochem. Photobiol. Sci. 2016, 15, 506–516. [Google Scholar] [CrossRef]
- Perni, S.; Prokopovich, P.; Parkin, I.P.; Wilson, M.; Pratten, J. Prevention of biofilm accumulation on a light-activated antimicrobial catheter material. J. Mat. Chem. 2010, 20, 8668–8673. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Characterization of a Conjugate between Rose Bengal and Chitosan for Targeted Antibiofilm and Tissue Stabilization Effects as a Potential Treatment of Infected Dentin. Antimicrob. Agents Ch. 2012, 56, 4876–4884. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Santos, A.; Mesquita, M.Q.; Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Advances in aPDT based on the combination of a porphyrinic formulation with potassium iodide: Effectiveness on bacteria and fungi planktonic/biofilm forms and viruses. J. Porphyrins Phthalocyanines 2019, 23, 1–12. [Google Scholar] [CrossRef]
- Cooper, A.; Oldinski, R.; Ma, H.Y.; Bryers, J.D.; Zhang, M.Q. Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohydr. Polym. 2013, 92, 254–259. [Google Scholar] [CrossRef]
- Li, Q.L.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kavitha, B.; Reddy, P.A.K.; Kim, K.H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Yin, R.; Hamblin, M.R. Antimicrobial Photosensitizers: Drug Discovery Under the Spotlight. Curr. Med. Chem. 2015, 22, 2159–2185. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.R.; Martins, V.d.C.a.o.A.; Plepis, A.M.d.G.; Perussi, J.R. Development of Chitosan Membranes Containing Photosensitizer for Water Disinfection. GJSFR-C: Biological Science 2017, 17, 5–12. [Google Scholar]
- Lourenço, L.M.O.; Sousa, A.; Gomes, M.C.; Faustino, M.A.F.; Almeida, A.; Silva, A.M.S.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cunha, Â.; Tomé, J.P.C. Inverted methoxypyridinium phthalocyanines for PDI of pathogenic bacteria. Photochem. Photobiol. Sci. 2015, 14, 1853–1863. [Google Scholar] [CrossRef]
- Pereira, J.B.; Carvalho, E.F.; Faustino, M.A.F.; Fernandes, R.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Gomes, N.C.M.; Cunha, Â.; Almeida, A.; Tomé, J.P.C. Phthalocyanine thio-pyridinium derivatives as antibacterial photosensitizers. Photochem. Photobiol. 2012, 88, 537–547. [Google Scholar] [CrossRef]
- Silva, E.M.P.; Giuntini, F.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.; Santana-Marques, M.G.; Ferrer-Correia, A.J.; Cavaleiro, J.A.S.; et al. Synthesis of cationic β-vinyl substituted meso-tetraphenylporphyrins and their in vitro activity against herpes simplex virus type 1. Bioorg. Med. Chem. Lett. 2005, 15, 3333–3337. [Google Scholar] [CrossRef]
- Gomes, M.C.; Woranovicz-Barreira, S.M.; Faustino, M.A.F.; Fernandes, R.; Neves, M.G.P.M.S.; Tomé, A.C.; Gomes, N.C.M.; Almeida, A.; Cavaleiro, J.A.; Cunha, Â.; Tomé, J.P.C. Photodynamic inactivation of Penicillium chrysogenum conidia by cationic porphyrins. Photochem. Photobiol. Sci. 2011, 10, 1735–1743. [Google Scholar] [CrossRef]
- Barata, J.F.; Pinto, R.J.; Vaz Serra, V.I.; Silvestre, A.J.; Trindade, T.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Daina, S.; Sadocco, P.; Freire, C.S. Fluorescent Bioactive Corrole Grafted-Chitosan Films. Biomacromolecules 2016, 17, 1395–1403. [Google Scholar] [CrossRef]
- Alves, E.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Mendo, S.; Almeida, A. Photodynamic inactivation of recombinant bioluminescent Escherichia coli by cationic porphyrins under artificial and solar irradiation. J. Ind. Microbiol. Biotechnol. 2008, 35, 1447–1454. [Google Scholar] [CrossRef]
- Alves, E.; Costa, L.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009, 9, 70. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Photodynamic antimicrobial chemotherapy in aquaculture: photoinactivation studies of Vibrio fischeri. PLoS ONE 2011, 6, e20970. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.; Almeida, A. Nucleic acid changes during photodynamic inactivation of bacteria by cationic porphyrins. Bioorg. Med. Chem. 2013, 21, 4311–4318. [Google Scholar] [CrossRef]
- Alves, E.; Santos, N.; Melo, T.; Maciel, E.; Dória, M.L.; Faustino, M.A.F.; Tomé, J.P.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cunha, A.; Helguero, L.A.; Domingues, P.; Almeida, A.; Domingues, M.R. Photodynamic oxidation of Escherichia coli membrane phospholipids: new insights based on lipidomics. Rapid Commun. Mass Sp. 2013, 27, 2717–2728. [Google Scholar] [CrossRef]
- Alves, E.; Esteves, A.C.; Correia, A.; Cunha, Â.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A. Protein profiles of Escherichia coli and Staphylococcus warneri are altered by photosensitization with cationic porphyrins. Photochem. Photobiol. Sci. 2015, 14, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Dias, S.R.; Carvalho, C.M.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.; Alves, E.; Almeida, A. Mechanisms of photodynamic inactivation of a Gram-negative recombinant bioluminescent bacterium by cationic porphyrins. Photochem. Photobiol. Sci. 2011, 10, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Moura, N.M.M.; Ramos, C.I.V.; Linhares, I.; Santos, S.M.; Faustino, M.A.F.; Almeida, A.; Cavaleiro, J.A.S.; Amado, F.M.L.; Lodeiro, C.; Neves, M.G.P.M.S. Synthesis, characterization and biological evaluation of cationic porphyrin-terpyridine derivatives. RSC Adv. 2016, 6, 110674–110685. [Google Scholar] [CrossRef]
- Caminos, D.A.; Spesia, M.B.; Durantini, E.N. Photodynamic inactivation of Escherichia coli by novel meso-substituted porphyrins by 4-(3-N,N,N-trimethylammoniumpropoxy)phenyl and 4-(trifluoromethyl)phenyl groups. Photochem. Photobiol. Sci. 2006, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Marciel, L.; Mesquita, M.Q.; Ferreira, R.; Moreira, B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An efficient formulation based on cationic porphyrins to photoinactivate Staphylococcus aureus and Escherichia coli. Future Med. Chem. 2018, 10, 1821–1833. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Ribeiro, R.R.; Wypych, F.; Nakagaki, S. Synthesis of new metalloporphyrin derivatives from [5,10,15,20-tetrakis (pentafluorophenyl)porphyrin] and 4-mercaptobenzoic acid for homogeneous and heterogeneous catalysis. Appl. Catal., A 2015, 503, 9–19. [Google Scholar] [CrossRef]
- Costa, J.I.T.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin: a versatile platform to novel porphyrinic materials. J. Porphyrins Phthalocyanines 2011, 15, 1116–1133. [Google Scholar] [CrossRef]
- Simões, C.; Gomes, M.C.; Neves, M.G.P.M.S.; Cunha, Â.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Faustino, M.A.F. Photodynamic inactivation of Escherichia coli with cationic meso-tetraarylporphyrins—The charge number and charge distribution effects. Catal. Today 2016, 266, 197–204. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of Porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Shumway, B.R.; Meldrum, D.R. A New Cross-Linkable Oxygen Sensor Covalently Bonded into Poly(2-hydroxyethyl methacrylate)-co-Polyacrylamide Thin Film for Dissolved Oxygen Sensing. Chem. Mater. 2010, 22, 2069–2078. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Nunez, C.; Santos, S.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Paz, F.A.A.; Neves, M.G.P.M.S.; Capelo, J.L.; Lodeiro, C. A New 3,5-Bisporphyrinylpyridine Derivative as a Fluorescent Ratiometric Probe for Zinc Ions. Chem. Eur. J. 2014, 20, 6684–6692. [Google Scholar] [CrossRef]
- Sharma, R.; Gautam, P.; Mobin, S.M.; Misra, R. β-Substituted ferrocenyl porphyrins: synthesis, structure, and properties. Dalton Trans. 2013, 42, 5539–5545. [Google Scholar] [CrossRef]
- Seybold, P.G.; Gouterman, M. Porphyrins: XIII: Fluorescence spectra and quantum yields. J. Mol. Spectrosc. 1969, 31, 1–13. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Wypych, F.; Nakagaki, S. Glycol metalloporphyrin derivatives in solution or immobilized on LDH and silica: synthesis, characterization and catalytic features in oxidation reactions. Catal. Sci. Technol. 2014, 4, 129–141. [Google Scholar] [CrossRef]
- Musselman, R.L.; Larsen, R.W.; Hoffman, B.M. Electronic spectra of porphyrins in the solid state: Newly observed transitions, collective and structural effects, and protein-mimicking environments. Coord. Chem. Rev. 2013, 257, 369–380. [Google Scholar] [CrossRef]
- Fontana, C.R.; dos Santos, D.S., Jr.; Bosco, J.M.; Spolidorio, D.M.; Chierici Marcantonio, R.A. Evaluation of chitosan gel as antibiotic and photosensitizer delivery. Drug Deliv. 2008, 15, 417–422. [Google Scholar] [CrossRef]
- Ogawa, K. Effect of Heating an Aqueous Suspension of Chitosan on the Crystallinity and Polymorphs. Agric. Biol. Chem. 1991, 55, 2375–2379. [Google Scholar] [CrossRef]
- Ogawa, K.; Yui, T.; Okuyama, K. Three D structures of chitosan. Int. J. Biol. Macromol. 2004, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Dai, Z.F.; Miura, A.; Tamai, N. Different back electron transfer from titanium dioxide nanoparticles to tetra (4-sulfonatophenyl) porphyrin monomer and its J-aggregate. Chem. Phys. Lett. 2001, 334, 257–264. [Google Scholar] [CrossRef]
- Nikolaou, V.; Angaridis, P.A.; Charalambidis, G.; Sharma, G.D.; Coutsolelos, A.G. A “click-chemistry” approach for the synthesis of porphyrin dyads as sensitizers for dye-sensitized solar cells. Dalton Trans. 2015, 44, 1734–1747. [Google Scholar] [CrossRef] [PubMed]
- Zervaki, G.E.; Tsaka, V.; Vatikioti, A.; Georgakaki, I.; Nikolaou, V.; Sharma, G.D.; Coutsolelos, A.G. A triazine di(carboxy)porphyrin dyad versus a triazine di(carboxy)porphyrin triad for sensitizers in DSSCs. Dalton Trans. 2015, 44, 13550–13564. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Segoviano, R.I.; Serratos, I.N.; Rojas-Gonzalez, F.; Tello-Solis, S.R.; Sosa-Fonseca, R.; Medina-Juarez, O.; Menchaca-Campos, C.; Garcia-Sanchez, M.A. On tuning the fluorescence emission of porphyrin free bases bonded to the pore walls of organo-modified silica. Molecules 2014, 19, 2261–2285. [Google Scholar] [CrossRef]
- Zhou, X.T.; Ji, H.B.; Huang, X.J. Photocatalytic degradation of methyl orange over metalloporphyrins supported on TiO2 Degussa P25. Molecules 2012, 17, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Moura, N.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Rakib, E.M.; Hannioui, A.; Mojahidi, S.; Hackbarth, S.; Roder, B.; Paz, F.A.A.; Silva, A.M.S.; Cavaleiro, J.A.S. Novel pyrazoline and pyrazole porphyrin derivatives: synthesis and photophysical properties. Tetrahedron 2012, 68, 8181–8193. [Google Scholar] [CrossRef]
- Mundt, J.M.; Rouse, L.; Van den Bossche, J.; Goodrich, R.P. Chemical and biological mechanisms of pathogen reduction technologies. Photochem. Photobiol. 2014, 90, 957–964. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Tomé, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef]
- Caminos, D.A.; Spesia, M.B.; Pons, P.; Durantini, E.N. Mechanisms of Escherichia coli photodynamic inactivation by an amphiphilic tricationic porphyrin and 5,10,15,20-tetra(4-N,N,N-trimethylammoniumphenyl) porphyrin. Photochem. Photobiol. Sci. 2008, 7, 1071–1078. [Google Scholar] [CrossRef]
- Salmon-Divon, M.; Nitzan, Y.; Malik, Z. Mechanistic aspects of Escherichia coli photodynamic inactivation by cationic tetra-meso(N-methylpyridyl)porphine. Photochem. Photobiol. Sci. 2004, 3, 423–429. [Google Scholar] [CrossRef]
- Alves, E.; Moreirinha, C.; Faustino, M.A.F.; Cunha, Â.; Delgadillo, I.; Neves, M.G.P.M.S.; Almeida, A. Overall biochemical changes in bacteria photosensitized with cationic porphyrins monitored by infrared spectroscopy. Future Med. Chem. 2016, 8, 613–628. [Google Scholar] [CrossRef]
- Gsponer, N.S.; Spesia, M.B.; Durantini, E.N. Effects of divalent cations, EDTA and chitosan on the uptake and photoinactivation of Escherichia coli mediated by cationic and anionic porphyrins. Photodiagnosis Photodyn. 2015, 12, 67–75. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E-coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; Gazzali, A.M. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Mitoraj, D.; Janczyk, A.; Strus, M.; Kisch, H.; Stochel, G.; Heczko, P.B.; Macyk, W. Visible light inactivation of bacteria and fungi by modified titanium dioxide. Photochem. Photobiol. Sci. 2007, 6, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Moura, N.M.M.; Cuerva, C.; Cavaleiro, J.A.S.; Mendes, R.F.; Paz, F.A.A.; Cano, M.; Neves, M.G.P.M.S.; Lodeiro, C. Metallomesogens with Luminescent Behaviour: Palladium Complexes Derived from Alkylamide Tetraarylporphyrins. ChemPlusChem 2016, 81, 262–273. [Google Scholar] [CrossRef]


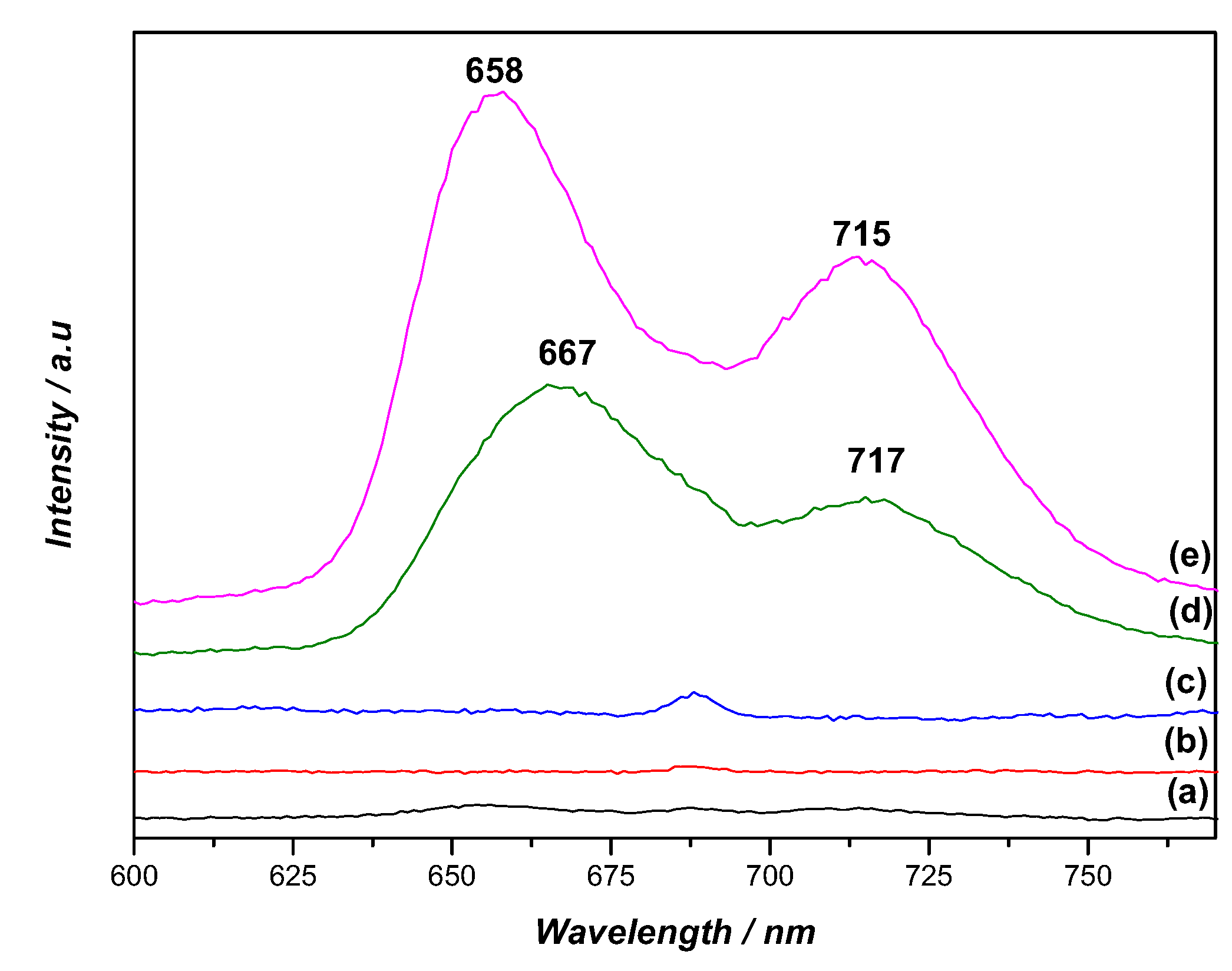
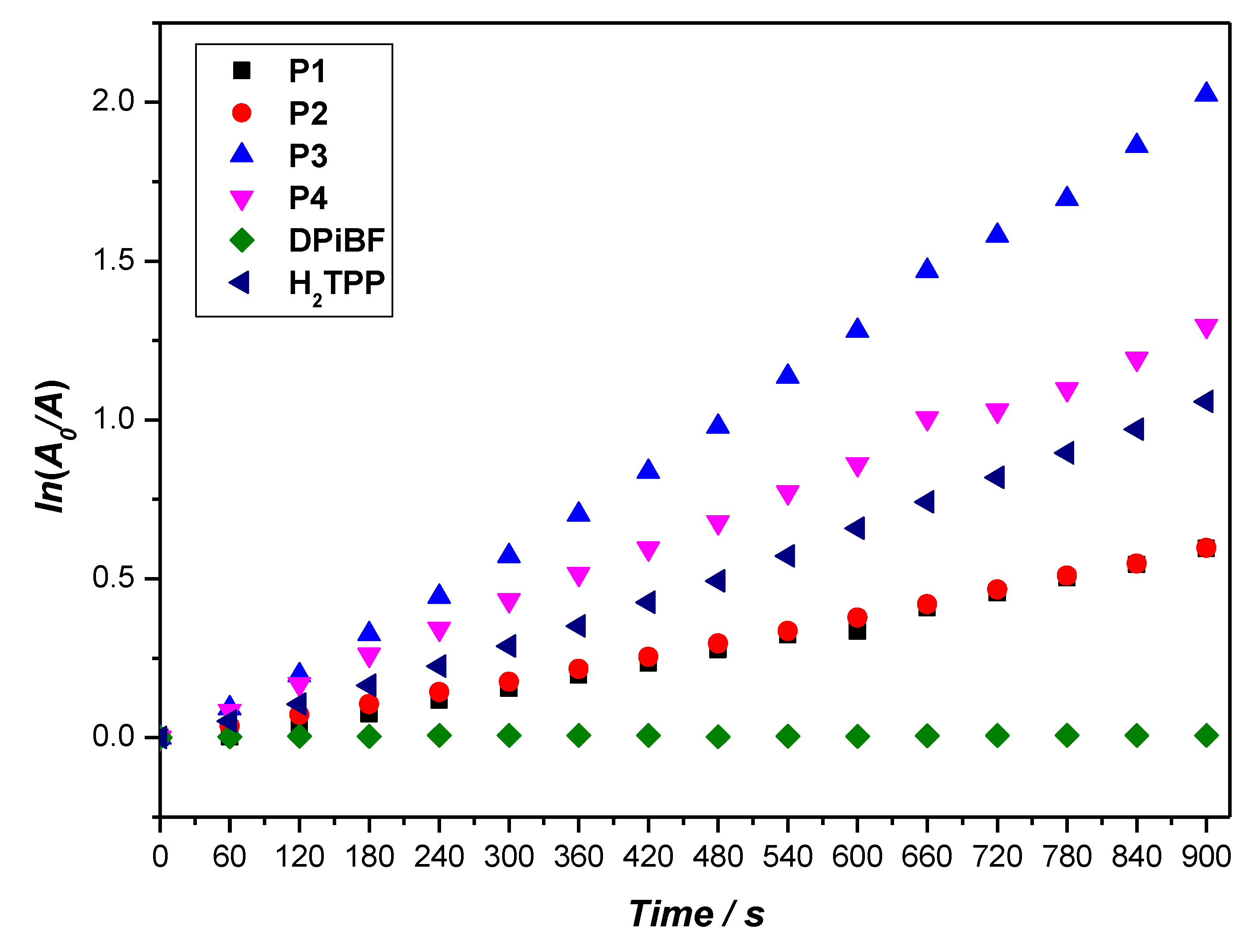
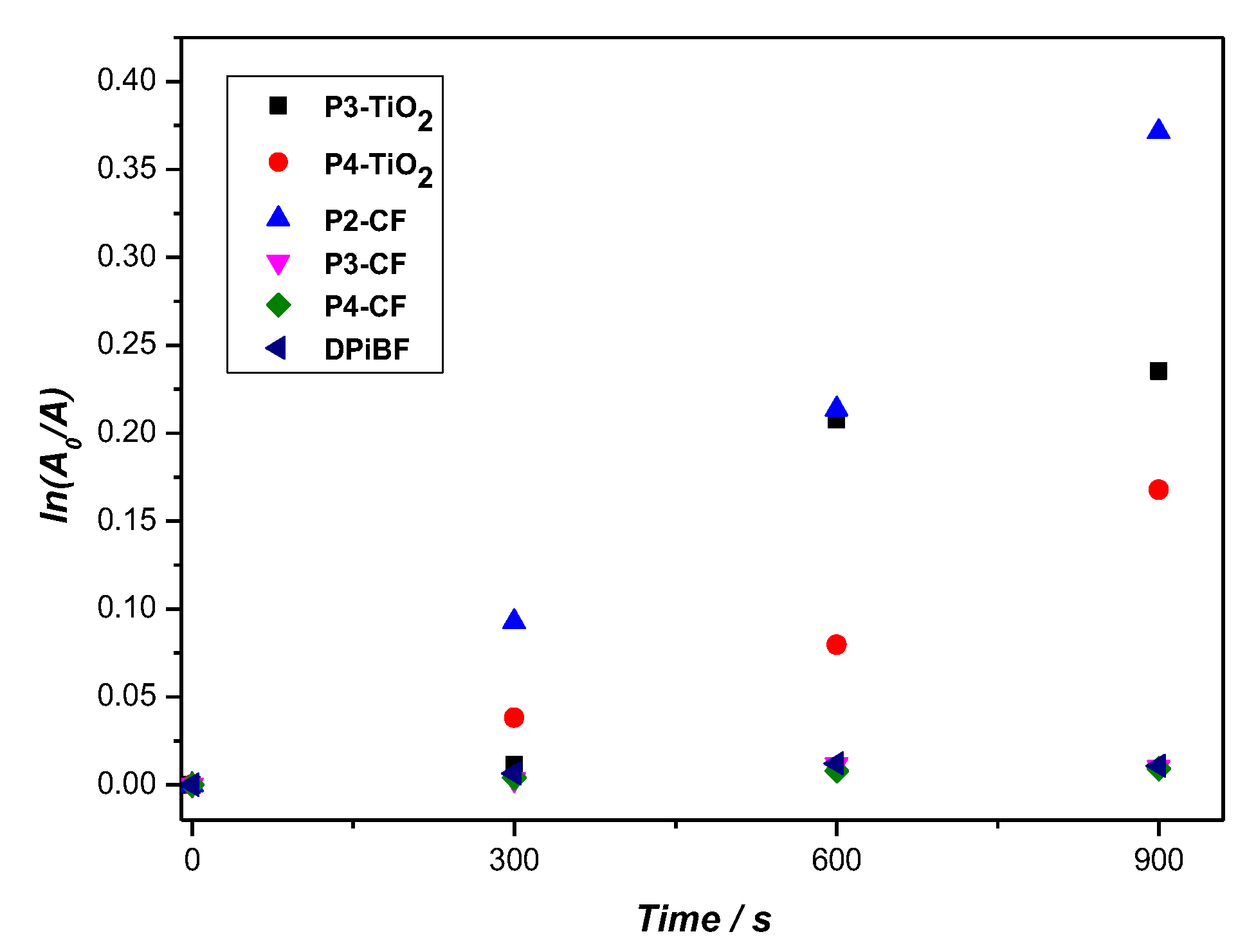

| Porphyrin | Soret Band λmax (nm) | Q Bands λmax (nm) | λemission (nm) a) | Stokes Shift (nm) | ΦF b) | |||
|---|---|---|---|---|---|---|---|---|
| P1 | 420 | 512 | 545 | 585 | 641 | 648/711 | 7 | 0.03 |
| P2 | 420 | 510 | 544 | 584 | 640 | 649/711 | 9 | 0.01 |
| P3 | 420 | 512 | 546 | 584 | 640 | 649/711 | 9 | 0.03 |
| P4 | 420 | 510 | 544 | 584 | 640 | 648/711 | 8 | 0.01 |
| Solid | Loading (mol·g−1) a) | w/w (%) b) | Immobilization (%) c) |
|---|---|---|---|
| P2-CF | 7.87 × 10−6 | 0.89 | 44% |
| P3-CF | 1.03 × 10−6 | 1.30 | 65% |
| P4-CF | 1.09 × 10−6 | 1.38 | 69% |
| Solid | Loading (Porphyrin Per Mass of Solid Prepared) (mol g−1) a) | w/wb) (%) | Immobilization c) (%) |
|---|---|---|---|
| P2-TiO2 | 0 d) | 0c | - |
| P3-TiO2 | 1.44 × 10−5 | 1.82 | 91% |
| P4-TiO2 | 1.55 × 10−5 | 1.96 | 96% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, K.A.D.F.; Moura, N.M.M.; Figueira, F.; Ferreira, R.I.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Silvestre, A.J.D.; Freire, C.S.R.; Tomé, J.P.C.; et al. New Materials Based on Cationic Porphyrins Conjugated to Chitosan or Titanium Dioxide: Synthesis, Characterization and Antimicrobial Efficacy. Int. J. Mol. Sci. 2019, 20, 2522. https://doi.org/10.3390/ijms20102522
Castro KADF, Moura NMM, Figueira F, Ferreira RI, Simões MMQ, Cavaleiro JAS, Faustino MAF, Silvestre AJD, Freire CSR, Tomé JPC, et al. New Materials Based on Cationic Porphyrins Conjugated to Chitosan or Titanium Dioxide: Synthesis, Characterization and Antimicrobial Efficacy. International Journal of Molecular Sciences. 2019; 20(10):2522. https://doi.org/10.3390/ijms20102522
Chicago/Turabian StyleCastro, Kelly A. D. F., Nuno M. M. Moura, Flávio Figueira, Rosalina I. Ferreira, Mário M. Q. Simões, José A. S. Cavaleiro, M. Amparo F. Faustino, Armando J. D. Silvestre, Carmen S. R. Freire, João P. C. Tomé, and et al. 2019. "New Materials Based on Cationic Porphyrins Conjugated to Chitosan or Titanium Dioxide: Synthesis, Characterization and Antimicrobial Efficacy" International Journal of Molecular Sciences 20, no. 10: 2522. https://doi.org/10.3390/ijms20102522
APA StyleCastro, K. A. D. F., Moura, N. M. M., Figueira, F., Ferreira, R. I., Simões, M. M. Q., Cavaleiro, J. A. S., Faustino, M. A. F., Silvestre, A. J. D., Freire, C. S. R., Tomé, J. P. C., Nakagaki, S., Almeida, A., & Neves, M. G. P. M. S. (2019). New Materials Based on Cationic Porphyrins Conjugated to Chitosan or Titanium Dioxide: Synthesis, Characterization and Antimicrobial Efficacy. International Journal of Molecular Sciences, 20(10), 2522. https://doi.org/10.3390/ijms20102522














